Abstract
This study assessed time-course changes of the small intestinal lesions during long-term treatment with diclofenac sodium plus omeprazole and the effects of irsogladine on such lesions. Thirty two healthy volunteers were treated with diclofenac sodium (75 mg/day) plus omeprazole (10 mg/day) for 6 weeks, with irsogladine (4 mg/day) added from weeks 6 to 10 (Group A) or with diclofenac sodium plus irsogladine for 6 weeks (Group B). Five volunteers received diclofenac sodium plus omeprazole for 10 weeks (Group C). Subjects underwent capsule endoscopy at each time. In Group A, the number of lesions remarkably increased at week 2, but the worse was not found at week 6 compared with week 2, whereas no exacerbation of lesions was observed in Group B. Additional treatment with irsogladine from weeks 6 to 10 in Group A significantly decreased the number of lesions at weeks 10 compared with Group C. In Group C, no significant change in lesions was observed since weeks 2. In conclusions, a PPI did not prevent the occurrence of small intestinal damage. However such lesions were not aggravated since weeks 2. These suggested mucosal adaptation may occur in the small intestine. Irsogladine was effective in both preventing and healing such lesions.
Keywords: NSAIDs, PPI, irsogladine, small bowel, capsule endoscopy
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are known to cause mucosal damage in both the stomach and small intestine.(1) Several questions regarding the pathogenesis and the best strategies to treat and prevent such mucosal damage in the small intestine remain to be answered. Animal studies have been used to elucidate the mechanisms underlying the mucosal damage induced by non-aspirin NSAIDs and suggest effective measures for its prevention.(1,2) A decrease in the level of endogenous prostaglandins (PGs) appears to play an important role in the development of NSAID-induced small intestinal mucosal damage. This could be due to decreased secretion of mucus, which results in abnormal motility, thereby causing mucosal microcirculatory disturbances in the small intestine. Such actions may then induce the release of inflammatory cytokines and neutrophil infiltration. Nitric oxide derived from inducible nitric oxide synthase as well as from enterobacteria (particularly gram-negative bacilli) may facilitate inflammation through a Toll-like receptor 4-dependent pathway.(3)
Previously, we evaluated the effects of a wide range of drugs used to treat peptic ulcers [PG analogs, mucoprotective agents, histamine-2 receptor antagonists, proton-pump inhibitors (PPIs)] on indomethacin-induced small intestinal mucosal damage in rats. We found that mucoprotective agents, such as rebamipide, teprenone, and irsogladine, as well as the PG analog misoprostol, significantly reduced small intestinal damage in a concentration-dependent manner.(4) Akagi et al.(5) reported that irsogladine increases intracellular cyclic adenosine 3',5-monophosphate content via nonselective inhibition of phosphodiesterase isozymes and exhibits gastric cytoprotection that is partly mediated by endogenous nitric oxide. These effects may account for a variety of actions of irsogladine in the gastrointestinal tract, including the facilitation of gap junctional intercellular communication, inhibition of reductions in the gastric mucosal blood flow response, and suppression of reactive oxygen species generation. Furthermore, Kamei et al.(6) reported that irsogladine protects the small intestine against indomethacin-induced lesions, and this effect may be associated with increased mucus secretion, probably because of the inhibitory actions of phosphodiesterase, resulting in the suppression of enterobacterial invasion and iNOS expression.
PPIs are standard treatments for NSAID-induced mucosal damage in the upper gastrointestinal tract. However, recent studies in rats suggested that PPIs may not prevent NSAID-induced mucosal damage in the small intestine and may even exacerbate it.(7,8) There are no reports regarding the effects of long-term PPI administration during NSAID treatment on the human small intestine. In this study, based on our study in healthy volunteers that compared the efficacy of a 2-week course of irsogladine with that of a PPI in preventing NSAID-induced small intestinal damage,(9) we designed a study to examine the time-course changes in capsule endoscopic findings of the small intestine during long-term treatment with an NSAID plus a PPI. In addition, we investigated whether this course is altered by the addition of irsogladine in healthy subjects.
