Abstract
Objectives/Hypothesis
To test the hypothesis that subligamental cordectomy produces superior acoustic outcome than subepithelial cordectomy for early (T1-2) glottic cancer that requires complete removal of the superficial lamina propria but does not involve the vocal ligament.
Study Design
Computer simulation
Methods
A computational tool for vocal fold surgical planning and simulation (the National Center for Voice and Speech Phonosurgery Optimizer-Simulator) was used to evaluate the acoustic output of alternative vocal fold morphologies. Four morphologies were simulated: normal, subepithelial cordectomy, subligamental cordectomy, and transligamental cordectomy (partial ligament resection). The primary outcome measure was the range of fundamental frequency (F0) and sound pressure level (SPL). A more restricted F0-SPL range was considered less favorable because of reduced acoustic possibilities given the same range of driving subglottic pressure and identical vocal fold posturing.
Results
Subligamental cordectomy generated solutions covering an F0-SPL range 82% of normal for a rectangular vocal fold. In contrast, transligamental and subepithelial cordectomies produced significantly smaller F0-SPL ranges, 57% and 19% of normal, respectively.
Conclusion
This study illustrates the use of the Phonosurgery Optimizer-Simulator to test a specific hypothesis regarding the merits of two surgical alternatives. These simulation results provide theoretical support for vocal ligament excision with maximum muscle preservation when superficial lamina propria resection is necessary but the vocal ligament can be spared on oncological grounds. The resection of more tissue may paradoxically allow the eventual recovery of a better speaking voice, assuming glottal width is restored. Application of this conclusion to surgical practice will require confirmatory clinical data.
Keywords: voice physiology, glottic carcinoma, vocal cord cancer, vocal fold cancer, cordectomy, transoral laser surgery, functional morphology, voice range profile, multiobjective optimization, patient-specific simulation, voice simulation, optimized simulation, NCVS simulator
Introduction
Voice outcome of surgical treatment for early (T1-2) glottic cancer is an important measure because the oncologic result is equivalent between surgery and radiation1–6, leaving functional outcome often an important consideration when choosing the treatment modality. The postoperative voice is generally poorer with deeper resection7–15, consistent with greater glottal insufficiency due to tissue loss. However, new data challenge the universality of this trend. Hillel et al.16 reported that patients who underwent subligamental cordectomy had better postoperative voice and stroboscopy scores than those who underwent subepithelial cordectomy. The authors concluded that if the tumor extends most of the thickness of the superficial layer of the lamina propria (SLLP) such that minimal SLLP can be preserved, the vocal ligament should be resected even if it is not oncologically necessary. This suggestion runs counter to conventional teaching of vocal fold microsurgery, which emphasizes the preservation of non-diseased tissue in order to maximally preserve phonatory function. Yet those findings echoed the experience of other surgeons who have had surprisingly good vocal outcomes from subligamental cordectomies.17
Comparing voice outcomes between subepithelial cordectomy that removes the entire SLLP and subligamental cordectomy is intrinsically challenging for several reasons. First, the comparison requires a specific subset of early glottic cancers, namely those that extend close to but do not involve the vocal ligament, for which either surgical option is oncologically sound. These tumors are not rare but are still uncommon enough that it is difficult for a study to accumulate large numbers of them. For example, only 4 patients were in the subepithelial cordectomy group in Hillel et al.16 Second, scarring following subepithelial or subligamental cordectomy is known to be highly variable.18 Third, there is substantial variability in vocal fold tissue structure and acoustic output even amongst normal subjects.19–21 To overcome these individual variations, an impractically large sample size would be required.
Computer simulation of vocal fold vibratory behavior can provide insight into clinical observations.22,23 Finite-element and finite-difference approaches have been reported over the past decade.24–27 Simulation has been used for planning medialization laryngoplasty28, for example. The goal of this study is to assess the relative acoustic merits of subepithelial cordectomy and subligamental cordectomy utilizing a new computational approach combining finite element model (FEM) voice simulation29 with multi-objective optimization30. A voice simulator produces one set of acoustic output variables given a defined input vocal fold geometry and a fixed subglottal pressure. A meaningful comparison between two different vocal fold geometries should entail a range of possible acoustic outputs given a clinically relevant range of subglottal pressures for each geometry. The comparison therefore requires thousands of simulation runs. To do this efficiently, a multi-objective optimization (MOO) algorithm is coupled to the simulator to fully explore the range of possible acoustic outputs, an approach implemented in the National Center for Voice and Speech (NCVS) Phonosurgery Optimizer-Simulator.30 In this study, the primary acoustic outcomes are the ranges of fundamental frequency (F0) and sound pressure level (SPL). F0 and SPL are of interest because they are fundamental acoustic parameters that characterize a voice. The ranges of F0 and SPL determine a speaker’s pitch range and loudness. Secondary simulation outcome variables are a physiological input parameter (subglottal pressure) and an output parameter (phonation onset time). We hypothesize that subligamental cordectomy produces a greater F0-SPL range than subepithelial cordectomy.
