Abstract
Background
Nonadherence to prescribed evidence-based medications after acute myocardial infarction (MI) can contribute to worse outcomes and higher costs. We sought to better understand the modifiable factors contributing to early nonadherence of evidence-based medications after acute MI.
Methods and Results
We assessed 7,425 acute MI patients treated with percutaneous coronary intervention (PCI) at 216 United States hospitals participating in TRANSLATE-ACS between 04/2010–05/2012. Using the validated Morisky instrument to assess cardiovascular medication adherence at 6 weeks post-MI, we stratified patients into self-reported high (score 8), moderate (score 6–7), and low (score <6) adherence groups. Moderate and low adherence was reported in 25% and 4% of patients, respectively. One-third of low adherence patients described missing doses of antiplatelet therapy at least twice a week after PCI. Signs of depression and patient-reported financial hardship due to medication expenses were independently associated with a higher likelihood of medication nonadherence. Patients were more likely to be adherent at 6 weeks if they had follow-up appointments made prior to discharge and had a provider explain potential side effects of their medications. Lower medication adherence may be associated with a higher risk of 3-month death/readmission (adjusted HR 1.35, 95% CI 0.98, 1.87) although this did not reach statistical significance.
Conclusions
Even early after MI, a substantial proportion of patients report suboptimal adherence to prescribed medications. Tailored patient education and pre-discharge planning may represent actionable opportunities to optimize patient adherence and clinical outcomes.
Clinical Trial Registry Information
clinical trial #NCT01088503; URL: https://clinicaltrials.gov/ct2/show/NCT01088503
Keywords: acute myocardial infarction, adherence, antiplatelet therapy, percutaneous coronary intervention
Cardiovascular disease is one of the leading causes of morbidity and mortality in the United States,1 yet rates of mortality associated with coronary artery disease have declined in recent years. This decline has been partially attributed to the use of evidence-based therapies, such as aspirin, adenosine diphosphate (ADP) receptor inhibitors, beta-blockers, and statins, that reduce risks of recurrent cardiovascular adverse events.2,3 National inpatient registries demonstrate high prescription rates of these medications at discharge from the index myocardial infarction (MI) hospital4, 5; however, a prescription does not necessarily translate into continued adherence to a prescribed regimen. Prior literature has shown that patient adherence to prescribed therapies remains poor, with more than 25% of patients not filling prescription medications within a week after discharge for an acute MI.6 Medication nonadherence is a widely recognized problem in healthcare and has been associated with worse patient outcomes and increased healthcare costs.7–9 Nonadherence to antiplatelet therapy after percutaneous coronary intervention (PCI) is of particular concern due to the increased risk of stent thrombosis.10 Therefore, a better understanding of modifiable factors contributing to nonadherence may help inform actionable opportunities to optimize longitudinal patient outcomes.
The TReatment with ADP receptor iNhibitorS: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) study is a longitudinal observational study of PCI-treated MI patients that rigorously assesses adherent behaviors via a validated, 8-question Morisky Medication Adherence Scale (MMAS).11–13 The reasons for poor medication adherence are likely multifactorial. TRANSLATE-ACS collects detailed information on patient sociodemographic, economic, and clinical factors, as well as assesses the quality of patient-provider interactions. As a result, TRANSLATE-ACS offers a unique opportunity to: 1) determine the incidence and degree of cardiovascular medication nonadherence early after hospital discharge in a contemporary PCI-treated MI population; 2) evaluate patient and provider factors independently associated with cardiovascular medication nonadherence; and 3) assess the association of medication nonadherence on subsequent mortality and readmission risk.
Methods
Study Design and Population
TRANSLATE-ACS is a longitudinal observational study of MI patients treated with PCI and antiplatelet therapy (clinical trial #NCT01088503). The study design, including a detailed description of patient follow-up and data collection, has been previously described.14 Briefly, this study of ST-segment elevation MI (STEMI) and non-STEMI (NSTEMI) patients who were treated with PCI and started on an ADP receptor inhibitor during the index hospitalization was broadly inclusive, excluding only those patients who were unable or unwilling to provide written informed consent for longitudinal follow-up, or who were simultaneously participating in another research study that specified use of a specific antiplatelet agent within the first 12 months post-MI. The institutional review board of each reporting hospital approved participation in TRANSLATE-ACS, and all data were collected prospectively.