Methods
Subjects
Subjects eligible for inclusion were healthy adults aged between 20 and 79 years at the time written informed consent was obtained, and who had not taken medications during the 1-month period before the start of the study. The exclusion criteria were as follows: (i) a history of peptic ulcers or gastrointestinal bleeding; (ii) significant hepatic, renal, heart, or respiratory disease; (iii) a history of gastrointestinal surgery other than appendectomy; (iv) oral use (or planned oral use) of a drug other than an anti-ulcer drug; (v) dependency on alcohol or chemicals; (vi) a history of intestinal obstruction or suspected gastrointestinal obstruction on other tests; (vii) a lack of consent for the surgery required if the capsule endoscope was retained within the body; and (viii) determination by the investigator (at his/her discretion) that the subject was ineligible for participation in the study for any other reason.
Protocol
Study 1: Thirty-two healthy volunteers were randomized into two groups to receive the following treatments: diclofenac sodium plus omeprazole for 6 weeks, with irsogladine added from weeks 6 to 10 (Group A); or diclofenac sodium plus irsogladine for 6 weeks (Group B) shown in Fig. 1. Subjects underwent capsule endoscopy at baseline and at weeks 2, 6, and 10 in Group A and at baseline and at weeks 2 and 6 in Group B. We evaluated time-course changes in capsule endoscopic findings of the small intestine in those groups. The study was conducted in accordance with the Declaration of Helsinki (1995). The protocol was approved by the Ethics Review Committee of Osaka Medical College (Osaka, Japan). A study of 32 healthy volunteers was conducted prospectively from January 2010 to March 2011 at Osaka Medical College Hospital. All subjects received oral and written explanations of the study before participation and provided written informed consent. The capsule endoscope did not reach into the cecum in one patient in both Groups A and B; thus, 15 subjects were analyzed in those groups.
Fig. 1.
Study design. Study 1: Thirty-two healthy volunteers were randomized into two groups to receive the following treatments: diclofenac sodium (75 mg/day) plus omeprazole (10 mg/day) for 6 weeks with irsogladine (4 mg/day) added from weeks 6 to 10 (Group A, n = 16) or diclofenac sodium (75 mg/day) plus irsogladine (4 mg/day) for 6 weeks (Group B, n = 16). Subjects underwent capsule endoscopy at baseline and at weeks 2, 6, and 10 in Group A and at baseline and at weeks 2 and 6 in Group B. The capsule endoscope did not reach into cecum in one patient each in Groups A and B; thus, 15 subjects were analyzed in each group. Study 2: Five healthy volunteers were enrolled as a control group to receive diclofenac sodium (75 mg/day) plus omeprazole (10 mg/day) for 10 weeks (Group C, n = 5). Subjects underwent capsule endoscopy at baseline and weeks 2, 6, and 10. CE, capsule endoscopy.
Study 2: Study 1 suggested possible adaptation to NSAIDs, even in the small intestine. We decided to add a control group in Study 2 and filed for protocol modification in March 2012 (Fig. 1). Because it was a simple addition of a control group, the Ethics Committee approved the continuation of our research. Furthermore, we registered the study in UMIN in March 2012 because we had not yet registered it at that time (Registry ID number; UMIN000007540). Five healthy volunteers were enrolled as the control group to receive diclofenac sodium plus omeprazole for 10 weeks (Group C) shown in Fig. 1. Subjects underwent capsule endoscopy at baseline and at weeks 2, 6, and 10. We evaluated time-course changes in capsule endoscopic findings of the small intestine of this group.
The dose of diclofenac sodium was determined based on the dose approved by the Japanese Ministry of Health and Welfare and the doses used in other clinical trials.(10–12) In general, the dose of a PPI used for the prevention of NSAID-induced gastric ulcers is half as that used in the treatment of gastric ulcers in Japan. On this basis, we determined that the appropriate dose of omeprazole should be 10 mg/day.
