Abstract
Functional foods that provide benefits beyond their traditional nutritional value have attracted much interest. Aim of the study was to evaluate the nutritional and the functional properties of a frozen ready-to-eat soup containing barley and pigmented vegetables. Both glycaemic index and the glyceamic load of ready-to-eat soup were evaluated in vivo. Moreover the bioavailability of carotenoids (lutein and beta-carotene) and the effect on lipid profile and lipid peroxidation were studied in 38 volunteers whose diet was supplemented for two weeks with a daily portion (250 g) of the ready-to-eat soup. Plasma levels of carotenoids (lutein and beta-carotene) and plasma total antioxidant capacity significantly increased after 2 weeks of treatment. Furthermore, we observed a decrease in the levels of lipids (total cholesterol and low density lipoprotein-cholesterol) and of markers of lipid peroxidation (oxidized low density lipoprotein and lipid hydroperoxides) in plasma of all subjects. The glyceamic index of the product was 36, therefore it could be considered a low glyceamic index food. An accurate selection of vegetable foods results in a palatable and healthy product that provides benefits on plasma lipids and lipid peroxidation (Protocol number 211525).
Keywords: oxidized-LDL, carotenoids, oxidative stress, functional food, glyceamic index
Introduction
Functional foods that provide benefits beyond their traditional nutritional value have attracted much interest. In fact several studies have shown that diet plays a protective role against the development of human diseases (diabetes, obesity, cardiovascular diseases and metabolic syndrome).(1–3) Among vegetable foods that could be defined as functional, there are pigmented vegetables such as black and blue cabbage, purple carrots, red spinach, red chard and red celery that contain bioactive molecules such as phytochemicals (polyphenols and carotenoids) and vitamins (vitamin C, folate, and provitamin A).(4–10) However their biological effects have been mainly investigated in vitro and in animals models.(11–17) The high content in soluble fiber, especially β-glucan, supports the role of barley as a functional food.(18) Health claims concerning the effect of barley β-glucan on plasma lipids, post prandial glucose and insulin response have been approved.(19,20) More recent studies have demonstrated that barley contains also other bioactive phytonutrients.(21–23) Therefore, it has been suggested that inclusion of whole barley grain and barley products in the daily diet could help mitigate oxidative stress related to disease conditions and in particular cardiovascular diseases. Studies in animal models confirm a significant effect of diet containing barley on lipid profiles and antioxidant system.(24,25) However it has to be stressed that it is not always clear whether the effects in animals can be extrapolated to humans.(26) In order to evaluate the nutritional properties of a frozen ready-to-eat soup containing barley and pigmented vegetables, the bioavailability of carotenoids (lutein and β-carotene) and the antioxidant potential were studied in 38 volunteers whose diet was supplemented for two weeks with a daily portion (250 g) of the product. Moreover we studied the product intake effect on plasma glucose and on markers of cardiovascular disease, such as plasma lipid profile and levels of oxidized LDL (ox-LDL).
Materials and Methods
Nutrient composition of barley-vegetable soup
“Barley-vegetable soup” contains 40% of barley and 60% of pigmented vegetables (black and blue cabbage, pumpkin, purple carrots, red spinach, red chard, red and yellow celery). Water-soluble vitamins were quantified by high-performance liquid chromatography/electrospray ionization-mass spectrometry.(27) Carotenoids were analyzed using high performance liquid chromatography (HPLC).(28) Total polyphenols were evaluated following Xu et al.(29) Total antioxidant potential was evaluated by Oxygen Radical Absorbance Capacity (ORAC) assay.(7) The compositional analysis was carried out in 2011 and 2013 to verify the stability and the variability from batch to batch of the products.
Macronutrients and micronutrients contained in the frozen ready-to-eat product containing barley and pigmented vegetables are summarized in Table 1. A portion of the product provides about 40% of recommended daily intake of dietary fiber (9.4 g/250 g). It contains several hydrophilic and lipophilic vitamins. A portion (250 g) of the product contains 5.3 mg of β-carotene, 5.2 mg of lutein and 700 mg of total phenols (Table 1). As shown in Table 1, among polyphenols, phenolics acids and flavonols are the main components in the vegetable product. Anthocyanins and flavones have also been identified (Table 1). Using ORAC assay to evaluate total antioxidant capacity, the product ranks highest among vegetables.(30) In fact a portion of the frozen product provides 3 mmol/TE.
Table 1.
Macronutrient, vitamin and phytochemical composition of frozen-ready to eat barley-vegetable soup (100 g)
| Constituent | Content in 100 g of products |
|---|---|
| ENERGY (kj) | 437 ± 8 |
| PROTEIN (g) | 2.2 ± 0.2 |
| Total CARBOHYDRATE (g) | 13.8 ± 0.3 |
| Starch (g) | 5.35 ± 0.11 |
| Amylose (g) | 2.02 ± 0.08 |
| Amylopectin (g) | 3.32 ± 0.10 |
| FIBERS (g) | 3.73 ± 0.03 |
| Soluble fibres (g) | 0.94 ± 0.01 |
| β-Glucans (g) | 0.77 ± 0.01 |
| Pectins (g) | 0.78 ± 0.02 |
| Non-soluble fibres (g) | 2.79 ± 0.03 |
| Total FAT (g) | 0.32 ± 0.01 |
| Saturated (g) | 0.051 ± 0.001 |
| Monounsaturated (g) | 0.024 ± 0.001 |
| Omega6 (g) | 0.083 ± 0.000 |
| Omega3 (g) | 0.026 ± 0.000 |
| VITAMINES | |
| Vitamin C (mg) | 10.7 ± 0.51 |
| Folate (mg) | 0.034 ± 0.002 |
| Pantothenic acid (mg) | 0.21 ± 0.04 |
| α-Tocopherol (mg) | 0.56 ± 0.04 |
| Phylloquinone (mg) | 0.050 ± 0.004 |
| Niacin (mg) | 1.02 ± 0.03 |
| Pyridoxine (mg) | 0.10 ± 0.02 |
| Retinol (mg) | 0.055 ± 0.03 |
| Riboflavin (mg) | 0.069 ± 0.01 |
| Thiamine (mg) | 0.064 ± 0.01 |
| Choline (mg) | 10.8 ± 0.01 |
| POLYPHENOLS | |
| Total phenols (mg) | 281 ± 8 |
| Phenolic acids | |
| Caffeic acid (mg) | 4.96 ± 0.06 |
| Chlorogenic acid (mg) | 1.10 ± 0.09 |
| Coumaric acid (mg) | 0.25 ± 0.01 |
| Ferulic acid (mg) | 4.85 ± 0.01 |
| Anthocyanines | |
| Cyanidin (mg) | 0.76 ± 0.09 |
| Delphinidin (mg) | 1.25 ± 0.11 |
| Peonidin (mg) | 0.22 ± 0.07 |
| Flavones | |
| Apigenin (mg) | 0.23 ± 0.04 |
| Luteolin (mg) | 0.51 ± 0.03 |
| Flavonones | |
| Naringenin (mg) | 0.12 ± 0.05 |
| Flavonols | |
| Kaempferol (mg) | 0.21 ± 0.07 |
| Quercetin (mg) | 0.21 ± 0.03 |
| Isorhamnetin (mg) | 0.09 ± 0.02 |
| CAROTENOIDS | |
| β-Carotene (mg) | 2.11 ± 0.08 |
| Zeaxanthin (mg) | 0.06 ± 0.02 |
| Lycopene (mg) | 0.003 ± 0.01 |
| Lutein (mg) | 2.06 ± 0.08 |
| Total ORAC (mmol (TE)) | 1.20 ± 0.14 |
Data are reported as mean (standard deviation) of data from analyses carried out in samples from different batch and different years (2011 and 2013).
