Abstract
Background
Novel oral anticoagulants (NOACs) have been shown to be at least as good as warfarin for preventing stroke or transient ischemic attack (TIA) in patients with atrial fibrillation (AF), yet diffusion of these therapies and patterns of use among AF patients with ischemic stroke and TIA have not been well characterized.
Methods and Results
Using data from Get With The Guidelines®–Stroke, we identified a cohort of 61,655 AF patients with ischemic stroke or TIA hospitalized between 10/2010–09/2012 and discharged on warfarin or NOAC (either dabigatran or rivaroxaban). Multivariable logistic regression was used to identify factors associated with NOAC versus warfarin therapy. In our study population, warfarin was prescribed to 88.9%, dabigatran to 9.6%, and rivaroxaban to 1.5%. NOAC use increased from 0.04% to a 16–17% plateau during the study period, though anticoagulation rates among eligible patients did not change appreciably (93.7% vs. 94.1% from first quarter 2011 to second quarter 2012), suggesting a trend of switching from warfarin to NOACs rather than increased rates of anticoagulation among eligible patients. Several bleeding risk factors and CHA2DS2-VASc scores were lower among those discharged on NOAC versus warfarin therapy (47.9% vs. 40.9% with CHA2DS2-VASc ≤5, p<0.001 for difference in CHA2DS2-VASc).
Conclusions
NOACs have had modest but growing uptake over time among AF patients hospitalized with stroke or TIA and are prescribed to patients with lower stroke risk compared to warfarin.
Keywords: anticoagulant, anticoagulation, stroke, fibrillation
Atrial fibrillation (AF) affects more than 2.5 million people in the United States, and the attributable risk for thromboembolic events ranges from 1.5% to 23.5% depending on a person’s age.1 Anticoagulation with warfarin reduces this risk by approximately 66%. For decades, warfarin was the only oral anticoagulant available for thromboembolic prophylaxis.2 Nevertheless, warfarin therapy is cumbersome for patients and medical providers due to numerous food and drug interactions, a narrow therapeutic index, and a need for frequent monitoring.
Recently, novel oral anticoagulants (NOACs) have become available for the prevention of thromboembolic events; specifically, the direct thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban and apixaban. Clinical trials show that these agents are at least non-inferior to warfarin with regard to thromboembolic prophylaxis for AF and potentially safer regarding the risk of intracranial hemorrhage.3–5 At the same time, these NOACs carry practical advantages including a decreased need for routine monitoring, a more predictable anticoagulant effect, and cost-effectiveness in high stroke risk populations.6,7 Yet provider inexperience and a lack of clear antidote for patients with bleeding complications on NOACs may have initially tempered enthusiasm for these drugs, even though clinical trials have demonstrated similar risks of major bleeding and significant reductions in intracranial hemorrhage compared with warfarin therapy.3–5 Little is known about the real-world uptake of these new anticoagulants, particularly in patients with ischemic stroke and transient ischemic attack (TIA).
To address this knowledge gap, we used data from Get With The Guidelines®-Stroke (GWTG-Stroke), a national quality improvement initiative and registry with >1800 participating hospitals, to characterize the prevalence, patterns, and predictors of NOAC versus warfarin therapy at discharge among AF patients hospitalized with ischemic stroke or TIA.
Methods
GWTG-Stroke
GWTG-Stroke is a national quality improvement initiative aimed at improving stroke care. Details of the GWTG-Stroke program design have been previously published.8 Hospitals that participate must receive approval through their local institutional review boards or a waiver of individual consent under the common rule. Trained personnel regularly review hospital records to identify patients admitted to participating centers with stroke or TIA. Medical history and demographic data from patient records are abstracted, and de-identified data are entered into a central database using a web-based patient management tool. Quintiles (Cambridge, MA) is the data collection coordination center for the American Heart Association/American Stroke Association Get With the Guidelines® programs. The Duke Clinical Research Institute (Durham, NC) serves as the data analysis center and has an agreement to analyze the aggregate, de-identified data for research purposes. The data are monitored and audited for quality and integrity.9
Study Population
Using GWTG-Stroke, we analyzed patients with AF who were hospitalized for ischemic stroke or TIA and discharged on warfarin or NOAC between October 19, 2010 and September 27, 2012. This time period spanned the two-year time interval after the Food and Drug Administration’s (FDA’s) approval of dabigatran (the first NOAC on the market for AF stroke prophylaxis), as well as the initial 15 months after the FDA’s approval of rivaroxaban. As a result, for the purposes of this study, NOAC refers to dabigatran and rivaroxaban only, since apixaban had not yet been approved for general use during the study period. We excluded sites with >25% missing information in the medical history panel, patients with hemorrhagic stroke or other documented contraindications to anticoagulant therapy, and patients who had no documented discharge destination or transferred out of the initial hospital. Of the 128,740 patients hospitalized with ischemic stroke/TIA and AF during the study period, 22,236 were excluded due to transferring out from the original facility or not having a documented discharge destination, 30,857 were excluded due to a documented contraindication to anticoagulant therapy, 4,771 were excluded due to lack of being discharged on any anticoagulant, 7,214 were excluded for anticoagulant choice other than NOAC or warfarin, and 2,007 were excluded due to conflicting or missing information. The final study population was comprised of 61,655 patients.