Capsule endoscopy
Mucosal breaks in the small intestine were defined as lesions with slough surrounded by erythema corresponding to grade 2 of the classification devised by Goldstein et al.(13) Typical examples of the bleeding, mucosal breaks, and reddened lesions found in this study are shown in Fig. 2. Reddened folds, denuded areas, and petechiae were grouped in a single classification termed ”reddened lesions.” Mucosal breaks, reddened lesions, and bleeding were identified and evaluated by independent reviewers blinded to the study protocol, as described later in the text. The number of mucosal breaks, reddened lesions, and sites of bleeding in the small intestine found at baseline, after 2 and 6 weeks, and after capsule endoscopy was calculated for each subject and compared among the three groups. Moreover, the distribution of small intestinal mucosal changes was analyzed. The changes in the mucosa of the small intestine were analyzed. The small intestine was equally divided into three segments (proximal, middle, and distal) on the basis the small intestinal transit time of each subject. Small intestinal transit time was defined as the time elapsed between the first duodenal image and the first cecal image.
Fig. 2.
Representative images of capsule endoscopy. Typical examples of the bleeding, mucosal breaks, and reddened lesions found in this study are shown. Reddened folds, denuded areas, and petechiae were grouped in a single classification termed ”reddened lesions.”
Investigators tasked with evaluating the results of capsule endoscopy of the small intestine (Y.K., T.K., T.T.) were required to attend a standardized training session on the use of the Given Diagnostic System. These three investigators independently assessed the capsule endoscopic images under blinded conditions.
Sample size
We recruited 16 subjects each for Groups A and B on the basis of sample size for our previous 2-week study that compared irsogladine with omeprazole.(9) The sample size in that study was based on our estimation of the proportion of subjects that would be expected to exhibit mucosal breaks by capsule endoscopy post-treatment. We estimated that the prevalence of mucosal injuries would be approximately 20% in the irsogladine group on the basis of a preliminary study by Niwa et al.(10) that illustrated that the prevalence of NSAID-induced small intestinal lesions was lower in subjects taking rebamipide every day (20%) than in those taking placebo (80%). In rats, irsogladine has been demonstrated to suppress the formation of indomethacin-induced small intestinal lesions as effectively as rebamipide.(4) In addition, we estimated that the prevalence of mucosal injuries would be approximately 70% in the control group because a recent study found small intestinal lesions in 55–68% of subjects taking NSAIDs.(1,3,14) Thus, we concluded that 15 subjects would need to be recruited to each group (30 subjects in total) for a chi-squared test, a significance level of 5% (two-sided), a power of 80%, and equal allocation. On the assumption that two subjects would not be able to complete the study, we concluded that a minimum of 32 subjects was required. For Group C, we had no basis for determination of the sample size and recruited a minimum of five subjects.
Randomization
A coordinator conducted a simple fixed-allocation randomization using a block randomization scheme. Random numbers were generated by SAS software (SAS Institute, Cary, NC).
Statistical analyses
For continuous or categorical variables, the statistical significance of differences between groups was determined with the t test or Wilcoxon rank-sum test. The statistical significance of differences within a group was determined using the Wilcoxon signed-rank test. For binary variables, the statistical significance of differences between groups was determined using Fisher’s exact test. All reported p values are two-sided, and p vales of <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS ver. 9.2 (SPSS, Chicago, IL).
Results
Characteristics of subjects
Baseline characteristics were not significantly different among the three groups (Table 1).
Table 1.