Evaluation of post prandial glucose response: glycaemic index (GI) and glycaemic load (GL)
The products were provided as breakfast to ten fasted subjects. In all subjects no co-morbidities such as type I or II diabetes or other diseases of glucose metabolism were observed, as demonstrated by serum levels of glycated hemoglobin (HbA1c<5%) (data not shown). The amount of carbohydrates in the product was used to calculate servings weight with 50 g of available carbohydrates. The portions were professionally prepared in the expected quantity. All samples were cooked following cooking instructions on the packages. All subjects completed the meal within 10 min. Each subject was asked to consume 50 g of available carbohydrate portions of test foods. Finger capillary blood samples were collected just before eating and 15, 30, 45, 60, 90 and 120 min after consumption. To determine differences in glucose kinetics, the 0–120 min incremental area under the blood glucose curve (iAUC) was calculated by using the trapezoidal rule.(31) The averages of fasting measurements were used as baseline values and areas below baseline were not included. The glycaemic index (GI) was calculated as the ratio between the iAUC of the glycaemic response obtained from product intake compared to iAUC reference food (glucose). From the value of GI we calculated the value of the glycaemic load (GL), expressed by the product of the amount of available carbohydrate, present in a portion of the product, for its GI value; GL = (GI/100*portion carbohydrate content). GL was calculated considering a portion of 250 g.
Experimental design to investigate the effect of product intake on plasma lipids and antioxidants
Subjects.
The study was conducted during September–November 2012. The inclusion criteria for subjects were: not taking vitamins, minerals, or other types of supplements during the previous 2 months; no-smoking; body mass index (BMI) within the normal range according to the World Health Organization criteria (18.5–25 kg/m2) and normal biochemical and haematological profile (cholesterol <6.8 mM, triacylglycerols <2.8 mM, glucose <6.11 mM). The exclusion criteria were: diagnosed diseases such as allergies, cancer, diabetes, obesity, hypertension, mental diseases, gastrointestinal or renal diseases, as well as intake of drugs related to these pathologies, alcohol consumption >30 g/day, vegetarian diet. None of the female subjects was pregnant or lactating. Volunteers were recruited in the Polytechnic University of Marche (UNIVPM), Italy. Thirty-eight subjects underwent barley diet. Their median age was 38.5 years (1st–3rd quartiles: 35–44), the median value of BMI was 22.3 kg/m2 (1st–3rd quartiles: 20.5–24.7) and 40% of subjects were males. The intervention phase consisted of a 2-week period which included a daily consumption of a portion (250 g) of “barley-vegetable soup” produced and supplied by Italsur s.r.l. (Notaresco, Teramo, Italy). The product was consumed after cooking and served with 2 tablespoons of extra virgin olive oil. Intake of the soup was included in the normal daily diet and no specific time of consumption or accompanying meal was established. Subjects were recommended to maintain their habitual dietary intake (especially as regard to their consumption of a provided list of foods with high carotenoids, polyphenol and vitamin C contents) and their usual physical activity or other lifestyle habits. Moreover, they were requested to record any sign of illnesses, medications, and any deviations from their experimental diets. Each subject was asked to complete a 15-day dietary record before and during the study intervention to evaluate their energy and nutrient intake. The energy and macronutrient and micronutrient intake of the subjects before and during the study were estimated by the software “MetaDieta,” using Italian food composition tables (MetaDieta software ver. 3.1, METEDA, Ascoli Piceno, Italy). No subject was reported to have side effects and thus, no one dropped out during the experimental period. At the beginning of the intervention (baseline, T0) and at the end (after 2 weeks, T15), fasting blood samples were collected. The study was performed in accordance with the Helsinki Declaration of Human Studies and approved by the Ethical Committee of the “Azienda Ospedaliero-Universitaria Ospedali Riuniti” Ancona (Italy) (Protocol number 211525). All participants signed an informed consent document. A limitation of the study is represented by the absence of a control group; however taking into account the short period of supplementation and strict record of other nutritional intake, other seasonal influences on analysed data can be excluded.
Analytical determination.
Fasting blood samples (10 ml) were collected from each subject by venipuncture from the antecubital vein: 5 ml were placed in heparin tubes for haematological measurements, while 5 ml were placed in tubes without any anticoagulant and centrifuged at 1,500 g for 10 min at 4°C for serum separation. Plasma and serum aliquots were prepared and stored at –80°C until analysis. Analyses did not commence until the full intervention study was completed and all samples from each subject were analysed within one batch to reduce inter-batch variability.
Plasma lipids and glycaemia.
Serum glucose, triacylglycerols (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) were analyzed by commercial kits (Chemadiagnostica, Jesi, Italy). Low density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula.
