Abstract
Cardiovascular disease is the principal complication and the leading cause of death for patients with diabetes (DM). The efficacy of anti-hyperglycemic treatments on cardiovascular disease risk remains uncertain. Cardiovascular risk factors are affected by anti-hyperglycemic medications, as are many intermediate markers of cardiovascular disease. Here we summarize the evidence assessing the cardiovascular effects of anti-hyperglycemic medications with regards to risk factors, intermediate markers of disease, and clinical outcomes.
Keywords: Diabetes, anti-hyperglycemic medications, cardiovascular risk, cardiovascular disease
INTRODUCTION
Diabetes mellitus (DM) affects over 366 million people worldwide, with the global prevalence expected to double in the next 15 years.(1) The principal complication of DM is cardiovascular disease (CVD), with 65% of patients with DM dying from CVD complications despite numerous advances in treatment.(2) Until recently, therapeutic options were quite limited for the treatment of hyperglycemia, with only 4 drug classes available through 1997 (insulin; sulfonylureas; metformin; α-glucosidase inhibitors), but a rapid expansion in the therapeutic arsenal has occurred in the past 20 years. At present, there are 13 classes of anti-hyperglycemic medications available for clinical use. Historically, anti-hyperglycemic medications were developed and approved based solely on their ability to lower glycosylated hemoglobin (HbA1c), the most widely used clinical measure of glycemic control, and to lower circulating glucose levels. This reliance on intermediate biomarkers without requirement of proof of morbidity and/or mortality benefits was driven by pressure to bring additional drugs to the clinic in order to address the unmet clinical need of such limited therapeutic options. While lowering blood glucose in patients with DM reduces the incidence and progression of microvascular disease, such as retinopathy, neuropathy, and nephropathy,(3) it remains unclear whether pharmacologically lowering blood glucose reduces the incidence of CVD, and the possibility remains that adverse cardiovascular effects of at least some anti-hyperglycemic medications may counter-balance or even reverse any CVD risk benefit deriving from glycemic control.(4-7)
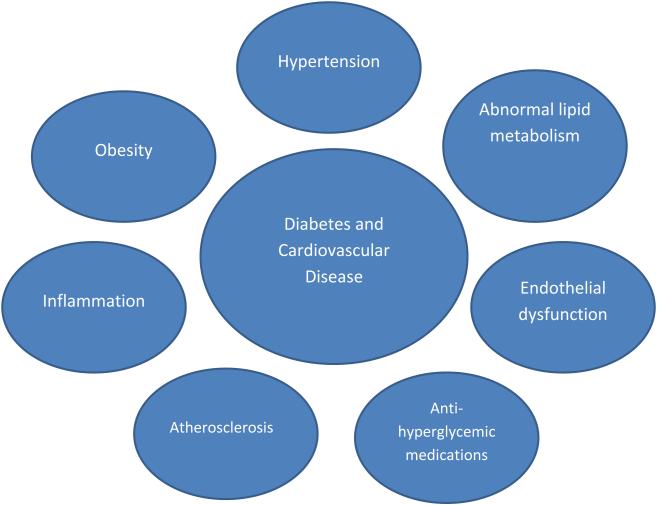
Figure 1 shows a proposed conceptual model of the pathobiologic interrelationships between DM and CVD, including the possibility for anti-hyperglycemic medications to either attenuate or increment CVD risk along each of the pathologic connections. Here we review CVD risk in patients with diabetes, and examine the effects of specific anti-hyperglycemic drugs/drug classes/strategies on traditional CV risk factors, intermediate measures of CV disease and CV outcomes.
Figure 1. Proposed conceptual model of demonstrating the relationship between diabetes mellitus and cardiovascular disease.
ANTI-HYPERGLYCEMIC DRUG EFFECTS ON CARDIOVASCULAR RISK FACTORS
Blood Pressure
The presence of hypertension in patients with diabetes is a strong risk factor for CV events.(8) In contrast to blood glucose lowering, there is a plethora of evidence proving that reducing blood pressure in patients with diabetes reduces the risk of macrovascular and microvascular disease complications, including mortality. Therefore, the effects of anti-hyperglycemic medications on blood pressure are an important clinical consideration.
The thiazolidinediones (TZDs; Table 1), which are peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, have demonstrated neutral to favorable effects on blood pressure. For example, the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) trial randomized 5,238 patients with T2DM and macrovascular disease to receive pioglitazone or placebo in addition to other glucose lowering medications.(9) Throughout the trial duration over a mean of 34.5 months of follow-up, there was a statistically significant average 3 mm Hg reduction in systolic blood pressure. In the Rosiglitazone evaluated for CV outcomes in oral agent combination therapy for type 2 diabetes (RECORD) trial that evaluated rosiglitazone vs. metformin/sulfonylurea combination in 4,447 patients with T2DM,(10) there was a non-significant 1.5mm Hg decrease in blood pressure in patients taking rosiglitazone. These neutral to favorable blood pressure effects are especially relevant in the context of observed incremental heart failure risk and volume expansion caused by the TZDs.(4)
Table 1.
Summary of Anti-diabetic drugs on cardiovascular risk factors
| Medication | Glucose | Weight | Blood Pressure |
LDL-C | HDL-C | Triglycerides | |
|---|---|---|---|---|---|---|---|
| Insulin | − − − | +++ | − | +/− | ++ | − − | |
| Glargine | |||||||
| Detemir | |||||||
| Degludec | |||||||
| Aspart | |||||||
| Glulisine | |||||||
| Lispro | |||||||
| NPH | |||||||
| Regular | |||||||
| Metformin | − − | +/− | +/− | − | +/− | − | |
| Sulfonylureas | − − | +++ | +/− | − | +/− | − | |
| Glipizide | |||||||
| Glyburide | |||||||
| (Glibenclamide) | |||||||
| Glimepiride | |||||||
| Gliclazide | |||||||
| Chlorpropamide | |||||||
| Tolbutamide | |||||||
| Meglitinides | − − | ++ | +/− | +/− | +/− | +/− | |
| Nateglinide | |||||||
| Repaglinide | |||||||
| Thiazolidinediones | |||||||
| Rosiglitazone | − − | +++ | +/− | ++ | + | + | |
| Pioglitazone | − − | +++ | − | + | ++ | − | |
| Alpha Glucosidase | − − | +/− | +/− | +/− | +/− | +/− | |
| Inhibitors | |||||||
| Acarbose | |||||||
| Miglitol | |||||||
| Voglibose | |||||||
| GLP-1R agonist | − − | − − | − | − | +/− | − | |
| Exenatide | |||||||
| Liraglutide | |||||||
| Dulaglutide | |||||||
| Albiglutide | |||||||
| DPP4 inhibitors | − | +/− | +/− | +/− | +/− | − | |
| Alogliptin | |||||||
| Sitagliptin | |||||||
| Saxagliptin | |||||||
| Linagliptin | |||||||
| Vildagliptin | |||||||
| SGLT2 inhibitors | − | − | − | + | + | − | |
| Canagliflozin | |||||||
| Dapagliflozin | |||||||
| Empagliflozin | |||||||
Legend: (−) indicates a reduction, (+) indicates an increase, (+/−) indicates no discernable change in parameter. NPH=Neutral Protamine Hagedorn.
Anti-hyperglycemic medications targeting the incretin system are being evaluated for their effects on blood pressure. Glucagon-like peptide-1 (GLP-1) is released from K cells in the small intestine mucosa in response to meals and binds to its related receptor on pancreatic ß-cells promoting glucose-appropriate insulin release.(5) GLP-1 has a very short half-life due to the proteolytic activity of circulating dipeptidyl peptidase 4 (DPP-4), and pharmacologically inhibiting DPP-4 potentiates endogenous GLP-1 activity. There are presently 4 GLP-1 receptor agonists and 4 DPP-4 inhibitors available for clinical use in the United States (Table 1). A meta-analysis of 16 clinical trials comprising 5,860 patients analyzed the blood pressure lowering effect of exenatide and liraglutide.(11) Compared with placebo, SBP was lowered 5.6 mm Hg by liraglutide and, 5.2 mm Hg by exenatide. Another meta-analysis evaluated the independent effect of GLP-1 agonists on blood pressure.(12) In these 33 trials that enrolled 12,469 patients randomized to exenatide or liraglutide versus active/placebo control, GLP-1 treatment demonstrated a 2.2 mm Hg lower SBP over comparator treatments. In the meta-regression analysis, SBP reduction was found to be independent of baseline blood pressure, weight loss, or improvement in HbA1c.
DPP-4 inhibitors have demonstrated a neutral to moderate lowering effect on blood pressure. A cross-over study of 19 patients randomized to sitagliptin or placebo found a non-statistically significant 2-3 mm Hg decrease in ambulatory SBP.(13) Sitagliptin was also evaluated in a prospective, randomized, cross-over study of 40 patients on stable doses of metformin.(14) Patients were randomized to sitagliptin 100mg or glyburide 5mg daily and followed for 12 weeks. At 16 weeks, patients were crossed over to the opposing treatment. Sitagliptin produced a 3.4 mm Hg decrease in SBP and 0.46 mmHg decrease in DBP from baseline, changes that were not significantly different from the changes in BP demonstrated with glyburide.
