Abstract
Advanced glycation end-products are toxic by-products of metabolism and are also acquired from high-temperature processed foods. They promote oxidative damage to proteins, lipids and nucleotides. Aging and chronic diseases are strongly associated with markers for oxidative stress, especially advanced glycation end-products, and resistance to peripheral insulin-mediated glucose uptake. Modifiable environmental factors including high levels of refined and simple carbohydrate diets, hypercaloric diets and sedentary lifestyles drive endogenous formation of advanced glycation end-products via accumulation of highly reactive glycolysis intermediates and activation of the polyol/aldose reductase pathway producing high intracellular fructose. High advanced glycation end-products overwhelm innate defenses of enzymes and receptor-mediated endocytosis and promote cell damage via the pro-inflammatory and pro-oxidant receptor for advanced glycation end-products. Oxidative stress disturbs cell signal transduction, especially insulin-mediated metabolic responses. Here we review emerging evidence that restriction of dietary advanced glycation end-products significantly reduces total systemic load and insulin resistance in animals and humans in diabetes, polycystic ovary syndrome, healthy populations and dementia. Of clinical importance, this insulin sensitizing effect is independent of physical activity, caloric intake and adiposity level.
Keywords: oxidative stress, insulin resistance, glycation, AGEs, Western diet
Introduction
Chronic illnesses account for about two-thirds of all premature deaths and 75% of all medical costs in the United States today.(1,2) Modifiable lifestyle factors play etiological roles in these diseases. Energy balance, micronutrient density of food, level of physical activity and exposure to tobacco smoke are known factors influencing health. Recent evidence demonstrates that food production, processing and cooking methods are critical to health outcomes as well. An important mechanism by which lifestyle influences loss of health and function is oxidative stress. Oxidative stress (OS) results in oxidized cell macromolecules and disturbs cell signal transduction. Metabolic insulin resistance (IR) remains a poorly understood phenomenon of cell stress associated with aging and chronic degenerative diseases.(3–5) Medical approaches focus on management of hyperglycemia, often at the expense of insulin-dependent cell stress.(6) Systemic advanced glycation end-products (AGEs) formed endogenously or acquired from high temperature-cooked foods and tobacco products are powerful pro-oxidants. Emerging research reveals the compelling contribution of dietary AGEs (dAGEs) to systemic load of AGEs, cell stress and IR. This review compiles research that demonstrates how dietary modifications, independent of calorie restriction, can regulate IR via modulating the AGEs load.
Redox Homeostasis
Aerobic biological systems require redox reactions for survival and have innate antioxidant systems to maintain tightly controlled redox homeostasis. These innate systems are enhanced by exogenous dietary antioxidants. The series of reactions involved in oxidizing carbohydrates and fats to claim stored energy in the readily usable form adenosine 5'-triphosphate (ATP) is an elegant example of the central role of redox reactions in human physiology. Mitochondria are the hub of metabolic redox activity where oxidative phosphorylation involves a series of redox reactions along the electron transport chain (ETC). Most ETC electrons go to cytochrome c oxidase where they are combined with oxygen and protons to form water. However, some electrons leak at complexes I and III and combine directly with oxygen to form superoxide (O2•−).(7,8) Mitochondrial O2•− is a “primary” reactive oxygen species (ROS) that reacts to form many other ROS and is estimated to be the origin of about 90% of all ROS in normal human physiology.(8–10) The proximity of mitochondrial DNA (mtDNA) to high concentrations of ROS and absence of DNA repair systems makes it particularly vulnerable to oxidative damage. Energy metabolism slows with age and cumulative oxidative damage to mitochondrial membranes, proteins and mtDNA is theorized to contribute to this process.(7) The aging process may then be slowed by lifestyle choices that enhance innate and exogenous antioxidants and limit endogenous and acquired pro-oxidants.
Cells synthesize beneficial ROS and reactive nitrogen species.(8) Peroxisomes produce hydrogen peroxide (H2O2) to metabolize long chain fatty acids and phagocytic immune cells produce the “respiratory burst” to kill pathogens. An important beneficial physiological role of free radicals is regulation of intracellular activities via signal transduction.(8,11) Hormones, cytokines, growth factors and neurotransmitters bind to cell surface receptors and stimulate release of free radicals like hydroxyl radical (HO•) or nitric oxide (NO) to regulate gene expression, nerve transmission, cell growth and muscle contraction. Transient changes in intracellular free radical status alter signal pathways like mitogen-activated protein kinase (MAPK) pathways and the survival factor protein deacetylase silent mating type information regulation 2 homolog 1 (SIRT1).(12–16) These regulate nuclear transcription factors activator protein-1 (AP-1) that induce mitogenic responses and nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) that induce inflammatory responses.(17,18) Free radicals can differentially activate and deactivate cell signal kinases and phosphatases at their oxidation-sensitive cysteine residues to carefully orchestrate energy metabolism, cell proliferation and apoptosis.(11,19–21)
Glutathione (GSH) is an abundant innate antioxidant in cytosol, mitochondria and nuclei of cells.(8) It is also involved in many conjugation and detoxification reactions.(22) Glutathione is synthesized from glycine, cysteine and glutamate. Oxidized GSH is glutathione disulphide (GSSG) and the GSH/GSSG ratio is a marker of OS. Glutathione peroxidase (GPX) catalyzes the oxidation of glutathione and reduction of H2O2 to water. Glutathione reductase reduces GSSG to GSH using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. Glutathione regenerates oxidized vitamins C and E. Cell cycle potently responds to GSH concentration.(23) Another intracellular redox buffering system is the dithiol thioredoxin (TRX).(8,24,25) Thioredoxin regenerates thioredoxin and glutathione peroxidases and reduces GSSH, vitamin C and CoQ10.(24) Oxidized TRX is regenerated by thioredoxin reductase.(24) A critical function of TRX is protecting mitochondria from H2O2.(26) Another function of TRX may be reducing and repairing oxidized proteins.(25) Thioredoxin is an intracellular signaling molecule known to inhibit apoptosis, promote cell growth and mediate inflammation.(24) It appears to be neuroprotective.(24,27) Transgenic mice that over-express TRX have significantly longer lifespans.(25)
While GSH and TRX protect the intracellular environment, uric acid is a key extracellular innate antioxidant.(28–30) It has deleterious consequences at very high serum concentrations by precipitating as monosodium urate crystals in joints producing gout.(31) Elevated serum uric acid is a risk factor for atherosclerosis and hypertension.(32) It is a classic example of substances having both antioxidant and pro-oxidant properties.(31,33) Serum OS that overwhelms other antioxidant systems may result in increased uric acid production.(34,35) Thus, elevated serum uric acid may be a valuable marker of OS in the extracellular fluid compartment relevant to cardiovascular disease (CVD).(29,30) High intake of red meat, chicken with the skin, fried food, shrimp, sweet beverages and ethanol increase serum urate and gout risk.(36–39) The current recommendation to limit purine-rich vegetables does not lower urate.(36,39) Higher intake of low-glycemic fruits and vegetables, nuts and seeds, coffee and vitamin C supplements are associated with lower uric acid.(36) Tart cherry juice inhibits xanthine oxidase activity, reducing endogenous purine synthesis and urate.(40)
Superoxide dismutase (SOD) is a crucial class of antioxidant enzymes that converts O2•− to H2O2 and prevents reaction with NO to form the highly toxic peroxynitrite (OONO•).(41) SOD1 acts in the cytoplasm, SOD2 in mitochondria and SOD3 in the nucleus and extracellular matrix. SOD1 and SOD3 contain copper and zinc (CuZn-SOD and EC-SOD) and SOD2 contains manganese (Mn-SOD).(42) Hyperglycemia-induced glycation of SOD1 and 3 impair function and increase ROS associated with neuron apoptosis and type 2 diabetes mellitus (T2DM) complications.
Many antioxidants are food-sourced, including vitamin A, ascorbic acid, α-tocopherol, mixed carotenoids, lipoic acid, bioflavonoids, coenzyme Q10, taurine, selenium and zinc.(8,22) Non-enzymatic antioxidants move oxidizing equivalents from lipid membranes to less damaging aqueous phase.(10,43) The most effective “chain breaker” in lipid phase is mixed tocopherols.(44,45) A steady-state of tocopheroxyl radical is maintained by water soluble vitamin C and thiols.(10) Carotenoids are efficient quenchers of the highly reactive hydroxyl radicals and singlet oxygen.(10,46) Fruits and vegetables are the richest sources of hydrophilic antioxidants and nuts and seeds are the best source of lipophilic antioxidants.
