Abstract
Reactive oxygen species not only cause damage but also have a physiological role in the protection against pathogens and in cell signalling. Mitochondrial nutrients, such as coenzyme Q10 and α-lipoic acid, beside their acknowledged antioxidant activities, show interesting features in relation to their redox state and consequent biological activity. In this study, we tested whether oral supplementation with 200 mg/day of coenzyme Q10 alone or in association with 200 mg/die of α-lipoic acid for 15 days on 16 healthy subjects was able to modulate the oxidative status into different compartments (plasma and cells), in basal condition and following an oxidative insult in peripheral blood lymphocytes exposed in vitro to H2O2. Data have shown that tested compounds produced antioxidant and bioenergetic effects improving oxidative status of the lipid compartment and mitochondrial functionality in peripheral blood lymphocytes. Simultaneously, an increased intracellular reactive oxygen species level was observed, although they did not lead to enhanced DNA oxidative damage. Coenzyme Q10 and α-lipoic acid produced beneficial effects also steering intracellular redox poise toward a pro-oxidant environment. In contrast with other antioxidant molecules, pro-oxidant activities of tested mitochondrial nutrients and consequent oxidant mediated signalling, could have important implications in promoting adaptive response to oxidative stress.
Keywords: Coenzyme Q10 , α-lipoic acid, reactive oxygen species, mitochondrial functionality, DNA damage
Introduction
An imbalance between oxidants and antioxidants in favour of the oxidants, potentially leading to damage, is termed “oxidative stress”. Oxidants are formed as a normal product of aerobic metabolism but can be produced at elevated rates under pathophysiological conditions.(1) Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen and they are generated as by-products during mitochondrial electron transport or during the defense process against infections. Moreover ROS are responsible for damage to biomolecules. In fact, many disease states are connected with ROS damage which is often associated with injure to bio-molecules, including carbohydrates, amino acids, fatty acids and nucleotides. These diseases include inflammations, infections, cancer, lifestyle-related diseases and metabolic diseases, such as diabetes mellitus, atherosclerosis and neurological disorders such as Alzheimer’s disease.(2) However, free radicals not only cause damage but also have a physiological role in the protection against pathogens and in cell signalling. Differently from other signalling molecules, ROS to a large extent arechemically reactive and some of them extremely unstable in biological systems. The specificity of ROS signalling functions depends on their chemical reactivity and production system.(2) ROS signalling process generally begins with an unstable primary ROS signal that is subsequently transformed into a more stable secondary signal. During this process, ubiquitous biological molecules (e.g., nucleic acids, nucleotides, lipids and reactive protein residues) serve as chemical sensors, which can effectively perceive ROS and are available in cells for a wide repertoire of reactions.(2) In healthy aerobes, production of ROS is approximately balanced with antioxidant defense systems. The balance is not always perfect, however, so that some ROS-mediated damage occurs frequently.(3) In order to preserve the proper oxidative balance in biological systems, the cell has several lines of endogenous antioxidant defenses. Moreover, antioxidant supplementation may contribute to sustain endogenous defenses promoting delaying of the aging process and prevention of age-associated diseases. Among these compounds, coenzyme Q10 (CoQ10) and α-lipoic acid (LA) are active both as cellular antioxidants and mitochondrial nutrients. Coenzyme Q is a key component of the mitochondrial respiratory chain and it was initially mainly known for its role in oxidative phosphorylation; subsequently it was demonstrated its presence in other sub-cellular fractions and in plasma lipoproteins, where it acts as a lipophilic antioxidant. Lately modulatory effects of CoQ10 on gene expression were also recognized.(4,5) These three functions underlie the rationale for its use in clinical practice and as a food supplement.(6) α-Lipoic acid is a naturally occurring dithiol compound synthesized enzymatically in the mitochondrion from octanoic acid.(7) Just like CoQ10, α-lipoic acid is also involved both in energy metabolism and as an antioxidant. In fact, LA is a necessary cofactor for mitochondrial α-keto acid dehydrogenase and, for this reason, plays a critical role in mitochondrial energy metabolism. Moreover, its antioxidant function is associated with the reduced form, dihydro lipoic acid (DHLA), a powerful mitochondrial antioxidant. Furthermore, DHLA recycles other cellular antioxidants including CoQ10 and vitamin C and is involved in iron and copper chelation.(8–11) These mitochondrial nutrients, beside their acknowledged antioxidant activities, show interesting features in relation to their redox state and consequent biological activity. Linnane et al.(5) proposed that CoQ10’s particular sub-cellular redox poise (ratio of ubiquinol to ubiquinone) represents a key determinant in regulating metabolic control function. Redox poise variations of this molecule are able to locally influence redox status, resulting in a signaling process. This hypothesis is in agreement with recently developed concepts in the field of free radical research such as nucleophilic tone and para-hormesis. Forman et al.(12) suggest the term nucleophilic tone to describe the cellular, tissue, organ, or even organism level of protection against electrophiles (including many free radicals and/or oxidants) by nucleophiles. This should be not only provided by antioxidants per se but could be modulated by non-toxic compounds that maintain an adaptive defense system by mimicking electrophiles and increasing the nucleophilic tone. They propose the name para-hormesis to describe this adaptive hormetic-like response that does not necessarily require an activating or initiating stress, remembering that hormesis refers to the positive adaptation effects of low-dose toxins, toxicants, or other stressors.
