Abstract
Secretory immunoglobulin A (sIgA) is produced from intestinal mucosa and is essential in preventing infection. We analyzed the influence of moderate exercise on intestinal sIgA production and antioxidative function under different carbohydrate nutritional conditions. Thirty-six mice were fed an experimental diet for 10 weeks—a high-carbohydrate (HC) diet, a low-carbohydrate (LC) diet, or a control (C) diet. After 1 week on the experimental diets, mice were divided into sedentary and exercise groups (n = 6/group), where the exercise consisted of treadmill running for 30 min/day at 11 m/min for 6 days/week in 9 consecutive weeks. Intestinal sIgA levels in the exercise groups fed C or LC diets were significantly lower compared with the parallel sedentary groups, or exercise-group mice fed HC diet. Expression of the polymeric immunoglobulin receptor (pIgR) in the small intestine was significantly higher in the exercise group fed a HC diet. Superoxide dismutase activity in the small intestine was higher in the exercise group than in the sedentary group, with no effects resulting from intake carbohydrate levels. Our results indicated that moderate exercise reduced the levels of intestinal sIgA depending on decreasing of carbohydrate intake, which is connected with the expression of pIgR.
Keywords: sIgA, moderate exercise, carbohydrate intake, pIgR, SOD
Introduction
Moderate exercise regulates various immune functions, such as antibody production by lymphocytes,(1–4) macrophage phagocytosis,(5) and intestinal secretory immunoglobulin A (sIgA) production.(6,7) However, previous studies have involved subjects that were provided nutritive diets, and it is uncertain whether similar effects of exercise occur under different carbohydrate nutritional conditions. Further studies are required to clearly demonstrate the optimum nutritional conditions required for enhanced immune functions under conditions of moderate exercise. In addition, low-carbohydrate diets are popular dietary approaches for weight control,(8) but concerns have also been raised regarding the risk of development of cardiovascular diseases owing to following such diets for long periods.(9) Although the effects of low-carbohydrate diets on immune functions are unclear, a high-carbohydrate diet (also considered a low-protein condition known as protein-energy malnutrition) is well known to reduce immune functions, especially in terms of secretory IgA (sIgA) production from the intestine.(10) Intestinal sIgA is secreted through the polymeric immunoglobulin receptor (pIgR) from intestinal mucosa, and plays a principal role in the immune system because it prevents infection at early stages by excluding bacteria and viruses from the gastrointestinal tract.(11) However, the effect of moderate exercise on intestinal sIgA production under different carbohydrate nourishment states is not well understood.
Immunity can be influenced by imbalances in nutritional conditions, which are associated with increased oxidative stress.(12) The level of dietary carbohydrate intake also regulate oxidative stress, which varies in different each organs.(13–16) Although Gu et al.(13) stated that a high-carbohydrate diet elevates oxidative stress in the intestine, the carbohydrate nutritional condition is not discussed enough of oxidative effectiveness to intestine. Exercise also causes effects on oxidative stress, differing depending on the intensity of exercise.(17,18) Several studies have shown that continuous moderate exercise reduces oxidative stress by stimulating the production of antioxidant enzymes,(17,19–21) such as superoxide dismutase (SOD). However, it is not clear that whether the reduction of oxidative stress alters the sIgA production in the intestine. Additionally, the influence of exercise may differ under different carbohydrate nourishment states.
In the present study, we analyzed the influence of moderate exercise on intestinal secretory IgA (sIgA) production and antioxidant capacities in mice under different carbohydrate nourishment states, namely a low-carbohydrate diet and high-carbohydrate diet.