Statistical Analysis
Contingency tables were generated to identify important covariables, including demographics, clinical data, medical history, and hospital characteristics. Descriptive data are reported as percentages or as medians with interquartile ranges (IQRs). Pearson chi-square and Wilcoxon tests were used to assess univariate differences among patients discharged on NOACs versus warfarin. Hospital and patient factors associated with discharge NOAC therapy were identified using a multivariable logistic regression model and reported as odds ratios (ORs) with 95% confidence intervals (CIs). The following variables were included in the initial model: age, sex, race, insurance status, stroke diagnosis (TIA vs. ischemic stroke), creatinine, systolic blood pressure, pre-admission anticoagulant use, heart failure, hypertension, diabetes, prior stroke/TIA, use of pre-admission anticoagulants, prosthetic heart valve, coronary disease/prior myocardial infarction, carotid stenosis, peripheral vascular disease, smoker, dyslipidemia, ambulatory status at discharge, aspirin or clopidogrel at discharge, discharge to home, hospital size, type, and region, and annual intravenous tissue plasminogen activator and stroke volumes. Patients missing information regarding creatinine, systolic blood pressure, ambulatory status, use of pre-admission anticoagulants, and site characteristics were excluded from the regression model for the main analysis (n=14,856 total exclusions). For patients included in logistic regression analysis, missing rates for remaining variables were less than 5%, with the exception of insurance status with a rate of 11.4%. Missing insurance status was imputed based on age as follows: if age ≥65 then insurance imputed to “Medicare,” if age <65 and insurance present then insurance imputed to “other.” If insurance was not documented then insurance status was imputed to “self-pay/no insurance.” Missing values for medical history were imputed to “no,” and missing race was imputed to “Caucasian.” Backward selection was used to eliminate highly insignificant factors (p-value >0.1), and generalized estimating equations were used to account for within-hospital clustering. Generalized estimating equation (GEE) methods posit a working correlation structure. For this study, we used exchangeable or compound symmetric working correlation and empirical standard errors (i.e., sandwich variance). Additionally, we performed a sensitivity analysis including patients with missing data for anticoagulant prior to admission, serum creatinine, systolic blood pressure, and ambulatory status using multiple imputation to handle missing data (total n=61,408 after excluding patients from sites with high missing rates). For categorical variables with k categories, we used a Wald test with k-1 degrees of freedom and report p-value for overall relationship across levels. Temporal changes of discharge anticoagulant use were assessed by calendar quarter using Cochran-Mantel-Haenszel statistics. In order to assess anticoagulation rates among eligible patients, patients discharged on no anticoagulant (n=4,771) or a non-NOAC/non-warfarin agent (n=7,214) (including low molecular weight heparin, unfractionated heparin, fondaparinux, intravenous direct thrombin inhibitors, or “other” anticoagulants) were also included in the temporal analysis. Additional descriptive data were generated, including CHADS2 (1 point each for C=congestive heart failure, H=hypertension, A=age ≥75, D=diabetes mellitus, 2 points for S2=prior stroke/TIA) and CHA2DS2-VASc (as with CHADS2 with exception of A2=2 points for Age ≥75, V=1 point for vascular disease, A=1 point for age 65–74, Sc=1 point for female sex) distribution among patients discharged on NOAC versus warfarin, and site-based utilization of NOAC and warfarin at hospital discharge. For descriptive data, missing rates for all variables were ≤3% with the exception of missing rates of >10% for admission medications, insurance status, and admission ambulatory status, as well as missing rates of >20% for admission laboratory and physical exam values, National Institute of Health (NIH) stroke scale, and ambulatory status at discharge. All statistical analyses were performed using SAS version 9.1 software (SAS Institute, Cary, NC).
Results
Overall Utilization
We identified a study population of 61,655 patients from 1,542 hospitals with AF/atrial flutter who were admitted with ischemic stroke or TIA and discharged on warfarin or NOAC during our study period; 79.1% of the population suffered ischemic stroke, and the remainder (20.9%) experienced TIA. Atrial fibrillation or atrial flutter was newly diagnosed in 33% of the study population, and this proportion did not differ among those discharged on NOAC or warfarin. Among those discharged on NOAC or warfarin, dabigatran was prescribed to 9.6% (n=5,925), rivaroxaban to 1.5% (n=904), and warfarin to 88.9% (n=54,820).
Patient Characteristics (Tables 1 and 2)
Table 1.