Materials and Methods
Finite-Element Models
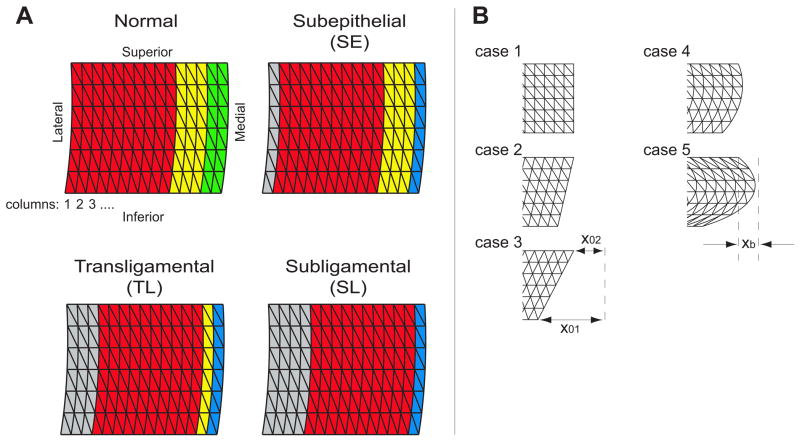
Each vocal fold was modeled in three layers: SLLP, ligament, and muscle. The SLLP layer was taken to represent SLLP plus epithelium, which was not explicitly modeled. Future work will include the epithelium as a discrete layer. Tissue was divided into triangular elements in the coronal plane and into rectangular layers in the anterior-posterior direction, along the length of the vocal fold. The FEM consisted of 15 vertical columns from lateral to medial, 6 horizontal layers from superior to inferior, and 5 anterior-posterior layers (Figure 1A). Each layer (SLLP, ligament and muscle) was represented by several columns of elements with mechanical properties common to the elements within that morphological layer (Table 1). Based on layer thickness in published histologic images31, the most medial 2 columns were designated as the SLLP, the next 3 columns the ligament, and the lateral 10 columns the muscle in a normal vocal fold model (Figure 1A). The critical viscoelastic parameters for each tissue column are μ, the shear modulus in a plane transverse to the fibers; μ′, the shear modulus in a plane that includes the fibers and their longitudinal tensions; and η, the viscosity. The Young’s modulus in the transverse plane is dependent on μ, and therefore does not need separate specification.29 Subscripts on these parameters define the tissue layer.
Figure 1.
A: The vocal fold finite element model consisted of fifteen columns representing three or four different layers. The normal vocal fold consisted of a muscle layer (red; 10 columns), ligament (yellow; 3 columns) and SLLP (green; 2 columns). Three excisions were modeled: Subepithelial cordectomy (SE), transligamental cordectomy (TL), and subligamental cordectomy (SL). In all three surgeries, a layer of scar is modeled (blue; 1 column). The remaining vocal fold tissue was medialized with an inelastic implant (gray) to maintain prephonatory glottal width. B: Five vocal fold medial surface shapes were tested. Case 1: rectangular; case 2: convergent; case 3: 2X convergent; case 4: convergent + bulging; case 5: 2X convergent + bulging. The convergence parameters X01, X02 and bulging parameter Xb are specified in Table 1.
Table 1.
Ranges of objective functions (F0, SPL, subglottal pressure) and decision variable (μ1) used for optimized simulation. Other parameters were kept constant. Three surgeries were simulated (see Figure 1). The subscripts denote the layer specified: layer 1: normal SLLP or scar; layer 2: ligament; layer 3: muscle; layer 4: implant. The material in the FEM was considered as a fiber-gel compound, fiber-like along the vocal fold longitudinally, but gel-like in the coronal plane, i.e. transversally isotropic. The longitudinal shear moduli of SLLP, ligament, and muscle were designated as , and , respectively. The transverse shear moduli of SLLP, ligament, and muscle were designated as μ1, μ2, and μ3, respectively, and reflect the gel properties. Viscosity is designated by η1, η2, and η3. For the scar layer, would increase with greater deposition of organized collagen in the longitudinal orientation. Since collagen fibers are randomly organized in scar45, was not altered. The lower boundary of μ1 was increased 3-fold to account for the higher collagen fiber content in scar. This was based on the observation that scarred vocal fold tissue demonstrates a smaller range for μ with an increased lower limit.46
| Normal vocal fold | Subepithelial | Transligamental | Subligamental | |
|---|---|---|---|---|
| F0 (Hz) | 120 (90–150) | 120 (90–150) | 120 (90–150) | 120 (90–150) |
|
| ||||
| SPL (dB) | 70 (60–80) | 70 (60–80) | 70 (60–80) | 70 (60–80) |
|
| ||||
| Subglottal pressure (kPa) | 0.01 to 2 | 0.01 to 2 | 0.01 to 2 | 0.01 to 2 |
|
| ||||
| μ′1 (dyn/cm2) | 5000 | 5000 | 5000 | 5000 |
|
| ||||
| μ′2 (dyn/cm2) | 20000 | 20000 | 20000 | n/a |
|
| ||||
| μ′3 (dyn/cm2) | 15000 | 15000 | 15000 | 15000 |
|
| ||||
| μ′4 (dyn/cm2) | n/a | 500000 | 500000 | 500000 |
|
| ||||
| μ1 (dyn/cm2) | 5000–50000 | 15000–50000 | 15000–50000 | 15000–50000 |
|
| ||||
| μ2 (dyn/cm2) | 5000 | 5000 | 5000 | n/a |
|
| ||||
| μ3 (dyn/cm2) | 5000 | 5000 | 5000 | 5000 |
|
| ||||
| μ4 (dyn/cm2) | n/a | 500000 | 500000 | 500000 |
|
| ||||
| η1 (poise) | 2 | 2 | 2 | 2 |
|
| ||||
| η2 (poise) | 2 | 2 | 2 | n/a |
|
| ||||
| η3 (poise) | 2 | 2 | 2 | 2 |
|
| ||||
| η4 (poise) | n/a | 50 | 50 | 50 |
|
| ||||
| Case 1 - Rectangular | ||||
|
| ||||
| VF convergence (cm) | x01 = 0.03 | x01 = 0.03 | x01 = 0.03 | x01 = 0.03 |
| x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | |
|
| ||||
| VF bulging (cm) | 0 | 0 | 0 | 0 |
|
| ||||
| Case 2 - 1X convergent | ||||
|
| ||||