Materials and Methods
Animals and diet
Thirty-six 6-week-old male C57BL/6J mice were obtained from Charles River Laboratory, Inc. (Kanagawa, Japan). Mice were randomly housed with 6 animals/cage in a temperature-controlled room at 24°C and maintained on a 12-h light: dark cycle, with the lights being turned on at 21:00. Mice received a control diet (based on AIN-93G, D10012G; Research Diets, Inc., Tokyo, Japan) for 1 week during the acclimation period. The animals were then divided into the following 3 experimental diet groups: a high-carbohydrate (HC) diet (D12052801, Research Diets, Inc.); a low-carbohydrate (LC) diet (D1205280s, Research Diets, Inc.); and a control (C) diet. The composition of the experimental diets is shown in Table 1. The carbohydrate content of the experimental diets was modified by using casein as a protein source. The mineral contents were adjusted to the same level in each of the 3 experimental diets. The mice were allowed free access to food and drinking water throughout the acclimation and experimental periods. Individual mouse weights and food consumption rates per cage were recorded twice weekly. The average food consumption rate per mouse was obtained by dividing by the number of mice per cage. This study complied with the guidelines of the Japanese Council on Animal Research at Daito Bunka University in Saitama, Japan.
Table 1.
Composition of the experimental diets
| Diet group | |||
|---|---|---|---|
| HC | C | LC | |
| Energy rate (% kcal) | |||
| Carbohydrate | 79 | 64 | 44 |
| Protein | 5 | 20 | 40 |
| Fat | 16 | 16 | 16 |
| Energy (kcal/gm) | 4.0 | 4.0 | 4.0 |
| Ingredient (g/kg) | |||
| Casein | 50.0 | 200.0 | 396.0 |
| Cornstarch | 549.5 | 397.5 | 198.5 |
| Maltodextrin 10 | 132.0 | 132.0 | 132.0 |
| Sucrose | 100.0 | 100.0 | 100.0 |
| cellulose | 50.0 | 50.0 | 50.0 |
| Soybean oil | 70.0 | 70.0 | 70.0 |
| Mineral mix S10022G† | 0 | 35.0 | 0 |
| Mineral mix S10022C† | 3.5 | 0 | 3.5 |
| Calcium carbonate | 10.0 | 0 | 12.5 |
| Potassium citrate | 1.0 | 0 | 8.0 |
| Potassium phosphate, monobasic | 8.7 | 0 | 0 |
| Potassium phosphate, dibasic | 3.4 | 0 | 0 |
| Sodium chloride | 2.6 | 0 | 2.6 |
| Vitamin mix V10037† | 10.0 | 10.0 | 10.0 |
| l-Cystine | 0.8 | 3.0 | 6.0 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| t-Butylthdroquinone (mg) | 140.0 | 140.0 | 140.0 |
HC, high-carbohydrate diet; C, control diet; LC, low-carbohydrate diet. †Research Diet, Inc.
Exercise protocol
After 1 week of feeding on the experimental diets, mice were divided into exercise and sedentary groups. All animals in the exercise group were adapted to treadmill running for 30 min/day at 8 m/min on a TMC-200 treadmill (Melquest, Japan) for 7 day prior to beginning the exercise training protocol. Subsequently, mice underwent moderate-intensity aerobic exercise training on a treadmill for 30 min/day at 11 m/min for 6 day/week, for a total of 8 weeks. Serum lactic acid levels were measured in a preliminary experiment to verify that the pace of treadmill running under moderate exercise conditions was suitable for the C57BL/6 mice. All sedentary mice were fasted during the running period of the exercise group. Running exercises were performed during dark cycle periods.
Preparation of fecal extracts
Fecal extracts were prepared as follows. Fresh feces were collected in cold phosphate-buffered saline (PBS; 1 mg of feces in 10 µl of PBS) containing Complete Protease Inhibitor Cocktail (Roche Diagnostics GmbH, Penzberg, Germany), and the mixture was stirred with a micropipette. Subsequently, fecal extracts were obtained by centrifuging samples at 6,000 rpm for 15 min at 4°C and collecting the supernatants. Fecal extracts were collected before the animals were sacrificed and were stored at –80°C until further use.