Patient Characteristics by Discharge NOAC and Warfarin Use
| Variable | Total Population (n=61655) |
Discharged on NOAC (n=6835) |
Discharged on Warfarin (n=54820) |
p-value |
|---|---|---|---|---|
| Female sex | 53.1 | 51.8 | 53.3 | 0.016 |
| Age | 79 (70–85) | 77 (69–84) | 79 (70–85) | <0.001 |
| ≥75 | 64.4 | 59.2 | 65.1 | <0.001 |
| Race/ethnicity | 0.001 | |||
| White | 81.0 | 82.7 | 80.8 | |
| Black | 8.7 | 7.3 | 8.9 | |
| Hispanic | 4.8 | 4.8 | 4.8 | |
| Asian | 2.0 | 1.9 | 2.0 | |
| Medical history | ||||
| Heart failure | 16.8 | 12.8 | 17.3 | <0.001 |
| Hypertension | 79.9 | 79.3 | 79.9 | 0.211 |
| Diabetes mellitus | 29.6 | 26.7 | 30.0 | <0.001 |
| Prior stroke/TIA | 34.7 | 33.6 | 34.9 | 0.041 |
| Prior atrial fibrillation/flutter | 77.0 | 77.1 | 77.0 | 0.911 |
| Coronary artery disease | 34.3 | 30.9 | 34.8 | <0.001 |
| Peripheral vascular disease | 5.7 | 4.9 | 5.9 | 0.001 |
| Carotid stenosis | 4.2 | 4.0 | 4.2 | 0.400 |
| Dyslipidemia | 48.5 | 48.6 | 48.5 | 0.957 |
| Prosthetic heart valve | 3.9 | 1.4 | 4.2 | <0.001 |
| Smoker | 8.2 | 8.0 | 8.2 | 0.528 |
| Admission medications | ||||
| Antiplatelet therapy | 40.4 | 45.4 | 39.8 | <0.001 |
| Anticoagulation | 45.1 | 33.1 | 46.6 | <0.001 |
| Admission exam/labs: | ||||
| Systolic BP (mmHg) | 150(133–170) | 151(134–172) | 150(133–169) | <0.001 |
| Diastolic BP (mmHg) | 80 (69–92) | 80 (70–92) | 80 (69–92) | <0.001 |
| Heart rate | 78 (68–92) | 77 (67–91) | 78 (68–92) | 0.001 |
| Body mass index | 27.0 (23.6–31.2) | 27.0 (23.8–31.2) | 27.0 (23.6–31.2) | 0.460 |
| Hemoglobin A1c | 6.9 (6.2–7.9) | 6.9 (6.2–7.9) | 6.9 (6.2–7.9) | 0.799 |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 1.0 (0.8–1.3) | <0.001 |
| GFR ≥30 mg/dL/1.73m2 | 73.1 | 75.2 | 72.9 | <0.001 |
| Total cholesterol | 151(126–179) | 152(128–181) | 150(126–179) | 0.002 |
| Median NIH stroke scale | 5 (2–11) | 3 (1–8) | 5 (2–11) | <0.001 |
| NIH stroke scale ≥11 | 18.3 | 14.0 | 18.8 | <0.001 |
| Insurance status | <0.001 | |||
| Medicare | 42.0 | 39.0 | 42.3 | |
| Medicaid | 6.2 | 5.4 | 6.3 | |
| Insured/HMO | 38.0 | 41.7 | 37.6 | |
| Self-pay | 2.1 | 1.4 | 2.2 |
Values represent percentages or medians (interquartile range).
BP indicates blood pressure; GFR, glomerular filtration rate; HMO, health maintenance organization; NIH, National Institute of Health (higher scores on NIH stroke scale indicate greater stroke severity); NOAC, novel oral anticoagulant
Table 2.
Discharge Patient and Hospital Characteristics by Discharge NOAC and Warfarin Use
| Variable | Total Population (n=61655) |
Discharged on NOAC (n=6835) |
Discharged on Warfarin (n=54820) |
p-value |
|---|---|---|---|---|
| Discharge medications | ||||
| Aspirin | 49.6 | 41.8 | 50.6 | <0.001 |
| Clopidogrel | 6.2 | 5.0 | 6.4 | <0.001 |
| Aspirin and clopidogrel | 2.7 | 2.6 | 2.8 | 0.57 |
| Length of stay (excluding transfers) | 4 (2–6) | 3 (2–5) | 4 (2–6) | <0.001 |
| Length of stay ≥5 days | 40.6 | 27.1 | 42.3 | <0.001 |
| Ambulatory status at discharge | <0.001 | |||
| Unable to ambulate | 10.7 | 6.2 | 11.2 | |
| Ambulate with assistance | 25.6 | 22.2 | 26.0 | |
| Ambulate independently (with or without device) | 40.1 | 47.5 | 39.2 | |
| Discharge destination | ||||
| Other health care facility | 46.2 | 35.0 | 47.6 | <0.001 |
| Home | 53.8 | 65.0 | 52.4 | <0.001 |
| Hospital factors | ||||
| Hospital size (mean no. beds) | 348 (243–503) | 349 (240–527) | 348 (243–503) | 0.261 |
| Annual stroke/TIA discharges | 0.157 | |||
| >300 | 41.4 | 40.9 | 41.4 | |
| 101–300 | 51.5 | 51.4 | 51.5 | |
| ≤100 | 7.1 | 7.7 | 7.1 | |
| Region | <0.001 | |||
| West | 19.6 | 17.6 | 19.8 | |
| South | 30.7 | 37.3 | 29.8 | |
| Midwest | 19.7 | 18.4 | 19.9 | |
| Northeast | 30.1 | 26.7 | 30.5 | |
| Location | 0.009 | |||
| Rural | 5.0 | 4.3 | 5.1 | |
| Urban | 94.2 | 95.1 | 94.1 | |
| Hospital type | 0.004 | |||
| Academic | 49.0 | 46.9 | 49.3 | |
| Non-academic | 40.1 | 41.2 | 40.0 |
Values represent percentages or medians (interquartile range).
TIA indicates transient ischemic attack; All other abbreviations can be found in Table 1.
For patients discharged on NOAC versus warfarin, 51.8% versus 53.3% (p=0.016) were female and the median age was 77 (IQR 69–84) versus 79 (IQR 70–85, p<0.001). The majority of patients discharged on NOAC or warfarin were white (82.7% vs. 80.8%, respectively, p=0.001), and slightly higher proportions of patients discharged on NOAC versus warfarin had private/health maintenance organization (HMO) insurance (41.7% vs. 37.6%) than Medicare (39.0% vs. 42.3%), Medicaid (5.4% vs. 6.3%), or self-pay (1.4% vs. 2.2%; p<0.001 for insurance status). Considerably fewer patients were receiving anticoagulation at hospital admission among those discharged on NOAC versus warfarin (33.1% vs. 46.6%, p<0.001). Among patients with non-missing information regarding anticoagulant use at admission and NOAC or warfarin use at discharge, use of NOAC versus warfarin was 8.1% versus 91.9% (n=2263 vs. 25562) for those on anticoagulation prior to hospitalization, and 13.9% versus 86.1% (n=3684 vs. 22908) for those not on anticoagulation prior to hospitalization.