| VF convergence (cm) | x01 = 0.06 | x01 = 0.06 | x01 = 0.06 | x01 = 0.06 |
| x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | |
|
| ||||
| VF bulging (cm) | 0 | 0 | 0 | 0 |
|
| ||||
| Case 3 - 2X convergent | ||||
|
| ||||
| VF convergence (cm) | x01 = 0.12 | x01 = 0.12 | x01 = 0.12 | x01 = 0.12 |
| x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | |
|
| ||||
| VF bulging (cm) | 0 | 0 | 0 | 0 |
|
| ||||
| Case 4 - 1X convergent with bulging | ||||
|
| ||||
| VF convergence (cm) | x01 = 0.06 | x01 = 0.06 | x01 = 0.06 | x01 = 0.06 |
| x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | |
|
| ||||
| VF bulging (cm) | 0.03 | 0.03 | 0.03 | 0.03 |
|
| ||||
| Case 5 – 2X convergent with bulging | ||||
|
| ||||
| VF convergence (cm) | x01 = 0.12 | x01 = 0.12 | x01 = 0.12 | x01 = 0.12 |
| x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | x02 = 0.03 | |
|
| ||||
| VF bulging (cm) | 0.07 | 0.07 | 0.07 | 0.07 |
To simulate the postoperative vocal fold anatomy and tissue properties following subepithelial cordectomy with complete excision of SLLP, a layer of surface scar was modeled with one column, with an increased lower bound for μ1 compared to normal SLLP (Table 1). This model will be referred to as SE (subepithelial). Subligamental cordectomy was modeled with the same surface scar as in SE, but with complete removal of the ligament (Figure 1). This model will be referred to as SL (subligamental). A third model simulated an intermediate scenario with transligamental excision (model TL), where a residual ligament of one column deep was present. The parameter μ, the shear modulus of the lamina propria “gel” component, was the main distinguishing feature between the layers. This parameter changes dramatically with scarred tissue.32
Glottal width was kept constant across the 4 scenarios (normal, SE, TL, SL) by incorporating an inelastic implant laterally to maintain a 15-column FEM (Figure 1). The inelastic implant was simulated by very large values for elastic moduli and viscosity. For simplicity, the simulations were carried out with symmetry, i.e. both vocal folds modeled identically.
Optimized Simulation
The implementation of FEM for voice simulation has been detailed previously30,33 and is briefly summarized in Appendix A. Multiobjective optimization (MOO) was integrated with the voice simulator to allow targeted exploration of acoustic possibilities from each vocal fold model, as detailed in Palaparthi et al.30 MOO aims to find solutions that simultaneously optimize multiple objective functions. The objective functions in this case were F0 and SPL. The acoustic requirements (objective functions) were set to mimic a male subject with an F0 target of 120 Hz and SPL target of 70 dB at 30 cm. To optimize these objective functions, two parameters (decision variables) were allowed to vary: subglottal pressure Ps, and transverse shear modulus of the surface scar, μ1. Ps was allowed to vary within a physiologic range, whereas μ1 was varied to reflect variability in scarring. The ranges in which the decision variables were allowed to vary are listed in Table 1. Vocal fold length L was set to 1.0 cm, thickness T to 0.5 cm and depth D to 0.5 cm.
The optimized simulation procedure is detailed in Appendix A. A total of 4000 solutions per vocal fold model were produced. Each solution was simulated to produce 400 ms of voice signal (Supplementary Figure 1, and video in Supporting Materials). The voicing was evaluated for periodicity, and a minimum of 6 cycles must be present for the voicing to be considered viable. Across the four vocal fold models, on average 50.1 ± 4.2% of the solutions (out of 4000 per vocal fold model) met the periodicity criterion and were used for further analysis.
Calculation of F0-SPL Range
The solutions for each vocal fold model were plotted on an F0-SPL scatter plot. The area covered by these solutions was computed as a measure of the acoustic capabilities of the respective vocal fold morphology. The area was calculated using a non-convex hull estimated using Delaunay triangulation.
Sensitivity Analysis
Parameters controlling the vocal fold medial surface shape were varied to perform a sensitivity analysis. Convergence was defined by lower and upper adduction at the vocal processes (x01 and x02 in Figure 1B), and the middle of the surface was allowed to bulge out convexly (xb in Figure 1B). Five different shapes were simulated (cases 1–5; Table 1 and Figure 1B).
Statistical Analysis
Statistical calculations were performed with Excel 2010 (Microsoft, Redmond, WA) and SAS 9.3 (SAS Institute Inc., Cary, NC). To determine if the acoustic parameters differed across the four surgical models, one-way analysis of variance (ANOVA) with Tukey’s test for pairwise comparison of means was performed, with α = 0.05.
