Abstract
There is little biological data available for diving birds because many live in hard-to-study, remote habitats. Only one species of diving bird, the black-footed penguin (Spheniscus demersus), has been studied in respect to auditory capabilities (Wever et al. 1969). We therefore measured in-air auditory threshold in ten species of diving birds, using the auditory brainstem response (ABR). The average audiogram obtained for each species followed the U-shape typical of birds and many other animals. All species tested shared a common region of greatest sensitivity, from 1000 to 3000 Hz, although audiograms differed significantly across species. Thresholds of all duck species tested were more similar to each other than to the two non-duck species tested. The red-throated loon (Gavia stellata) and northern gannet (Morus bassanus) exhibited the highest thresholds while the lowest thresholds belonged to the duck species, specifically the lesser scaup (Aythya affinis) and ruddy duck (Oxyura jamaicensis). Vocalization parameters were also measured for each species, and showed that with the exception of the common eider (Somateria mollisima), the peak frequency, i.e. frequency at the greatest intensity, of all species’ vocalizations measured here fell between 1000 and 3000 Hz, matching the bandwidth of the most sensitive hearing range.
Keywords: Diving birds, Auditory Brainstem Response, Hearing, Vocalization
Introduction
Hearing abilities have been measured in only approximately 50 of the 10,000 species of extant birds (Dooling 2000, 2002). Of these 50 species, only two are considered aquatic– the black-footed penguin (Spheniscus demersus; Wever et al. 1969), and the mallard duck (Anas platyrhynchos; Trainer 1946). These two species are from different taxonomic families, and vary in the habitat they occupy (penguins are exclusively marine and mallards are found throughout coastal and freshwater waterways), their aquatic lifestyle (penguins are adapted for swimming underwater and mallards live at the water’s surface), their social structure (penguins nest in dense colonies with males and females both incubating the eggs, while mallard nests are scattered throughout a range of environments and only females care for young), and foraging habits (penguins pursue live fish, while mallards eat grasses, seeds, and invertebrates). The divergence between these two species makes it difficult to determine if generalizations about aquatic bird hearing are possible, therefore, there is a fundamental need to extend our knowledge of hearing capabilities to other aquatic bird species to allow for potential phylogenetic, physiological, and life history comparisons.
Hearing in aquatic bird species may be impacted by general adaptations for living in an aquatic environment. Birds that dive more than a few meters may have adaptations to compensate for increasing water pressure on internal air spaces, such as the air-filled middle ear. Aquatic mammals may provide examples of how ear anatomy is adapted for diving. For example, the ear anatomy of the pinnipeds (seals, sea lions, and the walrus) has been adapted for diving in several ways. There is muscular control of the meatal opening to prevent water from entering the meatus, which is very narrow and waxy (Ramprashad et al. 1972; Kastalein et al. 1996; Welsch and Riedelsheimer 1997; Stenfors et al. 2000). In addition, the meatus and middle ear are lined with cavernous tissue, which is highly vascularized and fills with blood to compensate for increasing pressure on the tympanic membrane as the animal dives (Repenning 1972; Stenfors et al. 2000).
Although ear anatomy in aquatic birds is not well-investigated, some penguin species have similar adaptations for diving to those found in mammals, such as the cavernous tissue in the meatus and middle ear and active muscular control of the meatal opening (Sadè 2008). In addition, aquatic birds have specialized feather structure that creates a waterproof outer covering, including over the meatal opening (Rijke 1970). Any of these adaptations for diving could possibly impact auditory sensitivity in the air by changing tissue impedances and structural shape. For example, interlocking feathers over the meatus for waterproofing could impede sensitivity in the air by creating a sound barrier.
Aquatic bird families are scattered throughout the avian phylogeny and it is assumed that the aquatic lifestyle did not evolve from a common ancestor. Modern bird species are generally divided into Palaeognathae (tinamous and flightless ratites), Galloanseres (landfowl and waterfowl) and Neoaves (all other extant birds) (Jarvis et al. 2014). Of the species involved in this study (ducks, loons, and gannets), all are in different orders (anseriformes, gaviiformes, suliformes, respectively), with an ancient evolutionary split between the ducks and non-duck species (loons and gannets) (Jarvis et al. 2014). Diving abilities range greatly across aquatic bird families - with the diving ducks at the shallow end of the continuum (tens of meters) and the penguins at the other end (greater than 500 m) (Roberston and Savard 2002; Meir et al. 2008). Habitats for different species range from inland ponds and lakes to open ocean. Many aquatic bird species, especially marine-oriented seabirds (such as penguins, gannets, albatross, and auks) are colonial nesters, with thousands of nesting birds in one small area. Other aquatic bird species, such as the ducks or waterfowl, have low densities of nests scattered across a wide geographical area. These extensive differences in where birds live may have influenced sensory biology and it is important to examine auditory sensitivity across aquatic bird species that evolved separate adaptations to life on the water.
In addition to developing an understanding of aquatic bird hearing to compare to non-aquatic birds, such knowledge would also provide valuable information relevant to management issues, such as the introduction of man-made noise into flyways, critical stopover points during migration, or breeding areas. Aquatic birds are exposed to a variety of man-made noise sources, depending on their habitat. Species that occupy inland freshwater bodies, like some duck species, are exposed to noises typical in populated areas, such as traffic noise. Coastal birds are potentially impacted from sources such as recreational boating, commercial shipping, and coastal construction. Aquatic birds living farther from the coast could be most exposed to noise from commercial shipping and offshore energy development.
Increased noise levels in a bird’s habitat have the potential to cause a bird to alter its communication signals, mask communication signals or other biologically relevant sounds, cause avoidance of particular areas, decrease reproductive success, and increase physiological stress (Reijnen et al. 1996; Campo et al. 2005; Dooling and Popper 2007; Blickley et al. 2012; McClure et al. 2013; Naguib et al. 2013; Slabbekoorn 2013).
Given the lack of information available, it is important to conduct a comprehensive investigation into aquatic bird hearing. Electrophysiological and behavioral methods are commonly used in the laboratory to examine the avian auditory system. Behavioral audiograms generally produce thresholds that are more sensitive than those obtained using the ABR, but these studies require months of animal training, and work best with animals in captivity (Borg 1982; Borg and Engström 1983; Gorga et al. 1988; Brittan-Powell et al. 2002; Wolski et al. 2003; Yuen et al. 2005; Houser and Finneran 2006; Henry and Lucas 2008). Application of a time-efficient, minimally invasive technique such as the auditory brainstem response (ABR), can be a valuable physiological method to examine the auditory system in wild aquatic bird species. The ABR allows us to explore the auditory system more rapidly than behavioral techniques, in as little as one hour, and on wild-caught birds.
The ABR has been used as a tool for studying the functionality of the auditory system in a wide variety of animals, including several species of birds, such as budgerigars (Melopsittacus undulatus), screech owls (Megascops asio), barn owls (Tyto alba), several woodpecker species, and red-winged blackbirds (Agelaius phoeniceus) (Brittan-Powell et al. 2002, 2005; Koppl and Gleich 2007; Henry and Lucas 2010; Lohr et al. 2013). The ABR is a scalp-recorded potential resulting from synchronized neural discharge (population response), manifested as a series of four or more waves occurring within the first 10 ms following stimulation and representing the progressive propagation of auditory neural activity through the ascending auditory pathway (Katayama 1985; Hall 1992; Brittan-Powell et al. 2002).
The goal of this study was to evaluate the auditory abilities of a variety of aquatic birds to extend knowledge of bird hearing to aquatic species, and provide a baseline to facilitate future management actions concerning the introduction of noise into aquatic bird habitats. Objectives included: 1) comparing ABR sensitivity across various aquatic bird species, 2) evaluate the effects of different anesthetics on the ABR, and 3) investigate correlations between ABR sensitivity and vocalization characteristics for each species.
Materials and Methods
Subjects
This study included ten species of birds, with three to ten individuals tested per species, based on availability (Table 1). The majority of the species tested were seaducks and diving ducks. While seaducks do dive, they can be distinguished from diving ducks by their inclusion in a separate subfamily within Anatidae (the waterfowl: ducks, swans, and geese) that are essentially marine outside of the breeding season. Descriptive details for each of the species follow:
Table 1.
Common name, scientific name, number of individuals, whether they were captive or wild-caught, and the average mass for the specimens of each species used in this study.
| Common Name | Scientific Name |
Number | Captive or Wild- Caught |
Average Mass (g) |
|---|---|---|---|---|
| Black scoter |
Melanitta americana |
3 | Captive | 1040 |
| Common eider |
Somateria mollisima |
10 | Wild-Caught | 1955 |
|
Harlequin duck |
Histrionicus histrionicus |
7 | Captive | 613 |
| Lesser scaup | Aythya affinis | 6 | Captive | 900 |
|
Long-tailed duck |
Clangula hyemalis |
7 | Wild-Caught | 750 |
|
Northern gannet |
Morus bassanus |
7 | Wild-Caught | 3000 |
|
Red-throated loon |
Gavia stellata | 6 | Wild-Caught | 1850 |
| Ruddy duck |
Oxyura jamaicensis |
6 | Captive | 564 |
| Surf scoter | Melanitta | 9 | Wild-Caught | 975 |
|
White-winged scoter |
Melanitta fusca |
6 | Captive | 1370 |
Lesser scaup
(Aythya affinis) are a medium-sized diving duck that feeds primarily on mollusks, crustaceans, and aquatic insects. They are capable of diving to depths of at least 15–18 m, for 2–25 seconds at a time. Both males and females vocalize throughout the year to signal to mates and offspring. (Austin et al. 1998).