In univariate analyses, rates of diabetes (26.7% vs. 30.0%, p<0.001), prior stroke/TIA (33.6% vs. 34.9%, p=0.041), and heart failure (12.8% vs. 17.3%, p<0.001)—all well-established risk factors for AF thromboembolism—were significantly lower among those discharged on NOAC versus warfarin. Moreover, patients discharged on NOAC had lower CHADS2 and CHA2DS2-VASc scores than those discharged on warfarin therapy (p<0.001, Figure 1), with 38.0% versus 31.3% of patients having CHADS2 score ≤3 and 47.9% versus 40.9% with CHA2DS2-VASc ≤5.
Figure 1. Distribution of CHADS2 and CHA2DS2-VASc Scores by Choice of Anticoagulation.
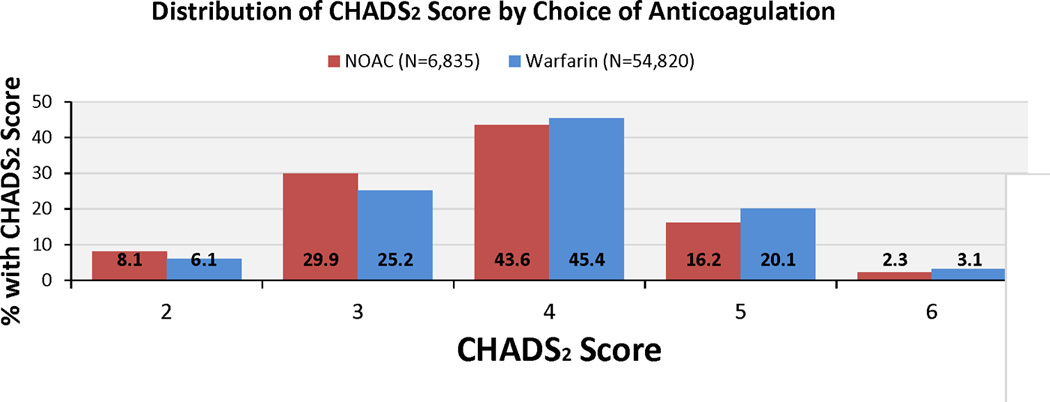
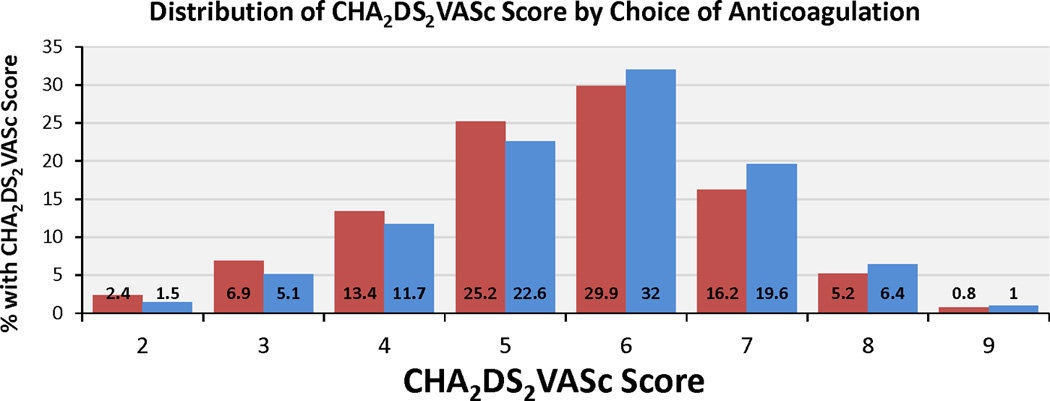
CHADS2 and CHA2DS2-VASc scores were lower among patients discharged on NOAC vs. warfarin therapy among AF patients hospitalized with ischemic stroke or TIA and discharged on either warfarin or NOAC (p<0.001 for difference in CHADS2 and CHA2DS2-VASc vs. warfarin).
AF indicates atrial fibrillation; NOAC, novel oral anticoagulant; CHADS2: C=congestive heart failure, H=hypertension, A=age ≥75, D=diabetes mellitus, S=prior stroke or TIA; CHA2DS2-VASc: CHADS2 plus V=vascular disease, A=age 65–74, S=female sex; TIA, transient ischemic attack
Patients discharged on NOAC versus warfarin had less severe ischemic stroke (median NIH stroke scale=3 [IQR 1–8] vs. 5 [IQR 2–11], p<0.001), shorter length of stay (3 [IQR 2–5] vs. 4 [IQR 2–6] days, p<0.001), and higher proportions of patients who could ambulate at admission (32.5% vs. 26.1%, p<0.001) and discharge (47.5% vs. 39.2%, p<0.001). More patients discharged on NOAC were discharged to home (65.0%) than a healthcare facility, compared with 52.4% of patients prescribed warfarin being discharged to home (p<0.001).