Sodium–glucose co-transporter 2 (SGLT2) inhibitors target transporter proteins in the kidneys to affect glucose lowering by inhibiting renal tubular glucose (and sodium) reabsorption and increasing urinary glucose excretion with a resultant diuretic effect (Figure 2).(15) A meta-analysis of 21 placebo-controlled studies evaluating SGLT-2 inhibitors demonstrated a mean difference in SBP of −3.8 mm Hg (95% CI, −4.7 to −2.9), while analysis of six active-controlled studies showed a mean difference of −4.5 mm Hg (95%CI, −5.7 to −3.2) compared with the active control group.(16) The mechanistic theory proposed for this decrease in BP is founded on osmotic diuresis of increased urinary glucose excretion along with natriuretic effects by inhibition of the sodium-glucose co-transporter, with some incremental BP efficacy over time possibly secondary to weight loss resulting from caloric losses of the urinary glucose.
Figure 2. Sodium–glucose co-transporter 2 mechanism of action and glucose handling in the kidney.
Na+/K+ ATPase creates a gradient that allows for the transport of sodium and glucose across the luminal border via SGLT2. Glucose is returned to the bloodstream via GLUT2. Ninety-seven percent of filtered glucose is reabsorbed through this mechanism. SGLT2 inhibitors block the action of SGLT2 allowing glucose to be eliminated by the kidneys. The remaining 3% of glucose that travels to the late proximal tubule is reabsorbed by SGLT1. Legend: Na+/K+ ATPase: sodium potassium adenosine triphosphatase active transporter; SGLT: sodium-glucose transporter; GLUT: facilitative glucose transporter; KCNE1: potassium voltage-gated channel Isk-related family member 1; KCNQ1: potassium voltage-gated channel KQT-like subfamily member 1.
Lipids
Patients with T2DM have abnormal lipid metabolism demonstrated by the atherogenic triad of reduced high-density lipoprotein cholesterol (HDL-C) concentration, small, dense low-density lipoprotein cholesterol (LDL-C), and hypertriglyceridemia. Given the associations between lipid disturbances and CVD risk and outcomes, especially LDL-C, effects of anti-hyperglycemic therapies on lipid profiles in general and specifically on LDL-C are of clinical interest.
Pioglitazone and rosiglitazone affect lipid parameters, with some important differences between these 2 drugs in those effects that are potentially clinically relevant. Pioglitazone increases HDL-C by ~10-20% from baseline with more modest changes in the same direction with rosiglitazone. (17) The increase in HDL-C observed with TZDs is thought to be due to an increase in hepatic apoA-1 synthesis. Pioglitazone decreases fasting triglyceride (TG) levels by 15-20%, and is associated with improvement in post-prandial chylomicron disposal, reductions in small dense LDL, and increased levels of larger, less atherogenic, LDL fractions. In a randomized trial that compared the lipid effects of pioglitazone vs. rosiglitazone, rosiglitazone increased TG levels by 13.1 mg/dL whereas pioglitazone decreased TG levels by 51.9 mg/dL (p<0.001 between treatments).(17) Moreover, the increase in HDL-C cholesterol was greater (5.2 +/− 0.5 vs. 2.4 +/− 0.5 mg/dl; p < 0.001) and the increase in LDL-C was less (12.3 +/− 1.6 vs. 21.3 +/− 1.6 mg/dl; p < 0.001) for pioglitazone compared with rosiglitazone, respectively. There is a clear difference between these two drugs within the same class; however, a mechanistic explanation for observed differences in lipid effects has not been fully elucidated.
GLP-1 agonists and DPP-4 inhibitors have beneficial effects on lipid parameters, proposed to be largely due to effects on post-prandial lipid metabolism. In a meta-analysis of placebo-controlled trials evaluating the effects of DPP-4 inhibitors on lipid profiles, the investigators found a decrease in total cholesterol (−7 mg/dL; p<0.001) and triglycerides (−17.1 mg/dL; p<0.001).(18) There was no significant change in HDL-C when compared with placebo. Monotherapy and add-on therapy with exenatide showed no significant changes in lipid measures compared with placebo.(19) In an 82-week open treatment study, a 16% reduction in TG and 12% increase in HDL-C from baseline was observed in patients taking exenatide.(20) Liraglutide trials have shown similar significant reductions in LDL-C, TG, and free fatty acids.(21)
Modest changes in lipid profiles have been described in clinical trials of SGLT2 inhibitors. Total cholesterol, LDL-C and HDL-C increased, while TG decreased with dapagliflozin in three clinical trials.(22-24) Canagliflozin and empagliflozin increased HDL-C and LDL-C with small and inconsistent changes in TG.(25, 26) The increase in LDL-C is notable and appears to occur with each medication in the class; however, it is a consistently modest increase and may be related to hemoconcentration associated with diuresis caused by these medication; the clinical relevance if any remains to be determined.
Weight
The association between DM and obesity is well established, and avoidance of weight gain or for those overweight or obese, weight loss, are cornerstones of lifestyle interventions in the population of patients with T2DM. Anti-hyperglycemic therapies for DM have effects on weight that are quite varied across the available classes of medications, and such effects often influence treatment choice.
Insulin is an anabolic hormone that increases glucose uptake in muscle and fat, with weight gain an expected effect, countering to some degree any decrease in HbA1c affected by insulin providing therapy. Insulin therapy can add 8 kg or more in a dose-dependent fashion in insulin naïve patients.(27) Sulfonylureas promote glucose-independent secretion of insulin from the pancreatic ß-cell, constitutively increasing net systemic insulin exposure and increasing weight up to 5 kilograms (kg) in controlled clinical trials.(28)
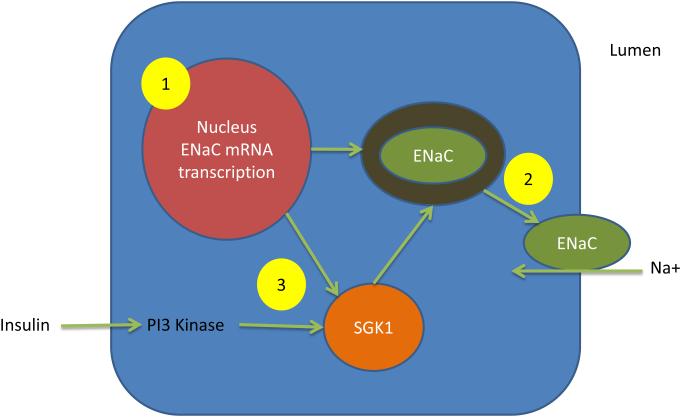
TZDs are associated with an approximate 4 kg weight gain in controlled clinical trials.(4) Weight gain with TZDs is exacerbated when used in combination with insulin or sulfonylureas and less so when used in combination with metformin. TZDs have a site-specific effect promoting differentiation of pre-adipocytes into mature insulin-sensitive adipocytes expanding the adipocyte organ for storage of caloric excesses. This adipocyte expansion is markedly enhanced in subcutaneous fat, with less effect in visceral fat. Weight gain is also affected by fluid retention that occurs with TZD treatment through a proposed mechanism of increased renal sodium reclamation due to TZD-induced increased expression of epithelial sodium channels (ENaC) in the distal convoluted tubule (Figure 3).(4)
Figure 3. Effect of thiazolidinediones on renal sodium reabsorption.
Legend: Thiazolidinediones (TZDs) enhance sodium reabsorption through the promotion of translocation of the epithelial sodium channel (ENaC) in the renal collecting ducts. TZDs promote 1) ENaC mRNA transcription in the nucleus, 2) ENaC translocation to the luminal surface, 3) phosphorylation of SGK1 which enhances ENaC translocation.
Metformin is widely considered to be weight neutral due to findings from UKPDS and the Diabetes Prevention Program (DPP) randomized trials.(29, 30) The UKPDS trial randomized 4075 patients with newly diagnosed T2DM to a policy of intensive glycemic control versus usual care, with mean follow-up of 10.7 years. The 1704 patients who were overweight or obese at trial entry were eligible to be randomized to metformin in the intensive control group (N=342). These patients experienced no significant change in weight from baseline. In the DPP, 3,234 patients with impaired fasting glucose/impaired glucose tolerance were randomized to treatment with metformin versus lifestyle intervention versus usual care, with up to 4 years of follow up. Similar to observations from the UKPDS, there was no significant change in weight in the metformin group during a mean 2.8 years of follow-up and no difference in weight at study end between the metformin and usual care groups.(30)
GLP-1 influences nutrient intake centrally by direct effects on CNS pathways controlling hunger, food intake and energy expenditure, and peripherally by reducing gastric emptying and gastric acid secretion leading to increased satiety, decreased hunger and decreased caloric intake.(31) GLP-1 based therapy likewise has been shown to suppress food intake and appetite, and promotes weight loss. In a meta-analysis of 25 trials, GLP-1 receptor agonists achieved a greater weight loss than control groups (−2.8 kg, 95% CI −3.4 to −2.3).(32) Liraglutide has a Food and Drug Administration (FDA) indication for weight loss at a dose of 3mg daily(33), a dose higher than the 1.2mg and 1.8 mg approved doses for the diabetes indication. Liraglutide was assessed in a randomized dose-finding trial against orlistat or placebo with the primary endpoint of weight loss in obese patients without T2DM (N=564).(34) More patients (n=70, 76%) lost more than 5% weight with liraglutide 3.0 mg that with placebo (n=29, 30%) or orlistat (n=42, 44%).