Long-tailed ducks
(Clangula hyemalis) are the deepest divers of all diving and seaducks, reaching at least 60 m of depth to search for crustaceans, fishes, and mollusks. Also long-tailed ducks may be the most vocal of the seaducks, having a distinctive and often incessant ow-owoolee male call. (Robertson and Savard 2002).
Surf scoters
(Melanitta perspicillata) are a seaduck species that are generally silent, but the male can make a gurgling call during courtship and the females a crow-like call when defending ducklings (Savard et al. 1998).
White-winged scoters
(Melanitta fusca) are the largest of all the scoters, and like the surf scoters, are not very vocal. Females protect the nest and ducklings with a whistle-like call (Brown and Fredrickson 1997).
Black scoters
(Melanitta americana) are the least studied of all scoters. They are the most vocal of all scoters, with the males frequently emitting a frequency-modulated melodious whistle (Bordage and Savard 2011).
Harlequin ducks
(Histrionicus histrionicus) are another seaduck species, often given the nickname of “sea mice,” because of their constant mouse-like squeak during courtship, agonistic interactions, and calls to ducklings (Robertson and Goudie 1999).
Ruddy ducks
(Oxyura jamaicensis) feed primarily on midge larvae and are distinct among all diving ducks, because of their unique courtship behavior, which consists of slapping their bill on their chest and producing a “belching” sound. These ducks are generally silent, except for a high-pitched peep and the courtship sound (Brua 2002).
Common eiders
(Somateria mollisima) are the largest duck found in the northern hemisphere and can weigh up to 3040 g. Their calls are hoarse, grating and cooing sounds (Goudie et al. 2000).
Red-throated loons
(Gavia stellata) pursue live fish underwater, including herring (Clupeidae), capelin (Mallotus villosus) and sculpin (superfamily Cottoidea). They do not “yodel” like other loon species, but instead use their “plesiosaur call” as a territorial duet (Barr et al. 2000).
Northern gannets
(Morus bassanus) are the largest indigenous seabirds in the North Atlantic, belonging to the family Sulidae (boobies and gannets). They catch live fish, mostly mackerel (Scombridae) and herring, through plunge diving, during which the bird starts from a height of 10–40m above the water and plunges into the water with speeds >100 km/hr. They breed in dense, noisy colonies on cliffs or islands (Mowbray 2002).
Subjects were all adult birds of both sexes, as determined by either captive history or plumage patterns. Captive subjects were raised from eggs at U.S. Geological Survey Patuxent Wildlife Research Center in Laurel, Maryland. Wild subjects were caught as part of on-going satellite telemetry studies (Beuth 2013; Bureau of Ocean Energy and Management 2013; Sea Duck Joint Venture 2012) from areas along the mid-Atlantic and New England coastline, transported to a veterinary hospital for testing, banding, transmitter attachment, and then released. ABR testing occurred before transmitter attachment surgery.
Experimental Procedures
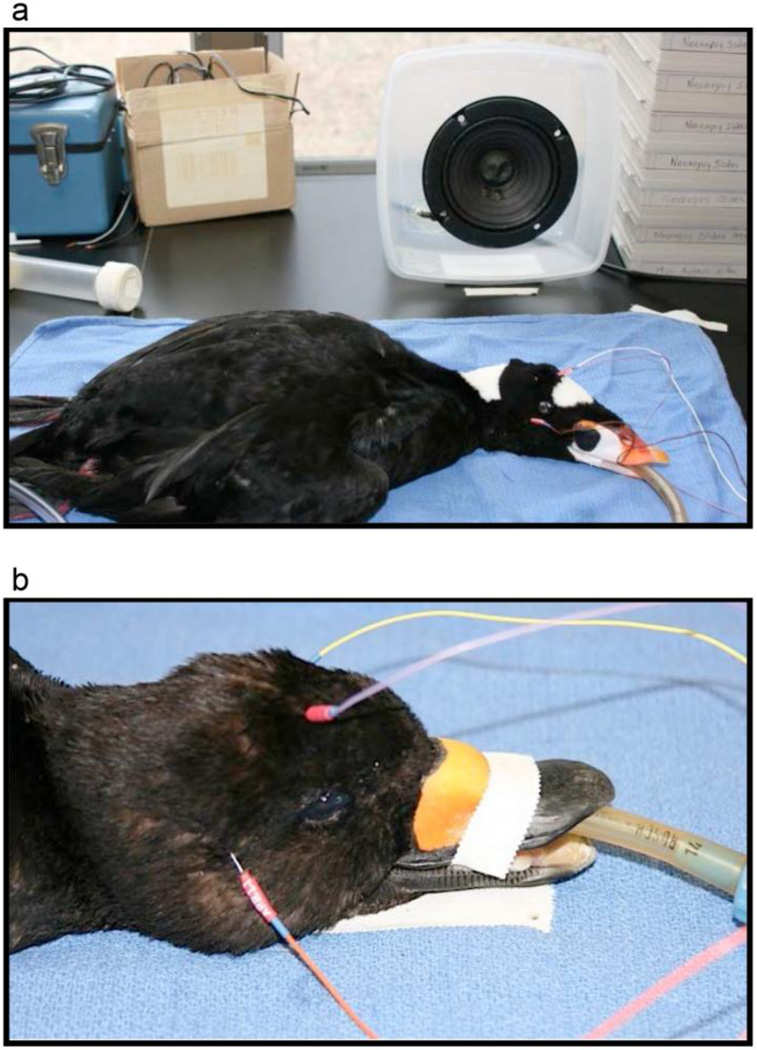
All subjects, whether wild-captured or captive, were tested using the same procedures and equipment, in a veterinary hospital. Birds were sedated with isoflurane (5% for induction, 2–4% for maintenance with oxygen at 1L/min/kg; the lowest possible percentage of isoflurane was used to prevent movement in the bird) prior to electrode placement. A mask was used to induce isoflurane anesthesia, and the bird was intubated once motionless. Electrodes were placed once the bird was motionless for several minutes. Body temperature was monitored with a Cooper-Atkins Electro-Therm thermistor probe (Model TM99A; Middlefield, CT), and remained between 38–40°C. The bird was positioned, on a table, so that the speaker (Pioneer B11EC80-02F 5-1/4”; Longbeach, CA; frequency response 320 – 6000 Hz) was 20 cm from the bird’s right ear (Fig. 1).
Fig. 1.
a: An intubated surf scoter prior to undergoing an ABR test, showing electrode and speaker placement. During testing, foam sheets were used to cover hard surfaces around the setup. b: Electrode placement on an intubated black scoter. Three electrodes were placed subdermally high on the bird’s forehead (active), directly behind the right ear canal (the ear ipsilateral to the speaker, reference), and behind the canal of the ear contralateral to stimulation (ground).
Stimuli
Subjects were presented with stimuli made up of tone bursts of 5 ms duration (1 ms cos2 rise / fall time and 3 ms steady-state) and 20 ms interstimulus intervals, for comparison with data from Brittan-Powell et al. (2002, 2005). Tone burst frequencies ranged from 500 – 5700 Hz and pressures from 30–90 dB re 20 µPa. Each stimulus set was composed of a train of nine single frequency tone bursts that increased successively in pressure and were presented at a rate of 4/s (see Brittan-Powell et al., 2002; 2005; 2010). Stimuli within each train increased in 5-dB steps from 35–55 dB, then in 10-dB steps from 60–90 dB. Sound frequencies were presented in random order. In addition to the tone bursts, click stimuli were presented to the bird at a constant pressure (80 dB re 20 µPa peak SPL) and with a repetition rate of 20/s, at the beginning of data collection and at the end to determine if ABR amplitude and latency changed over the course of the trial (due to anesthesia, physiological state, etc.). This additional click test was conducted only on those birds that were not going into surgery for satellite transmitter implantation after the ABR (n=28; all captive birds listed in table 1) in order to minimize the time that these birds were anesthetized.
Recording Equipment and Procedure
Three standard platinum alloy needle electrodes (Grass F-E2; West Warwick, RI) were placed subdermally high on the bird’s forehead (active), directly behind the right ear canal (the ear ipsilateral to the speaker, reference), and behind the canal of the ear contralateral to stimulation (ground), (Fig. 1) as in Brittan-Powell et al. (2002, 2005, 2010). Shielded electrode leads were twisted together to reduce electrical noise through common-mode rejection. To minimize reflections from hard surfaces near the bird, foam was placed around the setup.
The stimulus presentation and ABR acquisition were synchronized using a Tucker-Davis Technologies (TDT; Gainesville, FL, USA) mobile real-time processor (RM2) controlled by a Gateway PC (Irvine, CA). Sound stimulus waveforms were generated using OpenABR software (developed by Dr. Edward Smith, University of Maryland) and fed to the RM2 for D/A conversion, and then through an amplifier (Pyle PLMRMP1A; Brooklyn, NY) to drive the speaker. The electrodes were connected to a TDT RA4LI headstage and RA4PA Medusa preamplifier that amplified at 20X gain and digitized the signal before sending it over fiber optic cables to the TDT RM2, after which they were analyzed using OpenABR.
Each ABR represents the average response of 600 stimulus train presentations (alternating polarity/phase to cancel the cochlear microphonic), sampled at 20 kHz for 235 ms following onset of the stimulus. This allowed for 25 ms recording time for each stimulus. The biological signal was amplified and notch filtered at 60 Hz with the OpenABR software. The signal was bandpass filtered between 30 Hz and 3,000 Hz after collection using ABRomatic software (also developed by Dr. Edward Smith, University of Maryland).