Predictors of NOAC Therapy
Table 3 shows the results of a multivariable logistic regression model for prescription of NOAC versus warfarin therapy at hospital discharge. Anticoagulation prior to admission was associated with lower odds for NOAC versus warfarin therapy at discharge (OR 0.52, 95% CI 0.48–0.56, p<0.001). Consistent with the prevalent use of dabigatran among NOACs during the study period and safety recommendations, worsening creatinine and prosthetic heart valves were strongly associated with less NOAC therapy. Impaired ambulation, increasing age, discharge antiplatelet therapy, and Medicare or Medicaid insurance status were also associated with reduced NOAC use. Medical history of coronary disease had no association with NOAC therapy despite controversy regarding increased myocardial infarction risk with dabigatran therapy. A sensitivity analysis using multiple imputation for missing variables did not show appreciably different results, but did additionally identify female sex, heart failure, and ischemic stroke (vs. other type of stroke) to be associated with reduced NOAC use (Supplementary Table 1), which is consistent with the observation that higher stroke risk is associated with lower NOAC use. While hospitals in the south had higher odds of NOAC prescription, there was no association with hospital type (academic vs. not), bed number, or urban/rural location on NOAC therapy; however, considerable site-level variation in discharge NOAC utilization was observed. Out of 1,347 sites with >5 NOAC prescriptions during the study period, the median rate of discharge NOAC versus warfarin therapy was 9.6% (IQR 4.0–16.7), with a range from 0 to 62.5% (Figure 2).
Table 3.
Factors Associated with NOAC Use (vs. Warfarin Use): Multivariable GEE Analysis
| Variable | Odds Ratio |
Lower Limit of 95% CI |
Upper Limit of 95% CI |
p-value |
|---|---|---|---|---|
| Age (per 10 year increase) | 0.94 | 0.91 | 0.97 | <0.001 |
| Sex (female vs. male) | 0.94 | 0.88 | 1.01 | 0.100 |
| Race (white vs. other) | 1.20 | 1.09 | 1.32 | <0.001 |
| Insurance status (vs. other) | <0.001 | |||
| Medicaid | 0.84 | 0.72 | 0.97 | |
| Medicare | 0.91 | 0.84 | 0.99 | |
| No insurance | 0.45 | 0.35 | 0.57 | |
| Medical history | ||||
| Previous stroke/TIA | 1.13 | 1.05 | 1.21 | 0.001 |
| Carotid stenosis | 1.20 | 1.03 | 1.40 | 0.020 |
| Heart failure | 0.93 | 0.84 | 1.02 | 0.120 |
| Prosthetic heart valve | 0.40 | 0.31 | 0.53 | <0.001 |
| Smoking | 0.82 | 0.73 | 0.93 | 0.002 |
| Anticoagulant therapy prior to admission | 0.52 | 0.48 | 0.56 | <0.001 |
| Serum creatinine (per 1mg/dL unit increase) | 0.75 | 0.69 | 0.81 | <0.001 |
| Systolic BP (per 10 mmHg unit increase) | 1.02 | 1.00 | 1.03 | 0.010 |
| Discharge medications | ||||
| Aspirin | 0.72 | 0.66 | 0.77 | <0.001 |
| Clopidogrel | 0.70 | 0.60 | 0.82 | <0.001 |
| Discharge to home (vs. other) | 1.43 | 1.31 | 1.56 | <0.001 |
| Ambulatory status (vs. ambulate independently) | <0.001 | |||
| Unable to ambulate | 0.58 | 0.51 | 0.67 | |
| Ambulate with assistance | 0.87 | 0.80 | 0.95 | |
| Region (vs. Northeast) | 0.001 | |||
| Midwest | 1.05 | 0.89 | 1.25 | |
| South | 1.29 | 1.11 | 1.49 | |
| West | 0.91 | 0.75 | 1.11 | |
| Number of beds (per 100 increase) | 1.00 | 1.00 | 1.00 | 0.127 |
| Rural vs. urban | 0.75 | 0.55 | 1.04 | 0.080 |
CI indicates confidence interval; GEE, generalized estimating equations; All other abbreviations can be found in Tables 1 and 2; n=42,094
Figure 2. Hospital-level NOAC Use at Discharge.
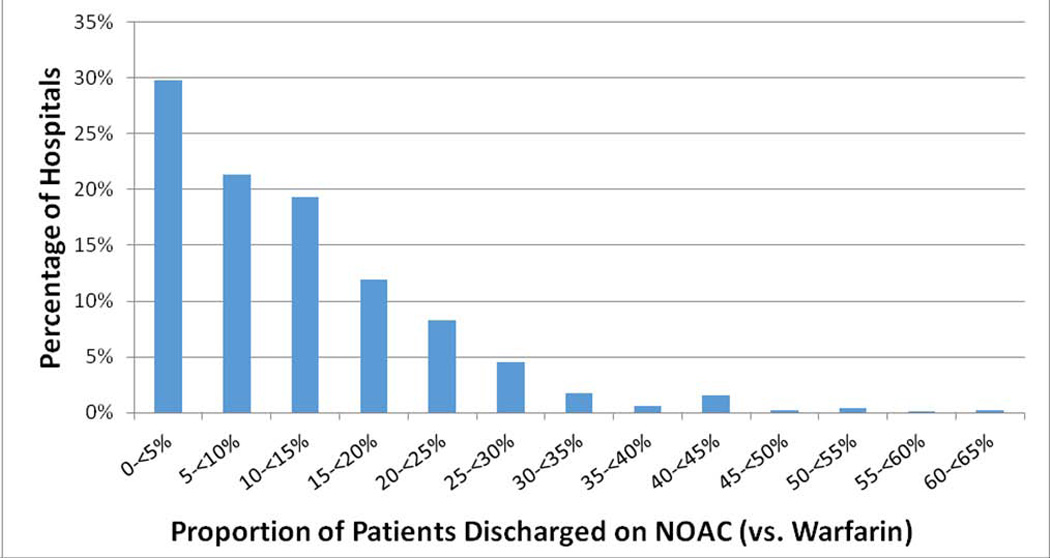
There was considerable site-level variation in NOAC use at hospital discharge among patients hospitalized with ischemic stroke/TIA and AF. Among 1347 sites with >5 NOAC prescriptions during the study period, the median rate of discharge NOAC versus warfarin therapy was 9.6% (IQR 4.0–16.7), with a range from 0 to 62.5%.