In this context, the aim of the study was to test whether mitochondrial nutrients, CoQ10 and LA, were able to modulate the oxidative status into different compartments (plasma and cells) in basal condition and following an oxidative insult in peripheral blood lymphocytes (PBL) exposed in vitro to H2O2.
Materials and Methods
Experimental design
Sixteen healthy subjects (8 male/8 female), aged 26.8 ± 2.8 with BMI 23.6 ± 1.4 volunteered in this study. Use of any type of dietary supplement or fortified food in the previous 3 months was considered an exclusion criterion. All participants were instructed to continue their normal life style without modifying their usual physical activities. Volunteers were divided in two homogeneous treatment groups: one group integrated its diet with 200 mg/day CoQ10 alone, while the other one took 200 mg/day CoQ10 in association with 200 mg/day of LA. Food supplements were taken in two doses during lunch and dinner for two weeks. CoQ10 and LA were provided by Pharmanord (Copenhagen, Denmark).
Blood samples (12 ml) were withdrawn from fasting volunteers and collected in ethylenediaminetetraacetic acid (EDTA) and heparin vacutainers at the beginning and after the two weeks of treatment with CoQ10 alone or in association with LA. The evening before the second blood withdrawal the volunteers did not take any food supplements. The study was performed in accordance with the Helsinki Declaration of human studies in 2009. All participants signed an informed consent document.
Plasma separation and lymphocyte isolation
Blood collected in a heparinized vacutainer was centrifuged at 1,600 g for 10 min at 4°C to separate plasma, which was subsequently kept at –80°C until used for assessment of CoQ10 content. 8 ml of blood were collected in vacutainers containing EDTA, washed twice with Phosphate Buffered Saline (PBS) and immediately used for PBL isolation by density-gradient centrifugation on lymphoprep (Nyegaard, Oslo, Norway). Isolated cells were washed twice in PBS. Lymphocyte count was determined using an hemocytometer chamber and cells number was adjusted to 1.2 × 106 cells/ml using RPMI medium (Sigma, Milan, Italy) pH 7.4 supplemented with 10% Fetal Bovine Serum (FBS) (Sigma, Milan, Italy).
Cell damage after oxidative stress induction
Lymphocyte samples (1.2 × 106 cells/ml) were incubated in the dark for 4 h at 37°C in complete medium alone (control) or with 100 and 200 µM H2O2 (Sigma).(13) At the end of the incubation, an aliquot of counted cells was washed in PBS and stored at –80°C for subsequent cellular CoQ10 analysis and DNA damage assessment by comet assay. Cellular integrity for DNA analysis was ensured by using a cryopreservation medium.(14) Flow cytometric investigations for the Mitochondrial Membrane Potential (MMP) and intracellular ROS levels were performed immediately.