Results
Simulation
Supplementary Figure 1 shows two example solutions produced by optimized simulation using the normal vocal fold model, highlighting some of the acoustic and aerodynamic features that could be compared between different solutions. Qualitative differences could be appreciated in the time of phonation onset, the time to reach steady state oscillation, and the amplitude of oscillation.
A video demonstrating the simulations is included in Supporting Materials.
Primary Outcome: F0-SPL Range
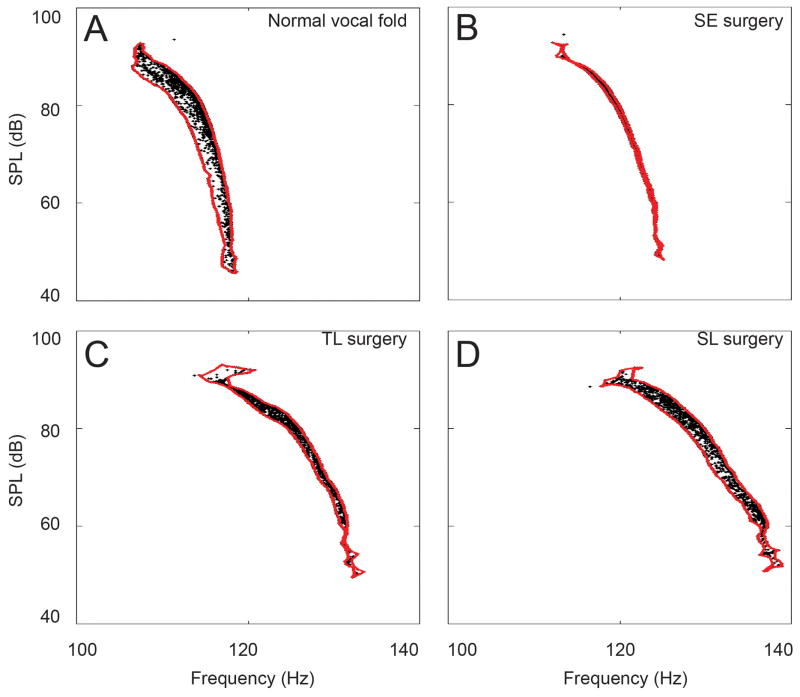
The acoustic merit of each vocal fold model was measured by the area in the F0-SPL plot occupied by the solutions (Figure 2). A larger area indicates greater pitch range and range of SPL that can be produced by the particular model, given an input range of subglottal pressure Ps and transverse shear modulus of the surface scar, μ1. Figure 2 shows the distribution of the solutions in F0-SPL space for the rectangular shaped vocal fold (case 1, Figure 1B) for each surgical scenario. The F0-SPL range was the largest for simulations with the normal vocal fold (Figure 2A). Solutions of subligamental cordectomy covered a range 82% of normal (Figure 2D), while those of subepithelial cordectomy covered a range only 19% of normal (Figure 2B). Solutions of transligamental cordectomy spread across an intermediate range (57% of normal; Figure 2C).
Figure 2.
Four sets of solutions spread in the F0-SPL space, each representing the acoustic output of a particular surgical morphology. A. Normal; B. Subepithelial cordectomy (SE); C. Transligamental cordectomy (TL); and D. Subligamental cordectomy (SL). The red outline shows the area covered by the respective set of solutions in F0-SPL space. The outline was estimated by a non-convex hull using Delaunay triangulation.
Sensitivity Analysis
The finite element model was defined by a set of geometric parameters, most of which were not targeted for optimization in this study. Before general interpretations can be made about the acoustic consequences of resection depth variation, it is important to examine the sensitivity of the results to the non-varied parameters. In particular, the prephonatory shape of the medial surface of the vocal folds is known to be an important determinant of phonatory flow and pressure.34–37 If the results remain qualitatively the same when the medial surface shape is altered, confidence is gained in the relative effects of resection depth and μ of scarred SLLP.
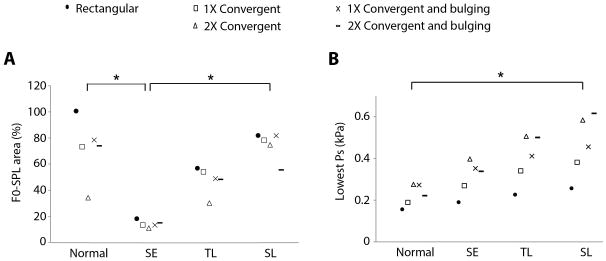
The bulging and convergence parameters that defined the prephonatory vocal fold medial surface shape were varied to produce 4 different convergent shapes as shown in Figure 1B. Each was used for optimized simulation as for the rectangular vocal fold above, and the results are shown in Figure 3A. One-way ANOVA comparing the F0-SPL ranges of the normal vocal fold and the three cordectomy models showed a significant difference, with F(3,16) = 19.5, P < .0001. A post-hoc Tukey test showed no significant difference between the normal vocal fold and subligamental cordectomy (P = 0.99). There was a significant difference between subepithelial cordectomy and normal (P < .0001), as well as between subepithelial cordectomy and subligamental cordectomy (P < .0001). The relative merits of the SE, TL, and SL models were unchanged by the medial surface shape based on F0-SPL range. These results support the hypothesis that more extensive ligament resection recovers a larger range for solutions.