AF indicates atrial fibrillation; IQR, interquartile range; NOAC, novel oral anticoagulant; TIA, transient ischemic attack
Temporal Variability
We did observe increased NOAC use over time (p for non-zero correlation <0.001, Figure 3). Dabigatran was FDA approved for AF stroke prophylaxis in the fourth quarter of 2010, yet NOAC use was nearly absent until the third quarter of 2011 when it increased to 6.4% among patients eligible for anticoagulation. This was the same quarter in which rivaroxaban was approved by the FDA for AF stroke prophylaxis and the quarter following the updated American College of Cardiology/American Heart Association/Heart Rhythm Society update on the management of patients with AF using dabigatran.10 There was subsequent rapid uptake with overall NOAC use leveling off in the 16–17% range for the remainder of the study period. Warfarin use and non-NOAC/non-warfarin anticoagulant use declined from the first quarter of 2011 to the second quarter of 2012 (78.8% to 72.4% for warfarin and 14.6% to 4.7% for non-NOAC/non-warfarin agents). Over these same quarters, the proportion of eligible patients who were not anticoagulated at hospital discharge did not change appreciably (from 6.3% to 5.9%).
Figure 3. Discharge Anticoagulant Use by Calendar Quarter.
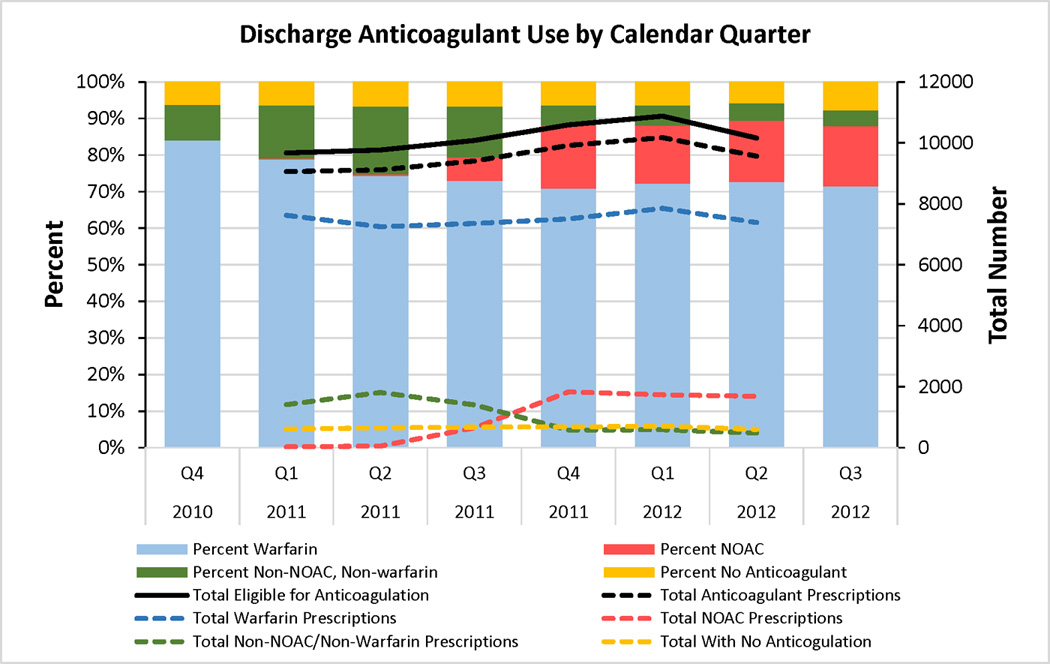
NOAC use increased over time from 0.3 to 16.6% from first quarter of 2011 to second quarter of 2012 (p for non-zero correlation <0.001). Warfarin use and non-NOAC/non-warfarin anticoagulant use declined during the same time period (78.8% to 72.4% for warfarin and 14.6% to 4.7% for non-NOAC/non-warfarin agents). The proportion of eligible patients who were not anticoagulated at hospital discharge did not change appreciably (from 6.3% to 5.9%), suggesting that NOAC use did not increase the proportion of patients anticoagulated at hospital discharge, but were used in place of other anticoagulation strategies. Note: The total number lines were not extended to Q4 2010 and Q3 2012 because these quarters were truncated by our pre-specified study period, thereby limiting the number of patients included for those quarters.
NOAC indicates novel oral anticoagulant; Q, quarter
Discussion
In this study, we examined the diffusion of NOACs in a high stroke risk population using the largest available registry of ischemic stroke and TIA patients. While other studies have also demonstrated a modest uptake in NOAC use, this is the largest study to evaluate the use of NOACs in a high-risk population with confirmed stroke or TIA.11–14 We also observed considerable variations in NOAC use among hospitals and important differences in risk for recurrent stroke/TIA and several bleeding risk factors among patients receiving discharge NOAC versus warfarin therapy. Moreover, we found that unique non-traditional factors, such as ambulatory status and discharge destination, are significant predictors of NOAC use.