Plasma and cellular levels of CoQ10
CoQ10 content and its oxidative status in plasma were assayed by High-Performance Liquid Chromatography (HPLC) with Electro-Chemical Detector (ECD) by Shiseido Co. Ltd. (Tokyo, Japan). Mobile phases were prepared as described,(15) auto-sampler Model 3033, switch valve Model 3012, concentration column CQC and separation column CQS, pump one and two were Model 3001 (Shiseido Co. Ltd., Tokyo, Japan). The system is characterized by a post-separation reducing column (Shiseido CQR) capable of fully reducing the peak of ubiquinone. The oxidation potential for ECD was 650 mV. Plasma and cellular oxidative status were expressed as percentage of ubiquinone/total CoQ10, while plasma levels of CoQ10 were expressed as µg/ml.(16)
Mitochondrial membrane potential
MMP was analyzed using the Nernstian dye 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolcarbocyanine iodide (JC-1) (Invitrogen, Monza, Italy). This cyanine dye is a lipophilic cation that is able to selectively enter in the mitochondria. The probe exists in a monomeric form emitting at 530 nm (green) upon excitation at 488 nm. However, with increasing MMP, JC-1 is able to form J-aggregates that are associated with a large shift in emission to 590 nm (red/orange) and mitochondrial membrane appears to be more polarized (above values about 80–100 mV the colour of the dye changes reversibly from green to orange).(17) After incubation with H2O2, lymphocyte samples were washed twice in PBS and incubated in the dark for 15 min at 37°C with JC-1 (10 µg/ml) in complete medium. Subsequently cells were washed in PBS and analyzed using EPICS XL flow cytometer (Beckmancoulter, Milan, Italy). Emissions at 579 nm (FL1) and 590 nm (FL4) were detected. Photomultiplier tubes (PMTs) were set at 511 V and 938 V respectively. Compensation was set at FL1-FL4 10% and FL4-FL1 35%. A minimum of 10,000 events were recorded. Mitochondrial depolarization was evaluated in terms of percentage of cells showing low red fluorescence, proportional to MMP, using Win MDI. For bulk determination of the percentage of oxidative induced depolarization, 1 × 106 lymphocytes/well were transferred to a 96 wells microplate, centrifuged at 400 g for 5 min in a Heraeusmultifuge 3S+ (Thermo Scientific, Milan, Italy) equipped with microplate rotor, to obtain a homogeneous distribution of cells on the bottom of plate. JC-1 fluorescence was assessed by using a microplate reader (Synergy HT, Winooski, VT) exciting the cells at 485/20 nm and reading the fluorescence emitted in the green at 528/20 nm and in the red at 590/20 nm. Mitochondrial depolarization was indicated by an increase in the green/red fluorescence intensity ratio. As a positive control of depolarization, cells were incubated with the uncoupling agent CCCP (200 µM) that produced 100% of depolarization.
Intracellular ROS assay
Intracellular ROS levels were assessed by means of DCFH-DA (2'-7'-dichlorodihydrofluorescein diacetate), a non-polar probe, which readily diffuses across cell membranes where it is hydrolyzed by intracellular esterases to the polar derivative, DCFH. In its reduced form DCFH is not fluorescent but in the presence of ROS, it is oxidized to DCF which is highly fluorescent and whose emission maximum can be monitored at 520 nm.(18) After incubation with H2O2, lymphocytes were washed with PBS, the supernatant was removed and a suspension of 3.6 × 105 cells was incubated with DCFH-DA (10 µM) at 37°C for 15 min in the dark. Then, cells were washed in PBS, resuspended in 1 ml of buffer, transferred to cytometry tubes and kept on ice avoiding light until assay. Prior to flow cytometry analysis, propidium iodide (20 µg/ml) was added to cell suspensions in order to label cells with compromised plasma membrane which would have produced false negative results. Fluorescence of the labelled cells was measured on an EPICS XL flow cytometer (Coulter) using an excitation wavelength of 488 nm. In order to exclude false negative due to dead cells, green fluorescence was gated on the cells negative to propidium iodide and 10,000 events from each sample were measured. Results were analyzed using Win MDI.