Tissue sampling
Mice were anaesthetized with diethyl ether and exsanguinated through the orbital cavity. Sera were separated by centrifugation. Immediately after the mice were sacrificed, the kidneys, spleens, livers, thymus, and small intestines were removed and weighted. The lengths of the small intestines were also measured. After small intestines were homogenized with 1% Triton-X (Roche Diagnostics GmbH) lysis buffer containing Complete Protease Inhibitor Cocktail. Subsequently, the homogenates were centrifuged at 5,000 rpm for 15 min at 4°C, and the supernatants were removed. Supernatants were stored in 50 µl aliquots at −80°C until protein analyses were performed.
Determination of total IgA and sIgA levels in fecal extracts
Total IgA and sIgA levels, reflecting specific and non-specific responses, respectively, were determined in enzyme-linked immunosorbent assays (ELISAs). ELISAs were performed using rabbit anti-mouse IgA (Bethyl Laboratories, Montgomery, TX) to coat the plates and biotinylated goat anti-mouse IgA (Bethyl Laboratories) or anti-mouse polymeric immunoglobulin receptor (pIgR; R&D systems, Minneapolis, MN) antibodies for detection. Total IgA levels were estimated by comparison with serial dilutions of mouse IgA (Sigma-Aldrich Corp., St. Louis, MO) as the standard.
Western blot analysis of pIgR expression in the small intestine
The bicinchoninic acid (BCA) protein assay method was used for protein quantification, as described.(18) Homogenized supernatants from small intestines were denatured at 100°C for 5 min with sodium dodecyl sulfate and β-mercaptoethanol, and 80 µg of total protein/sample was separated on 10% denaturing polyacrylamide gels by electrophoresis at 500 V for 1.5 h at room temperature. The proteins were transferred to a polyvinylidene fluoride membrane for 1.5 h at room temperature using Tris-glycine buffer containing 20% methanol. Membranes were blocked with 5% non-fat dry milk in Tris buffer containing 0.05% Tween-20 overnight at 4°C. Membranes were incubated for 1 h at room temperature with constant agitation, using a goat anti-mouse pIgR (1: 4,000 dilution) as the primary antibody. The membranes were washed and incubated for 1 h at room temperature with constant agitation with peroxidase-conjugated anti-goat IgG (H+L; Vector Laboratories, Burlingame, CA) at a 1: 10,000 dilution. After washing, the membranes were incubated for 1 min with Pierce Western Blotting Substrate (Thermo Scientific), and bands were detected using an ImageQuant LAS 4000 mini (Fujifilm, Tokyo, Japan) with an exposure time of 600 s. Densitometric measurements of protein bands were analyzed and quantified using MultiGauge software (Fujifilm).
SOD and thiobarbituric acid-reactive substance (TBARS) assays
SOD production was measured using the chemiluminescent probe 2-methyl-6-p-methoxyphenylethynyl imidazopyrazinone (MPEC; ATTO CO., Tokyo, Japan).(22) Homogenized supernatants from small intestines (10 µl/sample) were mixed with 290 µl of assay buffer containing 10 µM MPEC, placed in an AccuFLEX Lumi 400 Luminometer (Aloka Co., Ltd., Tokyo, Japan), and the relative luminescence intensity per second was measured.
Peroxidative damage to lipid membrane constituents was determined by measuring the production of TBARS (a product of membrane lipid peroxidation), as described by Kikugawa et al.(23) Briefly, homogenates were incubated for 1 h with reaction mixtures at 95°C. Chromogen reaction products were extracted in n-butanol and their concentrations were determined using a Sunrise spectrophotometer (Tecan Japan Co., Ltd, Kanagawa, Japan) at 532 nm. Results are expressed as nmoles/ml.
Statistical analysis
Results are expressed as mean ± SEM. Two-way analyses of variance (ANOVA) were performed to determine statistically significant effects and relationships between exercise and the 3 experimental diets. In cases where the relationship between exercise and carbohydrate diets was statistically significant, the Bonferroni’s post-hoc multiple comparison test was performed to compare groups. All p values of <0.05 were considered statistically significant. All statistical calculations were performed using SPSS ver. 19.0.