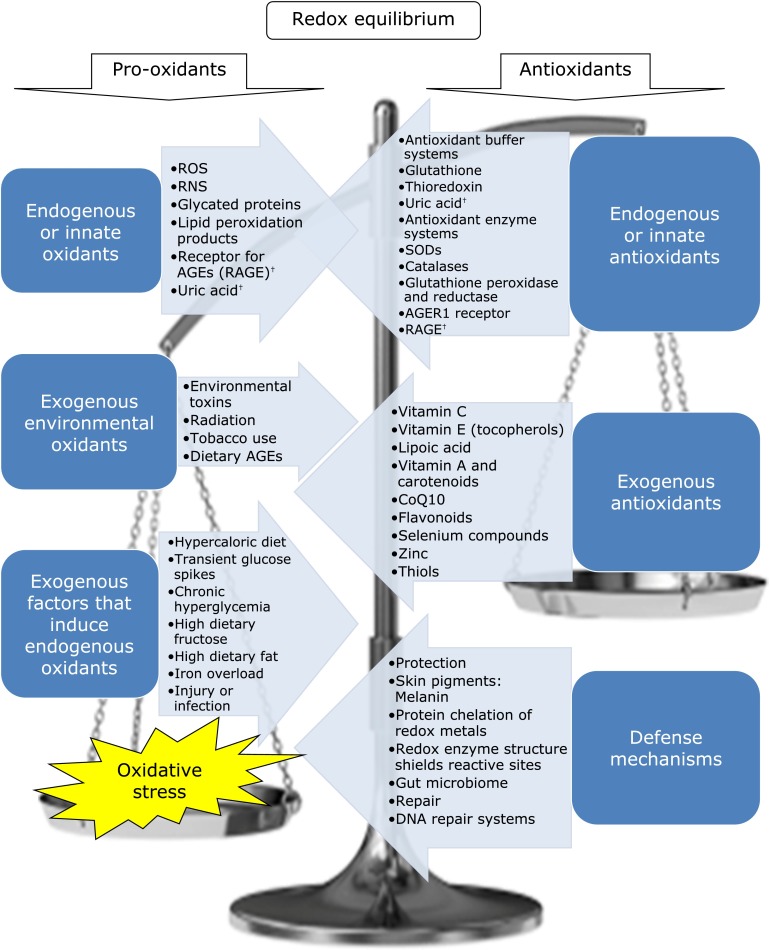
Oxidative stress is “a disturbance in the equilibrium status of pro-oxidant/antioxidant reactions in living organisms”.(8) See Fig. 1 for a graphic depiction of factors involved in dynamic oxidative balance. High ROS can exceed regulatory capacity and results in irreversible changes to proteins, lipids and DNA. Proteins are most vulnerable to oxidation at their cysteine and methionine residues.(47,48) Polyunsaturated fatty acid residues in phospholipid membranes are extremely vulnerable to oxidative damage.(8,49) Level of ROS is influenced by many modifiable factors, especially nutrition.(50–53) Until recently, the only animal model intervention that extended lifespan was calorie restriction.(54) Restriction of dAGEs is more practical and may be more powerful than calorie restriction. A mouse model study found that the longevity benefits of a hypocaloric diet are completely negated if the diet is high in pro-oxidant AGEs.(55) In fact, calorie-restricted high dAGEs fed mice had a lower lifespan than unrestricted regular diet mice.(55,56) Excess calorie intake is associated with elevated serum and tissue AGEs and calorie restriction may in part extend lifespan by reducing endogenous AGEs formation. Human muscle immunostaining of the AGE carboxymethyl-lysine (CML) and pro-inflammatory receptor for AGEs (RAGE) significantly and positively correlates with weight and age, and CML significantly correlates with OS and inflammation.(57) In addition, calorie restriction alone in overweight and obese adults lowers serum AGEs.(58) Serum AGEs significantly positively correlate with triglycerides (TG), waist circumference and body mass index.(58)
Fig. 1.
Redox Homeostasis and Oxidative Stress. Many known factors contribute to redox homeostasis in human physiology. On the left are those that increase oxidation with opposing anti-oxidant mechanisms on the right. †Uric acid and RAGE are antioxidants at low concentrations and pro-oxidant and markers of oxidative stress at high concentrations.
Cell signal transduction disturbances induced by OS may rival oxidative damage of cell components in mediating aging and chronic diseases.(17,59) An emerging theory suggests that excess ROS may disturb cell signal transduction, protein transcription and post-transcriptional processing.(11,59–61) Molecular mechanisms of IR involve OS-induced signal transduction disturbances including activation of protein kinases C (PKC) and changes in the insulin signal pathways phosphatidylinositol 3-kinase (PI3K) and MAPK with opposing protective effects by SIRT1.(15,17,62,63) Protein kinases C are a class of regulatory serine/threonine kinase enzymes that are activated by oxidants.(19,64,65) They have cysteine-rich regulatory regions in zinc fingers and catalytic sites that are vulnerable to oxidation.(19,64,65) The redox regulation of PKC, expression of specific isoforms of PKC and localization within cells is cell-specific.(64–66) Oxidative activation of PKC in muscle results in serine phosphorylation of insulin receptor substrate-1 (IRS1) in the PI3K pathway producing IR and activation of NFκB producing an inflammatory response.(63) Normal levels of ROS sustain SIRT1 activity which deacetylates the p65 subunit of NFκB, suppressing inflammation and ROS production, increasing innate antioxidant expression, maintaining energy homeostasis and normalizing hepatic lipid metabolism.(62,67–69)
Advanced Glycation End-Products
Advanced glycation end-products (AGEs) are a complex class of compounds produced by the Maillard reaction in food and in the human body.(70–73) Maillard products enhance aroma and flavor of food and produce the brown pigments formed during cooking. The Maillard reaction is the non-enzymatic condensation of reducing sugars with amino groups of proteins in foods. The importance of endogenous formation of AGEs began to be recognized in the 1970’s when glycated hemoglobin (HbA1c) was found to be associated with hyperglycemia in diabetes.(74,75) Unlike the rapid, irreversible formation of AGEs in cooked food, the initial condensation step in vivo is reversible and depends on reducing sugar concentration, resulting in formation of unstable intermediates referred to as Schiff bases or glycosylamines.(56,76) Schiff bases undergo rearrangements to form more stable but also reversible Amadori products, also called ketosamines, deoxyketoses or deoxyaldoses. In physiological conditions of temperature and pH, endogenous formation of AGEs beyond this step is time dependent, thus only long-lived proteins proceed to the irreversible third step.(77) After several dehydration, cyclization, oxidation, cross-linking and/or polymerization reactions they form the stable heterogeneous class of compounds referred to as melanoidins or AGEs. Endogenous formation of AGEs involve glucose, fructose, galactose, mannose, ribose and reactive triose intermediates of energy metabolism.(51,72,78) Lysine, arginine and sulfur-containing amino acids are particularly vulnerable to glycoxidation.(71,72)
The most studied AGEs or intermediates include HbA1c, 3-deoxyglucosone (3-DG), pentosidine, CML, methylglyoxal (MG) and malondialdehyde (MDA).(8,56,72,79) Most AGEs of carbohydrate origin involve lysine residues of target proteins while most AGEs of lipid peroxidation origin involve arginine residues (imidazolones).(48,73) Lipid peroxidation AGEs are occasionally referred to as advanced lipoxidation end-products (ALEs), and have been linked to kidney disease and complications of diabetes and appear to be particularly pathogenic.(51,72,79) Glyoxal, MDA and hydroxynonenal (HNE) are products of peroxidation lipids.(72,80,81) Table 1 outlines the various classes of AGEs.(73,82,83) It is now known that endogenous AGEs contribute to aging, CVD, kidney disease, diabetes, Alzheimer’s disease (AD), cataracts, autoimmune diseases, allergies, endocrine disorders and gastrointestinal disturbances.(41,51,56,79,84)
Table 1.
Classification of AGEs
| Fluorescence and protein crosslinking | ||
|---|---|---|
| Fluorescent | Non-fluorescent | |
| Protein crosslinking | Pentosidine | Glucosepane |
| Crossline | ||
| MRX | ||
| Vesperlysine | ||
| Glyoxal-lysine dimmer | ||
| Methylglyoxal-lysine dimmer | ||
| GOLDIC | ||
| MOLDIC | ||
| Non crosslinking | CML | |
| CEL | ||
| Pyrraline | ||
| Argpyrimidine | ||
| MG-imidizolones | ||
| 3-DG-imidizolones | ||
| GA-pyridine | ||
| Oxidized substrate | ||
|---|---|---|
| Lipid peroxidation | Amino acid metabolism by myeloperoxidases | Carbohydrate and ascorbate |
| MDA | Glyoxal (non-specific) | Glyoxal |
| Hydroxynonenal | Methylglyoxal | Methylglyoxal |
| Acrolein (non-specific) | Acrolein (non-specific) | 3-DG (Fructose) |
| Glyoxal (non-specific) | Glycoaldehyde (non-specific) | Arabinose |
| Glycolaldehyde | ||
| Dehydroascorbate | ||
| Source/Synthesis pathway | ||
|---|---|---|
| Class | Source or pathway | Important intermediate |
| AGEs 1 | Glucose direct, maillard reaction | Glucose |
| AGEs 2 | Glycolysis, fructose metabolism and polyol pathways | Glyceraldehyde (α-hydroxyaldehyde) |
| AGEs 3 | Maillard reaction Schiff bases | Glycolaldehyde |
| AGEs 4 | Glyceraldehyde (glycolysis intermediate triose) | Methylglyoxal (dicarbonyl) |
| AGEs 5 | Glucose and glycolaldehyde | Glyoxal (dicarbonyl) |
| AGEs 6 | Fructose (polyol pathway, dietary) | 3-DG (dicarbonyl) |
Typical advanced glycation end-products in three classification methods, by their fluorescent properties, the substrate from which it is derived and synthesis pathway.
Endogenous Formation of AGEs: Hyperglycemia, Energy Balance and IR
Endogenous-sourced AGEs accumulate in the body over time and are associated with physiological changes that characterize aging, especially IR.(41,51,56,79,85) Serum levels of AGEs in diabetes patients are about 50% greater than that of healthy age-matched controls.(86,87) Both transient glucose spikes and chronic hyperglycemia accelerate endogenous production of AGEs. Mitochondrial ROS production is accelerated by hypercaloric and high refined carbohydrate diets.(88) Chronic hyperglycemia of diabetes is known to accelerate virtually all degenerative processes associated with aging.(51,76,77,89,90) HbA1c averages 0.40% in healthy subjects and about 0.75% in diabetes and does not proceed to toxic AGEs due to the moderately short 6 to 12 week half-life of hemoglobin.(22,56,79,84) Non-insulin dependent tissues including erythrocytes, peripheral nerves, endothelial cells, lens and kidneys are especially prone to hyperglycemia-mediated damage.(56)
A critical role of the liver is to act as a gatekeeper for systemic energy supply.(91–94) Liver and muscle mitochondria adapt to ATP energy demands of physical activity which alters glucose and fat oxidation capacity.(7,70,95) Damage to liver mitochondria is accelerated with a hypercaloric diet and slowed with a slight hypocaloric diet.(96,97) The cumulative effect of a sedentary lifestyle and high refined carbohydrate, hypercaloric diet is glucose and fat in cells exceeding the oxidative capacity of mitochondria. When this occurs, glycolysis intermediates, TG, free fatty acids, acyl-CoA and ceramides in liver and muscle cells accumulate and induce OS and AGEs formation.(7,94,98) Stressed myocytes become IR, a protective mechanism that non-insulin sensitive cells do not have. Liver steatosis and muscle IR develop coincident with and are predictive of metabolic syndrome (MetS), a constellation of risk factors for CVD and T2DM believed to reflect IR and inflammation.(70,94) Compensatory hyperinsulinemia causes upregulation of hormone-sensitive lipase in adipocytes that maintains an elevated level of circulating free fatty acids leading to further lipid accumulation in hepatocytes. Elevated insulin also acts directly on hepatocytes to stimulate lipogenesis. The accumulation of lipids in the liver under OS leads to lipid peroxidation AGEs. The interaction of AGEs with RAGE induces additional ROS production via activation of NADPH oxidase and release of inflammatory cytokine tumor necrosis factor-α (TNF-α).(7,94,99) Elevated TNF-α produces outer mitochondrial membrane permeability which increases O2•− formation. This creates vicious cycles of oxidative and inflammatory damage to liver cells.