Comet assay
Analysis was conducted on cryopreserved cells as described above. Immediately after thawing aliquots containing 1,500 cells were washed in PBS. The supernatant was removed and cells were resuspended in Low Melting Agarose (LMA 0.7%) from which 50 µl were rapidly placed on high-throughput comet slides (Trevigen) equipped with silicone barrier delimiting 20 samples area per slide, pre-coated with 1% Normal Melting Agarose (NMA 1%). Subsequently microgels were lysed for at least 1 h at 4°C, in the dark in a freshly prepared lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris-HCl, 1% Triton X-100, and 10% DMSO, adjusted to pH 10). Finally DNA was allowed to unwind in alkaline buffer (1 mM EDTA, 300 mM NaOH, pH 13) for 30 min, in the dark and electrophoresed for 20 min at 1 V/cm in a refrigerated room. After electrophoresis, slides were washed with 0.4 M Tris-HCl buffer (pH 7.5) for 5 min, dehydrated in 75% methanol and dried at 45°C for 20 min about. Finally slides were stained by adding 15 µl of ethidium bromide (20 µg/ml) and analyzed in fluorescence microscopy. For each sample, 50 comets on two different slides (i.e., 100 comets/sample) were assayed in each experiment. DNA damage was evaluated by a custom-made computer-aided scoring and image analysis software developed by our Department in collaboration with the Engineering Department of the Polytechnic University of Marche. This software has several advantages over commercial software, allowing a semi-automatic analysis which greatly reduces operator-dependent variability.(14) Results are reported as percentage of DNA in the comet tail or tail intensity (TI) for each cell expressed as means of the median at different experimental points.
Statistical analysis
Mean, median and quartile values were calculated and data are presented as box plots where the cross (x), the box and the bars represents respectively the median, the 50% and 25% of the measurements of each parameters. Homogeneity of variances was verified using the Cochran C-test, and data were appropriately transformed when necessary.(19) A two-way analysis of variance was conducted for samples within each stress condition (ctrl, 100 and 200 µM H2O2) with treatments (CoQ10 vs CoQ10 + LA) as main experimental unit and time of observation (pre vs post) as the smallest experimental unit. P<0.01 was considered statistically highly significant, p<0.05 significant, and p<0.1 approaching significance.
Results
CoQ10 bioavailability
Supplementation with 200 mg/day CoQ10 for 2 weeks produced a significant increase in plasma levels with median increase from 0.56 to 1.91 µg/ml (+241%, p<0.01) in the overall studied population (Fig. 1A). Co-supplementation with LA did not influence CoQ10 bioavailability. Increased levels of plasma CoQ10 were associated with a change in its oxidative status. As shown in Fig. 1B, supplementation with CoQ10 produced a significant decrease of the percentage of ubiquinone/total CoQ10 with median decrease (from 15% to 8%, –47%, p<0.05) indicating an increase of plasma antioxidant defenses. Also in this case, co-supplementation with LA did not have a significant influence on CoQ10 oxidative status.
Fig. 1.
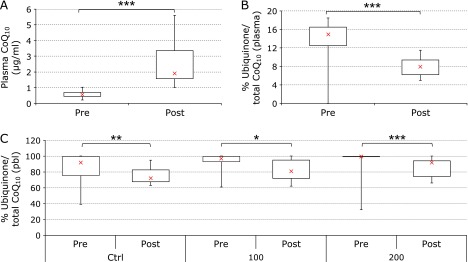
CoQ10 concentrations and oxidative status in plasma and in peripheral blood leukocytes (PBL). Plasma CoQ10 levels at study entry (pre) and following 2-week supplementation (post) (A); % of ubiquinone/total CoQ10 in plasma (B) and in PBL (C). Distribution of data relative to untreated control (ctrl), cells exposed to 100 µM (100) or 200 µM (200) H2O2. Data relative to 16 subjects are reported as box plot diagram where the cross (x), the box and the bars represent respectively the median, the 50% and 25% of data distribution.***p<0.01 highly significant, **p<0.05 significant and *p<0.1 approaching significance compared to study entry.
At cellular level, in basal conditions independently of supplementation, there is a significant decrease in the percentage of ubiquinone/total CoQ10 with median decrease (from 92% to 72%, –22%, p<0.05) (Fig. 1C). Moreover, this improvement of CoQ10 oxidative status was associated with a decreased susceptibility toward H2O2-mediated oxidation of CoQ10. Exposure of PBL to 100 and 200 µM H2O2 for 4 h at 37°C produced a dose-dependent oxidation of endogenous CoQ10. Supplementation was effective in protecting cellular ubiquinol from oxidation; in fact in the samples treated with 100 µM of H2O2 the percentage of ubiquinone decreased, in terms of median values by 16% (from 97% to 81%); however remarkable inter-individual variability at this concentration made this difference only approaching significance. On the contrary, in those incubated with the higher dose of H2O2, the extent of protection was highly significant (from 100% to 92%, –8%, p<0.01).