Plasma carotenoids.
Carotenoids (β-carotene and lutein) were quantified in plasma of 26 subjects by high-performance liquid chromatography (HPLC) system, using a single dilution step after extraction with propanol (1:5, v/v) and vigorous vortexing of 250 µl of extraction mixture. This mix was centrifuged for 2 min at 20,000 g at 4°C. Forty microlitres of supernatant were injected into the HPLC with electrochemical detector (ECD) by Shiseido (Tokyo, Japan), using a pre-separation concentrating column 50 × 2.0 mm ID 5 µm, separation C18 column 150 × 2.0 mm ID 3 µm, and a post-separation reducing column CQR 20 × 2.0 mm, all from Shiseido. For each carotenoid quantified, two mobile phases were used. Mobile phase 1 for loading and concentrating the sample (50 mM sodium perchlorate in methanol/water 95:5, v/v) was the same for both molecules, while mobile phase 2 was 50 mM sodium perchlorate in methanol/isopropanol (80:20, v/v) for lutein and 50 mM sodium perchlorate in methanol/ isopropanol (98:2, v/v) for β-carotene. Moreover, flow rate was 200 µl/min for phase 1 in both analyses. Flow rates for phase 2 were 300 and 80 µl/min for β-carotene and lutein, respectively. Total chromatographic run times and retention times were 24 min/12.3 min for β carotene and 21 min/9.8 min for lutein.(32)
Plasma total antioxidant capacity.
Plasma total antioxidant capacity was measured using oxygen radical absorbance capacity (ORAC) adapted for semi-automated measurement on a 96-well microplate reader (Synergy HT; BioTek,Winooski, VT).(33)
Markers of lipid peroxidation.
Ox-LDL were determined in plasma by a sandwich ELISA procedure using the murine monoclonal antibody mAB-4E6 as the capture antibody, and a peroxidase conjugated antibody against oxidized apolipoprotein B bound to the solid phase (ox-LDL, Mercodia AB, Uppsala, Sweden). Intra and inter-assay CVs were 2.82 and 7.29%, respectively. As LDL-C is considered a major determinant of absolute ox-LDL levels, plasma values of ox-LDL (U/L) were adjusted by the plasma levels of LDL-C (mmol/L) by calculating their ratio (units of ox-LDL per mmol of LDL-C), in agreement with Zuliani et al.(34) In the same subjects, plasma lipid hydroperoxide levels were analyzed using the ferrous oxidation-xylenol orange (FOX) assay. Variation for individual plasma using the FOX assay was <10%.(35)
Statistical analysis
Variables were not found normally distributed at Shapiro test, for this reason, a non-parametric approach was chosen for the statistical analysis. All subjects’ characteristics were evaluated at baseline (T0). Quantitative variables were summarized using percentiles, median and interquartile range and median values were estimated by means of 95% confidence interval (95% CI); qualitative variables were summarized using absolute and percentage frequencies. Absolute variations between T0 and after 15 days of diet supplemented with barley-vegetable soup were evaluated for the plasma lipid profile (TC, LDL-C, HDL-C and TG), plasma levels of glucose, of biochemical markers of oxidative damage (lipid hydroperoxides, ox-LDL, ox-LDL/LDL levels) and of the antioxidant variables (ORAC values and levels of lutein and β-carotene); positive values indicated a decrease of their levels. Median values of the absolute variations were estimated by means of 95% CI and differences between T0 and T15 were evaluated by Wilcoxon signed-rank test. Besides, glucose and each variable of the lipid profile, biochemical markers of oxidative damage and of the antioxidant variables measured at T0 were dichotomized and comparisons on the absolute variation of the variables between subjects with basal values under and over the median value were carried out by Wilcoxon rank sum test. The statistical significance was assessed at a level of probability of 0.05 and Benjamini and Hochberg procedure was used to adjust p values since multiple testing on the same data set were performed. Quantile regression analysis was fitted to evaluate the effect of variables associated to the absolute variation of the lipid profile, glucose, biochemical markers of oxidative damage and of the antioxidant variables, considered as dependent variables for each regression model. Each dependent variable dichotomized at their basal median value was used as independent variable in each regression model and age, gender and BMI as covariates. When lipid hydroperoxides, ORAC and ox-LDL were investigated, the percentage variation on plasma levels of lutein and β-carotene after 15 days of diet were added as covariates. The results of the quantile regression analysis were expressed as point and interval estimates of regression coefficients; when the 95% CIs did not included 0 value, the regression coefficients were considered statistically significant. All statistical analyses were performed using R statistical package, ver. 3.0.2.
Results
Post prandial glucose response
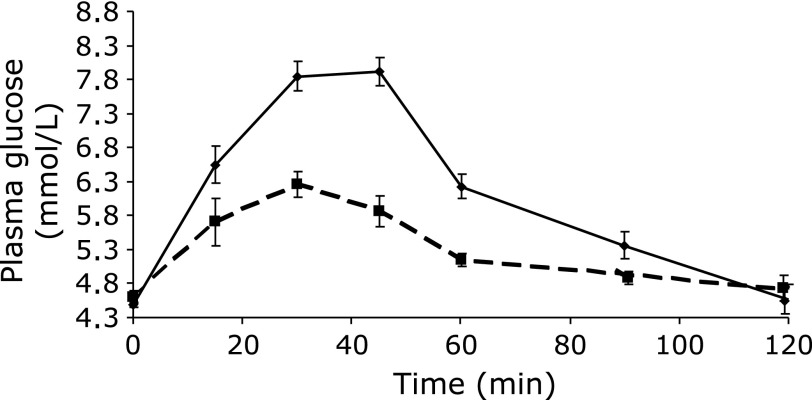
Fasting blood glucose concentrations in healthy subjects were similar before each test meal (4.47 ± 0.07 mM). Fig. 1 shows the mean blood-post-prandial glucose response following consumption of the product and glucose. The post-prandial glucose response demonstrated a peak value at 30 min and a return to baseline within two hours (Fig. 1). The increase of glyceamia was lower after intake of the product and the glyceamic index (GI) and the glyceamic load (GL) values were 36.3 ± 3.7% and 9.2 ± 0.9%, respectively.
Fig. 1.