Characteristics of subjects at baseline who underwent complete evaluation
| Group A | Group B | Group C | |
|---|---|---|---|
| Number of subjects | 16 | 16 | 5 |
| Mean (SD) age (years) | 25.1 ± 4.3 | 24.9 ± 4.3 | 23.6 ± 3.3 |
| Male/female | 11/5 | 10/6 | 5/0 |
| Hemoglobin concentration (mg/dl) | 14.38 ± 1.20 | 14.08 ± 1.99 | 14.60 ± 0.80 |
| No. mucosal breaks [mean (SD)] | 0.1 ± 0.3 | 0.3 ± 0.8 | 0.2 ± 0.4 |
| No. reddened lesions [mean (SD)] | 0.6 ± 0.8 | 0.2 ± 0.4 | 0.2 ± 0.4 |
| No. bleeds [mean (SD)] | 0.1 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Values were expressed as the mean ± SD. The differences in age were analyzed by the t test. The differences in sex were analyzed by Fisher’s exact test. Others were analyzed by Wilcoxon’s rank-sum test.
The time-course changes in capsule endoscopic findings
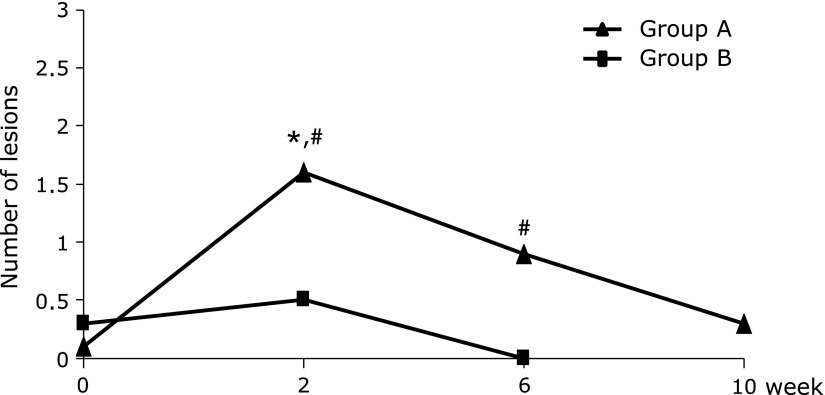
Study 1: In Group A, the number of small intestinal mucosal breaks detected by capsule endoscopy was significantly higher at week 2 than at baseline (p<0.05, Fig. 3), but the change for the worse of lesions was not found at week 6 compared with at week 2. In Group B, no exacerbations of mucosal lesions were found at weeks 2 and 6, and few lesions were found at week 6. Subjects in Group A had significantly more mucosal breaks at weeks 2 and 6 than those in Group B (p<0.05, Fig. 3). Mucosal breaks decreased during the add-on treatment with irsogladine from weeks 6 to 10.
Fig. 3.
Number of mucosal breaks in the small intestine in Study 1. In Group A, the number of small intestinal mucosal breaks detected by capsule endoscopy was significantly higher at week 2 (*p<0.05, Wilcoxon’s signed-rank test) than at baseline, but the change for the worse of lesions was not found at week 6 compared with at week 2. Subjects in Group A had significantly more mucosal breaks at weeks 2 and 6 than those in Group B (#p<0.05, Wilcoxon’s rank-sum test). Mucosal breaks decreased further during add-on treatment with irsogladine from weeks 6 to 10.
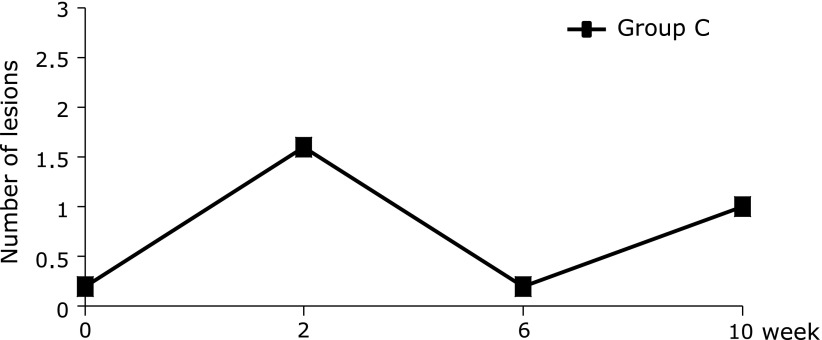
Study 2: The number of mucosal breaks detected in the small intestine by capsule endoscopy increased at week 2. No significant change in the number of mucosal breaks was observed from weeks 2 to 10 (Fig. 4).