Stimulus pressures were calibrated in the free field by placing a ¼” microphone (Earthworks M30-Calibrated; Milford, NH) at the approximate position of the animal’s ear (20 cm from the speaker). The microphone was connected to an iPad in an Alesis IO Dock (Cumberland, RI) running Signal Scope Pro software SPL module (Faber Acoustical; Santaquin, UT) that displayed the sound pressure level of calibration tones. The microphone and Signal Scope software were calibrated prior to each testing session by playing a known SPL tone through the system with a CEM SC-05 calibrator (Shenzhen, China). Because the microphone was a free-field microphone, a correction factor (provided with the calibrated microphone’s documentation) was entered into the Signal Scope software during this closed-field calibration. Calibration through the OpenABR software consisted of playbacks of one-second tones which were then measured using the fast-weighting flat setting in Signal Scope, and the dB levels entered into OpenABR for adjustment.
At the end of the experiment, the electrodes were removed. Birds remained isolated in a crate and monitored until they showed normal alertness (head held upright, eyes remaining open, normal preening behavior; usually 1–2 hours). Birds were then returned to the captive flock or released at the capture location. Captive birds were checked throughout the next day (identified by unique leg bands) to ensure good health and recovery. The health of the wild birds was monitored using a body temperature sensor incorporated into the implanted telemetry device. No morbidity or mortality was found associated with the ABR testing.
Statistical analyses were performed using GraphPad Prism statistical software (GraphPad Software, Inc., La Jolla, 2013). All statistical tests were considered significant at the 5% level.
Latency and Amplitude
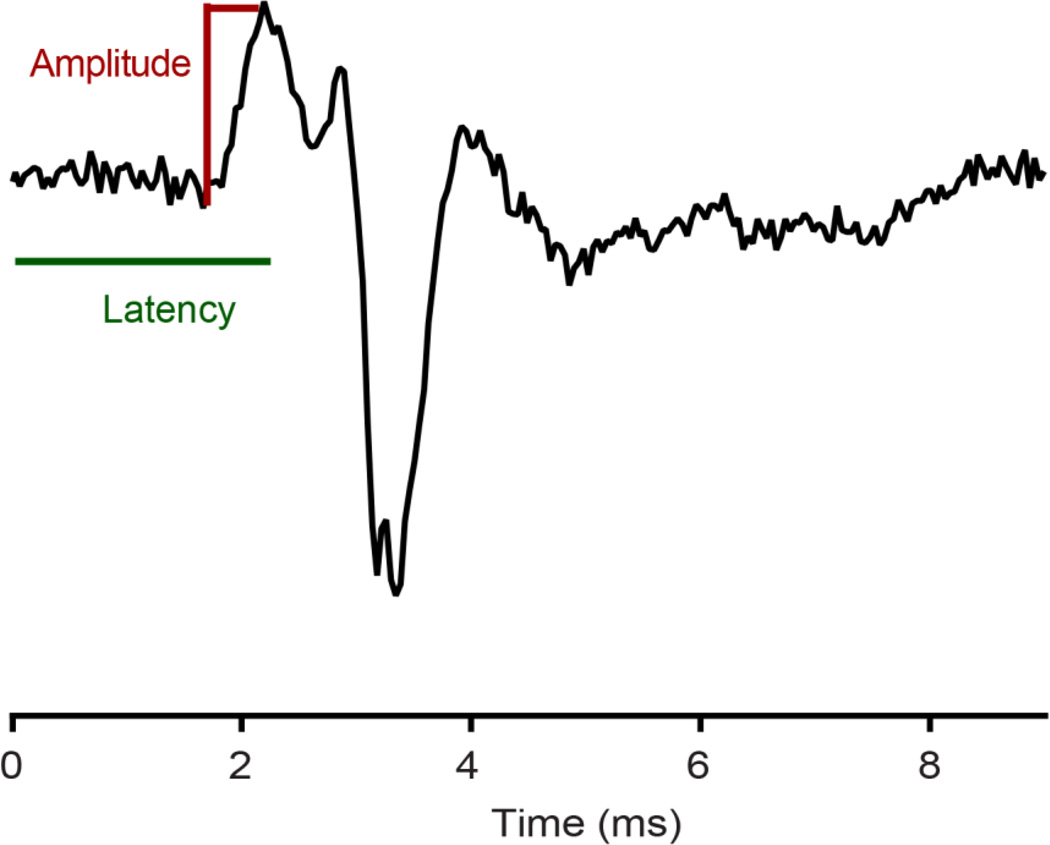
The amplitude and latency of the first peak of the ABR was measured for all stimulus frequencies and pressures tested (Fig. 2). The latency was corrected for the acoustic delay between the speaker and the bird’s ear (0.59 ms). The amplitude of the first peak was determined by averaging the section of the waveform before the response began (0–1.5 ms after the stimulus was played) and subtracting this average from the peak (peak to baseline measurement). Brittan-Powell et al. (2002) showed that the first negative deflection of the compound action potential (CAP) corresponded well to the first positive deflection of the ABR waveform, and so may represent the auditory nerve component of the ABR, while selective block of the neural responses in barn owls confirmed the CAP was of neural origin (Koppl and Gleich, 2007).
Fig. 2.
Amplitude and latency measurements on a lesser scaup ABR. Amplitude was measured as peak to baseline. Latency was corrected for the delay between the speaker and the bird’s ear. Positive peaks were above baseline.
Threshold Estimation
Threshold was defined using two methods: visual detection and linear regression. In the visual detection technique, the first 10ms of all ABR waveforms was examined visually by observers (who had no prior experience analyzing ABR data) for a response. These observers were trained to identify threshold as the level one half step below the lowest stimulus level at which a response could be visually detected on the trace (as in Brittan-Powell and Dooling 2004; Brittan-Powell et al. 2005, 2010; Lohr et al. 2013). To test if the observers varied in their analysis, 40 files were chosen at random and analyzed by both observers. A paired t-test was conducted to look for significant differences in thresholds across observers.
Thresholds were also estimated using linear regression analysis on lesser scaup data. The amplitude of the first positive peak was obtained across all frequency and stimulus levels and an amplitude-pressure function was generated. Threshold was defined as the 0 µV crossing of a line produced with linear regression. Techniques for estimating thresholds (visual detection vs. linear regression) within one species (six female lesser scaup) were evaluated, using repeated measures ANOVA.
To compare measures across species and frequencies, repeated-measures two-way analysis of variances (ANOVAs) were conducted. Differences between sexes were not tested due to limited power to detect differences from small sample sizes within sexes for each species.
Anesthesia Comparisons
Isoflurane was chosen as the anesthetic for these experiments because of its reputation of reliability and safety in waterfowl (Machin 2004; Carpenter 2013). Experiments on four additional lesser scaup were conducted to compare ABR results between two types of anesthesia: Isoflurane vs. a combination of ketamine and midazolam (Machin and Caulkett 1998; Carpenter 2013). Each duck received both treatments, with order of anesthetic determined by a randomized schedule and with a minimum of two weeks between treatments for a washout period (time for the anesthetic to be eliminated from the animal’s system). All equipment and stimulus procedures were as previously described except for ketamine/midazolam delivery, which required a single intramuscular injection of ketamine (40 mg/kg) and midazolam (2 mg/kg) to produce a sufficient level of anesthesia similar to the isoflurane for a period (usually 20–30 min) long enough to complete the ABR trial.
Vocalization Analysis
Vocalizations from eight of the ten species were obtained from Cornell University’s Macaulay Library collection. It was not possible to obtain vocalizations from surf scoters or white-winged scoters because neither is very vocal. Spectrographic analyses of peak frequency (the frequency of the greatest relative power) were performed on 10 individual calls of each species using Raven Lite 1.0 (Cornell Lab of Ornithology; Ithaca, New York). These measurements were conducted using a cursor on the spectrogram (by placing the cursor at the point of brightest color on the spectrogram, signifying the greatest relative power). These values were then compared to the most sensitive hearing frequency (i.e. the frequency with the lowest threshold), which was determined by using the audiogram points for each species to calculate a best-fit third-order polynomial in 100-Hz frequency steps for the range of frequencies tested (as in Gleich et al. 2005).