Figure 3.
Effect of resection depth on A. the distribution of solutions in F0-SPL space (F0-SPL area), and B. lowest subglottal pressure, Ps. In A, data are plotted as a percentage of the F0-SPL area occupied by solutions of the normal vocal fold with rectangular geometry. Pairwise comparisons with statistical significance are denoted with asterisks. The other pairwise comparisons did not reach statistical significance.
Secondary Outcomes
Two vocal input parameters were investigated. There was a statistically significant difference in mean subglottal pressure (Ps) between the four surgical models as determined by one-way ANOVA (F(3,16) = 14.2, P < .0001), with the mean Ps progressively higher as the resection depth increased (Supplementary Figure 2A). Tukey post-hoc test showed the differences were statistically significant between normal vocal fold and transligamental cordectomy (P= .0013), normal and subligamental cordectomy (P = .0001), and subepithelial and subligamental cordectomies (P = .0051). As the simulation did not explicitly evaluate the phonation threshold pressure, a surrogate measure was obtained by averaging the 10 lowest Ps values amongst the solutions for each model (Figure 3B). There was a statistically significant difference in the lowest Ps between the four surgical models as determined by one-way ANOVA (F(3,16) = 4.7, P = 0.015). The only statistically significant difference was between the normal vocal fold and subligamental cordectomy (P = 0.014).
Three vocal output parameters were investigated. F0 increased with resection depth (Supplementary Figure 2B; one-way ANOVA F(3,16) = 61.8, P < .0001, with significant differences between all pairwise comparisons). There was no statistically significant difference in the mean SPL or phonation onset time across the 4 surgical models (Supplementary Figure 2C–D).
Discussion
The relative acoustic merits of different extents of cordectomies were investigated with computer simulation using a novel optimized simulation strategy. Specifically, the role of the vocal ligament in the setting of mandatory SLLP resection was examined. A tenet in modern vocal fold microsurgery is the maximal preservation of the superficial layer of the lamina propria.38 This pliable layer facilitates easy conversion of aerodynamic energy into kinetic energy by flow-induced self-sustained oscillations.34 Minimizing the resection of oncologically uninvolved, deeper tissue (such as the ligament and muscle) should reduce the loss of glottal contact and is generally observed as a principle of vocal fold microsurgery. The simulations here suggest a possible exception to this principle. When cancer resection leaves little to no SLLP but the ligament can be spared oncologically, it may be advantageous to resect the ligament to enable more favorable voice production. The ligament is much stiffer than the SLLP and helps maintain stability when normal vocal folds are stretched longitudinally to generate higher F0 with considerably higher subglottal pressure. However, if the mucosa is thinner and less pliable due to scarring, the ligament can hinder tissue oscillation in the F0 range of the normal speaking voice. Since thyroarytenoid (TA) muscle activation is low in the phonatory position during speech39,40, the muscle contributes less longitudinal stiffness than the ligament. Complete resection of the ligament could therefore be a better option to maintain an adequate speaking voice.
An important caveat to this interpretation is that this study deliberately did not address the glottal gap due to variable healing and filling-in of the surgical defect. We chose to focus on the tissue properties as the variables in this investigation. Our findings were predicated on maintaining a constant glottal width across the different surgical scenarios in the simulations in order to exclude the effect of glottal width. In reality, the reduction of vocal fold depth in the transverse plane after subligamental cordectomy results in a glottal gap or loss of vertical contact. While this could be partly compensated by hypertrophy or herniation of the TA muscle16, granulation tissue formation with collagen deposition, or medialization laryngoplasty17,41, the end result is likely more variable. The extent of ligament resection inferiorly along the conus elasticus also differs among surgeons, adding to less predictable vocal outcomes in the clinical setting after subligamental cordectomy. The effect of variable glottal gap size can be studied in future investigations using the same optimized simulation approach, for example by assigning more layers of the finite element model to muscle in order to simulate muscle expansion following subligamental cordectomy.
A substantially reduced F0-SPL range after subepithelial cordectomy means that with the same range of driving subglottal pressures, the dynamic range of pitch and loudness is reduced. One consequence is decreased inflection in speech. Another consequence is that the speaker may have to adapt to altered vocal fold posturing in order to phonate in the more restricted range, i.e. it requires motor learning (including somatosensory control) of the new settings. Vocal fold posturing entails control of adduction/abduction, vocal fold length, and muscle activation. For each patient, as the attainable F0-SPL range narrows, there may be a point beyond which it becomes quite challenging to produce just the right vocal fold posture to speak. The optimized simulations suggest a tradeoff between greater coordination of vocal fold posturing versus higher subglottal pressure. We suspect for most patients it is easier to generate slightly higher subglottal pressure (as after subligamental cordectomy) than to change their habitual vocal fold posturing to target a much smaller target F0-SPL range (as after subepithelial cordectomy). The increase in subglottal pressure required appears relatively small (0.2 kPa greater for subligamental cordectomy than for subepithelial cordectomy) and therefore incurs only a minor cost.