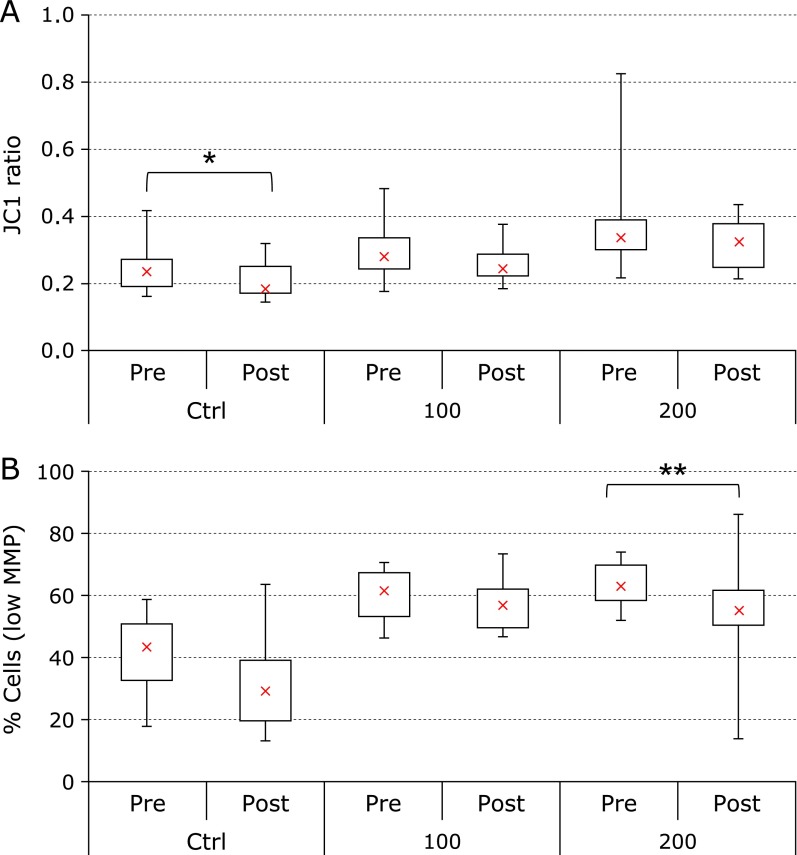
Mitochondrial membrane potential
Incubation of PBL with JC-1 allowed determination of the MMP-dependent decrease in fluorescence associated with H2O2 exposure (Fig. 2A and B). Supplementation with CoQ10 alone produced an improvement of mitochondrial functionality, in terms of membrane potential, as shown by a decrease of the ratio between green (JC-1 monomers) and red fluorescence (J-aggregates) in basal condition (median values changed from 0.24 to 0.19, –22% that approached significance p<0.1, Fig. 2A). These observations, conducted on the bulk population of cells using a fluorescence microplate reader, were further investigated at cellular level, by means of flow cytometric technique, in order to evaluate cellular population distributions in relation to MMP (Fig. 2B). Data are substantially in agreement with the bulk analysis, showing a decrease in the population of cells with low MMP following supplementation with antioxidants. In particular, this effect was more pronounced following oxidative insult (200 µM), where supplementation was able to significantly decrease the percentage of cells with low mitochondrial membrane potential in terms of median (from 63.4% to 55.5%, –12%, p<0.05). Factorial Anova did not show any significant difference in time between CoQ10 alone treatment and co-supplementation CoQ10 + LA.
Fig. 2.
Fluorescence signal proportional to mitochondrial membrane potential. Bulk measurement of nernstian dye JC-1 fluorescence in a microplate reader (A). Ratio green/red of JC-1 emission at study entry (pre) and following 2-week supplementation (post). Flow cytometric determination of JC-1 fluorescence at cellular level (B). Percentage of cells showing low MMP at study entry (pre) and following 2-week supplementation (post). Distribution of data relative to untreated control (ctrl), cells exposed to 100 µM (100) or 200 µM (200) H2O2 are reported. Data relativities to 16 subjects are reported as box plot diagram where the cross (x), the box and the bars represent respectively the median, the 50% and 25% of data distribution.***p<0.01 highly significant, **p<0.05 significant and *p<0.1 approaching significance compared to study entry.