Results
Body weights, organ weights, and small intestine length
Ten weeks after experimental diets, body weights of mice fed the HC diet were less than those of mice fed C or LC diets, in both the exercise and sedentary groups, as determined by 2-way ANOVA (Table 2, Diet; p<0.001). Mice in the exercise group weighed significantly less than those in the sedentary group (2-way ANOVA result, Ex = 0.004).
Table 2.
Body weights, organ weights, and small intestine lengths
| Body weights (g) |
Organ weights (mg) |
Small intestine length (cm) | |||||
|---|---|---|---|---|---|---|---|
| Group | 0 week | 10 weeks | Kidney | Spleen | Liver | Thymus | |
| Exercise group | |||||||
| HC | 20.2 ± 0.3 | 24.87 ± 0.5 | 219.0 ± 8.3 | 62.5 ± 6.2 | 816.7 ± 45.1 | 42.0 ± 2.0 | 31.1 ± 0.5 |
| C | 19.7 ± 0.5 | 28.62 ± 1.0 | 322.0 ± 30.0 | 99.7 ± 19.8 | 1123.7 ± 36.2 | 49.5 ± 4.9 | 31.2 ± 1.1 |
| LC | 19.9 ± 0.3 | 28.09 ± 1.1 | 337.0 ± 5.2 | 66.7 ± 3.9 | 1310.0 ± 39.0 | 45.8 ± 5.3 | 31.8 ± 0.7 |
| Sedentary group | |||||||
| HC | 20.5 ± 0.3 | 26.47 ± 1.0 | 243.5 ± 11.6 | 88.8 ± 7.0 | 821.0 ± 72.4 | 46.2 ± 2.8 | 30.8 ± 0.7 |
| C | 20.0 ± 0.4 | 31.32 ± 1.0 | 314.7 ± 16.7 | 91.0 ± 10.4 | 1236.3 ± 68.6 | 48.5 ± 5.6 | 32.4 ± 0.7 |
| LC | 20.0 ± 0.3 | 31.83 ± 1.5 | 332.7 ± 5.6 | 77.7 ± 2.4 | 1350.0 ± 51.3 | 50.0 ± 2.8 | 31.5 ± 1.1 |
| Two-way-ANOVA† | |||||||
| Diet | 0.702 | <0.001 | <0.001 | 0.128 | <0.001 | 0.590 | 0.728 |
| Ex | 0.522 | 0.004 | 0.758 | 0.315 | 0.461 | 0.363 | 0.674 |
| Diet × Ex | 0.491 | 0.603 | 0.659 | 0.357 | 0.606 | 0.665 | 0.745 |
The values shown are mean ± SEM. HC, high-carbohydrate diet; C, control diet; LC, low-carbohydrate diet.
†Two-way ANOVA (p values) for the effects of various protein diets (Diet), the effect of exercise (Ex), and their interactive effects (Diet × Ex).
Kidney and liver weights were significantly lower in both exercise and sedentary group mice fed the HC diet (Table 2, 2-way ANOVA result, Diet: p<0.001), compared to mice in either group that were fed other diets. Carbohydrate intake and exercise did not influence spleen or thymus weights, or small intestine lengths.
Total IgA and sIgA production in fecal extracts
The effects of diet and exercise on IgA levels in fecal extracts were explored. Total IgA was shown the same tendency on carbohydrate intake in the exercise group and sedentary group. Total IgA levels were markedly lower in both the exercise and sedentary mouse groups fed the LC diet, compared with mice fed HC and C diets (Fig. 1A, 2-way ANOVA result, Diet: p = 0.011). The impact of carbohydrate intake on sIgA production differed between the exercise group and sedentary group. In the exercise group, sIgA levels were significantly higher in mice fed the HC diet than in mice fed the C or LC diets (Fig. 1B, p<0.001). In the sedentary group, no significant differences in sIgA levels were observed, irrespective of their diets. In addition, exercise group mice fed either the LC or C diet had significantly lower sIgA levels than did parallel mice in the sedentary group. (Fig. 1B, p = 0.01).