Diet alone can induce IR without genetic predisposition in animal models.(100,101) Insulin resistance and MetS were induced in genetically normal healthy Fischer rats by ad libitum feeding a diet high in fat and simple carbohydrates (HFS).(100,101) Oxidative stress was significantly increased in the HFS rats compared to the rats fed a low fat and high complex carbohydrate (LFHC) diet. Also, NADPH oxidase was significantly upgregulated in the HFS rats compared to rats fed a LFHC diet. This increase in NADPH oxidase was associated with increased MDA. The HFS diet also induced a downregulation of innate antioxidants.
Induction of the polyol (aldose reductase) pathway is a primary route for AGEs synthesis in hyperglycemia.(102–104) Both chronic hyperglycemia of diabetes and transient hyperglycemia with high refined carbohydrate and hypercaloric meals activate the polyol pathway.(56,103,105–107) The polyol pathway converts glucose to sorbitol and then to fructose by the enzymes aldose reductase and sorbitol dehydrogenase.(56,108) Enzymes of the polyol pathway are found in high concentrations in non-insulin-independent tissues including kidney, lens, nerve, brain, erythrocytes and immune cells.(78,108) In these tissues, intracellular fructose concentrations equal that of serum glucose in diabetes.(108–112) Blocking the polyol pathway with an aldose reductase inhibitor prevents formation of MG.(108,113) Thus, fructose is the route of synthesis of MG-derived AGEs. Fructose is seven times more reactive than glucose in endogenous formation of AGEs.(79,108,114) Dietary fructose may augment endogenous production of AGEs.(108) A population study found that individuals who have both non-alcoholic fatty liver disease (NAFLD) and MetS drink an average of 4.25 soft drinks per day, individuals with NAFLD but not MetS drink 5.5 soft drinks per day and healthy people drink an average of 0.75 soft drinks per day.(114,115) Animal and human experiments have demonstrated that high sweetened beverage intake induces de novo lipogenesis, hypertriglyceridemia, IR and AGEs production.(116–119) However, fructose is metabolized to fructose-3-phosphate and further to the glyceraldehyde-3-phosphate and dihydroxyacetone-3-phosphate.(56,78,108) Highly reactive trioses from the polyol pathway and intermediates of anaerobic glycolysis are the primary source of endogenous AGEs formation.(78,120–122) These trioses are 200 times more reactive than glucose in AGEs formation.(78,108) Toxic triose AGEs are central to diabetes complications, kidney disease and AD.(78,108,121,123–125)
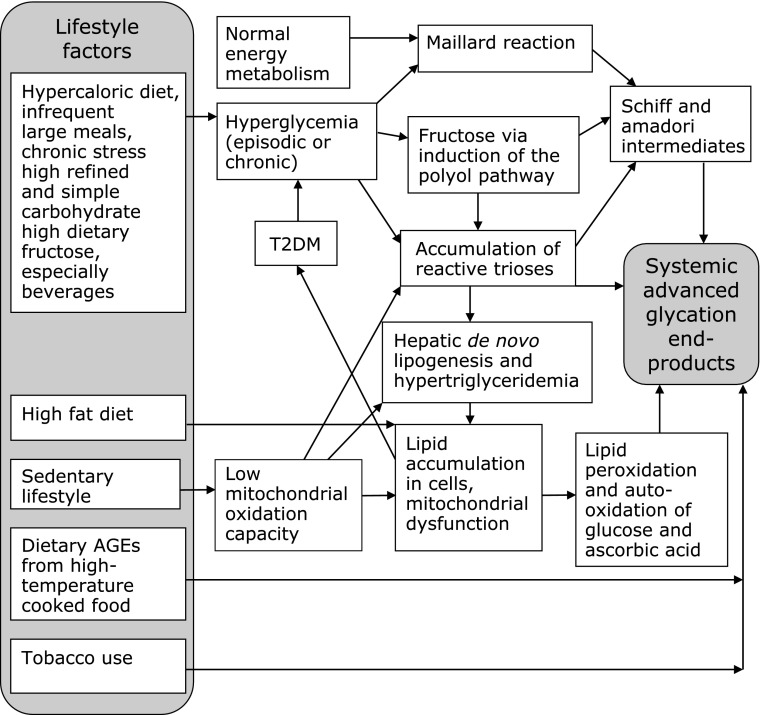
Diet-Derived AGEs
Not all T2DM patients are obese, suggesting etiology of IR beyond hypercaloric intake and endogenous AGEs.(126) Exogenous AGEs are acquired from tobacco and food.(56,76,79) About 10% of AGEs in food are absorbed and only about 1/3 are excreted by the kidneys.(50,127,128) Thus, about 6% of dAGEs consumed are retained and add to body’s total load of AGEs. Fig. 2 illustrates the lifestyle origins of total body load of AGEs. The “Western diet” foods are frequently prepared at high temperatures or highly processed.(129) Emerging evidence suggests that dAGEs make a dominant contribution to the total body pool of AGEs and the pathology of IR. Databases are now available for AGEs content of foods and the cooking and processing conditions that promote their formation.(129,130) The first database measured CML in 250 common foods by enzyme-linked immunosorbent assay.(130) Foods high in fat and protein contain the highest amount of AGEs. Higher cooking temperature, longer cooking time, absence of moisture and presence of metals increases AGEs formation. Carbohydrate and dairy foods tended to be low in AGEs, however, processing greatly increased AGEs content in these categories. Some ready-to-eat breakfast cereals can have more than 10-fold the amount of AGEs of less processed grains and baby formula has 100-fold more AGEs than human breast milk or bovine milk.(130) The next study expanded the database to 549 foods, tested both MG and CML content, and compared a range of cooking methods, temperatures and times.(129) The CML level correlated closely with MG content. Lower cooking times and temperatures, moist cooking methods, and use of acid marinades significantly reduce AGEs formation. A chicken breast with the skin, breaded and deep-fried contains about 10,000 kU/100 g while a poached skinless chicken breast contains about 1,000 kU/100 g and raw chicken breast contains about 800 kU/100 g. Separately, the European ICARE project directly measured four food AGEs: CML, Amadori products, acrylamide and 5-hydroxymethylfufural (HMF) by gas chromatography mass spectrometry in samples of common commercial food products.(131) They found that the relative amounts of different AGEs varied substantially between food types and grain-based cereals and baked goods were a substantial source of CML. Additionally, they collected samples of foods common in children’s diet: infant formula, grain-based baked goods, and potato chips and found an extraordinary variation in AGEs content within single food types representing ranges in processing, temperature and shelf time.(132)
Fig. 2.
Sources of Systemic AGEs. Known pathways to physiologic load of toxic advanced glycation end-products (AGEs). Hyperglycemia and hypercaloric diets drive endogenous formation while dietary AGEs enter the system pre-formed with heat-treated foods.