Post-discharge study follow-up was conducted via centralized telephone interviews by trained personnel at the Duke Clinical Research Institute. During the 6-week interview, patients were administered the 8 MMAS questions to evaluate self-reported medication adherence. At both 6-week and 6-month interviews, patients were asked to report any rehospitalizations. All self-reported rehospitalizations were verified by the collection of hospital bills. As a safeguard against under-reporting, all enrolling hospitals were queried at 12 months for any rehospitalizations that may not have been reported by the patient. For this analysis, we included all patients enrolled in TRANSLATE-ACS among 216 United States hospitals from April 2010 to May 2012 who were alive to complete the follow-up interviews at 6 weeks (n=8,488). We excluded patients who had incomplete answers to the MMAS questions (n=625), those who were lost to follow-up at 6 months (n=280), and those whose reported rehospitalizations that could not be validated by medical bill collection (n=158). Our final study population consisted of 7,425 patients.
Data Collection and Definitions
Participating hospitals collected information on baseline demographic and clinical characteristics, processes of care, and in-hospital outcomes using a standardized set of data elements and definitions, aligned with those used by the National Cardiovascular Data Registry®.14 Patients were contacted by telephone at 6 weeks and at 6 months post-discharge in order to collect additional information on medication use, patient-provider communication, quality of life, and follow-up care. We evaluated patient self-reported adherence to prescribed cardiovascular medications at the 6-week telephone interview by administering the MMAS.11–13 We assessed functional status using the validated EuroQol-5 Dimensions (EQ-5D) score15 while depression was measured using the Patient Health Questionnaire-2 (PHQ-2).16 Three previously validated questions assessing health literacy (listed in Table 1) were added to version 2 of the 6-week follow-up interview case report form17,18; consequently, data on health literacy were captured in 54% of the study population. All rehospitalizations were verified by the collection of hospital bills and/or medical records. All deaths were verified by hospital bills, medical records, or the national death index.
Table 1.
Patient Characteristics
| High Adherence (n=5,278) |
Moderate Adherence (n=1,845) |
Low Adherence (n=302) |
p-value* | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age (years)† | 60 (53, 69) | 59 (51, 66) | 55 (49, 63) | <0.001 |
| Female | 27.9% | 26.7% | 25.2% | 0.40 |
| Race | ||||
| White | 89.5% | 89.1% | 86.8% | 0.26 |
| African American | 7.7% | 8.2% | 10.6% | 0.18 |
| Asian | 1.3% | 0.8% | 1.3% | 0.27 |
| American Indian/Alaskan | 0.7% | 0.8% | 1.0% | 0.82 |
| Native Hawaiian/Pacific Islander | 0.1% | 0.2% | 0.0% | 0.77 |
| Hispanic | 2.6% | 3.0% | 4.0% | 0.27 |
| Employed (full/part) | 49.2% | 53.7% | 55.3% | 0.001 |
| Married | 66.7% | 62.6% | 56.6% | <0.0001 |
| High school graduate and beyond | 53.6% | 52.6% | 42.9% | 0.03 |
| Insurance | ||||
| Private | 66.90% | 66.4% | 59.3% | <0.02 |
| Medicare | 36.4% | 31.4% | 24.8% | <0.0001 |
| Medicaid | 5.0% | 6.2% | 8.3% | <.01 |
| Military | 3.2% | 2.1% | 3.3% | 0.04 |
| Indian health service | 0% | 0.1% | 0.3% | 0.12 |
| State specific (non-Medicaid) | 1.4% | 1.4% | 1.3% | 0.99 |
| Uninsured | 12.2% | 14.9% | 17.2% | 0.001 |
| Baseline clinical characteristics | ||||
| Diabetes | 24.5% | 26.6% | 31.8% | 0.006 |
| Hypertension | 67.9% | 64.7% | 66.9% | 0.03 |
| Dyslipidemia | 67.1% | 66.3% | 74.5% | 0.02 |
| Current smoker | 35.1% | 38.6% | 49.3% | <0.0001 |