Fig. 1.
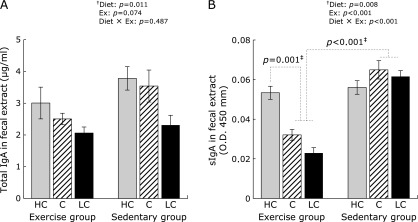
Determination of total IgA and secretory (sIgA) levels in fecal extracts. The levels of total IgA (A) and sIgA (B) in fecal extract were determined by enzyme-linked immunosorbent assays (ELISAs). Fecal extracts were collected just before mice were sacrificed. Data shown are mean ± SEM. HC, high-carbohydrate diet; C, control diet; LC, low-carbohydrate diet. †Two-way analysis of variance results (p values) comparing the effects of various carbohydrate diets (Diet), exercise (Ex), and their interactive effects (Diet × Ex) are displayed in the upper right panel. ‡The multiple-comparison Bonferroni’s post-hoc test was used to compare each group in cases where the interactive effect (Diet × Ex) was statistically significant.
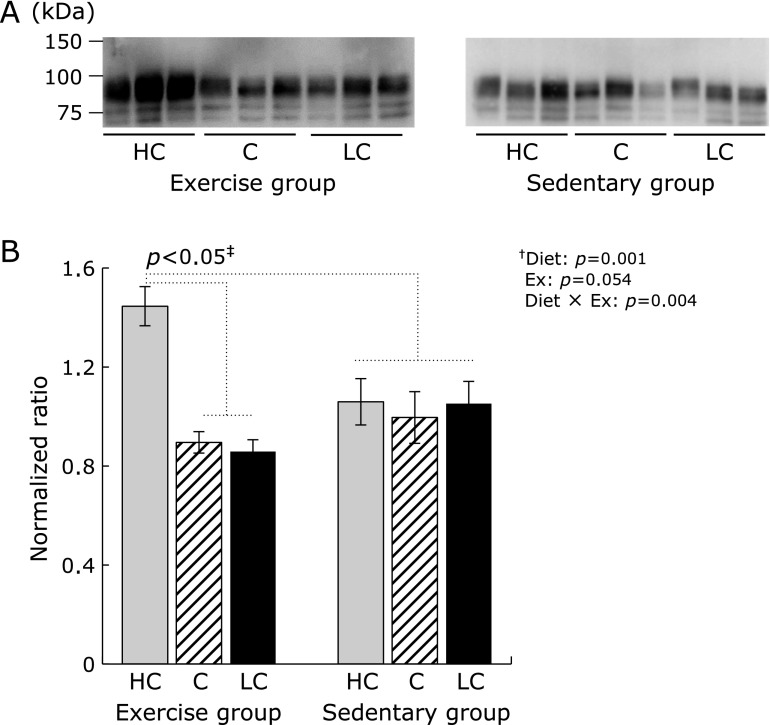
pIgR expression in the small intestine
Differences in pIgR expression in the small intestines depended upon carbohydrate intake and exercise status (i.e., exercise vs sedentary group mice). In the exercise group, pIgR expression was significantly higher in mice fed an HC diet compared to mice fed other diets (Fig. 2, p<0.05). In the sedentary group, no significant differences in pIgR levels were observed, irrespective of their diets. Additionally, pIgR expression, in mice in the sedentary group regardless of experimental diets, showed a significantly low levels compared to mice fed HC diet of exercise group (p<0.05).
Fig. 2.
Expression of the polymeric immunoglobulin receptor (pIgR) in the small intestine. HC, high-carbohydrate diet; C, control diet; LC, low-carbohydrate diet. (A) pIgR expression in the small intestine was determined by western blot analysis. Small intestines were collected after the mice were sacrificed. pIgR bands were detected at ~80 kDa. (B) Data shown are the mean ± SEM. †Two-way analysis of variance results (p values) of the effects of various carbohydrate diets (Diet), exercise (Ex), and their interactive effects (Diet × Ex) are displayed in the upper right panel. ‡The multiple-comparison Bonferroni’s post-hoc test was used to compare each group in cases where the interactive effect (Diet × Ex) was statistically significant.