The effects on weight of the DPP-4 inhibitors were assessed in a Cochrane review of 25 studies evaluating sitagliptin and vildagliptin.(35) These drugs did not produce any significant changes in weight in the overall analysis; however, when limited to only placebo controlled trials, sitagliptin was associated with a 0.7 kg loss and vildagliptin a 0.8 kg loss. GLP-1 agonists offer superior weight loss compared with DPP-4 inhibitors, likely because of the supraphysiological levels of the GLP-1 provided in comparison with the physiological concentrations of GLP-1 achieved with the DPP-4 inhibitors.
Increased urinary glucose excretion caused by SGLT2 inhibitors results in a loss of 200-300 calories per day. The results of clinical trials have consistently shown weight loss in patients with T2DM treated with SGLT2 inhibitors. In studies of 4 to 26 weeks in duration, in which SGLT2 inhibitors were used as monotherapy, placebo-adjusted weight reductions of approximately 1 to 2.9 kg have been reported.(36)
Table 1 summarizes the effects of anti-hyperglycemic medications on CV risk factors. Some drugs have favorable effects on CV risk while others have deleterious effects, and such effects are often independent of glycometabolic effects. Clinicians must balance these effects when treating patients with differing risk factors, and survey selected risk factors during anti-hyperglycemic treatment.
ANTI-HYPERGLYCEMIC DRUG EFFECTS ON INTERMEDIATES OF CARDIOVASCULAR DISEASE
Endothelial function
Vascular injury is a paramount complication in the development of hypertension and CVD in patients with DM. One of the main components of vascular injury is endothelial dysfunction and often precedes significant atherosclerotic disease, with research assessment most commonly using assessment of brachial artery flow-mediated, endothelium-dependent vasodilation (FMD). Several anti-hyperglycemic medications have demonstrated improvement in FMD, including rosiglitazone, pioglitazone, and metformin with improvements independent of effects on glucose.(37) Neither exenatide nor lirglutide affected FMD after 6 months of treatment,(38) but compared with glimepiride, FMD was significantly better with exenatide.(39) The clinical relevance of changes in FMD remains uncertain, at best.
Carotid Intima-media Thickness
Carotid intima-media thickness (CIMT) is being used more frequently as an intermediate marker for atherosclerosis risk. Ultrasound measurements correlate well with histology, and increased CIMT is associated with vascular risk factors, the presence of more advanced atherosclerosis, and independently associates with CVD risk including coronary artery disease. The prevalence of DM, its duration, and degree of glycemic control are each well correlated with CIMT measures.(40)
Evidence exists for favorable effects on CIMT with TZDs, DPP-4 inhibitor, α-glucosidase inhibitor, and metformin. The effects of pioglitazone versus glimepiride were assessed in a large multi-center randomized controlled trial.(41) Absolute change from baseline to the final trial visit in mean posterior-wall CIMT of the left and right common carotid arteries measured by carotid ultrasound imaging was the primary outcome. The progression of mean CIMT was less with pioglitazone compared with glimepiride (−0.001 mm vs +0.012 mm, respectively; difference, −0.013 mm; 95% CI, −0.024 to −0.002). Pioglitazone also slowed progression of maximum CIMT compared with glimepiride (0.002 mm vs 0.026 mm, respectively; difference, −0.024 mm; 95% CI, −0.042 to −0.006). The favorable effect of pioglitazone on mean CIMT was similar across subgroups based on age, sex, systolic blood pressure, duration of DM, body mass index, HbA1c, and statin use. The effect of the DPP-4 inhibitor sitagliptin on CIMT was assessed in comparison with diet control in 76 patients with clinically stable CAD and newly diagnosed T2DM.(42) CIMT was measured at baseline and at 12 months in this open-label randomized controlled trial in Japan. Sitagliptin was associated with regression of CIMT, 1.11 (+/−0.43) mm at baseline to 1.09 (+/−0.42) mm, while the diet controlled patients had an increase in CIMT, mean of 1.02 (+/−0.44) mm to 1.07 (+/−0.41) mm at 12-month follow-up (p=0.02 for differences). The effect of the α-glucosidase inhibitor acarbose on CIMT was evaluated in a subgroup analysis of a placebo-controlled randomized trial of patients with impaired glucose tolerance.(43) CIMT increased by 0.02 (+/−0.07) mm in the acarbose group versus 0.05 (+/−0.06) mm in the placebo group (p=0.027). The investigators theorized that post-prandial hyperglycemia may influence atherosclerosis; although, more research is required. Finally, in the CAMERA study where changes in CIMT was evaluated in 173 coronary artery disease patients without DM, randomized to receive either metformin or placebo.(44) No statistical difference was observed in CIMT between the groups over 1.5 years of follow-up.
Coronary Atherosclerosis and In-stent Restenosis
The largest randomized trial evaluating the effects of an anti-hyperglycemic medication on coronary atherosclerosis compared pioglitazone and glimepiride in 543 patients with CAD and DM.(45) The primary outcome was change in percent atheroma volume (PAV) from baseline to study completion based on coronary artery intra-vascular ultrasound (IVUS). Least squares mean PAV increased 0.73% (95% CI, 0.33% to 1.12%) with glimepiride and decreased 0.16% (95% CI, −0.57% to 0.25%) with pioglitazone (p = .002).
With recent advances in stent technology and the development and proliferation of drug-eluting stents available, the occurrence of in-stent restenosis (ISR) has markedly decreased; however, it is still problematic for patients with DM, affecting 10-20% of such patients. The effects of selected anti-hyperglycemic medications on markers and measures of post-coronary stent restenosis have been evaluated, including effects on neointimal proliferation, late lumen loss, binary ISR, and target-lesion and target-vessel revascularization.
PPAR-gamma appears to play an important role in stent restenosis, and the TZDs have been shown to affect several measures of ISR. In a trial that randomized 55 patients to troglitazone 400mg daily versus conventional anti-hyperglycemic treatment, troglitazone was associated with reduced angiographic in-stent restenosis and neointimal tissue proliferation.(46) Meta-analyses demonstrated significant reduction with the TZDs in the risk of ISR in patients with or without DM.(47) These analyses also demonstrated a reduction in late lumen loss, percent diameter stenosis, neointimal area/volume, and target lesion revascularization. These results could not be reproduced in a randomized trial of rosiglitazone in 65 patients with DM, where therapy did not lower in-stent late lumen loss or reduce angiographic progression of nonculprit coronary artery lesions.(48)
Cardiac structure, function, and integrated cardiopulmonary performance
CV disease and DM often co-exist and diabetic cardiac dysfunction-both systolic and diastolic- is a common and early manifestation. Common co-morbidities in patients with DM directly contribute to this association, such as prevalent ischemic heart disease and hypertension, with additional contribution to cardiac dysfunction underpinned by derangements of myocardial energy substrate metabolism.(49) The potential effects of anti-hyperglycemic medications on ventricular function and mass gained much attention after TZD-related increases in the risk for HF were observed in clinical trials.(4) The effects of troglitazone versus placebo on cardiac structure and function were assessed in a randomized trial of 48-weeks duration in patients with T2DM.(50) Echocardiography was used to assess left ventricular mass index (LVMI), cardiac index (CI), and stroke volume index (SVI). LVMI was not significantly changed with troglitazone compared with placebo (p>0.05); SVI and CI were significantly improved with troglitazone (p<0.05 for each). The effects of rosiglitazone on cardiac structure and function were studied with echocardiography in patients with T2DM and mild HF including NYHA class I or II in a 1 year randomized trial.(51) The LVEF was not different between the groups at baseline (rosiglitazone 35.3 +/− 6.2%, placebo 35.7 +/− 7.8%) or after 52 weeks of treatment (mean difference 1.5%; p = 0.1), despite an increase in adjudicated CHF events in the rosiglitazone group compared with placebo (25.5 vs. 8.8%). Rosiglitazone was also compared with glyburide in a 52-week, randomized, open-label, controlled trial of 203 patients.(52) LV mass index, LVEF, and LV end-diastolic volume were assessed by echocardiography at baseline and weeks 12, 28, and 52. Neither treatment produced an increase in LV mass index that exceeded 1 standard deviation, LVEF did not change in either group, and both groups had clinically insignificant increases in LV end-diastolic volume.
Rosiglitazone was evaluated versus placebo in a randomized double-blind controlled trial of 150 patients with T2DM and CVD or clustered CVD risk factors, with the primary outcome of peak oxygen uptake indexed to fat-free mass (VO2peak-FFM) during maximum treadmill exercise.(53) A subset analysis was also performed in 102 patients who underwent cardiac magnetic resonance imaging (cMRI). No significant differences were observed in mean VO2peak-FFM between rosiglitazone and placebo (26.1 +/− 7.0 vs. 27.6 +/− 6.6 mL/kg-FFM/min; p = 0.26). In the cMRI substudy, compared with placebo, the rosiglitazone group had larger end-diastolic volume (128.1 vs. 112.0 mL; P = 0.01) and stroke volume (83.7 vs. 72.9 mL; p = 0.01), with no difference in ejection fraction.