Results
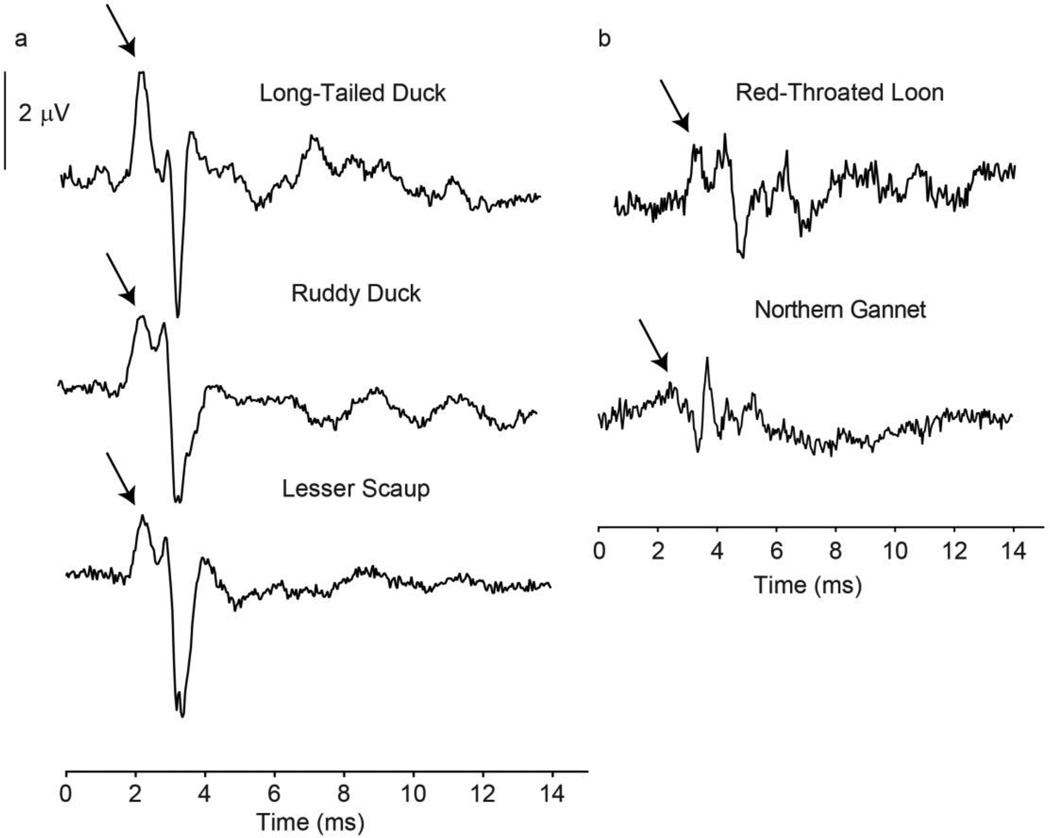
All species tested showed prominent ABR peaks within 4–5 ms after the stimulus reached the bird’s ear canal. Waveform morphology was very similar across all eight duck species tested (Fig. 3a). The two non-duck species (northern gannet and red-throated loon) also had similar peak patterns and smaller amplitudes (Fig. 3b). These peak patterns were stable across frequencies and pressure levels. As the level of stimulation increased, ABR amplitudes increased and peak latencies decreased (Fig. 4).
Fig. 3.
ABR waveform morphology examples from three duck species (a) and two non-duck seabirds (b). All waveforms were responses to 90 dB tone pips at 2860 Hz, the frequency at which the highest amplitude responses were recorded for most species. The stimulus was presented at time=0. Arrows point to first positive peak, which was used for amplitude and latency measurements.
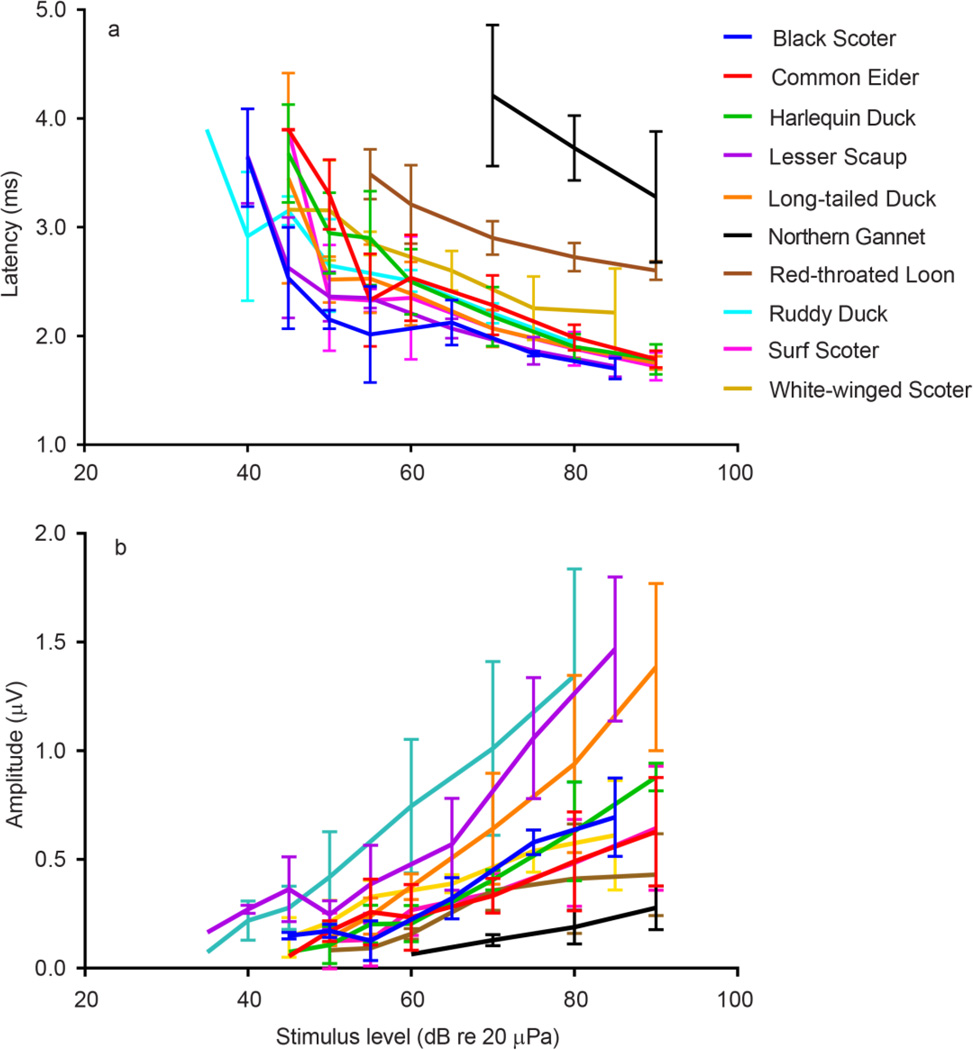
Fig. 4.
The latency (a) and amplitude (b) of the first peak as a function of increasing stimulus level at 2860 Hz, the frequency at which the highest amplitude responses were recorded for most species. Latencies are corrected for the delay from the speaker to the bird’s ear canal. Vertical bars represent +/− one standard deviation.
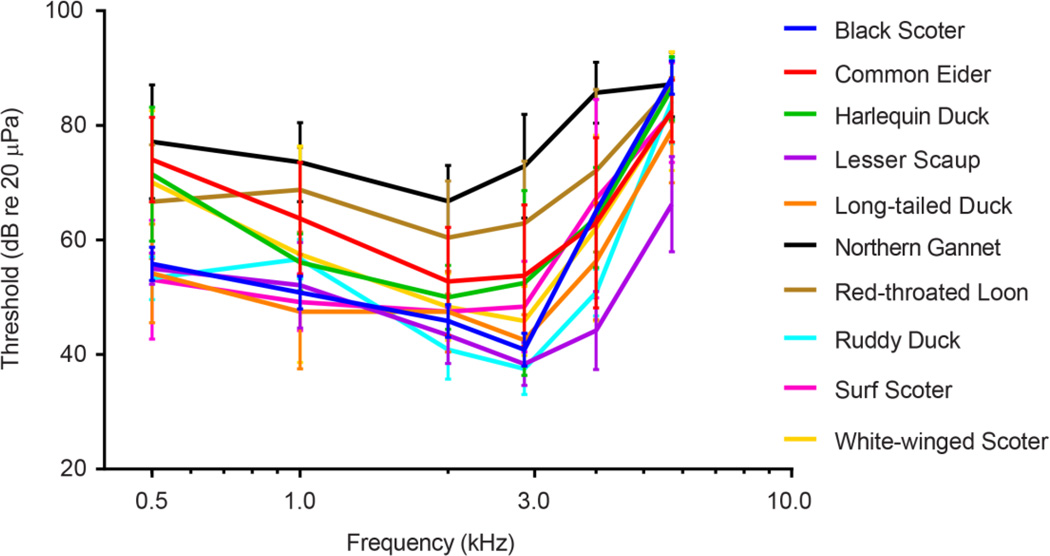
The measured audiograms for all birds were U-shaped (Fig. 5). Sensitivity peaked between 1000–3000 Hz, with a steep high-frequency roll-off after 4000 Hz. A two-way repeated measures ANOVA found significant effects of frequency (F(5;225) = 114.4, p < 0.0001), species (F(7,45) = 7.281, p <0.0001) and frequency by species interaction (F(35,225) = 2.165, P = 0.0004). There were differences in average audiograms across species (Fig. 5) with an apparent segregation occurring between waterfowl species and non-waterfowl species. The highest thresholds were found in the northern gannet and red-throated loon and the lowest to the lesser scaup and ruddy duck (Fig. 6). At the lower frequencies the harlequin duck, common eider, and white-winged scoter exhibited similar thresholds as the two non-waterfowl species, the red-throated loon and northern gannet. However, as the frequencies increased, all three species diverged away from the non-waterfowl species and resembled more closely the other waterfowl species thresholds. Within the waterfowl species, the common eider showed the highest thresholds across all frequencies closely followed by the harlequin duck and the white-winged scoter. At the highest frequency, all species except the lesser scaup converged to a similar threshold around 80 dB re 20 µPa.
Fig. 5.
Average ABR audiograms from all species tested. Vertical bars represent +/− one standard deviation.