Antioxidant capacities in the small intestine
As an index of oxidative stress, we studied SOD activity as a measure of the antioxidant capacity, with lipid peroxide as an example of oxidation products. SOD activity in the small intestine was higher in the exercise group than in the sedentary group (Fig. 3A, 2-way ANOVA result, Ex: p<0.001). In the exercise group, no significant differences in SOD activities were observed, irrespective of their diets. In the sedentary group, the lowest SOD activities were measured in mice fed the C diet. TBARS production in the small intestine decreased upon exercise, although these differences were not statistically significant (Fig. 3B, 2-way ANOVA result, Ex: p = 0.055).
Fig. 3.
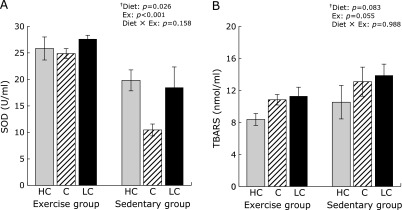
Antioxidant activity in the small intestine. Superoxide dismutase (SOD) activity (A) and thiobarbituric acid-reactive substance (TBARS) production (B) in the small intestine. Data shown are mean ± SEM. HC, high-carbohydrate diet; C, control diet; LC, low-carbohydrate diet. †Two-way ANOVA results (p values) of the effects of various carbohydrate diets (Diet), exercise (Ex), and their interactive effects (Diet × Ex) are displayed in the upper right panel. ‡The multiple-comparison Bonferroni’s post-hoc test was used to compare each group in cases where the interactive effect (Diet × Ex) was statistically significant.
Discussion
In the present study, we analyzed the effects of exercise and differing dietary carbohydrate intake levels on intestinal sIgA production in C57BL/6J mice. The major findings of this study were as follows: (i) the impact of carbohydrate intake on intestinal sIgA production was different between exercise group and sedentary group mice, and (ii) moderate exercise caused a decrease in intestinal sIgA production, depending on the reduction of carbohydrate intake, which was related to decreased pIgR expression.
sIgA serves important roles in preventing infection by excluding bacteria and viruses, and regulates homeostasis of intestinal immune functions.(11,24) Our results with mice fed the standard C diet showed that sIgA levels were decreased in the exercise group, compared with the sedentary group. These data suggest that moderate exercise may cause sIgA decomposition, thereby increasing the risks for infection. In contrast, previous studies showed that moderate exercise led to increased sIgA production in the small intestines of mice and in human mucosal sIgA levels.(1,2,6,7) These findings differed from our results, which may indicate that sIgA production is affected by exercise level and duration. The decrease in sIgA resulting from moderate exercise could be attributed to from increased oxidative stress, which can negatively affect lymphocyte function.(12,25) However, SOD activities and TBARS levels were not affected by exercise in mice fed the C diet, showing that sIgA production does not appear to affect antioxidant capacity. Because we measured only the SOD activity of antioxidant enzymes, it is possible that other enzymes such as glutathione peroxidase and glutathione-S-transferase were affected by moderate exercise and caused a decrease in sIgA levels.(17,26) Only limited studies into the relationship between antioxidant enzymes and sIgA, as well as the effect of exercise on intestinal sIgA have been performed. Thus, this area of research requires further examination.
In exercising mice, the decrease in IgA production levels, especially those of sIgA, was proportional to a decline of carbohydrate intake. sIgA levels were highest in exercising mice fed the HC diet and correlated with increased pIgR expression. pIgR stabilizes sIgA production in the small intestine by protecting sIgA molecules against proteases(24,26,27) and is associated with TNF-α expression.(28) We suggest that TNF-α enhances pIgR expression, because TNF-α has been reported to increase with moderate exercise(9) and high-carbohydrate conditions.(29) Therefore, it will be important to clarify the relationship between TNF-α and pIgR expression under moderate exercise conditions in future studies.