The effect of sitagliptin on peak stress ejection fraction was assessed in a study of 14 patients with coronary artery disease and preserved left ventricular function awaiting revascularization.(54) After a single dose of sitagliptin 100 mg versus placebo, glucose 75 mg was given orally to promote GLP-1 secretion and dobutamine stress echocardiography was conducted at rest, immediately after peak stress, and 30 minutes later. Sitagliptin -treated patients had higher average ejection fraction at peak stress compared with placebo (73.0 +/− 7.0% vs. 64.0 +/− 8.0%; p = 0.0001).
Anti-hyperglycemic medications effect intermediate markers of CVD, with some variability both between and within classes of medications. Although it is not entirely clear, the favorable or adverse effects of these drugs on such intermediate markers may provide a mechanistic explanation to results observed in CVD outcomes trials.
ANTI-HYPERGLYCEMIC THERAPY EFFECTS ON CARDIOVASCULAR OUTCOMES
Intensity of Blood Glucose Control
DM is a disease characterized on the basis of abnormally elevated blood glucose and sustained exposure to hyperglycemia in the setting of insulin resistance/hyperinsulinemia (type 2 diabetes; T2DM) or complete insulin deficiency (type 1 DM; T1DM). Given the clear associations between hyperglycemia and incremental CVD risk deriving from analyses of observational data, prevalent thought existed for decades that lowering blood glucose in patients with DM would result in a reduction in CVD risk and events; however, this has not born out in the more recent randomized controlled trials completed to date.
Long-term follow-up studies of UKPDS and DCCT/EDIC suggested that intense glucose control decreased the risk of MI and CVD, respectively.(55, 56). Based on these results, three large-scale randomized controlled clinical trials were designed and executed to determine if more intensive glucose control versus contemporary targets for HbA1c would affect risk for major adverse CVD events.(57-59) The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial randomized 10,251 high CV risk patients with T2DM to intensive glucose control with HbA1c target < 6% vs. standard therapy with HbA1c target 7.0-7.9%.(57) The primary outcome was time to the first event of a composite of nonfatal MI, nonfatal stroke, or death from CV causes. The trial was stopped early (mean 3.5 years of follow-up) due to increased mortality in the intensive therapy group (hazard ratio [HR]= 1.22, 95% CI 1.01-1.46). The mean HbA1c in the intensive therapy group was 6.4% vs. 7.5% in the standard therapy group. The explanation for this observed incremental mortality signal remains elusive, but is not clearly attributable to any specific drug class or to hypoglycemia events, and with no clear relationship with achieved HbA1c.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial also assessed the effect of more versus less intensive glycemic control on major CVD events in patients with T2DM.(58) The investigators randomly assigned 11,140 patients with T2DM, 32% of whom had a history of major CVD at study entry, to either standard glucose control with HbA1c target set by local guidelines versus intensive control using gliclazide first and other drugs as needed at the discretion of the treating provider to target HbA1c<6.5%. After a median follow-up of 5 years, the mean HbA1c was 6.5% in the intensive therapy group vs. 7.3% in the standard therapy group. There was no difference in major macrovascular events, defined as death from CV causes, nonfatal MI, or nonfatal stroke, between the two groups (HR=0.94; 95% CI 0.84-1.06). The findings regarding death were similar to the overall primary outcome results (HR=0.93, 95% CI 0.83-1.06); importantly, no incremental hazard for CV or all-cause mortality was observed as had been the case in the ACCORD trial.
The Veterans Affairs Diabetes Trial (VADT) trial also assessed the effects of more vs. less intensive glucose control on CVD risk in patients with T2DM.(59) A total of 1,791 patients with or at high risk for CVD were randomized to intensive vs. standard glucose lowering therapy. The primary outcome was the time to the first occurrence of MI, stroke, death from CV causes, new or worsening HF, surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease; and amputation for ischemic gangrene. There was no statistical difference for intensive versus standard glucose control in the primary outcome (HR=0.88, 95% CI 0.74-1.05), death from CV causes (HR=1.32, 95% CI 0.81-2.14), or death from any cause (HR=1.07, 95% CI 0.81-1.42). It is notable that CV mortality tended in the wrong direction, quantitatively similar to the ACCORD observations, though the VADT small sample size and relatively few CV death events for analysis, there was inadequate power in VADT to statistically discern the intervention effects on CV or all-cause mortality.
The discrepant differences between the UKPDS and EDIC studies compared with the three most recent studies could at least partially be attributed to the very different study population enrolled. UKPDS and DCCT enrolled patients with newly diagnosed diabetes, while ACCORD, ADVANCE and VADT enrolled high risk patients with preexistent cardiovascular disease, longer disease duration, and with a more advanced age. It is plausible that early intervention with intensive glycemic control has a primary prevention role, but it clearly established now that glycemic intensification late in the course of the disease is not beneficial and could potentially be harmful.
Insulin
Insulin is the most potent anti-hyperglycemic agent clinically available and is used by over 30% of patients with diabetes. The long-debated controversy of the CV effects of treatment with insulin has been diminished by the ORIGIN trial.(60) This was the largest and longest CV study reported to date, which enrolled 12,537 patients with CV risk factors and pre-diabetes or early T2DM. Patients were randomized to receive once daily injection of insulin glargine with a target fasting glucose <95 mg/dl versus standard of care, which in this population was primarily lifestyle modification, and/or metformin and/or sulfonylurea treatment. After a median follow-up of 6.2 years, both co-primary outcomes showed no statistical differences in composite CV disease outcomes for insulin glargine vs. control: HR 1.02 (95%CI 0.94-1.11) for the composite of MI, stroke, and CV death; HR 1.04 (95%CI 0.97-1.11) for the expanded composite that also included revascularization procedures and HF hospitalizations.
While it is safe to conclude that treatment with insulin has a neutral effect on CV outcomes based on the results from ORIGIN and from UKPDS, we are eagerly awaiting results from the first pre-marketing CV trial mandated by the FDA for an insulin product-the Trial Comparing Cardiovascular Safety of Inuslin Degludec vs. Insulin Glargine (DEVOTE) evaluating insulin degludec vs. insulin glargine (NCT01959529). In meta-analyses of data accumulated in the phase 3 trials program of degludec presented as part of FDA new drug application review, this insulin was noted to have a safety signal for possible increased CVD risk. However, it is important to note that none of the trials contributing the meta-analysis were designed to evaluate CVD and events were captured through adverse event reporting and not adjudicated, so completeness of event collection and accuracy of cardiovascular event reported were not certain. The DEVOTE trial is a large randomized clinical trial comparing insulin degludec vs. insulin glargine designed to assess non-inferiority of the 2 insulins with regard to effects on major adverse cardiovascular events, with the trial ongoing with enrollment complete comprising 7644 patients with T2DM.
Sulfonylureas/meglitinides
The controversy surrounding the effects of sulfonylureas on CV outcomes was established with the University Group Program (UGDP) study.(61) This trial enrolled 823 patients with T2DM and evaluated the effects on clinical outcomes of five randomized glucose treatment strategies. Patients were randomized to an insulin variable dose group-titrated as needed to achieve intensive glycemic control vs. insulin standard dose group-fixed dosing at very low dose vs. once daily tolbutamide vs. placebo; a fifth arm was randomized to once daily phenformin, but that arm of the trial was stopped when phenformin was withdrawn from the market due to excess lactic acidosis. The most concerning finding over the course of this study was that there was a statistically higher incidence of mortality-both all-cause and CV-in the tolbutamide group compared with all the other treatment groups (all p<0.005). This resulted in an early termination of the tolbutamide treatment arm of the study, and strong CV safety cautionary product labeling that remains in all sulfonylurea product labels to the present day.
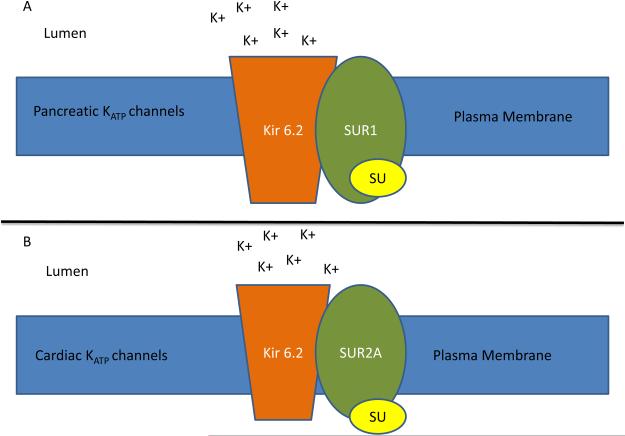
After the results of the UGDP trial, researchers worked to elucidate the mechanism of how sulfonylureas might adversely affect CV events. It was discovered that the ATP-dependent potassium channel (KATP) present in both pancreatic β-cells and cardiac myocytes, had the potential to be blocked by sulfonylureas. The importance of the opening of the KATP channel in cardiac myocytes relates to cardiac ischemic preconditioning, a phenomenon by which antecedent episodes of ischemia dramatically reduce myonecrosis during subsequent infarction leading to markedly reduced infarct size, less impact on systolic dysfunction, and reduced arrhythmic substrate.(62) Therefore, if the opening of these channels could be blocked by sulfonylureas, the myocardium could potentially lose the benefits of ischemic preconditioning and become more susceptible to life-threatening damage in the setting of an acute coronary syndrome event (Figure 4).