Mean blood glucose concentrations in healthy volunteers (n = 10) following ingestion of glucose (50 g) and a portion of barley-vegetable soup containing 50 g of available carbohydrate (values are mean ± SEM).
Effect of short-term consumption of the product
The basal levels of glucose, lipids, markers of lipid peroxidation (lipid hydroperoxides and ox-LDL) and carotenoids (β-carotene and lutein) in plasma of subjects are shown in Table 2. The results of the short-term consumption of the product on plasma glucose, plasma lipids and antioxidants are shown in Table 3. During the diet supplemented with vegetable product, both dietary energy and macronutrient intake of the participants were well balanced according to the Italian recommendations for adults (Table 4). Changes in BMI were not significant during the study (data not shown).
Table 2.
Subjects’ characteristics measured at baseline
| Variables measured at baseline | n | Median (1°–3° quartiles) [95% CI] |
|---|---|---|
| Total cholesterol (mmol/L) | 38 | 4.8 (4.5–5.3) [4.6–5.1] |
| LDL-C (mmol/L) | 38 | 3 (2.6–3.3) [2.8–3.1] |
| HDL-C (mmol/L) | 38 | 1.5 (1.2–1.6) [1.4–1.6] |
| Triacylglycerol (mmol/L) | 38 | 0.8 (0.6–1.1) [0.7–0.9] |
| Glucose (mmol/L) | 38 | 5.1 (4.5–5.3) [4.9–5.2] |
| Lipid hydroperoxides (µmol/L) | 38 | 2.2 (1.8–2.6) [2–2.4] |
| ORAC [µmol(TE)/L] | 38 | 16,523.7 (14,404.4–18,933.4) [15,362.9–17,684.5] |
| ox-LDL (U/L) | 38 | 46.8 (31.7–56.1) [40.6–53.1] |
| ox-LDL/LDL (U/mmol) | 38 | 14.5 (12.2–17.8) [13.1–16] |
| Lutein (µg/ml) | 26 | 0.2 (0.2–0.3) [0.2–0.3] |
| β-Carotene (µg/ml) | 26 | 0.2 (0.1–0.3) [0.2–0.3] |
Table 3.
Absolute variation (T0–T15) of subjects’ lipid profile, glucose levels, biochemical markers of oxidative damage and of the antioxidant variables
| Variables | n | median (1°–3° quartiles) [95% CI] | p |
|---|---|---|---|
| Total cholesterol (mM) | 38 | 0.3 (0.2–0.4) [0.2–0.4] | <0.001 |
| LDL-C (mM) | 38 | 0.3 (0.1–0.5) [0.2–0.4] | <0.001 |
| HDL-C (mM) | 38 | 0 (–0.2–0.1) [–0.1–0.1] | 0.262 |
| Triacylglycerol (mM) | 38 | 0.1 (0–0.2) [0–0.1] | <0.001 |
| Glucose (mM) | 38 | 0.2 (0.1–0.4) [0.1–0.3] | <0.001 |
| Lipid hydroperoxides (µM) | 38 | 0.4 (0.2–0.7) [0.3–0.5] | <0.001 |
| ORAC [µM (TE)] | 38 | –1,008.3 (–1,767.8– –319.1) [–1,379.6– –636.9] | <0.001 |
| ox-LDL (U/L) | 38 | 8 (5.1–13) [6–10] | <0.001 |
| ox-LDL/LDL (U/mmol) | 35 | 1.5 (1–1.9) [1.3–1.7] | <0.001 |
| Lutein (µg/ml) | 26 | –0.1 (–0.2– –0.1) [–0.1– –0.1] | <0.001 |
| β-Carotene (µg/ml) | 26 | –0.1 (–0.1–0) [–0.1–0] | <0.001 |
p values refer to Wilcoxon rank sum test, adjusted for multiple testing by Benjamini and Hochberg.
Table 4.
Energy, macronutrient and micronutrient intake of the subjects through the baseline diet and contribution of the vegetable product daily dose evaluated by 15-day dietary record.
| Diet | LARN | |
|---|---|---|
| Energy (kcal/day) | 1,649 ± 349 | 2,000 |
| Protein (g/day) | 64.1 ± 15.9 | 63 |
| Carbohydrate (g/day) | 209 ± 39 | 250 |
| Fiber (g/day) | 20.4 ± 5.5 | 25 |
| Fat (g/day) | 56.4 ± 14.1 | 66 |
| saturated (g/day) | 15.3 ± 5.6 | <22 |
| monounsaturated (g/day) | 25.1 ± 6.3 | |
| Vitamin C (mg/day) | 64.4 ± 25.2 | 95 |
| Folate (mg/day) | 0.18 ± 0.05 | 0.4 |
| α-Tocopherol (mg/day) | 8.4 ± 2.5 | 12 |
| Niacin (mg/day) | 15.3 ± 4.3 | 18 |
| Retinol (mg/day) | 0.8 ± 0.2 | 0.7 |
| Riboflavin (mg/day) | 1.14 ± 0.27 | 1.4 |
| Thiamine (mg/day) | 0.972 ± 0.17 | 1.1 |
Plasma lipid and glucose.
Absolute variations in the plasma lipid profile and plasma glucose levels after 15 days of diet are presented in Table 3. Significant reductions were found in the plasma levels of total cholesterol (mean percentage decrease, 6.5%) and LDL cholesterol (mean percentage decrease, 10.8%), triacylglycerol (mean percentage decrease, 8.3%) and glucose (mean percentage decrease, 4.6%) (p<0.001). No significant difference was found in the absolute variation of HDL cholesterol levels (p = 0.262).
Markers on lipid peroxidation.
The basal levels of ox-LDL and lipid hydroperoxides were similar to those reported by us and other authors in previous studies on healthy subjects (Table 2).(32,34,35) Significant decrease of the plasma levels of lipid hydroperoxides, ox-LDL and ox-LDL/LDL ratio was found after 15 days of supplemented diet (Table 3).
Plasma concentrations of carotenoids and total antioxidant capacity.