Animal-derived food AGEs may produce more toxic effects than AGEs from plant-derived foods.(73,133) There is some limited in vitro evidence of beneficial health effects of dAGEs. Some CHO-derived AGEs exhibit anti-carcinogenic properties and a casein-lactose AGE inhibits Helicobacter pylori.(134,135) Roasted coffee AGEs exhibit antioxidant properties and some glucose-based laboratory AGEs inhibit LDL oxidation.(136,137) Still, even high-heat-treated plant foods produce the extremely toxic and carcinogenic acrylamide, especially French fries, and studies that evaluate the health impact of the whole food matrix in vivo are more relevant.(131)
Protection against Toxic AGEs
Defense systems to maintain AGEs homeostasis include innate defenses, enzymatic degradation, renal clearance and receptor-mediated cell uptake and degradation. Innate defense against AGEs include skin pigmentation, chelation of redox metals and structural conformation of enzymes that shield reactive sites. The many benefits of the gut microbiome may include metabolizing exogenous AGEs.(138) Enzymes that deglycate proteins at the first or second step of the Maillard reaction or reduce dicarbonyls include fructosamine-3-kinase, amadoriase (fructosamine oxidase), 2-oxoaldehyde reductase and carbonyl reductase.(48,56,73,85) Enzymes that degrade AGEs include the glyoxalase I and II systems, aldo-ketoreductases and aldehyde dehydrogenases.(48,73,80,84,85,108) Glyoxalase I catalyzes metabolism of dicarbonyls and prevents their binding with proteins to form AGEs. Paraoxonase prevents oxidation of low density lipoprotein cholesterol (LDL).(79,139)
Kidneys are both a biological defense against AGEs and a target of their damage. Levels of serum and tissue AGEs positively correlate with degree of nephropathy.(50,56,72,85) Restriction of dAGEs significantly slows renal damage.(140–142) The formation of AGEs on matrix proteins increases albumin permeability and impairs degradation by metalloproteinases leading to basement membrane thickening.(77,143) Activation of RAGE activates PKC, increasing the MAPK ERK1/2 pathway and this stimulates NFκB leading to increased production of type IV collagen, laminin and fibronectin in mesangial cells.(84,144)
There are two classes of receptors for AGEs, receptors that mediate endocytosis and degradation of AGEs and a receptor that activates cell responses. The receptors that bind AGEs and induce endocytosis and degradation include AGE receptor-1 (AGER1), AGE receptor-2 (AGER2), galectin-3 or AGE receptor-3 (AGER3), cluster of differentiation 36 (CD36) and macrophage scavenger receptors I and II.(73,78,84,85,90,145–147) Antioxidant and anti-inflammatory properties of AGER1 are associated with suppression of RAGE expression and resultant suppression of NFκB, epidermal growth factor receptor, MAPK stress pathways and p66Shc. In health, expression of AGER1 correlates with levels of circulating AGEs and AGER1 to RAGE ratio is high.(146) However, in old age, diabetes and OS, the AGER1 to RAGE ratio is low. Mice fed a diet high in AGEs by heat-treatment of standard chow, both ad libitum and calorie restricted, develop OS, IR, lower AGER1 and elevated RAGE and p66Shc.(55) In contrast, old mice fed an ad libitum low AGEs diet or calorie restricted moderate AGEs diet had longer lifespans, were significantly less IR, had a low stable level of RAGE and higher AGER1.(55,148) There is a threshold body load of AGEs below which AGER1 can maintain homeostasis and suppress RAGE. When dAGEs cause total AGEs to exceed this threshold, AGER1 is suppressed and RAGE expression and OS are elevated.(85) The AGER1 receptor protects against AGEs-induced production of ROS by suppression of NADPH oxidase and prevention of activation of PKCδ.(146)
The receptor AGER3 does not have a membrane-spanning domain and is found throughout the cytosol and is secreted into extracellular space.(84) It mediates endocytosis of circulating AGEs and oxidized LDL.(149) The scavenger receptor CD36 mediates lipid uptake and is expressed on mononuclear phagocytes, adipocytes, hepatocytes, myocytes, platelets and some epithelia.(150) On phagocytes, CD36 binds oxidized LDL and phospholipids and promotes OS and inflammatory processes. Macrophage scavenger receptors initiate the atherosclerotic process by binding and uptake of AGE-modified and oxidized LDL particles to produce foam cells.(79,151)
Binding of AGEs with the signal transducing receptor RAGE stimulates OS, inflammation and disturbed cell signals.(41,50,72) The receptor RAGE is a member of the immunoglobulin superfamily of cell surface molecules.(152) Interaction of AGEs with RAGE produces rapid activation of NFκB, PKC and MAPK signaling cascades. Activation of NFκB increases expression of RAGE.(50,84) The NFκB activation promotes transcription of NADPH oxidase inducing synthesis of O2•−, procoagulation factors, TNF-α, IL-6 and CRP. Increased production of ROS promotes further production of AGEs and self-induced expression of RAGE sets up vicious cycles of OS and inflammation. Obesity-induced IR and adipokine synthesis is now known to be RAGE activation-dependent.(153)
Activation of RAGE by dAGEs induces IR in the absence of a hypercaloric diet. A dual in vitro and in vivo experiment investigated the effect of exposure of human L6 myotubes and C57/BL6 mouse muscle cells in vivo to high levels of AGEs.(152) Mice were randomized to either a standard chow or a nutritionally similar chow treated at high temperature. At 20 weeks, the high dAGEs (HdAGEs) mice ate the same amount of food and had equal weight but their circulating AGEs were 3 times that of the low AGEs (LdAGEs) fed mice. The HdAGEs mice had a fasting glucose level 1.5 times that of the LdAGEs mice and significant IR and hypertriglyceridemia not seen in the LdAGEs mice. The HdAGEs mice tibialis muscle had reduced Akt/PKB phosphorylation and a 2.5-fold increase in PKCα activity. The cultured human muscle cells treated with glycated hemoglobin also had increased PKCα activity. Receptor for AGEs activation resulted in the formation of a complex of Src with RAGE, PKCα and IRS1. Pharmacological inhibitors of PI3K and ERK1/2 did not block activation of PKCα. However, blocking Src reduced PKCα activation by 70% in cultured muscle cells and 80% in mouse muscle cells. Also, silencing of IRS1 abolished the RAGE activation of PKCα. In non-insulin dependent tissues, AGEs-RAGE interaction induced vascular permeability in rat retinal endothelial cells by mechanisms that depend on activation of PLC, PKCδ and rapid activation of NADPH oxidase.(154)
Do Exogenous Diet-derived AGEs Promote IR?
Animal models
Several diabetes mouse model studies demonstrate that restriction of dAGEs significantly reduces serum AGE, reduces IR and slows weight gain. In db/db mice, a diet high in AGEs (H-dAGEs) induces increased IR and 13% more weight gain with the same calorie intake compared to low dAGEs (L-dAGEs).(155) The mice were randomized to a L-dAGEs diet or a diet 3.4-fold higher in dAGEs. The L-dAGEs mice had half the serum AGEs of the H-dAGEs mice after 20 weeks. The L-dAGEs mice had lower fasting insulin levels throughout the study. At the end of 20 weeks, the L-dAGEs mice had no β-cell damage whereas the H-dAGEs mice had significant β-cell damage. The L-dAGEs mice had significantly lower HDL and 2-fold better insulin-stimulated glucose uptake than the H-dAGEs mice. Similarly, restriction of dAGEs reduces IR and improves lifespan in autoimmune T1DM (NOD) mice.(156) Control and NOD mice were randomized to L-dAGEs or 5-fold higher dAGEs for life. In both control and NOD mice the H-dAGES mice had significantly higher serum and urine AGEs than the L-dAGEs fed mice. Serum AGEs increased with time in the H-dAGEs mice and decreased with time in the L-dAGEs mice. At 16 weeks, the L-dAGEs mice had significantly lower fasting glucose and insulin and significantly lower glucose response and better insulin response to intraperitoneal glucose tolerance test than H-dAGEs mice. By 25 weeks, 95% of the H-dAGEs NOD mice had become diabetic and only 33% of the L-dAGEs NOD mice had become diabetic. At 56 weeks, 76% of the L-dAGEs founder mice were alive and none of the H-dAGEs fed mice survived past 44 weeks. Restriction of dAGEs in DM mouse models also reduces serum and kidney AGEs and is associated with improved wound healing, slower development of diabetic nephropathy and extended lifespan.(157–159) Further, restricting dAGEs in mouse models slow progression of CVD in health and in diabetes.(160,161)
A study of four generations of healthy normal mice randomized to isocaloric H-dAGEs or L-dAGEs found H-dAGEs significantly induced more obesity and premature IR than L-dAGEs.(162) The H-dAGEs mice had significant deficiencies of protective AGER1 and SIRT1 and elevated RAGE in muscle, adipose tissue and liver not observed in L-dAGEs mice. There was reduced insulin receptor, IRS1 and AKT activation in the H-dAGEs mice compared to L-dAGEs mice. Restriction of dAGEs in high fat-fed mice suggests IR is not induced by a high fat diet per se but by AGEs produced by oxidized and heat-treated fats.(163) Normal C57/BL6 mice were randomized to a high fat, high AGEs diet (HF-HdAGEs) by heat treatment, a low AGEs high fat diet (HF-LdAGEs) or a regular control diet. At 6 months, 75% of the HF-HdAGEs normal mice had diabetes and none of the HF-LdAGEs mice had diabetes. The HF-HdAGEs mice had significantly higher serum AGEs, fasting insulin, fasting glucose and body weight than control mice. The HF-HdAGEs mice had significantly greater IR and had pancreatic β-cell damage not seen in HF-LdAGEs and controls. Although both HF groups were similarly overweight, the HF-HdAGEs mice had 2 to 4-fold greater visceral fat than HF-LdAGEs mice.
Conditions characterized by IR can be induced in healthy animals by H-dAGEs. In healthy female rats, a model of polycystic ovary syndrome with IR and hyperandrogenism, is induced by H-dAGEs.(164) Polycystic ovary syndrome (PCOS) is a condition that exhibits elevated OS, high serum AGEs, an intrinsic IR and hyperandrogenism.(165,166) Female Wistar rats were randomized to H-dAGEs or L-dAGEs for 3 months. The H-dAGEs group had significantly greater insulin, glucose, serum AGEs, and testosterone and reduced estradiol and progesterone. Alzheimer’s disease is associated with diabetes, MetS and OS with AGEs deposition in the brain.(123,167) Wild type mice were randomized to H-dAGEs, L-dAGEs or regular chow and assessed for cognitive deficits, brain AGEs deposits and MetS.(168) The H-dAGEs mice and regular fed older mice developed MetS, cognitive deficits, amyloid β and AGEs deposits in the brain and the L-dAGEs group did not. The H-dAGEs group had suppressed SIRT1, AGER1 and PPARγ.