Figure 4. Sulfonylurea effect on ischemic preconditioning.
Legend: Potassium ATP (KATP) channel is a heterodimer composed of sulfonylurea receptor (SUR) and Kir6.2, a pore forming subunit, that assemble in the plasma membrane. SUR1 found in the pancreas (A) shares 68% identity to the SUR2A isoform found in cardiac tissue (B). Sulfonylureas (S) inhibit channel activity responsible for potassium influx. This inhibition is thought to effect ischemic preconditioning in cardiac myocytes.
Tolbutamide largely fell out of favor after the publication of the UGDP trial, leading to the development of newer formulations of sulfonylureas. The net clinical efficacy and safety of two of these 2nd generation sulfonylureas, chlorpropamide and glibenclamide (known in the US as glyburide) were tested in the UKPDS, noting however that the UKDPS trial was neither designed nor powered to assess the effects of the various randomized treatment strategies specifically on CV mortality or CVD events.(3) This trial assessed the composite incidence of microvascular, macrovascular and non-vascular diabetes-related outcomes in 3867 patients with newly diagnosed T2DM at study entry. Patients who were enrolled in the trial were randomized to a policy of intensive glucose control vs. standard care with dietary and lifestyle modifications. Those randomized to receive intensive treatment were secondarily randomized to treatment with insulin, glibenclamide (known in the US as glyburide), or chlopropamide (with randomization to metformin available for those overweight or obese at study entry). The study patients, with a median age of 54 years at study entry, were followed over a mean 10 year period. The incidence of fatal or non-fatal MI, angina, HF, stroke, diabetes-related death, and all-cause mortality were aggregated along with intermediate measures of microvascular disease and non-vascular outcomes into the primary composite endpoint, with sub-analysis of each component and permuted composites of these endpoints reported as well. The study found no differences in the rates of diabetes-related deaths, MI, or stroke (p=0.34, 0.052, 0.52 respectively) between intensive treatment groups.
The UKPDS results suggests improved safety of subsequent generation sulfonylureas contrasted with tolbutamide in the treatment of T2DM, with no mortality signal for either chlorpropamide or glibenclamide, but definitive conclusions cannot be drawn given the lack of statistical power in UKPDS for assessment of CVD risk. Importantly, if the adverse effects of SUs are mediated through inhibition of ischemic preconditioning, then analysis of such a low-risk cohort as the UKPDS with enrollment at the time of DM diagnosis may not have adequate numbers of acute coronary syndrome events to detect a signal of adversity. Further studies have continued to investigate the impact of this class of medication on CV outcomes.
The efficacy of glyburide as compared with metformin and rosiglitazone was studied in the A Diabetes Outcome Progression Trial (ADOPT).(63) This trial followed 4,360 patients from 2000 to 2006 and evaluated the time to failure of glycemic control of one of three monotherapy agents. Patients were randomized to receive rosiglitazone, metformin, or glyburide and were treated and monitored for a median duration of 4 years. CV outcomes were secondarily examined by the study investigators, and the incidence of CV events was not statistically different between the rosiglitazone and metformin groups. In regards to glyburide, a significantly lower risk of CV events (specifically congestive HF) was observed with glyburide as compared with rosiglitazone (p< 0.05). This most likely occurred due to the increased risk of HF with rosiglitazone rather than a protective effect from glyburide. The majority of patients who were included in the trial was within the first year from diagnosis with diabetes mellitus and did not have other comorbid conditions that might have contributed to the incidence of CV events. Therefore, for similar reasons as discussed above in the context of the UKPDS, the ADOPT cohort may not have been adequately high-risk to discern safety signals deriving from acute coronary syndrome events. Also, importantly, CV events in ADOPT were derived from adverse event reporting without prospective data collection or adjudication, thus limiting analyses of CV effects in that trial.
Evaluation of the impact of a meglitinide on CV outcomes was addressed in the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial.(64) The purpose of this trial was to factorially compare each of these two drugs vs. placebo in their capacity to reduce the risk of incident diabetes and CV events. Patients enrolled in this trial had impaired glucose tolerance and either risk factors for or overt CV disease. This trial included 9306 patients who were followed for five years after randomization to nateglinide 60mg daily vs. placebo.
Two of the three primary outcomes of the NAVIGATOR trial focused the composite incidence of death from a CV cause, nonfatal MI, nonfatal stroke, hospitalization for HF or unstable angina, or arterial revascularization intervention. The impact of nateglinide on these two composite CV outcomes did not differ significantly compared with placebo (p=0.16 and p=0.43 respectively). Though not designed as a non-inferiority trial for CVD safety with trial design pre-dating the present regulatory guidance by almost a decade, the point estimate and upper confidence limit for nateglinide vs. placebo for the core composite CVD endpoint (HR= 0.94; 95% CI 0.82-1.09) comprising 752 prospectively collected and adjudicated events would satisfy even the most stringent contemporary guidelines for demonstration of CV safety for an anti-hyperglycemic medication. The NAVIGATOR trial demonstrated that the impact of meglitinides, specifically nateglinide, was largely benign in regards CV health, a potential differential effect from sulfonylureas.
Metformin
Metformin is the only agent in the biguanide class and exerts its main anti-hyperglycemic effect by opposing glucagon action and thus inhibiting hepatic gluconeogenesis. Given its insulin-sensitizing effects and effects on lipids in addition to glucose-lowering, it is plausible that metformin may have beneficial CV effects. CV benefit of metformin was in fact documented in UKPDS 34, where treatment with metformin in 342 newly diagnosed patients with T2DM was associated with a 42% decrease in diabetes-related death, 36% decrease in all-cause mortality, and 31% decrease in MI over 10 years of follow-up, compared with standard care.(29) However, these analyses are limited by the small number of patients in the metformin arm of the trial, and the absence of central adjudication of the CV events reported. No other prospective, randomized trial has assessed the CV efficacy of metformin with any rigor. In fact, meta-analyses concluded there is insufficient evidence to claim a cardioprotective effect of metformin.(65)
Alpha-glucosidase Inhibitors
The impact of alpha-glucosidase inhibitors on CV outcomes was described in the STOP-Noninsulin-Dependent-Diabetes-Mellitus (STOP-NIDDM) trial.(66) This was a prospective placebo-controlled trial conducted in 1,368 patients. The trial was designed primarily to evaluate if acarbose could prevent the development of T2DM in patients with already established IGT. The incidence of CV events was designated as a secondary outcome of this trial. Patients enrolled in the study were randomized to receive either acarbose 100mg or placebo three times a day for three year treatment duration. CV events included MI, new angina, HF, stroke, or revascularization procedures. Investigators found that there was a statistically significant reduction of incidence of major adverse CV events in the acarbose group compared with placebo with a relative risk reduction of 49% (p< 0.001). After multivariable adjustment for other risk factors, CV risk reduction with acarbose treatment remained statistically significant (p=0.02).
Thiazolidinediones
Thiazolidinediones (TZDs) activate the peroxisome proliferator-activated-gamma (PPAR gamma) receptor, a nuclear receptor expressed primarily in adipocytes, but also in hepatocytes, skeletal muscle, cardiac muscle, colonic epithelium, vascular endothelial cells, and renal collecting duct epithelium. At a molecular level, PPAR gamma forms a heterodimer with the retinoid X receptor, forming a complex that binds to PPAR gamma response elements in target genes, altering the their transcription. In vivo, these agents induce improvement in skeletal and hepatic insulin resistance, promote adipocyte differentiation, decrease leptin levels (therefore increasing appetite), increase adiponectin level, decrease triglycerides, and increase HDL-C. They also inhibit VEGF-induced angiogenesis, decrease certain pro-inflammatory interleukins, and have an antiproliferative effect.
Pioglitazone and rosiglitazone are the only two TZDs currently on the market, and are indicated exclusively for the treatment of T2DM. Their popularity took a major downturn in 2007 when a meta-analysis suggested that rosiglitazone was associated with an increase in the risk of MI (OR 1.43, p=0.03) and possibly death from CV events (OR 1.64, p=0.06).(67) The PROactive trial was designed to assess the effects of pioglitazone versus placebo on the risk of a composite outcome that included all-cause mortality, MI, stroke, acute coronary syndrome, coronary or leg revascularization, or leg amputation.(9) While the study narrowly missed its primary outcome (HR 0.9, 95%CI, 0.8-1.02; p=0.095), it showed a significant reduction in the composite of all-cause mortality, MI, and stroke (HR 0.80, p=0.027). The lack of statistical significance of the primary outcome, along with the known side effects of pioglitazone (weight gain, edema, HF) , has limited clinical use of pioglitazone, though the data for CV efficacy for PROactive remain the most robust to date in the field of T2DM.(4, 5) The only other trial designed to evaluate the CV benefits of pioglitazone is still ongoing; the Insulin Resistance after Stroke (IRAS) trial (NCT00091949) randomized 3936 non-diabetic patients with a prior TIA or stroke and insulin resistance (HOMA-IR >3) to pioglitazone or placebo. The 3 year follow-up results evaluating the composite of stroke and MI are expected late-2015.