| Prior MI | 17.5% | 20.5% | 30.8% | <0.0001 |
| Prior PCI | 19.5% | 22.2% | 39.4% | <0.0001 |
| Prior stroke/TIA | 5.0% | 5.3% | 6.0% | 0.68 |
| Atrial fib/flutter | 4.6% | 4.3% | 4.6% | 0.87 |
| Peripheral arterial disease | 6.2% | 6.1% | 5.6% | 0.93 |
| Prior heart failure | 5.2% | 5.9% | 8.0% | 0.10 |
| In-hospital features | ||||
| STEMI presentation | 52.3% | 50.8% | 51.0% | 0.49 |
| Cardiac arrest presentation | 3.3% | 2.3% | 2.7% | 0.08 |
| EQ-5D VAS score† | 75 (60, 85) | 75 (55, 85) | 70 (50, 84) | 0.0003 |
| PHQ2 depression score >3 | 6.4% | 8.9% | 12.3% | <0.0001 |
| Culprit previously treated | 6.3% | 7.9% | 16.2% | <0.0001 |
| Drug-eluting stent used during PCI | 71.3% | 70.6% | 69.2% | 0.68 |
| Health literacy‡ | ||||
| Need help reading hospital materials | 0.11 | |||
| Always | 14.8% | 15.1% | 13.5% | |
| Often | 5.3% | 4.1% | 4.1% | |
| Sometimes | 9.2% | 8.1% | 8.2% | |
| Occasionally | 12.3% | 10.1% | 16.5% | |
| Never | 58.3% | 62.7% | 57.7% | |
| Difficulty learning about my medical condition due to difficulty understanding written information? | 0.002 | |||
| Always | 2.2% | 3.5% | 4.8% | |
| Often | 2.2% | 2.2% | 4.8% | |
| Sometimes | 7.3% | 7.9% | 10.7% | |
| Occasionally | 10.2% | 13.5% | 14.9% | |
| Never | 78.1% | 73.0% | 64.9% | |
| Confident filling out health-related forms by myself? | 0.01 | |||
| Extremely | 61.3% | 57.3% | 50.3% | |
| Quite a bit | 19.1% | 20.3% | 25.4% | |
| Somewhat | 10.7% | 11.4% | 14.8% | |
| A little bit | 4.4% | 4.2% | 3.6% | |
| Not at all | 4.5% | 6.9% | 5.9% | |
Compared using Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Reported as median (25th and 75th percentiles).
Health literacy questionnaire introduced in version 2 of the follow-up interview, data available for 54% of this analysis population.
EQ5D VAS indicates EuroQol-5D visual analogue scale; MI, myocardial infarction; PCI, percutaneous coronary intervention; PHQ2, Patient Health Questionnaire-2 (depression); STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack
Statistical Methods
We used answers from the MMAS (1 point for each question shown in Table 2) to stratify the study population into high (score 8, answered “no” to all questions), moderate (score 6–7), and low (score <6) medication adherence groups.11 We chose the cutpoints of high, moderate, and low, since they have been previously shown in validated models to be clinically useful.11 Patient characteristics and outcomes in each group were described using frequencies and percentages for categorical variables and median values (with 25th and 75th percentiles) for continuous variables. Characteristics of patients in each group were compared using Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Overall, 7.4% of data was missing.
Table 2.
Morisky Questionnaire Responses
| High Adherence (n=5,278) |
Moderate Adherence (n=1,845) |
Low Adherence (n=302) |
|
|---|---|---|---|
| Morisky medication adherence score* | 8 (8, 8) | 7 (6, 7) | 5 (4, 5) |
| Sometimes forgets to take heart medications | 0 | 31.7% | 82.5% |
| Forgot to take heart medications in last 2 weeks | 0 | 17.0% | 64.2% |
| Did not take heart medications yesterday | 0 | 5.7% | 17.6% |
| Cut back or stopped taking heart medications without telling doctor because it makes you feel worse | 0 | 6.4% | 20.2% |
| Stopped taking heart medications because feel like heart condition is under control | 0 | 1.2% | 11.6% |
| Sometimes forgets to take heart medications when traveling | 0 | 8.8% | 28.8% |
| Feels like it is a hassle to stick with heart treatment plan | 0 | 35.4% | 47.0% |
| Never have difficulty remembering to take all heart medications | 100% | 79.3% | 23.5% |
Reported as median (25th and 75th percentiles).