Evidence in humans
Cross-sectional studies in humans show association between dAGEs and serum AGEs, IR, inflammation and OS. A cross-sectional study compared 50 L-dAGEs intake DM patients, 68 H-dAGEs intake DM patients and 74 healthy controls.(169) The healthy controls and the L-dAGEs DM group both consumed L-dAGEs and the H-dAGEs DM consumed about 2 times the dAGEs. Serum AGEs in the H-dAGEs DM patients were about twice that of the L-dAGEs DM patients and about 6 times that of controls. Although dAGEs were slightly lower in the L-dAGEs DM patients than in the healthy controls, serum AGEs were about 3 times that of the controls, suggesting significant endogenous AGEs contribution. The H-dAGEs DM patients had significantly higher HbA1c, LDL, 8-isoprostane, IL-1α, TNF-α, monocyte chemoattractant protein-1 (MCP-1) and significantly lower SOD activity than the L-dAGEs DM patients and the controls. Among the DM patients, dAGEs significantly positively correlated with serum AGEs, HbA1c, 8-isoprostane, IL-1α and MCP-1 and negatively correlated with SOD activity. In DM, hyperglycemia-sourced AGEs and dAGEs both contributed significantly to serum AGEs, OS and inflammation. A cross-sectional study in healthy adults found that acute insulin secretion during OGTT correlates with serum AGEs.(170) A cross-sectional study in elderly pre-DM and DM patients found serum AGEs strongly correlated with IR and oxidized LDL in DM patients.(171)
Restriction of dAGES in DM patients significantly reduces serum AGEs and is associated with reduction in IR and inflammation. An interventional study in 24 DM patients, 11 in a two week crossover study and 13 in a six week study, compared inflammatory markers in a L-dAGEs diet to a H-dAGEs diet.(172) In the two week crossover study, the H-dAGEs diet increased serum AGEs 64.5% over baseline and the L-dAGEs diet decreased serum AGEs 30%. The H-dAGEs group had significantly higher TNF-α and VCAM-1 than the L-dAGEs group. In the six week study, serum AGEs increased 28% in the H-dAGEs group and decreased 20% in the L-dAGEs group. In the H-dAGEs group, TNF-α increased 86% and CRP increased 35% while TNF-α and CRP decreased in the L-dAGEs group. Another interventional study found that a L-dAGEs diet significantly lowers IR, OS and inflammation in T2DM.(147) Eighteen T2DM patients and 18 healthy adult controls were randomly assigned to a standard H-dAGEs diet or a 50% lower dAGEs diet for four months. This study investigated the role of SIRT1 and AGER1 in AGE-induced IR. Both SIRT1 and AGER1 are suppressed in T2DM. After the 4 month intervention, the H-dAGEs groups had significantly higher serum CML and MG than the L-dAGEs groups in both T2DM and controls. Both L-dAGEs groups had significantly reduced 8-isoprostane. Plasma insulin, leptin and IR were reduced by about 30% from baseline by restriction of dAGEs in the DM group. Inflammatory NFκB p65 acetylation and TNF-α were also significantly reduced after 4 months of L-dAGEs in the DM group. Four months of L-dAGEs normalized SIRT1 and AGER1 mRNA and protein concentrations in the DM group. Another study of restriction of dAGEs in T2DM patients found significant reduction in serum AGEs and TNF-α but not IR.(173) Researchers attribute this contradiction to the relatively low baseline dAGEs of the population. Restriction of dAGEs in DM patients also revealed that dAGEs modified LDL is a strong activator of the insulin MAPK pathway, central to CVD complications.(174)
The impact of dAGEs on OS is rapid. Even a single H-dAGEs meal induces acute changes in serum AGEs and OS in T2DM patients.(175) Vascular dysfunction is induced more by a single H-dAGEs meal compared to a H-dAGEs meal after a 3 day treatment with benfotiamine in T2DM patients.(176) Benfotiamine is a more bioavailable lipid soluble form of thiamin used to treat diabetic neuropathy. After the H-dAGEs meal, serum AGEs and TBARS were increased and this effect was reduced by benfotiamine. In another study, in both healthy non-DM subjects and T2DM patients, a test beverage high in AGEs was created by concentrating to 1/10th a sugar and caffeine-free cola beverage.(177) After the single oral challenge of H-dAGEs, both DM patients and controls had elevated serum AGEs and signs of endothelial dysfunction.
A dual cross-sectional 2 year follow-up study and 4 month interventional study investigated the effect of restricting dAGEs on IR, OS, inflammation and AGER1 in healthy young and old subjects and chronic kidney disease (CKD) patients.(178) The cross-sectional study included 325 healthy adults, either young (18–45 years old) or older than 60 and 66 CKD patients. The 2-year follow-up also included healthy young and old adults and CKD patients. The cross-sectional study found that serum AGEs are higher in healthy older adults than younger adults and correlates with OS and inflammation markers independent of age.(179) Serum CML significantly correlated with 8-isoprostane and HOMA IR. In the two year follow-up, changes in CML correlated with changes in inflammatory markers TNF-α and VCAM-1. Further, changes in dAGEs intake patterns accompanied changes in serum AGEs. Expression of AGER1 was positively correlated with serum AGEs in healthy participants. Age was not a predictor of RAGE or p66Shc, both of which remained relatively low and unchanged in healthy individuals. In CKD, RAGE and p66Shc were 3 to 4-fold higher and AGER1 was lower than healthy individuals in spite of higher levels of serum AGEs. This supports the previously described threshold for systemic AGEs above which AGER1 declines and RAGE is elevated. The 4 month intervention included healthy subjects divided into young and old groups and CKD patients randomized to either L-dAGEs or H-dAGEs (usual), differing only in food cooking methods. In healthy subjects, the L-dAGEs diet significantly reduced serum AGEs, AGER1, RAGE, p66Shc, 8-isoprostanes, VCAM-1 and TNF-α compared to baseline. In the CKD patients, the L-dAGEs group had similar reductions in all parameters with one notable exception, instead of AGER1 decreasing, it increased by 60%. This is similar to values seen in the healthy young group.
Restriction of dAGEs for four weeks reduced insulin and IR in healthy overweight women.(180) Seventy four women were randomized to either H-dAGEs or L-dAGEs diet and either glucose sweetened drinks or fructose sweetened drinks. The sugar source had no effect on outcomes. The L-dAGEs group had lower urinary AGEs excretion, fasting insulin and IR. Restriction of dAGEs in healthy adults also enhances native defenses.(181) After four months of restricted dAGEs, healthy adults had increased SIRT1 and PPARγ levels and reduced serum AGEs, RAGE, 8-isoprostane and TNF-α. Dietary AGEs intake and not caloric intake correlated negatively with SIRT1 and positively with serum AGEs, OS markers, and TNF-α.
Measurable increases in IR, OS and inflammation can be induced in healthy young individuals by one month of ingestion of ubiquitous H-dAGEs. As part of the European ICARE project, an interventional crossover trial investigated the effect of a diet of food cooked by steam versus a diet of foods cooked at high temperature and dry conditions for one month each in 66 lean healthy volunteers aged 18 to 24.(182) The steamed diet contained an average of 2.2 mg CML/day and the high temperature cooked diet contained 5.4 mg CML/day determined by gas chromatograpy/mass spectrometry measurement. Plasma and urine CML levels were significantly higher after the H-dAGEs diet than after the L-dAGEs diet. Compared to one month on the steam-cooked diet, one month of consuming H-dAGEs produced significantly lower insulin sensitivity, lower serum omega-3 fatty acids and lower plasma vitamin C and vitamin E. Despite no significant differences in nutrients, the H-dAGEs resulted in a 5% higher cholesterol and 9% higher TG.
A recent cross-sectional study of healthy mothers in labor and their healthy infants at birth and 12 months demonstrate that systemic AGEs can be maternally transmitted to offspring and dAGEs can increase this effect to predispose offspring to diabetes.(183) Maternal serum CML, MG and 8-isoprostane levels correlated with infant serum CML, MG and 8-isoprostane levels at birth. At 6 months only CML correlation was retained and at 12 months neither was retained. Recall that the AGEs content of infant formula is about 100-fold higher than human and bovine milk. Infant foods are also highly processed and contain relatively high levels of AGEs. At 12 months, infant CML levels doubled and were similar to maternal and adult levels and infant MG levels exceeded their mother’s levels. Infant AGEs levels maintained a positive correlation with 8-isoprostane levels. These dramatic increases in serum AGEs coincided with increases in dAGEs. The authors noted that several infants in this study had serum MG levels comparable to that seen in DM and renal disease. Infants of mothers in the highest quartile for serum MG had significantly higher fasting insulin and HOMA IR than infants of mothers in the lowest serum MG quartile at 12 months. The previously described mouse model demonstrated similar maternal AGEs transmission to offspring over several generations.(156)
Restriction of dAGEs in PCOS reduces systemic AGEs, IR and androgens in this intrinsic IR patient group.(184) Women with PCOS were assigned to three consecutive two month dietary protocols, a hypocaloric diet with ad libitum dAGEs, eucaloric H-dAGEs, and eucaloric L-dAGEs. Fasting insulin and IR were significantly increased after the H-dAGEs period compared to baseline, the hypocaloric diet and the L-dAGEs diet. Fasting insulin was lower on L-dAGEs than the hypocaloric diet. Serum AGEs were only significantly decreased by L-dAGEs while weight was only decreased by the hypocaloric diet. Testosterone and androstenedione were reduced by hypocaloric diet and L-dAGEs suggesting restriction of dAGEs reduces androgen synthesis independent of adiposity. Oxidative stress was significantly reduced below baseline by L-dAGEs.