In the absence of definitive proof of CV efficacy deriving from the pioglitazone trials to date, several meta-analyses and observational studies have attempted to answer the important question of the CV benefits of pioglitazone. A meta-analysis of 19 controlled trials using pioglitazone was performed comprising data from 16,390 patients in randomized controlled trials, and showed that the composite endpoint of death, MI, and stroke was decreased in the pioglitazone group (HR 0.82, 95%CI 0.72-0.94, p=0.005) .(67)
The RECORD and DREAM trials are the largest studies that randomized patients to received rosiglitazone, but neither were dedicated CV trials. The DREAM trial was primarily a diabetes prevention trial; it followed 5269 patients with pre-diabetes for a median of 3 years.(68) The primary outcome was a composite of incident diabetes or death. The CV event rates were not statistically different between groups. The RECORD trial enrolled 4447 patients with uncontrolled T2DM on monotherapy with metformin or a sulfonylurea, who were randomized to the addition of rosiglitazone vs. addition of metformin/sulfonylurea and followed for a median of 5.5 years.(10) There were no statistical differences in CV outcomes between groups, including the composite primary outcome of CV hospitalization or CV death (321 events in the rosiglitazone group and 323 in the active comparator group; (HR 0.99 95%CI 0.85-1.16), or the secondary outcomes of CV death (HR 0.84, 95%CI 0.59-1.18), MI (HR 1.14, 95%CI 0.8-1.63), and stroke (HR 0.72, 95%CI 0.49-1.06). The risk of HF was double (HR 2.1 95%CI 1.35-3.27) in the rosiglitazone group, and more upper and distal limb fractures were observed in women treated with rosiglitazone.
A consistent increase in HF, both incident HF and worsening of prevalent HF , has been a consistent finding across the clinical trials evaluating TZDs. The reported absolute incremental risk of incident or worsening HF due to these drugs is 0.25-0.45% per year.(4) In the PROactive trial, the rate of adjudicated HF events was higher in the pioglitazone vs. placebo arm (5.5% vs 4.2%; p=0.007), respectively.(69) In the RECORD trial, the rate of adjudicated hospitalizations for HF was higher in the rosiglitazone vs. metformin/sulfonyurea group (1.7% vs 0.8%; p=0.001).(10) This excess risk of HF was also suggested in the DREAM trial of rosiglitazone vs. placebo (0.5% vs 0.1%, p=0.01), where younger patients with prediabetes were enrolled, and excluded for any preexistent cardiac conditions.(68)
In a meta-analysis of the incremental risk of incident or worsening HF associated with the TZDs, the net incremental risk for rosiglitazone was HR 2.18; 95% CI 1.44, 3.32; and for pioglitazone, HR 1.32; 95% CI 1.04, 1.68.(70) While the risk of new or worsening HF with TZD use has been demonstrated unequivocally with both available compounds, its clinical implications are still not fully reconciled as it appears that these medications are not associated with a higher risk of death, even in patients admitted for HF.(71) In addition, though the relative increments in HF risk range from 32% to 118% increase from meta-analysis, the absolute incremental risk appears much more modest at ~0.5%/year at the upper end of the reported incremental risk; i.e. 1 additional case of HF per 200 patients treated for a year. All efficacy signals would be placed in the context of a risk carrying such a small number-needed-to-treat for harm to occur.(4, 70)
Dipeptidyl peptidase 4 inhibitors
The first DPP-4 inhibitor approved for clinical use, sitagliptin, was introduced in the US in 2006, followed by saxagliptin, alogliptin, and linagliptin in the US; vildagliptin is approved in many countries around the world, but not in the US. In this setting, only 2 drugs in the class, alogliptin and saxagliptin, have thus far completed and reported results from CV outcomes studies.
The EXAMINE trial evaluating the CVD effects of alogliptin, was a multi-center, randomized, double-blind, placebo-controlled trial in 5380 patients with T2DM and recent acute coronary syndromes (ACS).(72) Patients enrolled in the trial were randomized to receive alogliptin (N=2701) or placebo (N= 2679) in addition to standard care for T2DM and CV disease. The primary end point was a composite of death from CV causes, nonfatal MI, or non-fatal stroke. After a median follow-up of 18 months, there was no difference in occurrence of the primary endpoint between the alogliptin and placebo groups (11.3% vs. 11.8%, respectively, P<0.001 for non-inferiority, P=0.32 for superiority; HR 0.96, upper boundary of one-sided repeated CI 1.16).
The SAVOR-TIMI 53 trial evaluated the CV effects of saxagliptin vs. placebo in a randomized, double-blind, placebo-controlled trial comprising of 16,492 patients with T2DM with either prevalent atherosclerotic CVD or multiple CVD risk factors.(73) The primary end point was a composite of CV death, nonfatal MI, or non-fatal ischemic stroke. After a median follow-up of 2.1 years, there was no difference in the occurrence of the primary endpoint between the saxagliptin and placebo groups (7.3% vs. 7.2%, respectively, p<0.001 for non-inferiority and p=0.99 for superiority; HR 1.00, 95% CI 0.89-1.12). Interestingly, when analyzing individual components of the composite secondary endpoint, more patients treated with saxagliptin were hospitalized for HF than those treated with placebo (3.5% vs. 2.8%, respectively, HR 1.27, [95% CI 1.07-1.51], P=0.007).(73, 74) Though not achieving statistical significance, the incidence of incident HF reported in EXAMINE was also numerically greater with alogliptin vs. placebo.
At this time, the reason for increased HF risks with saxagliptin specifically, and ongoing concerns for possible class-effects of the DPP-4 inhibitors, is unknown and merits further study. Of note, the annualized absolute incremental risk for HF from the SAVOR-TIMI 53 analyses of 0.35%/year is numerically comparable to the estimates of incremental risk of HF with the TZDs. A subgroup analysis by the SAVOR-TIMI 53 investigators noted that the increased risk of hospitalization was greatest in the first 12 months of therapy, and was more pronounced in patients with higher absolute risk of HF.(74) While longer than the EXAMINE trial, patients in the SAVOR-TIMI 53 trial were followed for just over 2 years, and the long-term CV outcomes associated with saxagliptin remain unclear. As with alogliptin, further studies will be needed to determine the long-term CV outcomes with saxagliptin. It is difficult to speculate a plausible mechanistic reason why these drugs would increment HF in patients with T2DM.
Glucagon-like peptide-1 receptor agonists
The CV safety of GLP-1 receptor agonists was evaluated in meta-analysis of randomized controlled trial data.(75) Major adverse CV events (MACE) were the primary outcome, on an intention-to-treat basis. GLP-1 receptor agonists did not portend any increased risk in MACE compared with both active control and placebo combined (OR 0.74, 95% CI 0.50-1.08; p = 0.12). When compared with placebo only, there was an association of benefit with GLP-1 receptor agonists (OR 0.46, 95% CI 0.25-0.83; p = 0.009). The authors noted that long term clinical trials are needed to verify the results.
Sodium-glucose cotransporter 2 inhibitors
There are currently no completed clinical trials evaluating CV safety of SGLT-2 inhibitors. Dapagliflozin was assessed in a meta-analysis of clinical trials, requested by the FDA, in patients with T2DM and high CV risk.(76) The study included 19 trials and had a primary endpoint of composite of time-to-first event of CV death, MI, stroke, and hospitalization for unstable angina. The investigators found no increased risk with dapagliflozin in this high-risk population (HR 0.82; 95% CI, 0.59 to 1.14). Canagliflozin had a similar analysis conducted at the request of the FDA.(77) A meta-analysis of 9 clinical trials was performed and MACE events were the primary endpoint. The analysis found no increased risk of CV events with canagliflozin (HR 0.65, 95%CI 0.35-1.21).
In general, anti-hyperglycemic medications presently available have demonstrated neutral effects on most clinical CV outcomes, with much discordance and uncertainty in the accumulated data to date and selected important signals of advere CV effects of selected therapies. Table 2 summarizes the available data regarding CV outcomes by class/medication. Recent policy changes by the FDA and European Medicines Agency have recommended to industry a set of guidelines to evaluate CV safety prior to approval. Numerous trials assessing the CV effects of acarbose, thiazolidinediones, insulin degludec, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors are currently underway, summarized in Table 3. The results of these studies anticipated over the coming 5 years will further inform clinicians on the CV safety and potential efficacy of the broad spectrum of anti-hyperglycemic medications presently available.
Table 2.