Our results indicate that the impact of carbohydrate intake on sIgA production was affected by moderate exercise. The combination of moderate exercise and a LC diet resulted in decreased sIgA production that is associated with pIgR expression, indicating a decreased defense ability against infection. Therefore, we infer that, in the case of moderate exercise, a LC diet may not be suitable for regulating immune defenses involving sIgA. The mechanism by which sIgA levels decreases upon exercise by low-carbohydrate diets remains unknown; therefore, more in-depth analysis, including that of cytokine production, is necessary to determine suitable nutritional conditions for regulating immune functions during exercise.
Acknowledgments
The authors thank Mr. T. Narasaki and other members of the Nutrition Laboratory at Daito Bunka University for providing technical assistance. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to T.K., No. 40339479) and by a research grant from Daito Bunka University.
Abbreviations
- ANOVA
analyses of variance
- ELISAs
enzyme-linked immunosorbent assays
- IgA
immunoglobulin A
- pIgR
polymeric immunoglobulin receptor
- sIgA
secretory immunoglobulin A
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid-reactive substance
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mackinnon LT. Chronic exercise training effects on immune function. Med Sci Sports Exerc. 2000;32:S369–S376. doi: 10.1097/00005768-200007001-00001. [DOI] [PubMed] [Google Scholar]
- 2.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002;87:153–158. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 3.Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol (1985) 2004;97:491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu K, Kimura F, Akimoto T, Akama T, Kuno S, Kono I. Effect of free-living daily physical activity on salivary secretory IgA in elderly. Med Sci Sports Exerc. 2007;39:593–598. doi: 10.1249/mss.0b013e318031306d. [DOI] [PubMed] [Google Scholar]
- 5.Fehr HG, Lötzerich H, Michna H. The influence of physical exercise on peritoneal macrophage functions: histochemical and phagocytic studies. Int J Sports Med. 1988;9:77–81. doi: 10.1055/s-2007-1024983. [DOI] [PubMed] [Google Scholar]
- 6.Viloria M, Lara-Padilla E, Campos-Rodríguez R, et al. Effect of moderate exercise on IgA levels and lymphocyte count in mouse intestine. Immunol Invest. 2011;40:640–656. doi: 10.3109/08820139.2011.575425. [DOI] [PubMed] [Google Scholar]
- 7.Drago-Serrano ME, Godínez-Victoria M, Lara-Padilla E, et al. Moderate exercise enhances expression of SIgA in mouse ileum. Int J Sports Med. 2012;33:1020–1025. doi: 10.1055/s-0032-1312607. [DOI] [PubMed] [Google Scholar]
- 8.Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- 9.Lagiou P1, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;26: 344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha CL, Woodward B. Reduction in the quantity of the polymeric immunoglobulin receptor is sufficient to account for the low concentration of intestinal secretory immunoglobulin A in a weanling mouse model of wasting protein-energy malnutrition. J Nutr. 1997;127:427–435. doi: 10.1093/jn/127.3.427. [DOI] [PubMed] [Google Scholar]
- 11.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 12.Azenabor AA, Hoffman-Goetz L. Intrathymic and intrasplenic oxidative stress mediates thymocyte and splenocyte damage in acutely exercised mice. J Appl Physiol (1985) 1999;86:1823–1827. doi: 10.1152/jappl.1999.86.6.1823. [DOI] [PubMed] [Google Scholar]