Fig. 4.
Number of mucosal breaks in the small intestine in Study 2. The number of mucosal breaks detected in the small intestine by capsule endoscopy increased at week 2, but no further change in the number of mucosal breaks was observed from weeks 2 to 10 in Group C (week 2 vs baseline, p = 0.0625, Wilcoxon’s signed-rank test).
Changes in the number of lesions in each segment of the small intestine
In Groups A and C, among patients who received diclofenac sodium plus omeprazole (n = 16 + 5 at baseline and at weeks 2 and 6, n = 5 at week 10), the number of lesions decreased in the first segment and increased in the second and third segments at weeks 6 and 10. These findings suggest that in addition to a reduction in the total number of lesions throughout the small intestine because of mucosal adaptation, a change in the distribution of lesions may also occur during NSAID treatment (Fig. 5).
Fig. 5.
Changes in the number of lesions in each segment of the small intestine. In patients in Groups A and C who received diclofenac sodium plus omeprazole (n = 16 + 5 at baseline and weeks 2 and 6, n = 5 at week 10). The number of lesions decreased in the first segment and increased in the second and third segments after weeks 6 and 10.
Tolerability
Neither irsogladine nor omeprazole produced any side effects.
Discussion
It is known that NSAID-induced peptic ulcers in the stomach and duodenum, in which gastric acid is involved, can be prevented by PPIs.(15,16) Goldstein et al.(13) randomized healthy subjects into three groups to receive celecoxib, naproxen plus omeprazole, or placebo and followed them with capsule endoscopy. The percentages of subjects with small intestinal lesions after 2 weeks of treatment were 16%, 55%, and 7% in the respective groups, suggesting that suppression of acid secretion with a PPI could not prevent NSAID-induced small intestinal injury. Moreover, recent studies in rats suggested that PPIs and histamine-2 receptor antagonists may exacerbate NSAID-induced mucosal damage in the small intestine.(7,8) However, the influence of long-term PPI administration on NSAID-induced mucosal damage in the human small intestine has not been completely investigated.
In the present study, we examined the time-course changes of small intestinal lesions during long-term treatment with an NSAID and a PPI. The number of mucosal breaks increased at week 2 but no significant change was observed from weeks 2 to 10. These results suggest that mucosal adaptation to the damaging effects of NSAIDs (as observed in the stomach) may occurre in the small intestine. Gastric mucosal adaptation during repeated administration of NSAIDs (including aspirin) has been intermittently described since the 1960s. Several studies have investigated gastric adaptation to NSAID-induced damage and confirmed this phenomenon.(17–22) However, the mechanism of action or time course has not been clearly defined because of the different study designs (including the NSAID used, its dose, treatment period, and timing of evaluation).
We recorded findings which suggested mucosal adaptation in the small intestine during repeated administration of an NSAID, although a PPI was concomitantly administered. This means that during 10 weeks of treatment with diclofenac sodium (75 mg) and omeprazole (10 mg), the number of mucosal breaks (i.e., erosions and ulcers) in the small intestine increased in the first 2 weeks but did not increased by week 6, with no significant change until week 10. Thus, the small intestinal lesions that formed in the early stage did not worsen during long-term treatment through adaptation after approximately 1 month. In subjects who received diclofenac sodium and irsogladine, lesions almost disappeared by week 6. It is unknown whether the persistence of lesions after week 6 was caused by the coadministration of omeprazole because we did not include a diclofenac sodium-alone arm. Upper gastrointestinal lesions are known to be side effects in patients who take NSAIDs. Therefore, we could not create a diclofenac sodium-alone group.