Basal plasma levels of lutein ranged from 0.19 to 0.5 mg/ml and basal plasma levels of β-carotene ranged from 0.09 to 0.79 mg/ml, the median values are in agreement with other authors.(32,36) Table 3 shows the absolute variation in plasma concentrations of lutein and β-carotene after daily intake of the experimental product (250 g) containing 5.2 mg lutein and 5.3 mg β-carotene compared with baseline. A significant increase of plasma carotenoids (lutein and β-carotene) was found after 15 days of supplemented diet (p<0.001). The percentage increase was 48 and 59% for lutein and β-carotene, respectively. A significantly higher total antioxidant capacity has been also observed in subjects after supplemented diet (p<0.001).
Response to dietary intervention based on pre-intervention status.
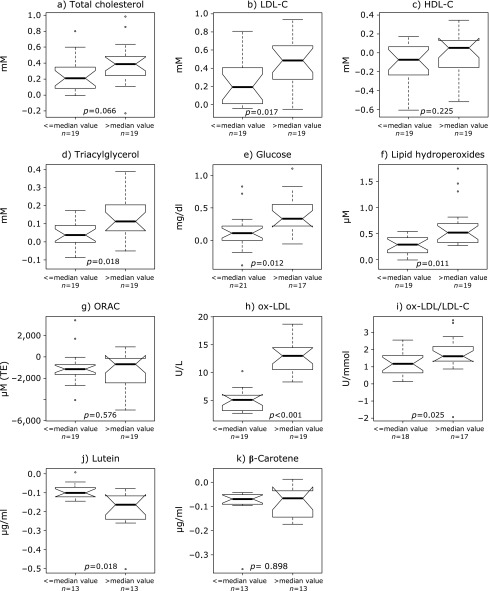
As shown in Fig. 2, subanalysis of data according to their baseline values highlighted a significantly higher increase of lutein levels and higher decrease of lipids (LDL-C, triacylglycerol), glucose levels as well as markers of lipid peroxidation (lipid hydroperoxides, ox-LDL, ox-LDL/LDL ratio) in those individuals that for the same parameters had baseline values over the median level. Results of the multiple quantile regression analysis are shown in Table 5. A significant higher absolute variation of TC, LDL-C, tryacilglycerol, glucose and ox-LDL was associated with the dichotomized variable; a higher reduction of the variable levels was associated with those individuals with basal values over the median level; higher increase of lutein values were associated with those individuals with basal values over the median level. Significant higher decrease of LDL-C and higher increase of β-carotene levels were associated with females, while higher decrease of HDL-C and triacylglycerol were associated with the male gender. The reduction of TC levels significantly decreased in older age individuals. No significant association was found between the percentage variation of lutein, β-carotene and the absolute variation of ORAC, ox-LDL and lipid hydroperoxides levels.
Fig. 2.
Absolute variation (T0–T15) of subjects’ lipid profile, glucose, biochemical markers of oxidative damage and of anti-oxidant variables in individuals with baseline values under and over the median of the plotted variable. P values refer to Wilcoxon rank sum test, adjusted for multiple testing by Benjamini and Hochberg.
Table 5.
Effect of the variables that influenced the absolute variation (T0–T15) of lipid profile, glucose, biochemical markers of oxidative damage and of anti-oxidant variables
| Dependent variables (absolute variation) | Dependent variable at baseline (> vs <= median values) Regression coefficiden (95% CI) | Gender (Male vs Female) Regression coefficiden (95% CI) | Age (10 years) Regression coefficiden (95% CI) | BMI (kg/m2) Regression coefficiden (95% CI) | Lutein variation (%) Regression coefficiden (95% CI) | β-carotene variation (%) Regression coefficiden (95% CI) |
|---|---|---|---|---|---|---|
| Total cholesterol (mM) | 0.16 (0.12–0.28) | –0.01 (–0.11–0.14) | –0.06 (–0.13– –0.04) | –0.03 (–0.03–0.03) | ||
| LDL-C (mM) | 0.37 (0.2–0.53) | –0.15 (–0.37– –0.08) | 0.01 (–0.12–0.03) | –0.04 (–0.04–0.03) | ||
| HDL-C (mM) | 0.01 (–0.08–0.11) | 0.13 (0.08–0.3) | 0 (–0.05–0.22) | –0.01 (–0.05– –0.003) | ||
| Triacylglycerol (mM) | 0.08 (0.06–0.13) | 0.06 (0.01–0.11) | –0.01 (–0.03–0.04) | 0.01 (–0.02–0.01) | ||
| Glucose (mM) | 0.25 (0.08–0.62) | –0.05 (–0.44–0.19) | 0 (–0.01–0.1) | 0.04 (–0.01–0.06) | ||
| ox-LDL/LDL (U/mmol) | 0.93 (0–1.24) | 0.07 (–0.26–1.05) | 0.03 (–0.18–0.52) | 0.06 (–0.07–0.07) | ||
| Lutein (µg/ml) | –0.05 (–0.09– –0.01) | 0 (–0.02–0.06) | –0.02 (–0.08–0.03) | 0 (0–0.01) | ||
| β-Carotene (µg/ml) | 0 (–0.07–0.05) | 0.01 (0.004–0.07) | –0.02 (–0.04–0) | 0 (–0.03–0) | ||
| ORAC [µM (TE)] | 360.67 (–1,246.77–2,023.27) | 203.05 (–1,029.25–1,785.27) | –384.67 (–954.97–2,163.23) | 61.36 (–900.47–126.26) | –4.61 (–27.2–26.47) | –4.75 (–17.8–39.99) |
| ox-LDL (U/L) | 9.29 (5.89–11.74) | 0.16 (–3.09–1.48) | –1.43 (–3.6–0.38) | 0.17 (–0.33–0.92) | 0.02 (–0.01–0.05) | –0.01 (–0.02–0.05) |
| Lipid hydroperoxides (µM) | 0.22 (–0.02–0.62) | –0.7 (–2.77–1.47) | 0.01 (–0.15–0.3) | –0.03 (–0.13–0.06) | 0 (–0.01–0) | 0 (–0.01–0.01) |
Discussion and Conclusion
Since 1981, when Jenkins showed that complex carbohydrates are digested more slowly and raise blood glucose less than simple sugars,(37) many studies have been performed to investigate the physio-pathological relevance of postprandial hyperglycaemia.(38,39) There is much scientific and popular interest in the role of low glycaemic index foods. Several public health organizations have recently integrated consideration of the glycaemic index in their nutritional recommendations for patients with metabolic diseases and for the general population.(40,41) Increasing attention has been addressed also to vegetable foods for their key role against the development of human chronic degenerative disease due to their peculiar composition in phytochemicals. The glyceamic index of the product containing a combination of barley and pigmented vegetables is 36 therefore it could be considered a low glyceamic index food.(31) The present study shows also that the short-term daily consumption of the low glycaemic product produces a significant decrease of plasma glucose levels. Moreover a significant decrease of plasma lipids (TC, LDL-C and triacylglycerols) and plasma levels of biochemical markers of lipid peroxidation (lipid hydroperoxides and ox-LDL) has been demonstrated. The effect on plasma lipids and plasma glucose is likely related to the peculiar chemical composition of the vegetable soup that contains 9.3 g of fibres with about one fourth (2.3 g) represented by soluble ones. Furthermore, a portion of the vegetable soup provides about 2 g of pectins and 2 g of β-glucans, the effect of both these nutritional factors on plasma cholesterol and glucose levels has been previously demonstrated.