Adoption of new therapies may take well over a decade—even when clinical practice guidelines with robust evidence are available.15,16 For example, in the first several years following publication of pivotal trials demonstrating warfarin’s efficacy in stroke prevention, such as Atrial Fibrillation, Aspirin, Anticoagulation Study, 1989 (AFASAK) and Stroke Prevention in Atrial Fibrillation Study, 1991 (SPAF), there were only modest increases in warfarin utilization among AF patients from 13% in 1989 to near 25% in 1991. While utilization of anticoagulation for stroke prevention in AF has slowly increased over time, the current anticoagulation rate near 60% suggests there remain opportunities for improvement.12 Despite potential practical advantages of NOACs over warfarin, such as reduced need for monitoring and lower intracranial hemorrhage rates, our study shows similarly modest early adoption rates for NOAC therapy. Our observed rate of uptake at 16–17% falls in the upper range of estimates among studies describing early utilization patterns. A report from the Danish National Register showed 5.2% uptake by fourth quarter 2011, while reports from the United States have shown higher early adoption rates—12% in the Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF) Registry by August 2011 to as high as 16.9% in a report from the National Disease and Therapeutic Index survey by December 2011.13,14
In part, the modest adoption rate of NOACs in the acute ischemic stroke and TIA population may be due to differences in the patient population encountered in acute ischemic stroke and TIA care compared with the pivotal AF trials evaluating NOACs. Compared with the patients studied in those clinical trials, our study population was older (~77–79 vs. ~71–73), with a higher proportion of women (~48.6–62.5% vs. ~33.1–46.6%), and with higher mean CHADS2 scores (3.87 vs. 2.10 in the Randomized Evaluation of Long-Term Anticoagulation [RE-LY] and 3.48 in Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in AF [ROCKET-AF]), consistent with our high-risk confirmed ischemic stroke/TIA population.3–5,17 Therefore, it is not surprising that the use of NOACs is different given the differences in treating inpatients hospitalized with stroke or TIA compared with the trial populations, which treated predominantly ambulatory patients with AF.
Previous reports from ORBIT-AF, the National Disease and Therapeutic Index, and the Danish National Patient Register also focused on outpatients or a mix of outpatients/inpatients with AF. In contrast, our patient population was exclusively inpatient and had higher CHADS2 and CHA2DS2-VASc stroke risk scores.13,14 Nevertheless, our data agree with these prior studies by suggesting cautious uptake of NOAC therapy by physicians, mirroring the risk-treatment mismatch previously observed for warfarin therapy in AF, with higher age, bleeding risk, and stroke risk being associated with less warfarin use.18–20 NOACs confer equal or better protection from stroke than warfarin with a lower intracerebral hemorrhage risk and no increase in major bleeding rates,3–5 and our current analysis showed patients discharged on NOACs had lower CHADS2 stroke risk scores. Moreover, NOAC use had less association with bleeding risk factors such as older age, concurrent antiplatelet therapy, and impaired ambulation.21–23 While bleeding risk is an important clinical consideration, reports of real-world dabigatran use compared with warfarin in United States- and Denmark-based registries showed no increases in bleeding. In fact, these reports found lower intracerebral hemorrhage rates.24,25 Although information on real-world rivaroxaban use is limited, these reports suggest that bleeding events described in case studies and series may overstate the actual bleeding risk compared with clinical trials and population-based registries, highlighting the need for further investigation into the real-world efficacy and bleeding risk for NOAC-treated patients.
Similar to ORBIT-AF, we also identified racial and financial differences in anticoagulant choice. We found that white patients had a higher odds of NOAC prescription than other races, echoing racial disparities previously described for AF anticoagulation.14,19,26 Closely related to this, it would appear that financial access to NOACs was a major determinant of their use, since lack of health insurance, Medicaid, and Medicare were associated with a lower odds of NOAC therapy compared with warfarin. High out-of-pocket costs are a deterrent to new prescription drug adherence, and even with health insurance, affordability may have been a concern due to substantial co-pays or cumbersome pre-approvals.27 Given the favorable risk-benefit profile of NOACs compared with warfarin, further work is needed to close the gap conferred by financial and social barriers.
One question of interest is whether patients who are being treated with NOACs are newly initiated or switched from warfarin to NOAC therapy. GWTG-Stroke does not collect information on the specific admission anticoagulant, but it is reasonable to assume that patients on anticoagulation prior to hospitalization were likely on warfarin therapy during this study period. Using this assumption, as many as 38% of patients could have been warfarin to NOAC switchers, whereas 62% were possibly new starts. While discharge NOAC use increased during this study period, the use of warfarin (and especially non-NOAC/non-warfarin agents such as low molecular weight heparins) decreased markedly. Nonetheless, the total percentage of eligible patients discharged to home without an anticoagulant did not change appreciably (from 6.6% to 5.9%). Therefore, rather than “growing the pie” of patients anticoagulated for AF, NOACs may be preferentially prescribed in place of non-NOAC/non-warfarin anticoagulants, and to a lesser degree, in place of warfarin. Lastly, 1.4% of patients discharged on NOACs had prosthetic heart valves and 30.9% had coronary artery disease despite the possibility of adverse events while on dabigatran, which was the predominant NOAC during this study period.3,28,29 Further education regarding the risks and benefits of NOAC therapy may be needed to increase familiarity with these drugs and prevent risk-treatment mismatches or adverse events.
Our study had several limitations. First, GWTG-Stroke is a voluntary quality improvement program and may not represent prescribing patterns at non-participating hospitals. For example, GWTG hospitals compared with American Hospital Association Hospitals were more likely to be teaching hospitals (45.2 vs. 23.7%, p<0.001), less likely to be rural (12.5 vs. 35.1%, p<0.001), and were larger (median bed size 255 vs. 88, p<0.001). Second, prescription patterns among those being discharged after ischemic stroke or TIA could differ from those of ambulatory patients with AF treated in the outpatient setting. Moreover, prior exposure to anticoagulants may have been an important confounder in the decision to either discharge on anticoagulation or on the choice of anticoagulant. Third, we only evaluated those patients discharged on oral anticoagulant therapy, and available data did not allow us to assess patterns of prescribing for new starts at hospital follow-up. Fourth, we were unable to assess adherence to discharge anticoagulant therapy after hospital discharge. Fifth, it must be noted that rivaroxaban was only available for the latter 15 months of the study period, and with current availability of apixaban and the ongoing dissemination of information regarding NOACs, current usage patterns may differ. Sixth, we were unable to perform a formal bleeding risk assessment because we lacked data for key components of well-validated bleeding risk models such as prior bleeding episodes, and we did not include a formal assessment of stroke severity in logistic regression modeling since the NIH stroke scale had a high missing rate (29%).21,23,30 Finally, residual measured and unmeasured confounders may still exist, particularly since we were unable to determine all clinical and social circumstances that could have influenced treatment selection from the registry such as patient choice, prescribing physician specialty, or payer mix and decisions.