The F0 was noted to increase with progressive resection of the ligament in these simulations. Elevated F0 is a common finding after cordectomy and is typically attributed to the reduction in vibratory mass due to cancer resection.9,13,42 However, unlike a simple mass coupled to a spring, the vocal fold consists of several layers of tissue so that a simple relationship between mass and F0 does not exist.43 A major reason for the increase in F0 following cordectomy is likely to be scar formation and scar constituting a significant part of the new vibratory tissue, which increases the stiffness of the vibratory vocal fold. It is the increase in stiffness, not the decrease in mass, that raises F0.43 The relatively thin muscle layer in our FEM likely exaggerated the magnitude of F0 increase as a function of resection depth. A thicker layer of muscle (at the cost of increased computation time for the higher number of FEM columns) would “shield” the material properties of the lateral implant, which increased the effective stiffness of the vocal fold in the SE, TL, and SL models as the vocal fold depth progressively decreased.
At first glance, the sacrifice of vocal ligament for vocal gain may seem counterintuitive, because the ligament plays a unique role in human vocalization by regulating the passive stress in the vocal fold and allowing the SLLP to remain pliable at high frequencies.44 Loss of the ligament reduces the capability of phonation at high frequencies, e.g. singing. However, the necessary SLLP sacrifice will already lead to a significant reduction in F0 dynamical range. The additional loss of ligament is unlikely to impose additional morbidity in the normal speaking F0 range. What is gained is a broader F0-SPL range for the speaking voice.
While this study was motivated by the reported difference in clinical vocal outcome between subepithelial and subligamental cordectomies16, at least one study reported no such difference. Ledda et al.9 found that the jitter, shimmer, and harmonic-to-noise ratio of voices after either subepithelial cordectomy or subligamental cordectomy were not statistically different from those of non-operated vocally healthy controls. In contrast, Hillel et al.16 found differences in voice-related quality of life scores, auditory perceptual ratings, and stroboscopic parameters between the two types of cordectomies. The two studies are not necessarily at odds since they assessed different dimensions of voice, which may explain the divergent interpretations. Furthermore, no direct comparison was made between subepithelial and subligamental cordectomy in Ledda et al.9 More clinical data comparing the vocal outcomes of the two types of cordectomies are clearly needed to verify the merits of ligament resection.
We wish to emphasize that this study is not a simple endorsement of subligamental cordectomy for all early glottic tumors for which subepithelial cordectomy is adequate. According to the European Laryngological Society cordectomy classification system45, a subepithelial cordectomy passes through the superficial layer of the lamina propria. This would include a superficial subepithelial cordectomy where most of the SLLP is preserved, or a deep subepithelial cordectomy where most or all of the SLLP is removed. The type of subepithelial cordectomy relevant to the study question is the deep version, where most of the SLLP requires sacrifice. A superficial epithelial lesion that could be removed via a superficial subepithelial cordectomy would not justify a subligamental cordectomy, since the voice outcome of a superficial subepithelial cordectomy should be close to normal.
Conclusion
Computer simulation utilizing a novel optimized simulation strategy shows greater F0-SPL range for subligamental cordectomy than subepithelial cordectomy with complete or near-complete resection of SLLP. These results provide theoretical support for the resection of the vocal ligament in cases where most of the SLLP requires sacrifice but the ligament could be spared on oncological grounds. More clinical data comparing the voice outcomes following subepithelial and subligamental cordectomies are needed to corroborate these simulation results to guide surgical intervention.
Supplementary Material
Appendix A. Optimized Simulation
For voice simulation, the FEM construct allows solution of the partial differential equations that govern tissue mechanics. Airflow was calculated using a modified Bernoulli flow calculation that included a correction for flow separation from walls.29 A wave reflection algorithm was used to solve all the acoustic wave pressures in the vocal tract, modeled as a uniform tube of 17.5 cm length and 2 cm diameter.29 Pressures in the glottis were solved with Bernoulli’s energy equation below flow detachment and with jet stream equations above flow detachment.29
The optimized simulation procedure is explained in detail in Palaparthi et al.30 Briefly, the optimization was a two-step process which was repeated 200 times. In a first step, 20 (‘parental’) solutions were identified which were within the boundaries defined in Table 1. In the second step, using Gaussian mutation and arithmetic crossover functions, 20 offspring solutions were generated based on the characteristics of the initial 20 parental solutions. Out of those now 40 solutions (20 parental plus 20 offspring solutions), 20 best solutions were recruited to form a new parental generation, which reenters the two-step process. The 20 new parental solutions were, again, within the boundaries defined in Table 1. However, the 20 offspring solutions were not necessarily within these boundaries. This openness beyond the initial boundaries allowed a better exploration of the parameter space surrounding the target (here: F0 = 120 Hz, and SPL = 70 dB). The offspring solutions from each generation entered the final data set which was analyzed in this study. Repeating the procedure 200 times, i.e. 200 generations, produced a total of 4000 solutions per vocal fold model.
Footnotes
This work has been accepted for podium presentation at the 2015 Annual Meeting of the American Laryngological Association in Boston in April, 2015.
Level of Evidence: N/A
Conflict of Interest: None.
Financial Disclosure: This work was supported by NIDCD grant R01 DC008612. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness and Other Communication Disorders or the National Institutes of Health.