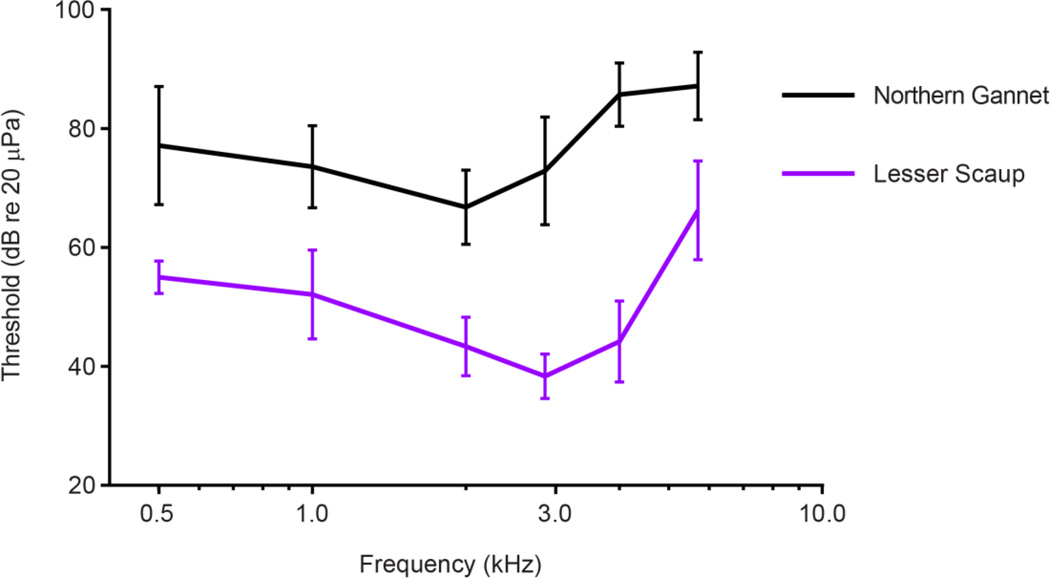
Fig. 6.
Average ABR audiograms from the species with the highest average thresholds (northern gannet) and the lowest average thresholds (lesser scaup). Vertical bars represent +/− one standard deviation.
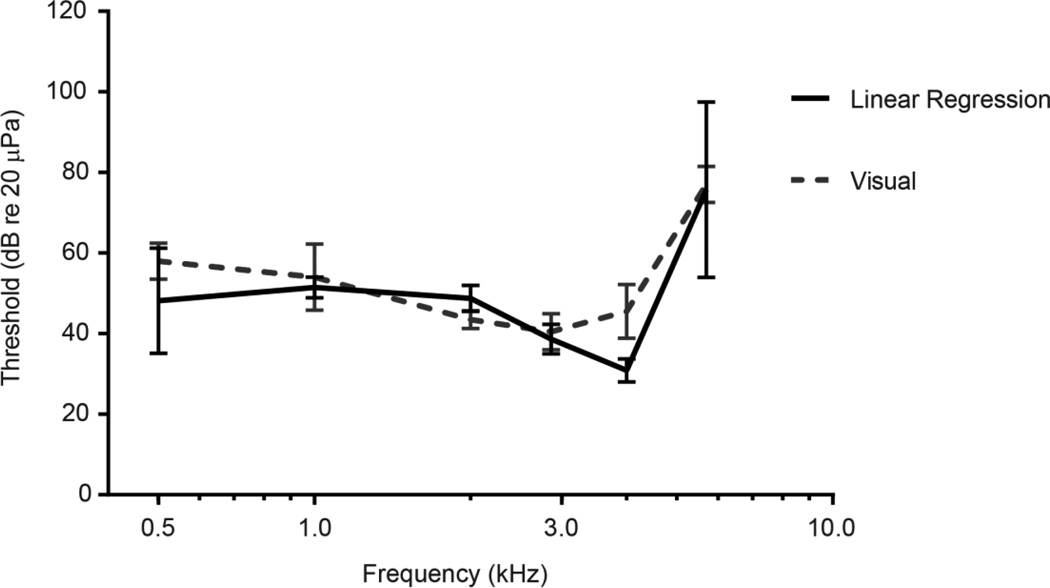
Threshold estimates for six female lesser scaup did not differ between the visual inspection method and the linear regression method across frequencies (F(1,8) = 2.524, p = 0.15; Fig. 7). Thresholds also did not differ significantly between visual observers (t=1.38, df = 39, p = 0.18). The amplitude and latency of clicks from the 28 captive birds tested did not differ from the beginning to the end of a testing session (amplitude: t = 0.4786, df = 27, p = 0.6361; latency: t = 1.980, df = 27, p = 0.0616).
Fig. 7.
Audiograms derived from the two methods of analysis: the visual inspection method, and the linear regression method. Vertical bars represent +/− one standard deviation.
Anesthesia Analysis
ABR thresholds were compared across two types of anesthesia: the inhalant isoflurane and injectable ketamine/midazolam combination. Four individual lesser scaup were given each treatment (with a two-week washout period between) and their ABRs were measured for each treatment. The anesthesia type did not have a significant effect on thresholds across frequencies (F(1,6) = 2.02, p =0.1975). However, the ketamine/midazolam recovery time was much longer (approximately four hours) than that for isoflurane (less than one hour).
Vocalization Analysis
Ten individual calls from Cornell University’s Macaulay Library were measured for eight species (Table 2). Samples from two call types for the red-throated loon (the “quark” and the “cry”) and from both male and female lesser scaup were analyzed. All other listed species are male calls. All species had average peak frequencies between 1000 and 3000 Hz, with the exception of the common eider at 443 Hz (Table 2). Maximum frequency ranged from 1053 Hz (common eider) to 18865 Hz (northern gannet).
Table 2.
Average peak frequency (frequency at greatest relative power) of vocalizations and best hearing frequency for each species.
| Species | Peak Frequency (Hz) | Best Hearing Frequency (Hz) |
|---|---|---|
| Common eider | 443 | 2400 |
| Red-throated loon Quark | 1528 | 1900 |
| Red-throated loon Cry | 1983 | 1900 |
| Black scoter | 1714 | 1900 |
| Long-tailed duck | 1723 | 2100 |
| Lesser scaup Male | 1779 | 3000 |
| Lesser scaup Female | 2736 | 3000 |
| Northern gannet | 2173 | 1700 |
| Harlequin duck | 2346 | 2100 |
Discussion
ABR waveforms were very similar across duck species tested. These duck ABR waveforms were similar to those exhibited by other birds tested with the ABR technique, such as budgerigars, screech owls, Carolina chickadees (Poecile carolinensis), red-winged blackbirds and brown-headed cowbirds (Molothrus ater) (Brittan-Powell et al. 2002, 2005; Henry and Lucas 2010; Gall et al. 2011). The pattern of evoked peaks differed in the two non-duck species (red-throated loons and northern gannets), but still exhibited at least two prominent peaks within 5 ms of stimulus onset.
Other measured characteristics of the ABR responses of all birds measured here also resembled those of other birds and mammals. Response latency of the first peak increased and the amplitude decreased with decreasing stimulus level, typical of other ABR studies (gerbil, Meriones unguiculatus, Burkard and Voigt 1989; budgerigars, Brittan-Powell et al. 2002; screech owls, Brittan-Powell et al. 2005; Belgian waterslager canaries, Serinus canaria domestica, Brittan-Powell et al. 2010; Lohr et al. 2013).
ABR audiograms obtained for each species tested here conformed to the U-shape typical of birds and many other animals. This U-shape is consistent across many species regardless of method used to examine thresholds (electrophysiological or behavioral). However, ABR thresholds are often higher than behavioral thresholds, especially in avian species, due to a variety of factors including stimulus characteristics and measurement techniques used for each method (Borg 1982; Borg and Engström, 1983; Gorga et al. 1988; Brittan-Powell et al. 2002, 2005; Wolski et al. 2003; Yuen et al. 2005; Houser & Finneran 2006; Henry and Lucas 2008; Woolley and Rubel 1999).
All species tested shared a common region of greatest sensitivity, from 1000 to 3000 Hz, although the audiograms differed significantly across species and frequencies. The significant impact of frequency across the audiogram was expected because of its U-shaped dependence, but the species differences were not as easily explained. Species differences in hearing thresholds could be impacted by many factors, such as anatomical differences associated with phylogenetic history and/or specific adaptations in skull shape. The thresholds of all duck species tested were more similar to each other than to the two non-duck species tested. The red-throated loon and northern gannet exhibited the highest thresholds, while the lowest thresholds belonged to the ducks, specifically the lesser scaup and ruddy duck.
The northern gannet is the only species of plunge-diver tested here, and has unique adaptations to compensate for hitting the water at speeds up to 100 mph. CT scans done in conjunction with this project at Woods Hole Oceanographic Institution showed extra air spaces in the gannet head and neck to cushion its impact, and these air spaces could affect the ability to observe already small responses from the auditory brainstem (Ketten and Crowell, unpublished data). In these CT scans, the tympanic membrane of the gannet was thicker than the tympanic membrane of the swan, a similar sized bird (Ketten and Crowell, unpublished data). This thickening could be a protective mechanism for plunging and could potentially explain the higher thresholds in gannets that were measured in this study.
Gannets may also have the ability to close off their auditory meatus to further streamline the body and avoid the introduction of water into its ear. Induction of anesthesia, especially when using a mask, can cause diving birds to go into a dive response, when their respiratory rate and heart rate decrease (Machin 2004). Any other adaptations to diving, including the closing of the meatus to the external environment, could also occur and affect the ABR. Masks were, however, only used to induce the isoflurane anesthesia. Once a sufficient level of anesthesia was reached, all test birds were intubated and isoflurane delivered through the endotracheal tube. Thus, it is unlikely that diving responses were triggered or present during the actual ABR testing.