One report focusing on small intestinal adaptation supports our results. Mizukami et al.(23) repeated capsule endoscopy at multiple time points during a 4-week NSAID treatment. Eleven healthy subjects underwent capsule endoscopy at weeks 0, 1, and 4 of treatment with enteric-coated aspirin (100 mg/day) plus omeprazole (20 mg/day) in this study. In the ileum, there were only a few red spots at week 0, but the number increased to 68.0 ± 133.6 at week 1 and then decreased to 48.4 ± 67.7 at week 4. No subjects had multiple erosions in the ileum at week 0, but the percentage of subjects with multiple erosions in the ileum increased to 54% (6/11) at week 1 and then decreased to 36% (4/11) at week 4.
We also evaluated the distribution of lesions along the small intestine. When considering all types of lesions (including those other than erosions and ulcers), more lesions were located in the first segment of the small intestine than in the other segments at all time points from week 2 to week 10. The number of lesions decreased in the first segment and increased in the second and third segments after weeks 6 and 10. These findings suggested that in addition to a reduction in the total number of lesions throughout the small intestine because of mucosal adaptation, a change in the distribution of lesions may also occur during NSAID treatment (Fig. 5). However, there was no clear pattern of change in the distribution of lesions if only erosions and ulcers are considered. Six aforementioned studies evaluated the distribution of lesions along the small intestine, and they did not find a significant difference in the distribution within each treatment group.(10,13,24–27) In contrast, more lesions were located in the jejunum in studies by Shiotani et al.(28) and Smecuol et al.(29) in which enteric-coated aspirin (100 mg) was administered for 1 and 2 weeks. Allison et al.(30) examined autopsy results for 713 patients and identified 249 patients who had received any NSAID during the 6 months preceding death. Of these, three patients who died from gastrointestinal perforation (all long-term users of NSAIDs) had widespread ulceration in the ileum, and one subject also had perforations in the jejunum. At present, there are insufficient data to ascertain the distribution pattern of NSAID-induced mucosal lesions in the small intestine. However, our results do not rule out a time-dependent change in the distribution pattern of NSAID-induced mucosal lesions in the small intestine during NSAID treatment. Further studies are required to clarify this issue.
With respect to the prevention of NSAID-induced mucosal damage in the small intestine, clinical studies have demonstrated remarkable efficacy for rebamipide,(10,11,23) misoprostol,(12) and irsogladine.(9) For geranylgeranylacetone, studies have inconsistently supported its efficacy.(25,28) However there are few reports about such gastromucoprotective drugs for healing the small intestinal lesions. In Group A, the number of lesions remarkably increased at week 2, but the worse was not found at week 6 compared with week 2. Additional treatment with irsogladine from weeks 6 to 10 in Group A significantly decreased the number of lesions at weeks 10 compared with Group C. (Group A vs Group C at week 10, p = 0.0088, Wilcoxon’s rank-sum test, Fig. 6). This finding suggests that irsogladine may be effective in healing NSAID-induced small intestinal damage.
Fig. 6.
Number of mucosal breaks in the small intestine in Study 1 and Study 2. In subjects who received diclofenac sodium (75 mg/day) plus omeprazole (10 mg/day for 6 weeks), the number of small intestinal mucosal breaks remarkably increased by week 2 (#p<0.05, Wilcoxon’s signed-rank test), but the change for the worse of lesions was not found at week 6 compared with at week 2. Subjects who received diclofenac sodium (75 mg/day) plus omeprazole (10 mg/day for 6 weeks) as well as irsogladine (4 mg/day) from weeks 6 to 10 had significantly fewer erosions and ulcers than those for whom irsogladine was not added (*p<0.05, Wilcoxon’s rank-sum test).