(19,20,42) In the present study we demonstrated that after integrating the daily diet with a portion of “barley-vegetable soup” containing 5.2 mg lutein and 5.3 mg β-carotene, plasma lutein and β-carotene concentration increased respectively by 59% by 48%. Our results demonstrate that carotenoids are bioavailable in the proposed food matrix, although to a lower extent in comparison to other vegetable foods. In fact in our previous study we demonstrated that the daily intake for two weeks of a portion of the vegetable combination of black and red cabbage (containing 1 mg lutein and 0.6 mg β-carotene) increased plasma β-carotene and lutein concentration by 80 and 204%, respectively compared with the baseline.(32) This could be due to different proportions of carotenoids present in the tested food matrix stressing the role of their relative composition in modulating the bioavailability.(43) Moreover other dietary factors such soluble fiber (such as pectins) are known to influence the bioavailability of carotenoids.(44) Previous human studies have demonstrated that the increase of plasma carotenoids is modulated by their basal levels.(32,45) In agreement with these data, we observed a higher increase of lutein levels in plasma of those individuals that had baseline values over the median level. Moreover the decrease of markers of lipid peroxidation was greater in subjects whose basal levels were higher suggesting a relationship between basal level of oxidative stress and modification of plasma lipid peroxidation after dietary intake of vegetable soup, in agreement with other authors.(32,46–48)
Different factors may have contributed to the beneficial effect exerted by the short term intake of the product on plasma lipid peroxidation. Concerning the molecular mechanisms, previous studies have shown a relationship between consumption of fruit and vegetable, increase in serum antioxidants and decrease of markers of lipoprotein peroxidation (ox-LDL and F2-isoprostane).(49–55) We suggest that the decrease in oxidized LDL is the result of both a decrease in LDL-cholesterol concentrations and an increase in serum antioxidants, such as lutein and β-carotene. Beside the direct beneficial effects on the plasma lipid profile, a potential synergistic effect is exerted by low glyceamic index of the studied food on LDL oxidation could not be excluded, in agreement with previous reports.(49) In fact it is well known that postprandial hyperglycaemia increases oxidative stress in susceptible individuals.(56)
The frozen ready-to-eat vegetable product represents a good dietary source of bioactive compounds in a dose that has been demonstrated to be efficient in inducing in vivo a beneficial effect against lipid peroxidation and in improving lipid profile and glycaemia. These effects could be due to single components and/or to the adjunctive and synergistic effects of phytochemicals contained in barley and pigmented vegetables. High levels of LDL-cholesterol and of oxidized LDL are biochemical markers for atherosclerosis and other human chronic degenerative diseases.(57,58) Therefore we suggest that products rich in soluble fibers, carotenoids and other phytochemicals could be inserted in dietary intervention aimed to prevent dismetabolic diseases and their complications. Despite that dietary health effects are influenced also by genetic factors,(59) an accurate selection of vegetable foods results in palatable, convenient and healthy product with physiological effects on plasma lipids and lipid peroxidation. The growing consumers’ demand for healthier foods is focusing the interest of the food industry towards the area of functional foods and functional food ingredients. The results obtained in the present study could be useful in the development of new healthy plant food products characterized by low glycaemic index and high content of antioxidants.
Acknowledgments
The work has been supported by Ricerca Scientifica di Ateneo (ex 60%) (T.B., L.T., G.F.) and by financial support of Italsur s.r.l. (grant to S.M.).
Abbreviations
- BMI
body mass index
- FOX
ferrous oxidation-xylenol orange assay
- GI
glycaemic index
- GL
glycaemic load
- HDL-C
high density lipoprotein cholesterol
- iAUC
incremental area under the blood glucose curve
- LDL
low density lipoproteins
- LDL-C
low density lipoprotein cholesterol
- ox-LDL
oxidized low density lipoproteins
- ORAC
Oxygen Radical Absorbance Capacity
- TC
total cholesterol
- TG
triacylglycerols
Conflict of Interest
No potential conflicts of interest were disclosed.
Authors’ Contribution
All authors contributed to the intellectual development of this work and approved the final manuscript. T.B. and G.F. were responsible for the experimental design, coordination of research and preparation of the manuscript; T.B. and S.M. carried out evaluations of plasma biochemical parameters (plasma lipids, markers of lipid peroxidation) and participated in the preparation of the manuscript; D.T. was actively involved in the chemical and agronomic investigation of plant species used to make the frozen product; L.T., F.B., S.S. and P.O. carried out evaluations of plasma carotenoids and contributed to the preparation of the manuscript. R.G. and E.S. carried out the statistical analysis and contributed to the preparation of the manuscript.