The region of peak energy in animal vocalizations is often correlated with the frequency of best sensitivity and/or the bandwidth of the best hearing range (e.g. bats – Long and Schnitzler 1975; Neuweiler et al. 1980; birds – Konishi 1970; Dooling et al. 1971; Dooling and Saunders 1975; Dooling et al. 2000; elephants – Heffner and Heffner 1982; Payne et al. 1986; frogs – Megela-Simmons et al. 1985). Vocalizations of most of the species tested range from frequency-modulated whistles (black scoters), to purrs and whee-oos (lesser scaup), to loud yodel-like calls (long-tailed duck), to constant chirps (harlequin ducks), to wails (red-throated loons), and to generally silent (white-winged scoter) (Brown and Fredrickson 1997; Austin et al. 1998; Savard et al. 1998; Robertson and Goudie 1999; Barr et al. 2000; Goudie et al. 2000; Brua 2002; Robertson and Savard 2002; Bordage and Savard 2011). With the exception of the common eider, the peak frequency (frequency at the greatest intensity) of all species’ vocalizations measured here fell between 1000 and 3000 Hz, matching the bandwidth of the most sensitive hearing range. There are some exceptions; the peak frequency of the common eider vocalization (443 Hz) did not match the calculated best hearing sensitivity (2400 Hz). Konishi (1970) notes that the bird’s ear is not narrowly tuned to the species song, and that the most dominant vocal frequencies are located above the most sensitive range of hearing. He proposed that birds may be choosing the frequency range in which they can obtain the best signal/noise ratio.
Common eiders and northern gannets are the only colonial nesting species tested in this study. Northern gannets have only six colonies in North America, with the largest, on Bonaventure Island, Quebec, containing more than 73,000 individuals (G. Chapdelaine unpubl; Mowbray 2002). Common eiders nest in densities of up to 100–400 nests/ha (Chapdelaine et al. 1986). In addition, common eiders frequently form dense flocks of up to tens-of-thousands of individuals in the non-breeding season, in response to clumped food resources and possibly heat conservation (Guillemette et al. 1993). Dense, noisy aggregations may preclude the usefulness of long-distance vocalizations, and instead favor short-range, more complex auditory cues used for individual recognition amongst thousands of individuals (such as in colonial penguin and auk species – Beecher 1981; Jouventin 1982; Jones et al. 1987; Aubin et al. 2000; Lengagne et al. 2000). Like these other colonial seabirds, gannet vocalizations have individually distinctive amplitude envelopes and birds respond preferentially to playbacks of their mate’s vocalizations (Nelson 1978; Mowbray 2002). The comparatively poor hearing sensitivity of the northern gannet (least sensitive of all species tested) and common eider (least sensitive of all the ducks) may, therefore, reflect the extent that these species communicate across only short distances in a crowded colony.
The most sensitive hearing of all species tested belonged to the lesser scaup and ruddy duck. In the case of these species, ambient noise levels in the environment may have shaped hearing sensitivity. Of all species tested, ruddy ducks and lesser scaup spend the most time on inland, freshwater environments (Austin et al. 1998; Brua 2002). Ambient noise levels in stagnant freshwater habitats tend to be consistently lower than in coastal and marine habitats, which are dominated by wind and wave action (Wenz 1962; Bom 1969; Urick 1983; Nystuen 1986; McConnell et al. 1992; Greene 1995; Amoser and Ladich 2005). There is some evidence that exceptional hearing sensitivity in fishes, such as in the otophysines (carps and minnows, catfishes, characins, knifefishes), may have evolved in quiet freshwater or deep sea habitats (Popper 1980; Deng et al. 2002, Ladich and Bass 2003; Amoser and Ladich 2005). It is possible that sensitive hearing in the ruddy duck and lesser scaup is associated with quiet freshwater habitats, as opposed to the wind- and wave-swept environments occupied by the other birds studied.
Because all birds were tested using the same procedures, species differences in hearing sensitivity were not a result of procedural differences. However, it is possible that species or individuals could vary in their response to the anesthesia. Isoflurane, which was used for all birds in this study, has been shown to elevate thresholds compared to a ketamine/xylazine combination in rats and mice, with a reduction in sensitivity over the duration of anesthesia (Cederholm et al. 2012; Ruebhausen et al. 2012). Deep anesthesia with ketamine, nembutal and halothane reduced or eliminated both cochlear action potentials and distortion products in starlings and chickens (Kettembeil et al. 1995). Prolonged isoflurane anesthesia (8 hours) was also associated with elevated auditory thresholds in the American alligator (Carr et al. 2009).
Isoflurane was chosen, because of its history of effectiveness and safety in waterfowl (Machin 2004; Carpenter 2013). While efforts were made to keep the anesthesia level and duration consistent across species (the lowest level possible that would maintain the bird immobile), it is possible that each species could react differently to the anesthesia, or that the waterfowl species could react differently from the non-waterfowl species. In order to determine if thresholds were elevated by the choice of isoflurane, we carried out additional testing on anesthesia type (isoflurane vs. a ketamine/midazolam combination) on a subset of lesser scaup. This comparison of the effects of anesthetic on the ABR in birds demonstrated that at least within one species, thresholds did not differ depending on anesthesia type. However, recovery time for the ketamine/midazolam combination was much longer than for isoflurane, illustrating isoflurane’s utility for wild birds that have to be released within a short amount of time. Confidence intervals were evaluated to detect potential loss of power due to the small available sample size of birds. This anesthesia analysis involved a sample size of four birds, and 95% confidence intervals were +/− 13.19 dB of the mean difference. The variation in this test suggests that our results could be influenced by the small sample size and it would be beneficial to add individuals to this sample size if more birds become available.
In addition, the season in which the birds were tested, along with the resulting levels of sex hormones, could affect hearing thresholds or anesthesia requirements. Caras et al. (2010) simulated natural breeding or non-breeding conditions by manipulating hormone levels and photoperiod in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii), and observed shifts in auditory thresholds, but no significant differences between males and females. During simulated breeding conditions, ABR thresholds were elevated and peak latencies were prolonged compared to non-breeding conditions. While each species was tested within one season (usually within two weeks), the seasons across testing all of the species varied, providing another possible complication for interpreting species differences. In addition, a study on peripheral auditory processing in the bobtail lizards (Tiliqua rugosa) showed that the anesthesia requirements of the animals changed seasonally (Köppl et al. 1990).
The aquatic bird audiograms obtained, as estimated by the ABR, shared many similarities with other birds tested previously, and also showed considerable variation across species tested. Because there is so little known about the biology and behavior of these species, it would be useful to explore anatomical, behavioral, and evolutionary correlations with these species differences in hearing. Future directions should further investigate the characteristics and use of vocalizations as well as ear anatomy differences across species.
These data should be used in conjunction with behavioral and physiological investigations into how anthropogenic noise sources impact aquatic bird populations, to inform future management decisions. Colonial nesting species, such as the northern gannet and common eider, are particularly susceptible to human disturbance. When a population is concentrated in several small areas (instead of distributed across a wide range), the population is more susceptible to catastrophic damage as a result of human intrusion at these limited locations (Carney and Sydeman 1999; Sladen and Leresche 1970; Wilson et al. 1991). However, it is possible that the northern gannet and common eider have some amount of added protection against noise disturbance because of their less sensitive hearing, as demonstrated by this study, and are more severely impacted by other types of disturbance, such as the visual presence of visitors or aircraft.
It is, therefore, likely that the species with the most sensitive hearing, the lesser scaup and ruddy duck, are potentially the most impacted by the introduction of noise into their habitats. Because of their abundance throughout coastal and inland waterways, these two species also spend the most time in populated areas and are most susceptible to human noise sources, such as boat or road traffic and construction. In addition, although abundance of lesser scaup is still high, their numbers have been declining in recent years for unknown reasons (Austin et al. 1998). Future studies on impacts of noise on aquatic birds should focus on these species that may be most susceptible.
Acknowledgements
The Animal Care and Use Committees at both the University of Maryland and the U.S. Geological Survey Patuxent Wildlife Research Center (where the birds were housed and tested) approved all of the following procedures. We thank Edward Smith for the use of his software, Elizabeth Brittan-Powell for her training on ABR procedures, and Arthur Popper for his editing. Funding for this project was provided by several sources: National Institutes of Health (NIH) DC00436 to Catherine Carr, NIH P30 DC0466 to the University of Maryland Center for Comparative and Evolutionary Biology of Hearing, by training grant DC-000466 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health, and from U.S. Geological Survey Patuxent Wildlife Research Center.
References
- Amoser S, Ladich F. Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J Exp Biol. 2005;208:3533–3542. doi: 10.1242/jeb.01809. [DOI] [PubMed] [Google Scholar]
- Aubin T, Jouventin P, Hildebrand C. Penguins use the two-voice system to recognize each other. Proc Roy Soc B. 2000;267:1081–1087. doi: 10.1098/rspb.2000.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JE, Custer CM, Afton AD. Lesser scaup (Aythya affinis) [Accessed 10 March 2014];Birds of North America Online. 1998 http://bna.birds.cornell.edu.proxyum.researchport.umd.edu/bna/species/338.
- Barr JF, Eberl C, Mcintyre JW. Red-throated loon (Gavia stellata) [Accessed 10 March 2014];Birds of North America Online. 2000 http://bna.birds.Cornell.edu.proxy-um.researchport.umd.edu/bna/species/513.
- Beecher MD. Development of parent-offspring recognition in birds. In: Aslin RK, Alberts JR, Petersen MR, editors. Development of perception. Vol. 1. New York: Academic Press; 1981. pp. 45–66. [Google Scholar]
- Beuth JM. Body composition, movement phenology and habitat use of common eider along the southern New England coast. Open Access Master’s Theses. 2013 [Google Scholar]
- Blickley JL, Blackwood D, Patricelli GL. Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conserv Biol. 2012;26:461–471. doi: 10.1111/j.1523-1739.2012.01840.x. [DOI] [PubMed] [Google Scholar]
- Bom N. Effect of rain on underwater noise level. J Acoust Soc Am. 1969;45:150–156. [Google Scholar]
- Bordage D, Savard JPL. Black scoter (Melanitta americana) [Accessed 10 March 2014];Birds of North America Online. 2011 http://bna.birds.Cornell.edu.proxy-um.researchport.umd.edu/bna/species/177.