Conclusions
Dietary AGEs from high temperature-treated foods make a significant contribution to systemic AGEs load and OS. Lifestyle factors can cause both endogenous and exogenous AGEs to exceed homeostatic regulation and mediate disease processes by oxidative damage of macromolecules and stimulation of cell stress signal transduction changes. Metabolic IR is a cell stress response and an important early event in many chronic diseases. Elevated AGEs activates RAGE which induces activation of NFκB, inflammation and NADPH oxidase. This further enhances oxidation and suppresses protective survival systems AGER1 and SIRT1. High AGEs induce IR by oxidative activation of PKCs that phosphorylate regulatory serine residues on IRS1 in insulin sensitive tissues. Exogenous dAGEs activate cell stress responses and induce IR independent of obesity. The health effects of foods containing the essential macronutrients protein and fat may be more associated with the quality of these foods as determined by processing and cooking methods and resultant AGEs content than by relative proportions in the diet. Restricting dAGEs may significantly improve metabolic insulin response and reduce the risk of chronic diseases associated with modern lifestyles.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA, ; Centers for Desease Control and Prevention (CDC) CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005–2013. MMWR Surveill Summ. 2014;63 Suppl 4:3–27. [PubMed] [Google Scholar]
- 2.Thorpe KE. Treated disease prevalence and spending per treated case drove most of the growth in health care spending in 1987–2009. Health Aff (Millwood) 2013;32:851–858. doi: 10.1377/hlthaff.2012.0391. [DOI] [PubMed] [Google Scholar]
- 3.Hellgren MI, Daka B, Jansson PA, Lindblad U, Larsson CA.Insulin resistance predicts early cardiovascular morbidity in men without diabetes mellitus, with effect modification by physical activity Eur J Prev Cardiol 2014. DOI: 10.1177/2047487314537917 [DOI] [PubMed] [Google Scholar]
- 4.Morris JK, Vidoni ED, Perea RD, et al. Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience. 2014;270:139–147. doi: 10.1016/j.neuroscience.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krentz AJ, Viljoen A, Sinclair A. Insulin resistance: a risk marker for disease and disability in the older person. Diabet Med. 2013;30:535–548. doi: 10.1111/dme.12063. [DOI] [PubMed] [Google Scholar]
- 6.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 7.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22 Suppl 1:S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Pitocco D, Zaccardi F, Stasio E, et al. Oxidative stress, nitric oxide, and diabetes. Rev Diabetic Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 11.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao H, Sundar IK, Ahmad T, et al. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2014;306:L816–L828. doi: 10.1152/ajplung.00323.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond-Stanic MK, Marchionne EM, Teachey MK, Durazo DE, Kim JS, Henriksen EJ. Critical role of the transient activation of p38 MAPK in the etiology of skeletal muscle insulin resistance induced by low-level in vitro oxidant stress. Biochem Biophys Res Commun. 2011;405:439–444. doi: 10.1016/j.bbrc.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato A, Okada M, Shibuya K, et al. Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Res. 2014;12:119–131. doi: 10.1016/j.scr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183–1194. doi: 10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Zhang J, Yan C, et al. SIRT1 regulates CD40 expression induced by TNF-α via NF-κB pathway in endothelial cells. Cell Physiol Biochem. 2012;30:1287–1298. doi: 10.1159/000343318. [DOI] [PubMed] [Google Scholar]
- 17.Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci. 2013;14:3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren JH, Tao Y, Zhang ZZ, et al. Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J Virol. 2014;88:2442–2451. doi: 10.1128/JVI.02861-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bononi A, Agnoletto C, De Marchi E, et al. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011;2011:329098. doi: 10.4061/2011/329098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 21.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 22.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 23.Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. A nuclear glutathione cycle within the cell cycle. Biochem J. 2010;431:169–178. doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]
- 24.Burke-Gaffney A, Callister ME, Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26:398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 26.Miranda-Vizuete A, Damdimopoulos AE, Spyrou G. The mitochondrial thioredoxin system. Antioxid Redox Signal. 2000;2:801–810. doi: 10.1089/ars.2000.2.4-801. [DOI] [PubMed] [Google Scholar]
- 27.Kong L, Zhou X, Li F, Yodoi J, McGinnis J, Cao W. Neuroprotective effect of overexpression of thioredoxin on photoreceptor degeneration in Tubby mice. Neurobiol Dis. 2010;38:446–455. doi: 10.1016/j.nbd.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbrini E, Serafini M, Colic Baric, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63:976–981. doi: 10.2337/db13-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson RA, Horsley ET, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. J Lipid Res. 2003;44:512–521. doi: 10.1194/jlr.M200407-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Nyyssönen K, Porkkala-Sarataho E, Kaikkonen J, Salonen JT. Ascorbate and urate are the strongest determinants of plasma antioxidative capacity and serum lipid resistance to oxidation in Finnish men. Atherosclerosis. 1997;130:223–233. doi: 10.1016/s0021-9150(96)06064-9. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford) 2010;49:1229–1238. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 32.Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab (Lond) 2004;1:10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang DH, Ha SK. Uric acid puzzle: dual role as anti-oxidant and pro-oxidant. Electrolyte Blood Press. 2014;12:1–6. doi: 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcaino H, Greig D, Chiong M, et al. Serum uric acid correlates with extracellular superoxide dismutase activity in patients with chronic heart failure. Eur J Heart Fail. 2008;10:646–651. doi: 10.1016/j.ejheart.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392:1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Torralba KD, De Jesus E, Rachabattula S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: current and future directions. Int J Rheum Dis. 2012;15:499–506. doi: 10.1111/1756-185X.12010. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Yan S, Li C, et al. Risk factors for gout developed from hyperuricemia in China: a five-year prospective cohort study. Rheumatol Int. 2013;33:705–710. doi: 10.1007/s00296-012-2439-8. [DOI] [PubMed] [Google Scholar]
- 38.Chang WC. Dietary intake and the risk of hyperuricemia, gout and chronic kidney disease in elderly Taiwanese men. Aging Male. 2011;14:195–202. doi: 10.3109/13685538.2010.512372. [DOI] [PubMed] [Google Scholar]
- 39.Zgaga L, Theodoratou E, Kyle J, et al. The association of dietary intake of purine-rich vegetables, sugar-sweetened beverages and dairy with plasma urate, in a cross-sectional study. PLoS One. 2012;7:e38123. doi: 10.1371/journal.pone.0038123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haidari F, Jr, Mohammad Shahi, Keshavarz SA, Rashidi MR. Inhibitory effects of tart cherry (Prunus cerasus) juice on xanthine oxidoreductase activity and its hypouricemic and antioxidant effects on rats. Malays J Nutr. 2009;15:53–64. [PubMed] [Google Scholar]
- 41.Edeas M, Attaf D, Mailfert AS, Nasu M, Joubet R. Maillard reaction, mitochondria and oxidative stress: potential role of antioxidants. Pathol Biol (Paris) 2010;58:220–225. doi: 10.1016/j.patbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 43.Liebler DC, Kling DS, Reed DJ. Antioxidant protection of phospholipid bilayers by alpha-tocopherol. Control of alpha-tocopherol status and lipid peroxidation by ascorbic acid and glutathione. J Biol Chem. 1986;261:12114–12119. [PubMed] [Google Scholar]
- 44.Liu M, Wallin R, Wallmon A, Saldeen T. Mixed tocopherols have a stronger inhibitory effect on lipid peroxidation than alpha-tocopherol alone. J Cardiovasc Pharmacol. 2002;39:714–721. doi: 10.1097/00005344-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Wu CM, Cheng YL, Dai YH, Chen MF, Wang CC. α-Tocopherol protects keratinocytes against ultraviolet A irradiation by suppressing glutathione depletion, lipid peroxidation and reactive oxygen species generation. Biomed Rep. 2014;2:419–423. doi: 10.3892/br.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues E, Mariutti LR, Mercadante AZ. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar Drugs. 2012;10:1784–1798. doi: 10.3390/md10081784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baraibar MA, Ladouce R, Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J Proteomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Rabbani N, Thornalley PJ. Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress. Biochem Soc Trans. 2008;36:1045–1050. doi: 10.1042/BST0361045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meguid NA, Dardir AA, Abdel-Raouf ER, Hashish A. Evaluation of oxidative stress in autism: defective antioxidant enzymes and increased lipid peroxidation. Biol Trace Elem Res. 2011;143:58–65. doi: 10.1007/s12011-010-8840-9. [DOI] [PubMed] [Google Scholar]
- 50.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol Series A: Biolog Sci Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Jennings BJ, Ozanne SE, Hales CN. Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab. 2000;71:32–42. doi: 10.1006/mgme.2000.3077. [DOI] [PubMed] [Google Scholar]
- 53.Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22:895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr. 2007;137:1078–1086. doi: 10.1093/jn/137.4.1078. [DOI] [PubMed] [Google Scholar]
- 55.Cai W, He JC, Zhu L, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–336. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de la Maza MP, Uribarri J, Olivares D, et al. Weight increase is associated with skeletal muscle immunostaining for advanced glycation end products, receptor for advanced glycation end products, and oxidation injury. Rejuvenation Res. 2008;11:1041–1048. doi: 10.1089/rej.2008.0786. [DOI] [PubMed] [Google Scholar]
- 58.Gugliucci A, Kotani K, Taing J, et al. Short-term low calorie diet intervention reduces serum advanced glycation end products in healthy overweight or obese adults. Ann Nutr Metab. 2009;54:197–201. doi: 10.1159/000217817. [DOI] [PubMed] [Google Scholar]
- 59.Stuart JA, Maddalena LA, Merilovich M, Robb EL. A midlife crisis for the mitochondrial free radical theory of aging. Longev Healthspan. 2014;3:4. doi: 10.1186/2046-2395-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Labunskyy VM, Gerashchenko MV, Delaney JR, et al. Lifespan extension conferred by endoplasmic reticulum secretory pathway deficiency requires induction of the unfolded protein response. PLoS Genet. 2014;10:e1004019. doi: 10.1371/journal.pgen.1004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 62.Caron AZ, He X, Mottawea W, et al. The SIRT1 deacetylase protects mice against the symptoms of metabolic syndrome. Faseb J. 2014;28:1306–1316. doi: 10.1096/fj.13-243568. [DOI] [PubMed] [Google Scholar]