References
- 1.Baboota RK, Bishnoi M, Ambalam P, et al. Functional food ingredients for the management of obesity and associated co-morbidities - A review. J Funct Foods. 2013;5:997–1012. [Google Scholar]
- 2.Liu RH. Dietary bioactive compounds and their health implications. J Food Sci. 2013;78:A18–A25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 3.Magrone T, Perez de Heredia F, Jirillo E, Morabito G, Marcos A, Serafini M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can J Physiol Pharm. 2013;91:387–396. doi: 10.1139/cjpp-2012-0307. [DOI] [PubMed] [Google Scholar]
- 4.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 5.Kugler F, Stintzing FC, Carle R. Identification of betalains from petioles of differently colored Swiss chard (Beta vulgaris L. ssp. cicla [L.] Alef. Cv. Bright Lights) by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem. 2004;52:2975–2981. doi: 10.1021/jf035491w. [DOI] [PubMed] [Google Scholar]
- 6.Ninfali P, Bacchiocca M, Antonelli A, et al. Characterization and biological activity of the main flavonoids from Swiss Chard (Beta vulgaris subspecies cycla) Phytomedicine. 2007;14:216–221. doi: 10.1016/j.phymed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Ninfali P, Bacchiocca M. Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. J Agric Food Chem. 2003;51:2222–2226. doi: 10.1021/jf020936m. [DOI] [PubMed] [Google Scholar]
- 8.Proteggente AR, Pannala AS, Paganga G, et al. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic Res. 2002;36:217–233. doi: 10.1080/10715760290006484. [DOI] [PubMed] [Google Scholar]
- 9.Grassmann J, Schnitzler WH, Habegger R. Evaluation of different coloured carrot cultivars on antioxidative capacity based on their carotenoid and phenolic contents. Int J Food Sci Nutr. 2007;58:603–611. doi: 10.1080/09637480701359149. [DOI] [PubMed] [Google Scholar]
- 10.Kopsell DA, Kopsell DE, Lefsrud MG, Curran-Celentano J, Dukach LE. Variation in lutein, beta-carotene, and chlorophyll concentrations among Brassica oleracea cultigens and seasons. Hortscience. 2004;39:361–364. [Google Scholar]
- 11.Ko SH, Park JH, Kim SY, Lee SW, Chun SS, Park E. Antioxidant Effects of Spinach (Spinacia oleracea L.) Supplementation in Hyperlipidemic Rats. Prev Nutr Food Sci. 2014;19:19–26. doi: 10.3746/pnf.2014.19.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Son CW, Kim MY, et al. Red beet (Beta vulgaris L.) leaf supplementation improves antioxidant status in C57BL/6J mice fed high fat high cholesterol diet. Nutr Res Pract. 2009;3:114–121. doi: 10.4162/nrp.2009.3.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MY, Cheong SH, Kim MH, et al. Leafy vegetable mix supplementation improves lipid profiles and antioxidant status in C57BL/6J mice fed a high fat and high cholesterol diet. J Med Food. 2009;12:877–884. doi: 10.1089/jmf.2008.1125. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia AL, Jain M. Spinacia oleracea L. protects against gamma radiations: a study on glutathione and lipid peroxidation in mouse liver. Phytomedicine. 2004;11:607–615. doi: 10.1016/j.phymed.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104:1322–1332. doi: 10.1017/S0007114510002308. [DOI] [PubMed] [Google Scholar]
- 16.Kural BV, Kücük N, Yücesan FB, Orem A. Effects of kale (Brassica oleracea L. var. acephala DC) leaves extracts on the susceptibility of very low and low density lipoproteins to oxidation. Indian J Biochem Biophys. 2011;48:361–364. [PubMed] [Google Scholar]
- 17.Melega S, Canistro D, De Nicola GR, Lazzeri L, Sapone A, Paolini M. Protective effect of Tuscan black cabbage sprout extract against serum lipid increase and perturbations of liver antioxidant and detoxifying enzymes in rats fed a high-fat diet. Br J Nutr. 2013;113:988–997. doi: 10.1017/S0007114513000068. [DOI] [PubMed] [Google Scholar]
- 18.Pins JJ, Kaur H. A review of the effects of barley beta-glucan on cardiovascular and diabetic risk. Cereal Food World. 2006;51:8–11. [Google Scholar]
- 19.ESFA Panel on Dietetic Products, Nutrition and Allergics (NDA). Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 EFSA Journal 201192207 [Google Scholar]
- 20.ESFA Panel on Dietetic Products, Nutrition and Allergics (NDA). Scientific Opinion on the substantiation of a health claim related to barley beta-glucans and lowering of blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006 EFSA Journal 201192471 [Google Scholar]
- 21.Abdel-Aal ESM, Gamel TH. Effects of selected barley cultivars and their pearling fractions on inhibition of human LDL oxidation in vitro using a modified conjugated dienes method. Cereal Chem. 2008;85:730–737. [Google Scholar]
- 22.Gamel TH, Abdel-Aal EM. Phenolic acids and antioxidant properties of barley wholegrain and pearling fractions. Agr Food Sci. 2012;21:118–131. [Google Scholar]
- 23.Madhujith T, Shahidi F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L.) cultivars and their potential for inhibition of low-density lipoprotein (LDL) cholesterol oxidation. J Agric Food Chem. 2007;55:5018–5024. doi: 10.1021/jf070072a. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Shin JH, Lee SS. Cardioprotective effects of diet with different grains on lipid profiles and antioxidative system in obesity-induced rats. Int J Vitam Nutr Res. 2012;82:85–93. doi: 10.1024/0300-9831/a000097. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, Son BK, Lee SS. Effects of adlay, buckwheat, and barley on transit time and the antioxidative system in obesity induced rats. Nutr Res Pract. 2012;6:208–212. doi: 10.4162/nrp.2012.6.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldini M, Fabietti F, Giammarioli S, Onori R, Orefice L, Stacchini A. Rapporti ISTISAN 96/34. Metodi di analisi utilizzati per il controllo chimico degli alimenti. [Google Scholar]
- 28.Chen Z, Chen B, Yao SZ. High-performance liquid chromatography/electrospray ionization-mass spectrometry for simultaneous determination of taurine and 10 water-soluble vitamins in multivitamin tablets. Anal Chim Acta. 2006;569:169–175. [Google Scholar]
- 29.Xu BJ, Chang SKC. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Phaseolus vulgaris L.) as affected by thermal processing. J Agric Food Chem. 2009;57:4754–4764. doi: 10.1021/jf900695s. [DOI] [PubMed] [Google Scholar]