- 13.Gu C, Shi Y, Le G. Effect of dietary protein level and origin on the redox status in the digestive tract of mice. Int J Mol Sci. 2008;9:464–475. doi: 10.3390/ijms9040464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petzke KJ, Elsner A, Proll J, Thielecke F, Metges CC. Long-term high protein intake does not increase oxidative stress in rats. J Nutr. 2000;130:2889–2896. doi: 10.1093/jn/130.12.2889. [DOI] [PubMed] [Google Scholar]
- 15.Theys N, Clippe A, Bouckenooghe T, Reusens B, Remacle C. Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction. PLoS One. 2009;4:e6110. doi: 10.1371/journal.pone.0006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park KS, Kim SK, Kim MS, et al. Fetal and early postnatal protein malnutrition cause long-term changes in rat liver and muscle mitochondria. J Nutr. 2003;133:3085–3090. doi: 10.1093/jn/133.10.3085. [DOI] [PubMed] [Google Scholar]
- 17.Reddy Avula CP, Fernandes G. Modulation of antioxidant enzymes and lipid peroxidation in salivary gland and other tissues in mice by moderate treadmill exercise. Aging (Milano) 1999;11:246–252. doi: 10.1007/BF03339665. [DOI] [PubMed] [Google Scholar]
- 18.Paula FB, Gouvêa CM, Alfredo PP, Salgado I. Protective action of a hexane crude extract of Pterodon emarginatus fruits against oxidative and nitrosative stress induced by acute exercise in rats. BMC Complement Altern Med. 2005;5:17–25. doi: 10.1186/1472-6882-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alessio HM. Exercise-induced oxidative stress. Med Sci Sports Exerc. 1993;25:218–224. [PubMed] [Google Scholar]
- 20.Favier RJ, Ghaemmaghami F, Sempore B, et al. Skeletal muscle adaptation to physical training and beta-adrenergic blockade in spontaneously hypertensive rats. Eur J Appl Physiol Occup Physiol. 1989;58:652–660. doi: 10.1007/BF00418513. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol (1985) 1990;68:2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- 22.Shimomura O, Wu C, Murai A, Nakamura H. Evaluation of five imidazopyrazinone-type che.miluminescent superoxide probes and their application to the measurement of superoxide anion generated by Listeria monocytogenes. Anal Biochem. 1998;258:230–235. doi: 10.1006/abio.1998.2607. [DOI] [PubMed] [Google Scholar]
- 23.Kikugawa K, Yasuhara Y, Ando K, Koyama K, Hiramoto K, Suzuki M. Effect of supplementation of n-3 polyunsaturated fatty acids on oxidative stress-induced DNA damage of rat hepatocytes. Biol Pharm Bull. 2003;26:1239–1244. doi: 10.1248/bpb.26.1239. [DOI] [PubMed] [Google Scholar]
- 24.Shimada S, Kawaguchi-Miyashita M, Kushiro A, et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- 25.Miyazaki Y, Yamasaki M, Mishima H, Mansho K, Tachibana H, Yamada K. Oxidative stress by visible light irradiation suppresses immunoglobulin production in mouse spleen lymphocytes. Biosci Biotechnol Biochem. 2001;65:593–598. doi: 10.1271/bbb.65.593. [DOI] [PubMed] [Google Scholar]
- 26.Kunisawa J, Hashimoto E, Inoue A, et al. Regulation of intestinal IgA responses by dietary palmitic acid and its metabolism. J Immunol. 2014;193:1666–1671. doi: 10.4049/jimmunol.1302944. [DOI] [PubMed] [Google Scholar]
- 27.Asano M, Komiyama K. Polymeric immunoglobulin receptor. J Oral Sci. 2011;53:147–156. doi: 10.2334/josnusd.53.147. [DOI] [PubMed] [Google Scholar]
- 28.Takenouchi-Ohkubo N, Moro I, Mukae S, Kaneko Y, Komiyama K. Tumour necrosis factor-α-mediated human polymeric immunoglobulin receptor expression is regulated by both mitogen-activated protein kinase and phosphatidylinositol-3-kinase in HT-29 cell line. Immunology. 2008;123:500–507. doi: 10.1111/j.1365-2567.2007.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauerwein RW, Mulder JA, Mulder L, et al. Inflammatory mediators in children with protein-energy malnutrition. Am J Clin Nutr. 1997;65:1534–1539. doi: 10.1093/ajcn/65.5.1534. [DOI] [PubMed] [Google Scholar]