- 63.Ragheb R, Shanab GM, Medhat AM, Seoudi DM, Adeli K, Fantus IG. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem Biophys Res Commun. 2009;389:211–216. doi: 10.1016/j.bbrc.2009.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giorgi C, Agnoletto C, Baldini C, et al. Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid Redox Signal. 2010;13:1051–1085. doi: 10.1089/ars.2009.2825. [DOI] [PubMed] [Google Scholar]
- 65.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 66.Rimessi A, Rizzuto R, Pinton P. Differential recruitment of PKC isoforms in HeLa cells during redox stress. Cell Stress Chaperones. 2007;12:291–298. doi: 10.1379/CSC-211.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Jung TW, Lee KT, Lee MW, Ka KH. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem Biophys Res Commun. 2012;422:229–232. doi: 10.1016/j.bbrc.2012.04.129. [DOI] [PubMed] [Google Scholar]
- 69.Xu C, Bai B, Fan P, et al. Selective overexpression of human SIRT1 in adipose tissue enhances energy homeostasis and prevents the deterioration of insulin sensitivity with ageing in mice. Am J Transl Res. 2013;5:412–426. [PMC free article] [PubMed] [Google Scholar]
- 70.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 71.O'Brien J, Morrissey PA. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit Rev Food Sci Nutr. 1989;28:211–248. doi: 10.1080/10408398909527499. [DOI] [PubMed] [Google Scholar]
- 72.Bengmark S. Advanced glycation and lipoxidation end products--amplifiers of inflammation: the role of food. JPEN J Parenter Enteral Nutr. 2007;31:430–440. doi: 10.1177/0148607107031005430. [DOI] [PubMed] [Google Scholar]
- 73.Chuyen NV. Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs. Mol Nutr Food Res. 2006;50:1140–1149. doi: 10.1002/mnfr.200600144. [DOI] [PubMed] [Google Scholar]
- 74.Mortensen HB. Glycated hemoglobin. Reaction and biokinetic studies. Clinical application of hemoglobin A1c in the assessment of metabolic control in children with diabetes mellitus. Dan Med Bull. 1985;32:309–328. [PubMed] [Google Scholar]
- 75.Hindle EJ, Rostron GM, Gatt JA. The diagnostic value of glycated haemoglobin levels in post-mortem blood. Ann Clin Biochem. 1985;22 (Pt 2):144–147. doi: 10.1177/000456328502200206. [DOI] [PubMed] [Google Scholar]
- 76.Xanthis A, Hatzitolios A, Koliakos G, Tatola V. Advanced glycosylation end products and nutrition—a possible relation with diabetic atherosclerosis and how to prevent it. J Food Sci. 2007;72:R125–R129. doi: 10.1111/j.1750-3841.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 77.Yamagishi S. Advanced glycation end products and receptor-oxidative stress system in diabetic vascular complications. Ther Apher Dial. 2009;13:534–539. doi: 10.1111/j.1744-9987.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 78.Takeuchi M, Takino J, Yamagishi S. Involvement of TAGE-RAGE system in the pathogenesis of diabetic retinopathy. J Ophthalmol. 2010;2010:170393. doi: 10.1155/2010/170393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 80.Miyata T, Kurokawa K, Van Ypersele De Strihou C. Advanced glycation and lipoxidation end products: role of reactive carbonyl compounds generated during carbohydrate metabolism. J Am Soc Nephrol. 2000;11:1744–1752. doi: 10.1681/ASN.V1191744. [DOI] [PubMed] [Google Scholar]
- 81.Leuner B, Max M, Thamm K, et al. RAGE influences obesity in mice. Effects of the presence of RAGE on weight gain, AGE accumulation, and insulin levels in mice on a high fat diet. Z Gerontol Geriatr. 2012;45:102–108. doi: 10.1007/s00391-011-0279-x. [DOI] [PubMed] [Google Scholar]
- 82.Monnier VM, Sun W, Sell DR, Fan X, Nemet I, Genuth S. Glucosepane: a poorly understood advanced glycation end product of growing importance for diabetes and its complications. Clin Chem Lab Med. 2014;52:21–32. doi: 10.1515/cclm-2013-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poulsen MW, Hedegaard RV, Andersen JM, et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 84.Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 85.Vlassara H, Uribarri J, Cai W, Striker G. Advanced glycation end product homeostasis. Ann N Y Acad Sci. 2008;1126:46–52. doi: 10.1196/annals.1433.055. [DOI] [PubMed] [Google Scholar]
- 86.Su XD, Li SS, Tian YQ, Zhang ZY, Zhang GZ, Wang LX. Elevated serum levels of advanced glycation end products and their monocyte receptors in patients with type 2 diabetes. Arch Med Res. 2011;42:596–601. doi: 10.1016/j.arcmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. 1999;22:1543–1548. doi: 10.2337/diacare.22.9.1543. [DOI] [PubMed] [Google Scholar]
- 88.Masoro EJ, Katz MS, McMahan CA. Evidence for the glycation hypothesis of aging from food-restricted rodent model. J Gerontol. 1989;44:B20–B22. doi: 10.1093/geronj/44.1.b20. [DOI] [PubMed] [Google Scholar]
- 89.Sensi M, Pricci F, Andreani D, Di Mario U. Advanced nonenzymatic glycation endproducts (AGE): their relevance to aging and the pathogenesis of late diabetic complications. Diabetes Res. 1991;16:1–9. [PubMed] [Google Scholar]
- 90.Karasu C. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc Med J. 2010;4:240–256. doi: 10.2174/1874192401004010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 92.Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7:14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar R, Prakash S, Chhabra S, et al. Association of pro-inflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian J Med Res. 2012;136:229–236. [PMC free article] [PubMed] [Google Scholar]
- 94.Santos JC, Valentim IB, de Araujo OR, Ataide Tda R, Goulart MO. Development of nonalcoholic hepatopathy: contributions of oxidative stress and advanced glycation end products. Int J Mol Sci. 2013;14:19846–19866. doi: 10.3390/ijms141019846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lima FD, Stamm DN, Della-Pace ID, et al. Swimming training induces liver mitochondrial adaptations to oxidative stress in rats submitted to repeated exhaustive swimming bouts. PLoS One. 2013;8:e55668. doi: 10.1371/journal.pone.0055668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hagopian K, Harper ME, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–E684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- 97.Ascensão A, Martins MJ, Santos-Alves E, et al. Modulation of hepatic redox status and mitochondrial metabolism by exercise: therapeutic strategy for liver diseases. Mitochondrion. 2013;13:862–870. doi: 10.1016/j.mito.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Coen PM, Hames KC, Leachman EM, et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 2013;21:2362–2371. doi: 10.1002/oby.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leung C, Herath CB, Jia Z, et al. Dietary glycotoxins exacerbate progression of experimental fatty liver disease. J Hepatol. 2014;60:832–838. doi: 10.1016/j.jhep.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 100.Barnard RJ, Roberts CK, Varon SM, Berger JJ. Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J Appl Physiol (1995) 1998;84:1311–1315. doi: 10.1152/jappl.1998.84.4.1311. [DOI] [PubMed] [Google Scholar]
- 101.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Hamada Y, Araki N, Koh N, Nakamura J, Horiuchi S, Hotta N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem Biophys Res Commun. 1996;228:539–543. doi: 10.1006/bbrc.1996.1695. [DOI] [PubMed] [Google Scholar]
- 103.Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87. doi: 10.3389/fphar.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. Faseb J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 105.Tang WH, Cheng WT, Kravtsov GM, et al. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am J Physiol Cell Physiol. 2010;299:C643–C653. doi: 10.1152/ajpcell.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14 (8 Suppl 3):S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 107.Srivastava SK, Ansari NH, Hair GA, Jaspan J, Rao MB, Das B. Hyperglycemia-induced activation of human erythrocyte aldose reductase and alterations in kinetic properties. Biochim Biophys Acta. 1986;870:302–311. doi: 10.1016/0167-4838(86)90234-7. [DOI] [PubMed] [Google Scholar]
- 108.Schalkwijk CG, Stehouwer CDA, van Hinsbergh VW. Fructose-mediated non-enzymatic glycation: sweet coupling or bad modification. Diabet Metab Res Rev. 2004;20:369–382. doi: 10.1002/dmrr.488. [DOI] [PubMed] [Google Scholar]
- 109.Varma SD, Schocket SS, Richards RD. Implications of aldose reductase in cataracts in human diabetes. Invest Ophthalmol Vis Sci. 1979;18:237–241. [PubMed] [Google Scholar]
- 110.Lerner BC, Varma SD, Richards RD. Polyol pathway metabolites in human cataracts. Correlation of circulating glycosylated hemoglobin content and fasting blood glucose levels. Arch Ophthalmol. 1984;102:917–920. doi: 10.1001/archopht.1984.01040030737033. [DOI] [PubMed] [Google Scholar]
- 111.Dyck PJ, Zimmerman BR, Vilen TH, et al. Nerve glucose, fructose, sorbitol, myo-inositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med. 1988;319:542–548. doi: 10.1056/NEJM198809013190904. [DOI] [PubMed] [Google Scholar]
- 112.Mayhew JA, Gillon KR, Hawthorne JN. Free and lipid inositol, sorbitol and sugars in sciatic nerve obtained post-mortem from diabetic patients and control subjects. Diabetologia. 1983;24:13–15. doi: 10.1007/BF00275940. [DOI] [PubMed] [Google Scholar]
- 113.Hallam KM, Li Q, Ananthakrishnan R, et al. Aldose reductase and AGE-RAGE pathways: central roles in the pathogenesis of vascular dysfunction in aging rats. Aging Cell. 2010;9:776–784. doi: 10.1111/j.1474-9726.2010.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nseir W, Nassar F, Assy N. Soft drinks consumption and nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:2579–2588. doi: 10.3748/wjg.v16.i21.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol. 2009;51:918–924. doi: 10.1016/j.jhep.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 116.Crescenzo R, Bianco F, Falcone I, Coppola P, Liverini G, Iossa S. Increased hepatic de novo lipogenesis and mitochondrial efficiency in a model of obesity induced by diets rich in fructose. Eur J Nutr. 2013;52:537–545. doi: 10.1007/s00394-012-0356-y. [DOI] [PubMed] [Google Scholar]
- 117.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mastrocola R, Collino M, Rogazzo M, et al. Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G398–G407. doi: 10.1152/ajpgi.00450.2012. [DOI] [PubMed] [Google Scholar]
- 119.Lee O, Bruce WR, Dong Q, Bruce J, Mehta R, O'Brien PJ. Fructose and carbonyl metabolites as endogenous toxins. Chem Biol Interact. 2009;178:332–339. doi: 10.1016/j.cbi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 120.Takino J, Kobayashi Y, Takeuchi M. The formation of intracellular glyceraldehyde-derived advanced glycation end-products and cytotoxicity. J Gastroenterol. 2010;45:646–655. doi: 10.1007/s00535-009-0193-9. [DOI] [PubMed] [Google Scholar]
- 121.Ebata Y, Takino J, Tsuchiya H, et al. Presence of glyceraldehyde-derived advanced glycation end-products in the liver of insulin-resistant mice. Int J Vitam Nutr Res. 2013;83:137–141. doi: 10.1024/0300-9831/a000150. [DOI] [PubMed] [Google Scholar]
- 122.Kitahara Y, Takeuchi M, Miura K, Mine T, Matsui T, Yamagishi S. Glyceraldehyde-derived advanced glycation end products (AGEs). A novel biomarker of postprandial hyperglycaemia in diabetic rats. Clin Exp Med. 2008;8:175–177. doi: 10.1007/s10238-008-0176-9. [DOI] [PubMed] [Google Scholar]