- 30.Wu XL, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- 31.Food products. Determination of the glycaemic index (GI) and recommendation for food classification ISO 26642:2010 [Google Scholar]
- 32.Bacchetti T, Tullii D, Masciangelo S, et al. Effect of black and red cabbage on plasma carotenoid levels, lipid profile and oxidized low density lipoprotein. J Funct Foods. 2014;8:128–138. [Google Scholar]
- 33.Fernández-Pachón MS, Villaño D, Troncoso AM, García-Parrilla MC. Antioxidant capacity of plasma after red wine intake in human volunteers. J Agric Food Chem. 2005;53:5024–5029. doi: 10.1021/jf0501995. [DOI] [PubMed] [Google Scholar]
- 34.Zuliani G, Morieri ML, Volpato S, et al. Determinants and clinical significance of plasma oxidized LDLs in older individuals. A 9 years follow-up study. Atherosclerosis. 2013;226:201–207. doi: 10.1016/j.atherosclerosis.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee D, Madhusoodanan UK, Sharanabasappa M, Ghosh S, Jacob J. Measurement of plasma hydroperoxide concentration by FOX-1 assay in conjunction with triphenylphosphine. Clinica Chimica Acta. 2003;337:147–152. doi: 10.1016/j.cccn.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Al-Delaimy WK, van Kappel AL, Ferrari P, et al. Plasma levels of six carotenoids in nine European countries: report from the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2004;7:713–722. doi: 10.1079/phn2004598. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 38.Blaak EE, Antoine JM, Benton D, et al. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13:923–984. doi: 10.1111/j.1467-789X.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livesey G. Low-glycaemic diets and health: implications for obesity. Proc Nutr Soc. 2005;64:105–113. doi: 10.1079/pns2004400. [DOI] [PubMed] [Google Scholar]
- 40.Chiu CJ, Liu S, Willett WC, et al. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr Rev. 2011;69:231–242. doi: 10.1111/j.1753-4887.2011.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins DJ, Willett WC, Astrup A, et al. Glycaemic index: did Health Canada get it wrong? Position from the International Carbohydrate Quality Consortium (ICQC) Br J Nutr. 2014;111:380–382. doi: 10.1017/S0007114513003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ESFA Panel on Dietetic Products, Nutrition and Allergics (NDA). Scientific Opinion on the substantiation of health claims related to pectins and reduction of post-prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No 1924/20061 EFSA Journal 201081747 [Google Scholar]
- 43.Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995;62:604–610. doi: 10.1093/ajcn/62.3.604. [DOI] [PubMed] [Google Scholar]
- 44.Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr. 1999;129:2170–2176. doi: 10.1093/jn/129.12.2170. [DOI] [PubMed] [Google Scholar]
- 45.Riso P, Brusamolino A, Scalfi L, Porrini M. Bioavailability of carotenoids from spinach and tomatoes. Nutr Metab Cardiovasc Dis. 2004;14:150–156. doi: 10.1016/s0939-4753(04)80035-8. [DOI] [PubMed] [Google Scholar]
- 46.Schirrmacher G, Skurk T, Hauner H, Grassmann J. Effect of Spinacia oleraceae L. and Perilla frutescens L. on antioxidants and lipid peroxidation in an intervention study in healthy individuals. Plant Food Hum Nutr. 2010;65:71–76. doi: 10.1007/s11130-009-0152-x. [DOI] [PubMed] [Google Scholar]
- 47.Thompson HJ, Heimendinger J, Gillette C, et al. In vivo investigation of changes in biomarkers of oxidative stress induced by plant food rich diets. J Agric Food Chem. 2005;53:6126–6132. doi: 10.1021/jf050493x. [DOI] [PubMed] [Google Scholar]
- 48.Thompson HJ, Heimendinger J, Sedlacek S, et al. 8-Isoprostane F2alpha excretion is reduced in women by increased vegetable and fruit intake. Am J Clin Nutr. 2005;82:768–776. doi: 10.1093/ajcn/82.4.768. [DOI] [PubMed] [Google Scholar]
- 49.Barona J, Jones JJ, Kopec RE, et al. A Mediterranean-style low-glycemic-load diet increases plasma carotenoids and decreases LDL oxidation in women with metabolic syndrome. J Nutr Biochem. 2012;23:609–615. doi: 10.1016/j.jnutbio.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck J, Ferrucci L, Sun K, et al. Circulating oxidized low-density lipoproteins are associated with overweight, obesity, and low serum carotenoids in older community-dwelling women. Nutrition. 2008;24:964–968. doi: 10.1016/j.nut.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaskins AJ, Rovner AJ, Mumford SL, et al. Adherence to a Mediterranean diet and plasma concentrations of lipid peroxidation in premenopausal women. Am J Clin Nutr. 2010;92:1461–1467. doi: 10.3945/ajcn.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haegele AD, Gillette C, O'Neill C, et al. Plasma xanthophyll carotenoids correlate inversely with indices of oxidative DNA damage and lipid peroxidation. Cancer Epidemiol Biomakers Prev. 2000;9:421–425. [PubMed] [Google Scholar]
- 53.Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr. 2010;140:1093–1098. doi: 10.3945/jn.109.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sánchez-Moreno C, Cano MP, de Ancos B, et al. Pulsed electric fields-processed orange juice consumption increases plasma vitamin C and decreases F2-isoprostanes in healthy humans. J Nutr Biochem. 2004;15:601–607. doi: 10.1016/j.jnutbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Sánchez-Moreno C, Cano MP, de Ancos B, et al. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F2-isoprostanes, prostaglandin E2 and monocyte chemotactic protein-1 in healthy humans. J Nutr Biochem. 2006;17:183–189. doi: 10.1016/j.jnutbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 56.de Castro SH, Castro-Faria-Neto HC, Gomes MB. Association of postprandial hyperglycemia with in vitro LDL oxidation in non-smoking patients with type 1 diabetes--a cross-sectional study. Rev Diabet Stud. 2005;2:157–164. doi: 10.1900/RDS.2005.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR study) Arterioscler Thromb Vasc Biol. 2002;22:1162–1167. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- 58.Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- 59.Wang TT, Edwards AJ, Clevidence BA. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to beta-carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms. J Nutr Biochem. 2013;24:1538–1546. doi: 10.1016/j.jnutbio.2013.01.001. [DOI] [PubMed] [Google Scholar]