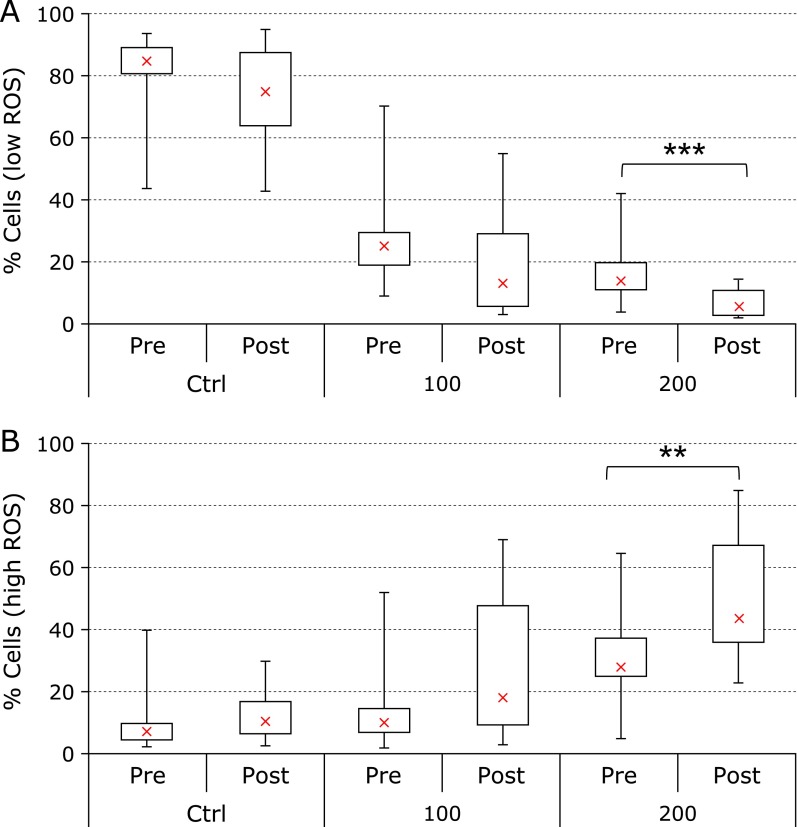
Intracellular ROS levels
Flow-cytometric analysis of intracellular DCFH-DA oxidation by endogenous ROS, allowed evaluation of the distribution of cells according to the intracellular oxidant status. As shown in Fig. 3A and B, exposure to H2O2 produced a dose-dependent decrease in cells characterized by low DCFH-DA fluorescence, associated with low content of intracellular ROS. Moreover, supplementation with tested mitochondrial nutrients significantly enhanced the oxidant-mediated increase in intracellular ROS. In particular, the extent of this decrease was highly significant following oxidative insult (200 µM) with median values changing from 13.9% to 5.8%, –58%, p<0.01). Similarly, also cells characterized by high content of intracellular ROS increased significantly following oxidative insult (200 µM) with a median change from 28.5% to 44.3%, +56%, p<0.05). Also in this case Factorial Anova did not show any significant difference in time between CoQ10 alone treatment and co-supplementation CoQ10 + LA.
Fig. 3.
Cytometric determination of intracellular-ROS quantified by DCFH-DA oxidation. Percentage of cells showing low (A) and high (B) intracellular ROS levels at study entry (pre) and following 2-week supplementation (post). Distribution of data relative to untreated control (ctrl), cells exposed to 100 µM (100) or 200 µM (200) H2O2 are reported. Data relative to 16 subjects are reported as box plot diagram where the cross (x), the box and the bars represent respectively the median, the 50% and 25% of data distribution. ***p<0.01 highly significant, **p<0.05 significant and *p<0.1 approaching significance compared to study entry.