- 123.Choei H, Sasaki N, Takeuchi M, et al. Glyceraldehyde-derived advanced glycation end products in Alzheimer’s disease. Acta Neuropathol. 2004;108:189–193. doi: 10.1007/s00401-004-0871-x. [DOI] [PubMed] [Google Scholar]
- 124.Usui T, Shizuuchi S, Watanabe H, Hayase F. Cytotoxicity and oxidative stress induced by the glyceraldehyde-related Maillard reaction products for HL-60 cells. Biosci Biotechnol Biochem. 2004;68:333–340. doi: 10.1271/bbb.68.333. [DOI] [PubMed] [Google Scholar]
- 125.Hamada Y, Nakamura J, Naruse K, et al. Epalrestat, an aldose reductase inhibitor, reduces the levels of Nε-(carboxymethyl)lysine protein adducts and their precursors in erythrocytes from diabetic patients. Diabetes Care. 2000;23:1539–1544. doi: 10.2337/diacare.23.10.1539. [DOI] [PubMed] [Google Scholar]
- 126.Coleman NJ, Miernik J, Philipson L, Fogelfeld L. Lean versus obese diabetes mellitus patients in the United States minority population. J Diabetes Complications. 2014;28:500–505. doi: 10.1016/j.jdiacomp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 127.Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Poc Natl Acad Sci U S A. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He C, Sabol J, Mitsuhashi T, Vlassara H. Dietary glycotoxins: inhibition of reactive products by aminoquanidine facilitates renal clearance and reduces tissue sequestration. Diabetes. 1999;48:1308–1315. doi: 10.2337/diabetes.48.6.1308. [DOI] [PubMed] [Google Scholar]
- 129.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916.e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goldberg T, Cai W, Peppa M, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 131.Tessier FJ, Birlouez-Aragon I. Health effects of dietary Maillard reaction products: the results of ICARE and other studies. Amino Acids. 2012;42:1119–1131. doi: 10.1007/s00726-010-0776-z. [DOI] [PubMed] [Google Scholar]
- 132.Birlouez-Aragon I, Morales F, Fogliano V, Pain JP. The health and technological implications of a better control of neoformed contaminants by the food industry. Pathol Biol (Paris) 2010;58:232–238. doi: 10.1016/j.patbio.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 133.Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002;8:337–346. [PMC free article] [PubMed] [Google Scholar]
- 134.Marko D, Habermeyer M, Kemény M, et al. Maillard reaction products modulating the growth of human tumor cells in vitro. Chem Res Toxicol. 2003;16:48–55. doi: 10.1021/tx025531a. [DOI] [PubMed] [Google Scholar]
- 135.Hiramoto S, Itoh K, Shizuuchi S, et al. Melanoidin, a food protein-derived advanced maillard reaction product, suppresses Helicobacter pylori in vitro and in vivo. Helicobacter. 2004;9:429–435. doi: 10.1111/j.1083-4389.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 136.Borrelli RC, Visconti A, Mennella C, Anese M, Fogliano V. Chemical characterization and antioxidant properties of coffee melanoidins. J Agric Food Chem. 2002;50:6527–6533. doi: 10.1021/jf025686o. [DOI] [PubMed] [Google Scholar]
- 137.Dittrich R, El-Massry F, Kunz K, et al. Maillard reaction products inhibit oxidation of human low-density lipoproteins in vitro. J Agric Food Chem. 2003;51:3900–3904. doi: 10.1021/jf026172s. [DOI] [PubMed] [Google Scholar]
- 138.Borrelli RC, Fogliano V. Bread crust melanoidins as potential prebiotic ingredients. Mol Nutr Food Res. 2005;49:673–678. doi: 10.1002/mnfr.200500011. [DOI] [PubMed] [Google Scholar]
- 139.Bacchetti T, Masciangelo S, Armeni T, Bicchiega V, Ferretti G. Glycation of human high density lipoprotein by methylglyoxal: effect on HDL-paraoxonase activity. Metabolism. 2014;63:307–311. doi: 10.1016/j.metabol.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 140.Uribarri J, Peppa M, Cai W, et al. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol. 2003;14:728–731. doi: 10.1097/01.asn.0000051593.41395.b9. [DOI] [PubMed] [Google Scholar]
- 141.Peppa M, Uribarri J, Cai W, Lu M, Vlassara H. Glycoxidation and inflammation in renal failure patients. Am J Kidney Dis. 2004;43:690–695. doi: 10.1053/j.ajkd.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 142.Ueda S, Yamagishi S, Takeuchi M, et al. Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol Med. 2006;12:180–184. doi: 10.2119/2005-00034.Ueda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mott JD, Khalifah RG, Nagase H, Shield CF, 3rd, Hudson JK, Hudson BG. Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int. 1997;52:1302–1312. doi: 10.1038/ki.1997.455. [DOI] [PubMed] [Google Scholar]
- 144.Pugliese G, Pricci F, Romeo G, et al. Upregulation of mesangial growth factor and extracellular matrix synthesis by advanced glycation end products via a receptor-mediated mechanism. Diabetes. 1997;46:1881–1887. doi: 10.2337/diab.46.11.1881. [DOI] [PubMed] [Google Scholar]
- 145.Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Nati Acad Sci U S A. 2006;103:13801–13806. doi: 10.1073/pnas.0600362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cai W, Torreggiani M, Zhu L, et al. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-delta: implications for vascular disease. Am J Physiol Cell Physiol. 2010;298:C624–C634. doi: 10.1152/ajpcell.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uribarri J, Cai W, Ramdas M, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34:1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cai W, He JC, Zhu L, et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol. 2007;170:1893–1902. doi: 10.2353/ajpath.2007.061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu W, Sano H, Nagai R, Fukuhara K, Miyazaki A, Horiuchi S. The role of galectin-3 in endocytosis of advanced glycation end products and modified low density lipoproteins. Biochem Biophys Res Commun. 2001;280:1183–1188. doi: 10.1006/bbrc.2001.4256. [DOI] [PubMed] [Google Scholar]
- 150.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50 Suppl:S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cassese A, Esposito I, Fiory F, et al. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J Biol Chem. 2008;283:36088–36099. doi: 10.1074/jbc.M801698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gaens KH, Goossens GH, Niessen PM, et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–1208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 154.Warboys CM, Toh HB, Fraser PA. Role of NADPH Oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci. 2009;50:1319–1328. doi: 10.1167/iovs.08-2730. [DOI] [PubMed] [Google Scholar]
- 155.Hofmann SM, Dong HJ, Li Z, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–2089. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- 156.Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H. Fetal or neonatal low-glycotoxin environment prevent autoimmune diabetes in NOD mice. Diabetes. 2003;52:1441–1448. doi: 10.2337/diabetes.52.6.1441. [DOI] [PubMed] [Google Scholar]
- 157.Peppa M, Brem H, Ehrlich P, et al. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52:2805–2813. doi: 10.2337/diabetes.52.11.2805. [DOI] [PubMed] [Google Scholar]
- 158.Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab Res Rev. 2002;18:224–237. doi: 10.1002/dmrr.283. [DOI] [PubMed] [Google Scholar]
- 159.Feng JX, Hou FF, Liang M, et al. Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int. 2007;71:901–911. doi: 10.1038/sj.ki.5002162. [DOI] [PubMed] [Google Scholar]
- 160.Lin RY, Reis ED, Dore AT, et al. Lowering of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis. 2002;163:303–311. doi: 10.1016/s0021-9150(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 161.Lin RY, Choudhury RP, Cai W, et al. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2003;168:213–220. doi: 10.1016/s0021-9150(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 162.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A. 2012;109:15888–15893. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54:2314–2319. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 164.Chatzigeorgiou A, Kandaraki E, Piperi C, et al. Dietary glycotoxins affect scavenger receptor expression and the hormonal profile of female rats. J Endocrinol. 2013;218:331–337. doi: 10.1530/JOE-13-0175. [DOI] [PubMed] [Google Scholar]
- 165.Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777–784. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 166.Diamanti-Kandarakis E, Katsikis I, Piperi C, et al. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2008;69:634–641. doi: 10.1111/j.1365-2265.2008.03247.x. [DOI] [PubMed] [Google Scholar]
- 167.Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta. 2014;1842:1693–1706. doi: 10.1016/j.bbadis.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cai W, Uribarri J, Zhu L, et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A. 2014;111:4940–4945. doi: 10.1073/pnas.1316013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chao PC, Huang CN, Hsu CC, Yin MC, Guo YR. Association of dietary AGEs with circulating AGEs, glycated LDL, IL-1α and MCP-1 levels in type 2 diabetic patients. Eur J Nutr. 2010;49:429–434. doi: 10.1007/s00394-010-0101-3. [DOI] [PubMed] [Google Scholar]
- 170.Forbes JM, Sourris KC, de Courten MP, et al. Advanced glycation end products (AGEs) are cross-sectionally associated with insulin secretion in healthy subjects. Amino Acids. 2014;46:321–326. doi: 10.1007/s00726-013-1542-9. [DOI] [PubMed] [Google Scholar]
- 171.Gradinaru D, Borsa C, Ionescu C, Margina D. Advanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetes. J Proteomics. 2013;92:313–322. doi: 10.1016/j.jprot.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 172.Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Nat Acad Sci U S A. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luévano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM, Wrobel K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52:22–26. doi: 10.3164/jcbn.12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cai W, He JC, Zhi L, et al. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;110:285–291. doi: 10.1161/01.CIR.0000135587.92455.0D. [DOI] [PubMed] [Google Scholar]
- 175.Negrean M, Stirban A, Stratmann B, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 176.Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29:2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 177.Uribarri J, Stirban A, Sander D, et al. Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and nondiabetic subjects. Diabetes Care. 2007;30:2579–2582. doi: 10.2337/dc07-0320. [DOI] [PubMed] [Google Scholar]
- 178.Vlassara H, Cai W, Goodman S, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uribarri J, Cai W, Peppa M, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mark AB, Poulsen MW, Andersen S, et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care. 2014;37:88–95. doi: 10.2337/dc13-0842. [DOI] [PubMed] [Google Scholar]
- 181.Uribarri J, Cai W, Pyzik R, et al. Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. 2014;46:301–309. doi: 10.1007/s00726-013-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Birlouez-Aragon I, Saavedra G, Tessier FJ, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91:1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 183.Mericq V, Piccardo C, Cai W, et al. Maternally transmitted and food-derived glycotoxins: a factor preconditioning the young to diabetes? Diabetes Care. 2010;33:2232–2237. doi: 10.2337/dc10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tantalaki E, Piperi C, Livadas S, et al. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS) Hormones (Athens) 2014;13:65–73. doi: 10.1007/BF03401321. [DOI] [PubMed] [Google Scholar]