The limitations of this study
The present study involved a small sample size and featured young healthy subjects. Therefore, the results cannot be extrapolated to elderly individuals with underlying medical conditions. And we did not include a control group (Group C) for comparisons with Group A of Study 1 in the original study protocol, because we did not expect the occurrence of mucosal adaptation to NSAIDs, To verify the mucosal adaptation in the small intestine, a diclofenac sodium-alone group is needed. However we could not create a diclofenac sodium-alone group because of the ethical point: that is upper gastrointestinal lesions are known to be side effects in patients who take NSAIDs.
In the future, we should confirm whether irsogladine suppresses the small intestinal lesions in patients who receive NSAIDs in a prospective randomized controlled trial. In particular, we would like to evaluate the healing efficacy of small intestinal lesions in patients who receive NSAIDs and PPIs plus irsogladine in a large clinical trial.
Conclusions
A PPI (which was used for the prevention of mucosal damage in the upper gastrointestinal tract) did not prevent the occurence of small intestinal lesions induced by NSAIDs. However the small intestinal lesions that formed in the early stage did not worsen during long-term coadoministration of NSAIDs and PPI. These results suggested that mucosal adaptation may occur in the small intestine. And irsogladine was effective in both preventing and healing such small intestinal mucosal lesions.
Acknowledgments
Arshad Makhdum, Ph.D. and Springer Healthcare provided assistance with English language editing and preparation of the manuscript for submission.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 2.Handa O, Naito Y, Fukui A, Omatsu T, Yoshikawa T. The impact of non-steroidal anti-inflammatory drugs on the small intestinal epithelium. J Clin Biochem Nutr. 2014;54:2–6. doi: 10.3164/jcbn.13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe T, Higuchi K, Kobata A, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–187. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 4.Yoda Y, Takeuchi K, Kato S, et al. Search for prophylactic drugs against NSAID-induced small intestinal lesions in rats. Gastroenterology. 2008;134(Suppl 1):A528. [Google Scholar]
- 5.Akagi M, Amagase K, Murakami T, Takeuchi K. Irsogladine: overview of the mechanisms of mucosal protective and healing-promoting actions in the gastrointestinal tract. Curr Pharm Des. 2013;19:106–114. doi: 10.2174/13816128130115. [DOI] [PubMed] [Google Scholar]
- 6.Kamei K, Kubo Y, Kato N, Hatazawa R, Amagase K, Takeuchi K. Prophylactic effecgt of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci. 2008;53:2657–2666. doi: 10.1007/s10620-008-0199-9. [DOI] [PubMed] [Google Scholar]
- 7.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 8.Satoh H, Amagase K, Takeuchi K. Exacerbation of nonsteroidal anti-inflammatory drug-induced small intestinal lesions by antisecretory durgs in rats: the role of intestinal motility. J Pharmacol Exp Therap. 2012;343:270–277. doi: 10.1124/jpet.112.197475. [DOI] [PubMed] [Google Scholar]
- 9.Kuramoto K, Umegaki E, Nouda S, et al. Preventive effect of irsogladine or omeprazole on non-steroidal anti-inflammatory drug-induced esophagitis, peptic ulcers, and small intestinal lesions in humans, a prospective randomized controlled study. BMC Gastroenterol. 2013;13:85. doi: 10.1186/1471-230X-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niwa Y, Nakamura M, Ohmiya N, et al. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injury in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled cross-over study. J Gstroenterol. 2008;43:270–276. doi: 10.1007/s00535-007-2155-4. [DOI] [PubMed] [Google Scholar]
- 11.Fujimori S, Takahashi Y, Gudis K, et al. Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: a double-blind, randomized, controlled trial evaluated by capsule endoscopy. J Gastroenterol. 2011;46:57–64. doi: 10.1007/s00535-010-0332-3. [DOI] [PubMed] [Google Scholar]