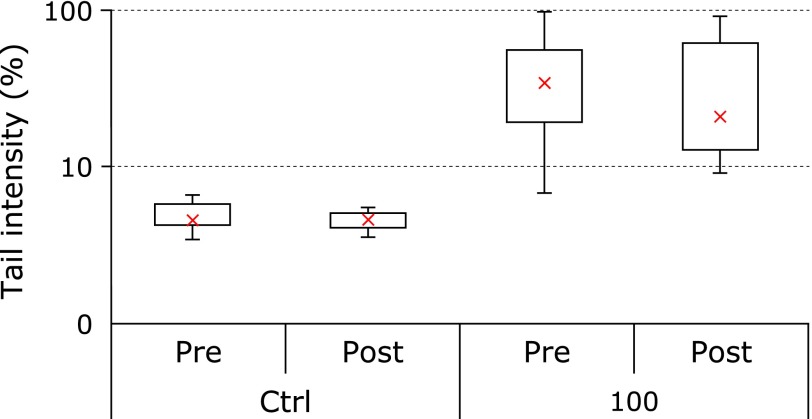
Oxidative DNA damage
Analysis by means of the alkaline version of the comet assay highlighted, as expected, low detectable levels of strand breaks in the DNA of healthy volunteers at study entry, with only 10% of the measured PBL producing comets with an average median of 5% of damaged DNA quantified as TI (Fig. 4). Incubation with 200 µM H2O2 for 4 h in complete medium produced very extensive DNA damage that did not allow quantification. Incubation with 100 µM H2O2 produced a highly significant increase of DNA damage showing a median TI of 34.6%. Supplementation with mitochondrial nutrients did not modify in a significant manner susceptibility of leukocyte DNA to oxidative stimuli, although a decrease of median TI value was observable. Factorial Anova did not show any significant difference in time between CoQ10 alone treatment and co-supplementation CoQ10 + LA.
Fig. 4.
Comet assay analysis of DNA damage (% comet tail) in lymphocytes either unexposed or challenged with 100 µM H2O2 at study entry (pre) and following 2-week supplementation (post). Median values of at least 150 cells for each sample are reported as Box plot diagrams where the cross (x), the box and the bars represent respectively the median, the 50% and 25% of data distribution.
Discussion
The present study focuses on oxidative stress and mitochondrial dysfunction in an in vitro model. In particular, we evaluated the effect of antioxidants with a clear mitochondrial target of preventing H2O2 mediated oxidative damage and mitochondrial depolarization. The tested antioxidants were clearly able to significantly improve antioxidant status of plasma in supplemented subjects. In fact, CoQ10 is known to be transported by plasma lipoproteins and our data show that ubiquinone levels were inversely related to total CoQ10 content; this result is widely confirmed in the literature.(19)
At cellular level, in PBL their antioxidant activity was more complex, in particular when cells were challenged with an oxidative stimulus. In fact, while the percentage of ubiquinone/total CoQ10 in cells was decreased in supplemented patients, as well as its susceptibility to oxidation following H2O2 exposure, intracellular levels of ROS were slightly increased in basal conditions and remarkably following oxidative insult. This conflicting result suggests also a pro-oxidant activity at cellular level of CoQ10 and α-lipoic acid.
A large body of evidence points out that most, if not all, of the classical routes to transcriptional activation are modulated by redox processes or even critically depend on oxidant signals.(20) Different redox-sensitive mammalian pathways of gene activation have been deeply investigated in the recent literature, in particular the Nrf2 and NF-κB systems. CoQ10 and lipoic acid have been shown to modulate the expression of these transcription factors and downstream pathways(21) and observed pro-oxidant activities might justify a redox trigger to improved stress response.
These data are in agreement with Linnane(22) report that highlighted a pro-oxidant effect of CoQ10 and LA; in this work he ascribed the beneficial effects of these molecules to a pro-oxidant effect that underlies adaptive responses in what could be defined as a para-hormetic mechanism involving radical signalling. In fact, despite an increase in intracellular ROS, in subjects supplemented with CoQ10 and LA, we were able to show both an improvement of basal mitochondrial functionality and a protective effect toward oxidant-induced mitochondrial depolarization. Moreover, basal ROS increase following supplementation did not reach an extent able to promote oxidative DNA damage, highlighting its compatibility with physiological processes. Factorial Anova analysis was not able to highlight in our experimental model any additive effect of lipoic acid in association with CoQ10; however large inter-individual variability might have contributed to an under-powered experimental design and data should be confirmed with a larger sample size.