Randomized controlled trials of anti-hyperglycemic medications and cardiovascular outcomes
| ORIGIN | UGDP | NAVIGATOR | UKPDS 34 | STOP-NIDDM | RECORD | PROactive | EXAMINE | SAVOR- TIMI 53 |
|
|---|---|---|---|---|---|---|---|---|---|
| Drug assessed |
Insulin glargine |
Tolbutamide | Nateglinide | Metformin | Acarbose | Rosiglitazone | Pioglitazone | Alogliptin | Saxagliptin |
| Patient population |
N=12,537 IFG, IGT, T2DM + CV risk factors |
N= 823 newly diagnosed T2DM |
N=9,306 IGT + CVD or CV risk factors |
N=1,704 Newly diagnosed T2DM + obese |
N=1,368 IGT |
N= 4,447 T2DM on metformin or SU |
N=5,238 T2DM + macrovascular disease |
N=2701 T2DM + previous ACS |
N=16,492 T2DM + CVD or high CV risk |
| Primary outcome |
Composite: Nonfatal MI, nonfatal stroke, or death from CV causes |
CV death, all-cause mortality |
Composite: death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for CHF |
All-cause mortality |
Composite: coronary heart disease, CV death, congestive heart failure, cerebrovascular event, and peripheral vascular disease |
CV death/CV hospitalization, MI, CV death |
Composite: Death, MI, Stroke, ACS, vascular intervention, amputation |
Composite: Death from CV causes, nonfatal MI, or nonfatal stroke. |
Composite: CV death, MI, or ischemic stroke |
| Follow up | 6.2 years | 5 years | 5 years | 10.7 years | 3.3 years | 5.5 years | 2.9 years | 3.3 years | 2.1 years |
| Results | Composite: 1.02 (95% CI 0.94- 1.11) |
CV death: 12.7% TOL vs. 4.9% PLB All-cause mortality: 14.7% TOL vs. 10.2 PLB |
Composite: 0.94 (95% CI 0.82-1.09) |
All-cause mortality: 36% RRR vs. conventional therapy |
Composite: 0.51 (95% CI 0.28-0.95) |
CV death/CV hospitalization:0.99 (95% CI 0.85-1.16) MI: 1.14 (95% CI 0.80-1.63) CV death: 0.84 (95% CI 0.59-1.18) |
Composite: 0.90 (95% CI 0.80-1.02) |
Composite: 0.96; upper boundary of the one-sided repeated confidence interval, 1.16; p<0.001 for noninferiority |
Composite: 1.00(95% CI 0.89 - 1.12) |
Legend: All results are hazard ratios/95% confidence intervals unless otherwise noted. Abbreviations: IFG=impaired fasting glucose, IGT=impaired glucose tolerance, T2DM=type 2 diabetes, CV=cardiovascular, MI=myocardial infarction, CI=confidence intervals, TOL=tolbutamide, PLB=placebo, CVD=cardiovascular disease, CHF=congestive heart failure, SU=sulfonylurea, RRR=relative risk reduction, ACS=acute coronary syndromes.
Table 3.
Ongoing clinical trials of anti-hyperglycemic medications evaluating cardiovascular safety and efficacy
| Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine in Subjects With Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE) (NCT01959529) |
The Sitagliptin Cardiovascular Outcome Study (TECOS) (NCT00790205) |
The Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA) (NCT01243424) |
The Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA) (NCT01897532) |
The Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL) (NCT01144338) |
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results - A Long Term Evaluation (LEADER) (NCT01179048) |
|
| Drug Assessed | Insulin degludec | Sitagliptin | Linagliptin | Linagliptin | Exenatide once weekly |
Liraglutide |
| Comparator | Insulin glargine | Placebo | Glimepiride | Placebo | Placebo | Placebo |
| Estimated Enrollment |
N=7,644 | N=14,724 | N=6,000 | N=8,300 | N=14,000 | N=9,340 |
| Primary Outcome | Composite: CV death, non-fatal MI, or non-fatal stroke |
composite endpoint: time to first confirmed CV-related death, nonfatal MI, or non-fatal ischemic stroke |
composite endpoint: CV death, non-fatal MI (excluding silent MI), non-fatal stroke and hospitalization for unstable angina pectoris |
composite endpoint: CV death, non-fatal MI, non-fatal stroke and hospitalization for unstable angina pectoris |
Composite: CV death, nonfatal MI, or nonfatal stroke |
Composite: CV death, non-fatal MI, or non-fatal stroke |
| Follow up | 5 years | 3 years | 7.7 years | 4 years | 7.5 years | 5 years |
| Anticipated completion |
2018 | June 2015 | September 2018 | January 2018 | April 2018 | October 2015 |
| Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) (NCT01394952) |
Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN 6) (NCT01720446) |
Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) (NCT01131676) |
CANagliflozin cardioVascular Assessment Study (CANVAS) (NCT01032629) |
Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE- TIMI 58) (NCT01730534) |
Cardiovascular Outcomes Following Treatment With Ertugliflozin in Participants With Type 2 Diabetes Mellitus and Established Vascular Disease (VERTIS) (NCT01986881) |
|
| Drug Assessed | Dulaglutide | Semaglutide | Empagliflozin | Canagliflozin | Dapagliflozin | Ertugliflozin |
| Comparator | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo |
| Estimated Enrollment |
N=9,622 | N=3,297 | N=7,000 | N=14,000 | N=17,150 | N=3,900 |
| Primary Outcome | Composite: CV death, non-fatal MI, or non-fatal stroke |
Composite: CV death, non-fatal MI, or non-fatal stroke |
Composite: CV death (including fatal stroke and fatal MI), non- fatal MI (excluding silent MI) and non- fatal stroke |
Composite: CV death, non-fatal MI, non-fatal stroke |
Composite: CV death, MI or ischemic stroke |
Composite: CV Death, Non-fatal MI, or Non-fatal Stroke |
| Follow up | 6.5 years | 2.8 years | 5 years | 7.5 years | 6 years | 6.3 years |
| Anticipated completion |
April 2019 | 2016 | April 2015 | June 2018 | April 2019 | May 2021 |
| Thiazolidinediones Or Sulphonylureas and Cardiovascular Accidents.Intervention Trial (TOSCA IT) (NCT00700856) |
Acarbose Cardiovascular Evaluation Trial (ACE) (NCT00829660) |
Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010 (Lixisenatide) (ELIXA) (NCT01147250) |
A Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine in Subjects With Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE) (NCT01959529) |
A Study of the Effects of Canagliflozin (JNJ- 28431754) on Renal Endpoints in Adult Participants With Type 2 Diabetes Mellitus (CANVAS-R) (NCT01989754) |
Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) (NCT02065791) |
|
| Drug Assessed | Pioglitazone | Acarbose | Lixisenatide | Insulin degludec | Canagliflozin | Canagliflozin |
| Comparator | Glibenclamide, gliclazide, or glimepiride |
Placebo | Placebo | Insulin glargine | Placebo | Placebo |
| Estimated Enrollment |
3,371 | 7,500 | 6,075 | 7,644 | 5,700 | 3,700 |
| Primary Outcome | Composite: all-cause mortality, non-fatal MI ( including silent MI), non-fatal stroke, unplanned coronary revascularization |
Composite: Cardiovascular death, Non-fatal MI, Non-fatal stroke |
Composite: CV death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina |
Composite: cardiovascular death, non-fatal MI, or non- fatal stroke |
Progression of albuminuria Secondary outcome: Composite: CV death, nonfatal MI, and nonfatal stroke |
Composite: ESKD, doubling of serum creatinine, renal or CV death |
| Follow up | 4 years | 4 years | 3.9 years | 5 years | 3 years | 5.5 years |
| Anticipated completion |
December 2018 | October 2014 | February 2015 | November 2018 | April 2017 | January 2020 |
Legend: CV=cardiovascular, MI=myocardial infarction, ESKD=End stage kidney disease
We attempted to review all relevant data and represent a balanced summary of the evidence to date, yet we did not execute a formal systematic review and thus the possibility of unintentional bias exists.
CONCLUSIONS
CVD and DM coexist frequently and cause significant morbidity and mortality worldwide. There are an abundance of treatment options presently available for patients with DM, with differential effects between and within the classes of available medications with regards to CVD risk factors, intermediate markers of cardiovascular disease, and CVD outcomes. Policy changes by the US FDA and by the European Medicines Agency in 2008 have mandated more rigorous assessment of the CV effects of anti-hyperglycemic medications, and the resultant explosion of randomized clinical trial assessments of these drugs will serve us well-the scientific and clinical community, and most importantly, the patients with T2DM under our care.
ACKNOWLEDGEMENTS
This work was supported (C.A.A.) by the National Institutes of Health (K08DK101602).
Dr Lingvay discloses research funding from NovoNordisk, Pfizer/Merck, GI Dynamics, honoraria for consultation from Novo Nordisk, Astra Zeneca and publication support from Sanofi, Novo Nordisk. Dr McGuire discloses honoraria for trial leadership and consultation from GlaxoSmithKline, Takeda, Novo Nordisk, Janssen, Eli Lilly, Bristol Myers Squibb, Astra Zeneca, Boehringer Ingelheim, Merck, Regeneron, Lexicon, Sanofi Aventis.
Footnotes
CONFLICT OF INTEREST
Drs Alvarez, Vuylsteke, and Koffarnus report no conflicts of interest relevant to this article.
AUTHOR CONTRIBUTIONS
CAA, VV, RLK, IL, and DKM wrote the manuscript and provided critical edits.