- Borg E. Auditory thresholds in rats of different age and strain. A behavioral and electrophysiological study. Hear Res. 1982;8(2):101–115. doi: 10.1016/0378-5955(82)90069-7. [DOI] [PubMed] [Google Scholar]
- Borg W, Engstrόm B. Hearing thresholds in the rabbit: A behavioral and electrophysiological study. Acta Otolaryngol. 1983;95:19–26. doi: 10.3109/00016488309130911. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ. Development of auditory sensitivity in budgerigars (Melopsittacus undulatus) J Acoust Soc Am. 2004;115:3092–3102. doi: 10.1121/1.1739479. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Lohr B, Hahn DC, Dooling RJ. Auditory brainstem responses in the Eastern Screech Owl: An estimate of auditory thresholds. J Acoust Soc Am. 2005;188(1):314–321. doi: 10.1121/1.1928767. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Gleich O. Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus) J Acoust Soc Am. 2002;112(3):999–1008. doi: 10.1121/1.1494807. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Ryals BM, Gleich O. Electrophysiological and morphological development of the inner ear in Belgian waterslager canaries. J Acoust Soc Am. 2010;118:314–321. doi: 10.1016/j.heares.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PW, Fredrickson LH. White-winged Scoter (Melanitta fusca) [Accessed 10 March 2014];Birds of North America Online. 1997 http://bna.birds.cornell.edu.proxy-um.researchport.umd.edu/bna/species/274.
- Brua RB. Ruddy duck (Oxyura jamaicensis) [Accessed 10 March 2014];Birds of North America Online. 2002 http://bna.birds.cornell.edu.proxy-um.researchport.umd.edu/bna/species/696.
- Bureau of Ocean Energy and Management. Determining offshore use by diving marine birds using satellite telemetry. [Accessed 12 February 2014];Environmental studies program. 2013 http://www.boem.gov/uploadedFiles/BOEM/Environmental_Stewardship/Environmental_Studies/Renewable_Energy/AT%2012-02%20Diving%20Birds.pdf.
- Burkard R, Voigt HF. Stimulus dependencies of the gerbil brainstem auditory evoked response (BAER): I. Effects of click level, rate and polarity. J Acoust Soc Am. 1989;85:2514–2525. doi: 10.1121/1.397746. [DOI] [PubMed] [Google Scholar]
- Campo JL, Gil MG, Davila SG. Effects of specific noise and music stimuli on stress and fear levels of laying hens of several breeds. Appl Anim Behav Sci. 2005;91:75–84. [Google Scholar]
- Carr CE, Soares D, Smolders J, Simon JZ. Detection of interaural time differences in the alligator. J Neurosci. 2009;29(25):7978–7990. doi: 10.1523/JNEUROSCI.6154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. J Comp Physiol A. 2010;196(8):581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney KM, Sydeman WJ. A review of the human disturbance effects on nesting colonial waterbirds. Waterbirds: Int J Waterbird Biol. 1999;22(1):68–79. [Google Scholar]
- Carpenter JW. Exotic animal formulary. St. Louis, MO: Elsevier; 2013. [Google Scholar]
- Cederholm JM, Froud KE, Wong AC, Ko M, Ryan AF, Housely GD. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hear Res. 2012;292(1–2):71–9. doi: 10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapdelaine G, Dupuis P, Reed A. Distribution, abondance at fluctuation des populations d’eider a duvet dans l’estuaire et le golfe du Saint-Laurent. In: Reed A, editor. Eider ducks in Canada. Ottawa: 1986. pp. 6–19. Can Wild Serv Rep Ser no 47. [Google Scholar]
- Corfield JR, Krilow JM, Ligt MNV, Iwaniuk AN. A quantitative morphological analysis of the inner ear of galliform birds. Hear Res. 2013;304(C):111–127. doi: 10.1016/j.heares.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Deng X, Wanger HJ, Popper AN. Messages from the bottom of the Atlantic Ocean: comparative studies of anatomy and ultrastructure of the inner ears of several gadiform deep-sea fishes. Abst Assn Res Otolaryngol. 2002;25:101. [Google Scholar]
- Dmitrieva LP, Gottlieb G. Development of brainstem auditory pathway in mallard duck embryos and hatchlings. J Comp Physiol A. 1992;171:665–671. doi: 10.1007/BF00194114. [DOI] [PubMed] [Google Scholar]
- Dooling RJ. Avian hearing and the avoidance of wind turbines. Colorado: National Renewable Energy Laboratory; 2002. [Google Scholar]
- Dooling RJ, Popper AN. The effects of highway noise on birds. Report prepared for the California Department of Transportation, Division of Environmental. Analysis under Contract # 43A0139. 2007 [Google Scholar]
- Dooling RJ, Saunders JC. Hearing in the parakeet (Melopsittacus undulatus): Absolute thresholds, critical ratios, frequency difference limens, and vocalizations. J Comp Physiol Psych. 1975;88(1):1–20. doi: 10.1037/h0076226. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B, Dent ML. Hearing in birds and reptiles. In: Dooling RJ, Popper AN, Fay RR, editors. Comparative hearing: Birds and reptiles. New York: Springer-Verlag; 2000. pp. 308–359. [Google Scholar]
- Fay RR. Hearing in vertebrates: a psychophysics data book. Winnetka, IL: Hill-Fay Associates; 1988. [Google Scholar]
- Gall MD, Brierley LE, Lucas JR. Species and sex effects on auditory processing in brown-headed cowbirds and red-winged blackbirds. Anim Behav. 2011;81(5):973–982. [Google Scholar]
- Gleich O, Dooling RJ, Manley GA. Audiogram, body mass, and basilar papilla length: Correlations in birds and predictions for extinct archosaurs. Naturwissenschaften. 2005;92:595–598. doi: 10.1007/s00114-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Goudie R, Gregory I, Robertson J, Reed A. Common eider (Somateria mollissima) [Accessed 10 March 2014];Birds of North America Online. 2000 http://bna.birds.Cornell.edu.proxy-um.researchport.umd.edu/bna/species/546.
- Gorga MP, Kaminski JR, Beauchaine KA, Jesteadt W. Auditory brainstem responses to tone bursts in normally hearing subjects. J Speech Lang Hear R. 1988;31:87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- Greene CR., Jr . Ambient noise. In: Richardson WJ, Green CR Jr, Malme CI, Thomson DH, editors. Marine mammals and noise. London: Academic Press; 1995. pp. 87–100. [Google Scholar]
- Guillemette M, Himmelman JH, Barette C. Habitat selection by common eiders in winter and its interaction with flock size. Can J Zool. 1993;71:1259–1266. [Google Scholar]
- Hall JW. Handbook of auditory evoked responses. Boston: Allyn & Bacon; 1992. [Google Scholar]
- Heffner RS, Heffner HE. Hearing in the elephant (Elephas maximus): Absolute sensitivity, frequency discrimination, and sound localization. J Comp Physiol Psych. 1982;96(6):926–944. [PubMed] [Google Scholar]
- Henry KS, Lucas JR. Coevolution of auditory sensitivity and temporal resolution with acoustic signal space in three songbirds. Anim Behav. 2008;76:1659–1671. [Google Scholar]
- Henry KS, Lucas JR. Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis. Anim Behav. 2010;80(3):497–507. [Google Scholar]
- Houser DS, Finneran JJ. A comparison of underwater hearing sensitivity in bottlenose dolphins (Tursiops truncatus) determined by eletrophysiological and behavioral methods. J Acoust Soc Am. 2006;120(3):1713–1722. doi: 10.1121/1.2229286. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, Ho SYW, et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science. 2014;346(6215):1320–1331. doi: 10.1126/science.1253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IL, Falls JB, Gaston AJ. Vocal recognition between parent and young of ancient murrelets, Synthliboramphus antiquus (Aves: Alcidae) Anim Behav. 1987;35:1405–1415. [Google Scholar]
- Jouventin P. Visual and vocal signals in penguins, their evolution and adaptive characters. Adv Ethol. 1982;24:1–149. [Google Scholar]
- Kastelein RA, Dubbeldam JL, de Bakker MAG, Gerrits NM. The anatomy of the walrus head (Odobenus rosmarus). Part 4: The ears and their function in aerial and underwater hearing. Aquat Mamm. 1996;22(2):95–125. [Google Scholar]
- Katayama A. Postnatal development of auditory function in the chicken revealed by auditory brain-stem responses (ABRs) Electroencephalogr Clin Neurophysiol. 1985;62:388–398. doi: 10.1016/0168-5597(85)90048-6. [DOI] [PubMed] [Google Scholar]
- Kettembeil S, Manley GA, Siegl E. Distortion-product otoacoustic emissions and their anaesthesia sensitivity in the European starling and the chicken. Hear Res. 1995;86(1–2):47–62. doi: 10.1016/0378-5955(95)00053-7. [DOI] [PubMed] [Google Scholar]
- Konishi M. Comparative neurophysiological studies of hearing and vocalization in songbirds. Z Vergl Physiol. 1970;66:257–272. [Google Scholar]