Summarizing, we show that CoQ10 and α-lipoic acid might have a double-edged behaviour in terms of its oxidative state that may vary according to the biological compartment considered. While antioxidant effect of ubiquinol in lipoproteins is widely verified and accepted, at cellular level, these so called mitochondrial nutrients, might shift their oxidative status modulating cellular redox-poise. In light of recent para-hormetic hypothesis this might contribute to promote the intracellular nucleophilic tone.(12) In this sense, mitochondrial nutrients with antioxidant activity such as CoQ10 and LA, differ from other antioxidant molecules such as vitamin C. In recent reports, it has been shown that high doses of these hydrophilic antioxidants are able to hamper ROS signalling induced by physical exercise abolishing adaptive response.(20) These authors extended their conclusions to all types of antioxidants including CoQ10 and LA, disregarding their chemical nature not mechanism of action.(21) In this sense, the present study propose some contrasting evidences in line with a report by Sun et al.(23) showing that in rats trained with endurance exercise, supplementation of the diet with mitochondrial nutrients, including antioxidants such as CoQ10 and LA, were able to enhance exercise-induced mitochondrial biogenesis. Pro-oxidant activities of tested mitochondrial nutrients and consequent oxidant mediated signalling, might explain beneficial aspects associated with modulation of gene expression in the elderly. In these patients Linnane et al.(24) showed that CoQ10 was able to drive muscle gene expression toward type II fiber, fast contracting fibers that decrease more rapidly during ageing.
In conclusion, our data point out that, differently from other antioxidants, CoQ10 and α-lipoic acid produce beneficial effects also steering intracellular redox poise toward a pro-oxidant environment. This interesting data deserves further investigations in an in vivo model of oxidative stress.
Acknowledgments
The authors are thankful to Pharmanord for supporting this research and providing the supplements. We also wish to thank the volunteers that participated in the study and Mrs. Monica Glebocki for her assistance in editing the manuscript and Dr. Annaluisa Tangorra for skillful technical assistance.
Conflicts of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 2.Akaike T, Nishida M, Fujii S. Regulation of redox signalling by an electrophilic cyclic nucleotide. J Biochem. 2012;153:131–138. doi: 10.1093/jb/mvs145. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 4.Groneberg DA, Kindermann B, Althammer M, et al. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol. 2005;37:1208–1218. doi: 10.1016/j.biocel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Linnane AW, Eastwood H. Cellular redox poise modulation; the role of coenzyme Q10, gene and metabolic regulation. Mitochondrion. 2004;4:779–789. doi: 10.1016/j.mito.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition. 2009;26:250–254. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 9.Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 10.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 11.Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 12.Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JS, Lin CT. Systemic hypoxia promotes lymphocyte apoptosis induced by oxidative stress during moderate exercise. Eur J Appl Physiol. 2009;108:371–382. doi: 10.1007/s00421-009-1231-2. [DOI] [PubMed] [Google Scholar]
- 14.Tiano L, Littarru GP, Principi F, et al. Assessment of DNA damage in Down Syndrome patients by means of a new, optimised single cell gel electrophoresis technique. Biofactors. 2005;25:187–195. doi: 10.1002/biof.5520250122. [DOI] [PubMed] [Google Scholar]
- 15.Sekine K, Ota N, Nishii M, Uetake T, Shimadzu M. Estimation of plasma and saliva levels of coenzyme Q10 and influence of oral supplementation. Biofactors. 2005;25:205–211. doi: 10.1002/biof.5520250125. [DOI] [PubMed] [Google Scholar]
- 16.Tiano L, Padella L, Carnevali P, et al. Coenzyme Q10 and oxidative imbalance in Down syndrome: biochemical and clinical aspects. Biofactors. 2008;32:161–167. doi: 10.1002/biof.5520320119. [DOI] [PubMed] [Google Scholar]
- 17.Tiano L, Ballarini P, Santoni G, Wozniak M, Falcioni G. Morphological and functional changes of mitochondria from density separated trout erythrocytes. Biochim Biophys Acta. 2000;1457:118–128. doi: 10.1016/s0005-2728(00)00071-2. [DOI] [PubMed] [Google Scholar]
- 18.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2',7'-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27:146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 19.Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37:31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 21.Anghel SA. Antioxidant not heaven-sent. Harv Sci Rev. 2010;23:32–34. [Google Scholar]
- 22.Linnane AW, Eastwood H. Cellular redox regulation and prooxidant signaling systems: a new perspective on the free radical theory of aging. Ann N Y Acad Sci. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- 23.Sun M, Qian F, Shen W, et al. Mitochondrial nutrients stimulate performance and mitochondrial biogenesis in exhaustively exercised rats. Scand J Med Sci Sports. 2011;22:764–775. doi: 10.1111/j.1600-0838.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 24.Linnane AW, Zhang C, Yarovaya N, et al. Human aging and global function of coenzyme Q10. Ann N Y Acad Sci. 2002;959:396–411. doi: 10.1111/j.1749-6632.2002.tb02110.x. [DOI] [PubMed] [Google Scholar]