- 12.Fujimori S, Seo T, Gudis K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339–1346. doi: 10.1016/j.gie.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JL, Eisen GM, Lewis B, Grainek IM, Ziotnick S, Fort JG, ; Investigators Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 15.Tytgat GN. Etiopathogenetic principles and peptic ulcer disease classification. Dig Dis. 2011;29:454–458. doi: 10.1159/000331520. [DOI] [PubMed] [Google Scholar]
- 16.Satoh H. Discovery of lansoprazole and its unique pharmacological properties independent from anti-secretory activity. Curr Pharm Des. 2013;19:67–75. doi: 10.2174/13816128130110. [DOI] [PubMed] [Google Scholar]
- 17.Kuwayama H, Eastwood G, Miyake S, Furukawa H. Adaptation of gastric mucosa to repeated indomethacin administration in the rat. Gastroenterology. 1986;90:1506. [Google Scholar]
- 18.Kawai T, Yamagishi T, Goto S. Circadian variations of gastrointestinal mucosal damage detected with transnasal endoscopy in apparently healthy subjects treated with low-dose aspirin for a short period. J Atheroscler Thromb. 2009;16:155–163. doi: 10.5551/jat.e615. [DOI] [PubMed] [Google Scholar]
- 19.Alderman BM, Ulaganathan M, Judd LM, et al. Insights into the mechanisms of gastric adaptation to aspirin-induced injury: a role for regenerating protein but not trefoil peptides. Lab Invest. 2003;83:1415–1425. doi: 10.1097/01.lab.0000092231.54761.cd. [DOI] [PubMed] [Google Scholar]
- 20.Konturek JW, Dembiński A, Konturek SJ, Domschke W. Helicobacter pylori and gastric adaptation to repeated aspirin administration in humans. J Physiol Pharmacol. 1997;48:383–391. [PubMed] [Google Scholar]
- 21.Lipscomb GR, Wallis N, Armstrong G, Goodman MJ, Rees WD. Influence of Helicobacter pylori on gastric mucosal adaptation to naproxen in man. Dig Dis Sci. 1996;41:1583–1588. doi: 10.1007/BF02087904. [DOI] [PubMed] [Google Scholar]
- 22.Shorrock CJ, Rees WD. Mucosal adaptation to indomethacin induced gastric damage in man--studies on morphology, blood flow and prostaglandin E2 metabolism. Gut. 1992;33:164–169. doi: 10.1136/gut.33.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizukami K, Murakami K, Abe T, et al. Aspirin-induced small bowel injuries and the preventive effect of rebamipide. World J Gastroenterol. 2011;17:5117–5122. doi: 10.3748/wjg.v17.i46.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo H, Hosono K, Inamori M, et al. Incidence of small bowel injury induced by low-dose aspirin: a crossover study using capsule endoscopy in healthy volunteers. Digestion. 2009;79:44–51. doi: 10.1159/000204465. [DOI] [PubMed] [Google Scholar]
- 25.Niwa Y, Nakamura M, Miyahara R, et al. Geranylgeranylacetone protects against diclofenac-induced gastric and small intestinal mucosal injuries in healthy subjects: a prospective randomized placebo-controlled double-blind cross-over study. Digestion. 2009;80:260–266. doi: 10.1159/000236032. [DOI] [PubMed] [Google Scholar]
- 26.Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JL, Eisen GM, Lewis B, et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007;25:1211–1222. doi: 10.1111/j.1365-2036.2007.03312.x. [DOI] [PubMed] [Google Scholar]
- 28.Shiotani A, Haruma K, Nishi R, et al. Randomized double-blind, pilot study of geranylgeranylacetone versus placebo in patients taking low-dose enteric-coated aspirin. Low-dose aspirin induced small bowel damage. Scand J Gastroenterol. 2010;45:292–298. doi: 10.3109/00365520903453182. [DOI] [PubMed] [Google Scholar]
- 29.Smecuol E, Pinto Sanchez MI, Suarez A, et al. Low-dose aspirin affects the small bowel mucosa: results of a pilot study with a multidimensional assessment. Clin Gastroenterol Hepatol. 2009;7:524–529. doi: 10.1016/j.cgh.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of nonsteroidal anti-inflammatory drugs. N Engl J Med. 1992;327:749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]