- Konishi M. Development of auditory neuronal responses in avian embryos. P Natl Acad Sci USA. 1973;70(6):1795–1798. doi: 10.1073/pnas.70.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C, Gleich O. Evoked cochlear potentials in the barn owl. J Comp Physiol A. 2007;193(6):601–612. doi: 10.1007/s00359-007-0215-0. [DOI] [PubMed] [Google Scholar]
- Köppl C, Nickel R. Prolonged maturation of cochlear function in the barn owl after hatching. J Comp Physiol A. 2007;193(6):613–624. doi: 10.1007/s00359-007-0216-z. [DOI] [PubMed] [Google Scholar]
- Köppl C, Manley GA, Johnstone BM. Peripheral auditory processing in the bobtail lizard Tiliqua rugosa. V, Seasonal effects of anaesthesia. J Comp Physiol. 1990;167(1):139–144. [Google Scholar]
- Ladich F, Bass AH. Underwater sound generation and acoustic reception in fishes with some notes on frogs. In: Collin SP, Marshall NJ, editors. Sensory processing in aquatic environments. New York: Springer; 2003. pp. 173–193. [Google Scholar]
- Lengagne T, Aubin T, Jouventin P, Lauga J. Perceptual salience of individually distinctive features in the calls of adult king penguins. J Acoust Soc Am. 2000;107:508–516. doi: 10.1121/1.428319. [DOI] [PubMed] [Google Scholar]
- Lohr B, Brittan-Powell EF, Dooling RJ. Auditory brainstem response and auditory thresholds in woodpeckers. J Acoust Soc Am. 2013;133(1):337–342. doi: 10.1121/1.4770255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GR, Schnitzler HU. Behavioural audiograms from the bat, Rhinolophus ferrumequinum. J Comp Physiol. 1975;100:211–219. [Google Scholar]
- Machin KL. Waterfowl anesthesia. Semin Avian Exot Pet. 2004;13(4):206–212. [Google Scholar]
- Machin KL, Caulkett NA. Effects of injectable anesthetics in mallard ducks (Anas platyrhynchos): A descriptive study. J Avian Med Surg. 1998;12:255–262. [Google Scholar]
- McClure CJ, Ware HE, Carlisle J, Kaltenecker G, Barber JR. An experimental investigation into the effects of traffic noise on distributions of birds: avoiding the phantom road. Proc Roy Sci B. 2013;280(1773):20132290. doi: 10.1098/rspb.2013.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SO, Schilt MP, Dworski JG. Ambient noise measurements from 100 Hz to 80 kHz in an Alaskan fjord. J Acoust Soc Am. 1992;91:1990–2003. doi: 10.1121/1.403683. [DOI] [PubMed] [Google Scholar]
- Megela-Simmons A, Moss CF, Daniel KM. Behavioral audiograms of the bullfrog (Rana catesbeiana) and the green tree frog (Hyla cinerea) J Acoust Soc Am. 1985;78:1236. doi: 10.1121/1.392892. [DOI] [PubMed] [Google Scholar]
- Meir JU, Stockard TK, Williams CL, Ponganis KV, Ponganis PJ. Heart rate regulation and extreme bradycardia in diving emperor penguins. J Exp Biol. 2008;211:1169–1179. doi: 10.1242/jeb.013235. [DOI] [PubMed] [Google Scholar]
- Mowbray TB. Northern gannet (Morus bassanus) [Accessed 10 March 2014];Birds of North America Online. 2002 http://bna.birds.cornell.edu.proxy-um.researchport.umd.edu/bna/species/693.
- Naguib M, van Oers K, Braakhuis A, Griffioen M, de Goede P, Waas JR. Noise annoys: effects of noise on breeding great tits depend on personality but not on noise characteristics. Anim Behav. 2013;85(5):949–956. [Google Scholar]
- Nelson JB. The gannet. Vermillion, SD: Buteo Books; 1978. [Google Scholar]
- Neuweiler G, Bruns V, Schuller G. Ears adapted for the detection of motion, or how echolocating bats have exploited the capacities of the mammalian auditory system. J Acoust Soc Am. 1980;68:741. [Google Scholar]
- Nystuen JA. Rainfall measurements using underwater ambient noise. J Acoust Soc Am. 1986;79:972–981. [Google Scholar]
- Payne KB, Langbauer WR, Thomas EM. Infrasonic calls of the Asian elephant (Elephas maximus) Behav Ecol Sociobiol. 1986;18:297–301. [Google Scholar]
- Popper AN. Scanning electron microscopic studies of the sacculus and lagena in several deep-sea fishes. Am J Anat. 1980;157:115–136. doi: 10.1002/aja.1001570202. [DOI] [PubMed] [Google Scholar]
- Ramprashad F, Money KE, Ronald K. The harp seal, Pagophilus groenlandicus (Erxlebel, 1777). XXI. The structure of the vestibular apparatus. Can J Zool. 1972;50:1357–1361. doi: 10.1139/z72-185. [DOI] [PubMed] [Google Scholar]
- Reijnen R, Foppen RUUD. The ecology of transportation: managing mobility for the environment. Netherlands: Springer Netherlands; 2006. Impact of road traffic on breeding bird populations; pp. 255–274. [Google Scholar]
- Reppening CA. Underwater hearing in seals: functional morphology. In: Harrison RJ, editor. Functional anatomy of marine mammals. London: Academic Press; 1972. pp. 307–331. [Google Scholar]
- Reubhausen MR, Brozoski TJ, Bauer CA. A comparison of the effects of isofluorane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hear Res. 2012;287(1–2):25–29. doi: 10.1016/j.heares.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Rijke AM. Wettability and phylogenetic development of feather structure in water birds. J Exp Biol. 1970;52(2):469–479. [Google Scholar]
- Robertson GJ, Goudie RI. Harlequin duck (Histrionicus histrionicus) [Accessed 10 March 2014];Birds of North America Online. 1999 http://bna.birds.Cornell.edu.proxy-um.researchport.umd.edu/bna/species/466.
- Robertson GJ, Savard JPL. Long-tailed duck (Clangula hyemalis) [Accessed 10 March 2014];Birds of North America Online. 2002 http://bna.birds.cornell.edu.proxy-um.researchport.umd.edu/bna/species/651.
- Sade J, Handrich Y, Bernheim J, Cohen D. Pressure equilibration in the penguin middle ear. Acta Otolaryngol. 2008;128:18–21. doi: 10.1080/00016480701299667. [DOI] [PubMed] [Google Scholar]
- Savard JPL, Bordage D, Reed A. Surf scoter (Melanitta perspicillata) [Accessed 10 March 2014];Birds of North America Online. 1998 http://bna.birds.cornell.edu.proxyum.researchport.umd.edu/bna/species/651.
- Sea Duck Joint Venture. Atlantic and Great Lakes sea duck migration study. [Accessed 24 February 2014];Progress Report. 2012 http://seaduckjv.org/atlantic_migration_study/atlantic_grlakes_sea_duck_rpt_november2012_compressed.pdf.
- Slabbekoorn H. Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim Behav. 2013;85:1089–1099. [Google Scholar]
- Sladen WL, Leresche RE. New and developing techniques in Antarctic ornithology. Antarct Ecol. 1970;1:585–596. [Google Scholar]
- Stenfors LE, Sade J, Hellström S, Anniko M, Folkow L. Exostoses and cavernous venous formation in the external auditory canal of the hooded seal as a functional physiological organ. Acta oto-laryngologica. 2000;120(8):940–943. doi: 10.1080/00016480050218663. [DOI] [PubMed] [Google Scholar]
- Trainer JE. The auditory acuity of certain birds. Doctoral dissertation, Cornell University. In: Fay RR, editor. Hearing in vertebrates: A psychological databook. Winnetka, IL: Hill-Fay Associates; 1946. 1988. [Google Scholar]
- Urick RJ. The noise background of the sea: ambient noise level. In: Urick RJ, editor. Principles of underwater sound. Los Altos, CA: Peninsula Publishing; 1983. pp. 202–236. [Google Scholar]
- Welsch U, Riedelsheimer B. Histophysiological observations on the external auditory meatus, middle, and inner ear of the Weddell seal (Leptonychotes weddelli) J Morphol. 1997;234(1):25–36. doi: 10.1002/(SICI)1097-4687(199710)234:1<25::AID-JMOR3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wenz GM. Acoustic ambient noise in the ocean: spectra and sources. J Acoust Soc Am. 1962;34:1936–1956. [Google Scholar]
- Wever EG, Herman PN, Simmons JA, Hertzler DR. Hearing in the blackfooted penguin, Spheniscus demerits, as represented by the cochlear potentials. Proc Natl Acad Sci USA. 1969;63:676–680. doi: 10.1073/pnas.63.3.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RP, Culik B, Danfeld R, Adelung D. People in Antarctica - how much do Adelie Penguins Pygoscelis adeliae care? Polar Biol. 1991;11:363–379. [Google Scholar]
- Wolski LF, Anderson RC, Bowles AE, Yochem PK. Measuring hearing in the harbor seal (Phoca vitulina): Comparison of behavioral and auditory brainstem response techniques. J Acoust Soc Am. 2003;113(1):629–637. doi: 10.1121/1.1527961. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Rubel EW. High-frequency auditory feedback is not required for adult song maintenance in Bengalese finches. J Neuroscil. 1999;9(1):358–371. doi: 10.1523/JNEUROSCI.19-01-00358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen MM, Nachtigall PE, Breese M, Supin AY. Behavioral and auditory evoked potential audiograms of a false killer whale (Pseudorca crassidens) J Acoust Soc Am. 2005;118(4):2688–2695. doi: 10.1121/1.2010350. [DOI] [PubMed] [Google Scholar]