REFERENCES
- (1).Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- (2).Grundy SM, Pasternak R, Greenland P, Smith S, Jr., Fuster V. AHA/ACC scientific statement: Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Journal of the American College of Cardiology. 1999;34:1348–59. doi: 10.1016/s0735-1097(99)00387-3. [DOI] [PubMed] [Google Scholar]
- (3).UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. UK Prospective Diabetes Study (UKPDS) Group. [PubMed] [Google Scholar]
- (4).McGuire DK, Inzucchi SE. New drugs for the treatment of diabetes mellitus: part I: Thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008;117:440–9. doi: 10.1161/CIRCULATIONAHA.107.704080. [DOI] [PubMed] [Google Scholar]
- (5).Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation. 2008;117:574–84. doi: 10.1161/CIRCULATIONAHA.107.735795. [DOI] [PubMed] [Google Scholar]
- (6).Gore MO, McGuire DK. Drugs for type 2 diabetes mellitus: the imperative for cardiovascular outcome assessment. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2012;9:85–8. doi: 10.1177/1479164112441527. [DOI] [PubMed] [Google Scholar]
- (7).Gore MO, McGuire DK. Resolving drug effects from class effects among drugs for type 2 diabetes mellitus: more support for cardiovascular outcome assessments. European heart journal. 2011;32:1832–4. doi: 10.1093/eurheartj/ehr019. [DOI] [PubMed] [Google Scholar]
- (8).Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. Jama. 2015;313:603–15. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- (9).Dormandy JA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- (10).Home PD, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- (11).Wang B, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes, obesity & metabolism. 2013;15:737–49. doi: 10.1111/dom.12085. [DOI] [PubMed] [Google Scholar]
- (12).Katout M, et al. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. American journal of hypertension. 2014;27:130–9. doi: 10.1093/ajh/hpt196. [DOI] [PubMed] [Google Scholar]
- (13).Mistry GC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. Journal of clinical pharmacology. 2008;48:592–8. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- (14).Koren S, et al. The effect of sitagliptin versus glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabetes technology & therapeutics. 2012;14:561–7. doi: 10.1089/dia.2011.0296. [DOI] [PubMed] [Google Scholar]
- (15).Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocrine reviews. 2011;32:515–31. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- (16).Vasilakou D, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Annals of internal medicine. 2013;159:262–74. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- (17).Goldberg RB, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes care. 2005;28:1547–54. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- (18).Monami M, et al. Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: meta-analysis of placebo-controlled trials. Advances in therapy. 2012;29:736–46. doi: 10.1007/s12325-012-0045-5. [DOI] [PubMed] [Google Scholar]
- (19).Buse JB, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- (20).Blonde L, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes, obesity & metabolism. 2006;8:436–47. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- (21).Buse JB, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- (22).Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–33. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- (23).Nauck MA, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes care. 2011;34:2015–22. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes care. 2012;35:1473–8. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Rosenstock J, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes care. 2012;35:1232–8. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).McGill JB. The SGLT2 Inhibitor Empagliflozin for the Treatment of Type 2 Diabetes Mellitus: a Bench to Bedside Review. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2014;5:43–63. doi: 10.1007/s13300-014-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).United Kingdom Prospective Diabetes Study Group United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Annals of internal medicine. 1998;128:165–75. doi: 10.7326/0003-4819-128-3-199802010-00001. United Kingdom Prospective Diabetes Study Group. [DOI] [PubMed] [Google Scholar]
- (28).Schade DS, Jovanovic L, Schneider J. A placebo-controlled, randomized study of glimepiride in patients with type 2 diabetes mellitus for whom diet therapy is unsuccessful. Journal of clinical pharmacology. 1998;38:636–41. doi: 10.1002/j.1552-4604.1998.tb04471.x. [DOI] [PubMed] [Google Scholar]
- (29).UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. UK Prospective Diabetes Study (UKPDS) Group. [PubMed] [Google Scholar]
- (30).Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–5. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. Bmj. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nature reviews Endocrinology. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- (34).Astrup A, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–16. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- (35).Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Db Syst Rev. 2008 doi: 10.1002/14651858.CD006739.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Inzucchi SE, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Walcher T, et al. Rapid effect of single-dose rosiglitazone treatment on endothelial function in healthy men with normal glucose tolerance: data from a randomised, placebo-controlled, double-blind study. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2010;7:178–85. doi: 10.1177/1479164110367812. [DOI] [PubMed] [Google Scholar]
- (38).Hopkins ND, et al. Effects of 6 months glucagon-like peptide-1 receptor agonist treatment on endothelial function in type 2 diabetes mellitus patients. Diabetes, obesity & metabolism. 2013;15:770–3. doi: 10.1111/dom.12089. [DOI] [PubMed] [Google Scholar]
- (39).Irace C, et al. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2013;10:72–7. doi: 10.1177/1479164112449562. [DOI] [PubMed] [Google Scholar]
- (40).Polak JF, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60:607–13. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Mazzone T, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296:2572–81. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- (42).Ishikawa S, et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. The American journal of cardiology. 2014;114:384–8. doi: 10.1016/j.amjcard.2014.04.050. [DOI] [PubMed] [Google Scholar]
- (43).Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35:1073–8. doi: 10.1161/01.STR.0000125864.01546.f2. [DOI] [PubMed] [Google Scholar]
- (44).Preiss D, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. The lancet Diabetes & endocrinology. 2014;2:116–24. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- (45).Nissen SE, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–73. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- (46).Takagi T, et al. Impact of troglitazone on coronary stent implantation using small stents in patients with type 2 diabetes mellitus. The American journal of cardiology. 2002;89:318–22. doi: 10.1016/s0002-9149(01)02232-9. [DOI] [PubMed] [Google Scholar]
- (47).Geng DF, Jin DM, Wu W, Wang Z, Wang JF. Effect of thiazolidinediones on in-stent restenosis in patients after coronary stenting: a meta-analysis of randomized controlled trials. Atherosclerosis. 2009;202:521–8. doi: 10.1016/j.atherosclerosis.2008.05.029. [DOI] [PubMed] [Google Scholar]
- (48).Finn AV, et al. Predictive factors for in-stent late loss and coronary lesion progression in patients with type 2 diabetes mellitus randomized to rosiglitazone or placebo. American heart journal. 2009;157:383 e1–8. doi: 10.1016/j.ahj.2008.11.013. [DOI] [PubMed] [Google Scholar]
- (49).Saunders J, Mathewkutty S, Drazner MH, McGuire DK. Cardiomyopathy in type 2 diabetes: update on pathophysiological mechanisms. Herz. 2008;33:184–90. doi: 10.1007/s00059-008-3115-3. [DOI] [PubMed] [Google Scholar]
- (50).Ghazzi MN, et al. The Troglitazone Study Group Cardiac and glycemic benefits of troglitazone treatment in NIDDM. Diabetes. 1997;46:433–9. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- (51).Dargie HJ, et al. A Randomized, Placebo-Controlled Trial Assessing the Effects of Rosiglitazone on Echocardiographic Function and Cardiac Status in Type 2 Diabetic Patients With New York Heart Association Functional Class I or II Heart Failure. Journal of the American College of Cardiology. 2007;49:1696–704. doi: 10.1016/j.jacc.2006.10.077. [DOI] [PubMed] [Google Scholar]
- (52).St John Sutton M, et al. A comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetes. Diabetes care. 2002;25:2058–64. doi: 10.2337/diacare.25.11.2058. [DOI] [PubMed] [Google Scholar]
- (53).McGuire DK, et al. Randomized comparison of the effects of rosiglitazone vs. placebo on peak integrated cardiovascular performance, cardiac structure, and function. European heart journal. 2010;31:2262–70. doi: 10.1093/eurheartj/ehq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circulation Cardiovascular imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- (55).Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England journal of medicine. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- (56).Nathan DM, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gerstein HC, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Patel A, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. The New England journal of medicine. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- (59).Duckworth W, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England journal of medicine. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- (60).Gerstein HC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. The New England journal of medicine. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- (61).Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA. 1971;218:1400–10. [PubMed] [Google Scholar]
- (62).Yellon DM, Hausenloy DJ. Realizing the clinical potential of ischemic preconditioning and postconditioning. Nature clinical practice Cardiovascular medicine. 2005;2:568–75. doi: 10.1038/ncpcardio0346. [DOI] [PubMed] [Google Scholar]
- (63).Kahn SE, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. The New England journal of medicine. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- (64).Holman RR, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. The New England journal of medicine. 2010;362:1463–76. doi: 10.1056/NEJMoa1001122. [DOI] [PubMed] [Google Scholar]
- (65).Boussageon R, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS medicine. 2012;9:e1001204. doi: 10.1371/journal.pmed.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Chiasson JL, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. Jama. 2003;290:486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- (67).Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298:1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- (68).Gerstein HC, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- (69).Ryden L, Thrainsdottir I, Swedberg K. Adjudication of serious heart failure in patients from PROactive. Lancet. 2007;369:189–90. doi: 10.1016/S0140-6736(07)60106-8. [DOI] [PubMed] [Google Scholar]
- (70).Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- (71).Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–90. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- (72).White WB, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. The New England journal of medicine. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- (73).Scirica BM, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England journal of medicine. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- (74).Scirica BM, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- (75).Monami M, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Experimental diabetes research. 2011;2011:215764. doi: 10.1155/2011/215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).FDA BRIEFING DOCUMENT NDA 202293 DAPAGLIFLOZIN ORAL TABLETS, 5 AND 10 MG. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM378076.pdf. Accessed 3-15-15.
- (77).FDA Briefing Document NDA 204042 Invokana (canagliflozin) Tablets. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf. Accessed 3-15-15.