References
- 1.Spector JG, Sessions DG, Chao KS, et al. Stage I (T1 N0 M0) squamous cell carcinoma of the laryngeal glottis: therapeutic results and voice preservation. Head Neck. 1999;21:707–717. doi: 10.1002/(sici)1097-0347(199912)21:8<707::aid-hed5>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Gallo A, de Vincentiis M, Manciocco V, Simonelli M, Fiorella ML, Shah JP. CO2 laser cordectomy for early-stage glottic carcinoma: a long-term follow-up of 156 cases. Laryngoscope. 2002;112:370–374. doi: 10.1097/00005537-200202000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Sigston E, de Mones E, Babin E, et al. Early-stage glottic cancer: oncological results and margins in laser cordectomy. Arch Otolaryngol Head Neck Surg. 2006;132:147–152. doi: 10.1001/archotol.132.2.147. [DOI] [PubMed] [Google Scholar]
- 4.Hartl DM, de Mones E, Hans S, Janot F, Brasnu D. Treatment of early-stage glottic cancer by transoral laser resection. Ann Otol Rhinol Laryngol. 2007;116:832–836. doi: 10.1177/000348940711601107. [DOI] [PubMed] [Google Scholar]
- 5.Yoo J, Lacchetti C, Hammond JA, Gilbert RW Head and Neck Cancer Disease Site Group. Role of endolaryngeal surgery (with or without laser) versus radiotherapy in the management of early (T1) glottic cancer: A systematic review. Head Neck. 2014;36:1807–1819. doi: 10.1002/hed.23504. [DOI] [PubMed] [Google Scholar]
- 6.Comert E, Tuncel U, Dizman A, Guney YY. Comparison of early oncological results of diode laser surgery with radiotherapy for early glottic carcinoma. Otolaryngol Head Neck Surg. 2014;150:818–823. doi: 10.1177/0194599814521775. [DOI] [PubMed] [Google Scholar]
- 7.Casiano RR, Cooper JD, Lundy DS, Chandler JR. Laser cordectomy for T1 glottic carcinoma: a 10-year experience and videostroboscopic findings. Otolaryngol Head Neck Surg. 1991;104:831–837. doi: 10.1177/019459989110400611. [DOI] [PubMed] [Google Scholar]
- 8.Peretti G, Piazza C, Balzanelli C, Mensi MC, Rossini M, Antonelli AR. Preoperative and postoperative voice in Tis-T1 glottic cancer treated by endoscopic cordectomy: an additional issue for patient counseling. Ann Otol Rhinol Laryngol. 2003;112:759–763. doi: 10.1177/000348940311200903. [DOI] [PubMed] [Google Scholar]
- 9.Ledda GP, Grover N, Pundir V, Masala E, Puxeddu R. Functional outcomes after CO2 laser treatment of early glottic carcinoma. Laryngoscope. 2006;116:1007–1011. doi: 10.1097/01.MLG.0000217557.45491.BD. [DOI] [PubMed] [Google Scholar]
- 10.Haddad L, Abrahao M, Cervantes O, et al. Vocal assessment in patients submited to CO2 laser cordectomy. Braz J Otorhinolaryngol. 2006;72:295–301. doi: 10.1016/S1808-8694(15)30960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roh JL, Kim DH, Kim SY, Park CI. Quality of life and voice in patients after laser cordectomy for Tis and T1 glottic carcinomas. Head Neck. 2007;29:1010–1016. doi: 10.1002/hed.20625. [DOI] [PubMed] [Google Scholar]
- 12.Piazza C, Bolzoni Villaret A, Redaelli De Zinis LO, Cattaneo A, Cocco D, Peretti G. Phonosurgery after endoscopic cordectomies. II. Delayed medialization techniques for major glottic incompetence after total and extended resections. Eur Arch Otorhinolaryngol. 2007;264:1185–1190. doi: 10.1007/s00405-007-0330-0. [DOI] [PubMed] [Google Scholar]
- 13.Vilaseca I, Huerta P, Blanch JL, Fernandez-Planas AM, Jimenez C, Bernal-Sprekelsen M. Voice quality after CO2 laser cordectomy--what can we really expect? Head Neck. 2008;30:43–49. doi: 10.1002/hed.20659. [DOI] [PubMed] [Google Scholar]
- 14.Galletti B, Freni F, Cammaroto G, Catalano N, Gangemi G, Galletti F. Vocal outcome after CO2 laser cordectomy performed on patients affected by early glottic carcinoma. J Voice. 2012;26:801–805. doi: 10.1016/j.jvoice.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Chu PY, Hsu YB, Lee TL, Fu S, Wang LM, Kao YC. Longitudinal analysis of voice quality in patients with early glottic cancer after transoral laser microsurgery. Head Neck. 2012;34:1294–1298. doi: 10.1002/hed.21914. [DOI] [PubMed] [Google Scholar]
- 16.Hillel AT, Johns MM, 3rd, Hapner ER, Shah M, Wise JC, Klein AM. Voice outcomes from subligamentous cordectomy for early glottic cancer. Ann Otol Rhinol Laryngol. 2013;122:190–196. doi: 10.1177/000348941312200308. [DOI] [PubMed] [Google Scholar]
- 17.Zeitels SM, Hillman RE, Franco RA, Bunting GW. Voice and treatment outcome from phonosurgical management of early glottic cancer. Ann Otol Rhinol Laryngol Suppl. 2002;190:3–20. doi: 10.1177/0003489402111s1202. [DOI] [PubMed] [Google Scholar]
- 18.Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Mau T, Muhlestein J, Callahan S, Weinheimer KT, Chan RW. Phonation threshold pressure and flow in excised human larynges. Laryngoscope. 2011;121:1743–1751. doi: 10.1002/lary.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plant RL. Aerodynamics of the human larynx during vocal fold vibration. Laryngoscope. 2005;115:2087–2100. doi: 10.1097/01.mlg.0000184324.45040.17. [DOI] [PubMed] [Google Scholar]