In conclusion, among AF patients hospitalized with ischemic stroke or TIA, use of NOACs have been modestly integrated into clinical practice, though they tend to be used in patients with lower stroke risk and better functional status. Use of NOACs varied widely by hospital and overall anticoagulation rates at hospital discharge among eligible stroke and TIA patients did not increase appreciably. Future investigations should consider linking to claims data to further explore adherence rates, longitudinal outcomes, and safety of NOACs in clinical practice.
Supplementary Material
Acknowledgements
The authors thank Erin Hanley, MS for her editorial assistance in the preparation of this manuscript. Ms. Hanley did not receive compensation for her assistance, apart from her employment at the institution where this study was conducted.
Funding Sources
This study was funded by an American Heart Association Young Investigator Database Research Seed Grant. Priyesh A. Patel received additional support from National Institutes of Health grant T32-HL007101.
Footnotes
Conflict of Interest Disclosures
PA Patel: Dr. Patel has no relevant disclosures to report.
X Zhao: Ms. Zhao has no relevant disclosures to report.
GC Fonarow: Dr. Fonarow reports research support from the Patent-Centered Outcome Research Institute (PCORI) significant and consulting with Janssen Pharmaceutical (modest).
BL Lytle: Ms. Lytle has no relevant disclosures to report.
EE Smith: Dr. Smith has no relevant disclosures to report.
Y Xian: Dr. Xian has no relevant disclosures to report.
DL Bhatt: Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Elsevier Practice Update Cardiology (modest), Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees (all modest): Duke Clinical Research Institute; Harvard Clinical Research Institute; Mayo Clinic; Population Health Research Institute; Honoraria (all significant): American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Associate Editor); Research Grants (all significant): Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company, Pfizer; Unfunded Research: FlowCo, PLx Pharma, Takeda.
ED Peterson: Dr. Peterson receives significant research funding from Janssen Pharmaceutical and modest consulting fees from Boehringer-Ingelheim.
LH Schwamm: Dr. Schwamm is chair of AHA GWTG-Stroke clinical workgroup..
AF Hernandez: Dr. Hernandez has no relevant disclosures to report.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005:CD001927. doi: 10.1002/14651858.CD001927.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 6.Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, Wang PJ, Turakhia MP. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- 7.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123:2562–2570. doi: 10.1161/CIRCULATIONAHA.110.985655. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y, LaBresh KA. Overview of the american heart association "get with the guidelines" programs: Coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. doi: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 9.Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, Schwamm LH, Smith EE. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am Heart J. 2012;163:392–398. doi: 10.1016/j.ahj.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Wann LS, Curtis AB, Ellenbogen KA, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Heuzey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Jacobs AK, Anderson JL, Albert N, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:1144–1150. [Google Scholar]
- 11.Carley B, Griesbach S, Larson T, Krueger K. Assessment of dabigatran utilization and prescribing patterns for atrial fibrillation in a physician group practice setting. Am J Cardiol. 2014;113:650–654. doi: 10.1016/j.amjcard.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the united states, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen R, Gislason G, Torp-Pedersen C, Olesen JB, Fosbol EL, Hvidtfeldt MW, Karasoy D, Lamberts M, Charlot M, Kober L, Weeke P, Lip GY, Hansen ML. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, Fonarow GC, Gersh B, Mahaffey KW, Kowey PR, Ezekowitz MD, Singer DE, Thomas L, Peterson ED, Hylek EM Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation. J Am Heart Assoc. 2013;2:e000535. doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garjon FJ, Azparren A, Vergara I, Azaola B, Loayssa JR. Adoption of new drugs by physicians: a survival analysis. BMC Health Serv Res. 2012;12:56. doi: 10.1186/1472-6963-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41:56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 17.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 19.Lewis WR, Fonarow GC, Grau-Sepulveda MV, Smith EE, Bhatt DL, Hernandez AF, Olson D, Peterson ED, Schwamm LH. Improvement in use of anticoagulation therapy in patients with ischemic stroke: results from Get With The Guidelines-Stroke. Am Heart J. 2011;162:692–699. e2. doi: 10.1016/j.ahj.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk--treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97:2046–2050. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- 21.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 23.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callreus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in "real-world" patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. doi: 10.1016/j.jacc.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013;368:1272–1274. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- 26.Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson ED, Fonarow GC Get With The Guidelines Steering Committee and Hospitals. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54:1280–1289. doi: 10.1016/j.jacc.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 27.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44:1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohnloser SH, Oldgren J, Yang S, Wallentin L, Ezekowitz M, Reilly P, Eikelboom J, Brueckmann M, Yusuf S, Connolly SJ. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation. 2012;125:669–676. doi: 10.1161/CIRCULATIONAHA.111.055970. [DOI] [PubMed] [Google Scholar]
- 29.Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397–402. doi: 10.1001/archinternmed.2011.1666. [DOI] [PubMed] [Google Scholar]
- 30.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