- 21.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaka K, Isshiki N. Computer simulation of pathological vocal-cord vibration. J Acoust Soc Am. 1976;60:1193–1198. doi: 10.1121/1.381221. [DOI] [PubMed] [Google Scholar]
- 23.Alipour F, Berry DA, Titze IR. A finite-element model of vocal-fold vibration. J Acoust Soc Am. 2000;108:3003–3012. doi: 10.1121/1.1324678. [DOI] [PubMed] [Google Scholar]
- 24.Tao C, Jiang JJ. A self-oscillating biophysical computer model of the elongated vocal fold. Comput Biol Med. 2008;38:1211–1217. doi: 10.1016/j.compbiomed.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson SL, Mongeau L, Frankel SH. Aerodynamic transfer of energy to the vocal folds. J Acoust Soc Am. 2005;118:1689–1700. doi: 10.1121/1.2000787. [DOI] [PubMed] [Google Scholar]
- 26.Zheng X, Xue Q, Mittal R, Beilamowicz S. A coupled sharp-interface immersed boundary-finite-element method for flow-structure interaction with application to human phonation. J Biomech Eng. 2010;132:111003. doi: 10.1115/1.4002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian FB, Dai H, Luo H, Doyle JF, Rousseau B. Fluid-structure interaction involving large deformations: 3D simulations and applications to biological systems. J Comput Phys. 2014;258 doi: 10.1016/j.jcp.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal R, Zheng X, Bhardwaj R, Seo JH, Xue Q, Bielamowicz S. Toward a simulation-based tool for the treatment of vocal fold paralysis. Front Physiol. 2011;2:19. doi: 10.3389/fphys.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titze IR. The Myoelastic Aerodynamic Theory of Phonation. Denver, CO: The National Center for Voice and Speech; 2006. [Google Scholar]
- 30.Palaparthi A, Riede T, Titze IR. Combining multiobjective optimization and cluster analysis to study vocal fold functional morphology. IEEE Trans Biomed Eng. 2014;61:2199–2208. doi: 10.1109/TBME.2014.2319194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirano M, Kurita S, Nakashima T. The structure of the vocal folds. In: Stevens KN, Hirano M, editors. Vocal Fold Physiology. Tokyo: University of Tokyo Press; 1981. pp. 33–41. [Google Scholar]
- 32.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 33.Titze IR, Riede T. A cervid vocal fold model suggests greater glottal efficiency in calling at high frequencies. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83:1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- 35.Berry DA, Clark MJ, Montequin DW, Titze IR. Characterization of the medial surface of the vocal folds. Ann Otol Rhinol Laryngol. 2001;110:470–477. doi: 10.1177/000348940111000514. [DOI] [PubMed] [Google Scholar]
- 36.Khosla S, Oren L, Ying J, Gutmark E. Direct simultaneous measurement of intraglottal geometry and velocity fields in excised larynges. Laryngoscope. 2014;124 (Suppl 2):S1–13. doi: 10.1002/lary.24512. [DOI] [PubMed] [Google Scholar]
- 37.Mau T, Muhlestein J, Callahan S, Chan RW. Modulating phonation through alteration of vocal fold medial surface contour. Laryngoscope. 2012;122:2005–2014. doi: 10.1002/lary.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courey MS, Gardner GM, Stone RE, Ossoff RH. Endoscopic vocal fold microflap: a three-year experience. Ann Otol Rhinol Laryngol. 1995;104:267–273. doi: 10.1177/000348949510400402. [DOI] [PubMed] [Google Scholar]
- 39.Poletto CJ, Verdun LP, Strominger R, Ludlow CL. Correspondence between laryngeal vocal fold movement and muscle activity during speech and nonspeech gestures. J Appl Physiol (1985) 2004;97:858–866. doi: 10.1152/japplphysiol.00087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillel AD. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope. 2001;111:1–47. doi: 10.1097/00005537-200104001-00001. [DOI] [PubMed] [Google Scholar]
- 41.Mastronikolis NS, Remacle M, Kiagiadaki D, Lawson G, Bachy V, Van Der Vorst S. Medialization thyroplasty for voice restoration after transoral cordectomy. Eur Arch Otorhinolaryngol. 2013;270:2071–2078. doi: 10.1007/s00405-013-2462-8. [DOI] [PubMed] [Google Scholar]
- 42.Luo CM, Fang TJ, Lin CY, et al. Transoral laser microsurgery elevates fundamental frequency in early glottic cancer. J Voice. 2012;26:596–601. doi: 10.1016/j.jvoice.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Titze IR. Vocal fold mass is not a useful quantity for describing F0 in vocalization. J Speech Lang Hear Res. 2011;54:520–522. doi: 10.1044/1092-4388(2010/09-0284). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Titze IR. Principles of Voice Production. Iowa City, IA: National Center for Voice and Speech; 2000. pp. 225–229. [Google Scholar]
- 45.Remacle M, Eckel HE, Antonelli A, et al. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur Arch Otorhinolaryngol. 2000;257:227–231. doi: 10.1007/s004050050228. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–124. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.