Multivariable logistic regression modeling was used to determine baseline demographic and discharge factors associated with patient-reported medication nonadherence at 6 weeks (MMAS score <8). We hypothesized that patient characteristics (particularly those related to socioeconomic and health status [e.g., physical function, depression]), as well as provider factors (such as those related to communication and follow-up), contribute significantly to the likelihood that a patient will be adherent to prescribed medications after discharge. Therefore, patient-level covariates selected for inclusion in the model were age, gender, black race, non-Hispanic ethnicity, educational level achieved, marital status, employment status, private insurance coverage, current smoker, EQ-5D score, PHQ-2 depression score, self-reported financial hardship obtaining prescription medications, and medication copayment assistance program participation. As a healthy adherer effect may be evident,19,20 we further adjusted for self-reported engagement in routine exercise (exercised at least 20 minutes daily). Pre-discharge provider-level factors entered into the model included: cardiac rehabilitation referral and follow-up appointment made prior to discharge, written list of discharge medication with instructions provided at hospital discharge, and provider explanation of medication rationale and potential side effects. Missing values of the continuous variables were imputed to the median and missing values of categorical variables were imputed to the mode.
We used a composite endpoint of death or all-cause readmission from the 6-week interview out to 6 months among patients with high, moderate, and low cardiovascular medication adherence. For time-to-event analyses, all events occurring prior to the 6-week interview were censored. The event rates for death or all-cause readmission were estimated using Kaplan-Meier analysis, and log-rank p-values were calculated to compare outcomes between groups. For adjusted analyses comparing outcomes between low adherence and other groups, multivariable Cox regression modeling was used to estimate the hazard ratio and 95% confidence intervals. Variables in the adjustment model were adapted from prior MI risk adjustment models.21,22
The TRANSLATE-ACS study was funded by Daiichi Sankyo, Inc. and Eli Lilly & Company. All data analyses were performed independently by statisticians at the Duke Clinical Research Institute using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Among 7,425 acute MI patients, 5,278 (71%) reported being highly adherent to prescribed cardiovascular medications, 1,845 (25%) reported moderate adherence (1,350 had a Morisky score of 7 and 495 patients had a Morisky score of 6), and 302 (4%) reported low adherence by 6 weeks after their MI. As shown in Table 2, 82% of patients with low medication adherence reported “sometimes forgetting to take his/her heart medications,” 47% felt that “it was a hassle to stick with” their cardiac treatment plan, 20% stated they might “cut back or stop medications without telling the doctor because it makes them feel worse,” and 12% reported they would stop taking heart medication because “they feel like their heart condition is under control.”
Baseline Characteristics and In-hospital Treatment
From a sociodemographic standpoint, patients with low cardiovascular medication adherence were more often younger, unmarried, or employed, and were less often high school graduates or had healthcare insurance, compared with patients with high medication adherence (Table 1). When compared with highly adherent patients, those with low medication adherence were more often smokers, and more often had diabetes or dyslipidemia, a prior MI or PCI, and a previously treated culprit lesion. During the index MI hospitalization, we found no difference in the use of drug-eluting stents between the three groups. Notably, patients with low medication adherence more often admitted limitations in their ability to learn about their medical condition, due to difficulty understanding written health information; this finding suggests that patients with low medication adherence have lower health literacy compared with patients with higher medication adherence.
Discharge and Post-discharge Processes
There were no significant differences in the number of medications prescribed at discharge between groups (Table 3). The large majority of patients (95.7%) received written discharge medications and instructions. Nevertheless, when compared with highly adherent patients, those with lower adherence were less likely to report a provider explaining the rationale and potential side effects for each medication.
Table 3.
Pre- and Post-discharge Care
| High Adherence (n=5,278) |
Moderate Adherence (n=1,845) |
Low Adherence (n=302) |
p-value* | |
|---|---|---|---|---|
| Pre-discharge | ||||
| Number of discharge medications† | 7 (6, 10) | 7 (6, 10) | 7(5, 9) | 0.11 |
| Prescribed at discharge | ||||
| Aspirin | 98.3% | 98.4% | 98.3% | 0.91 |
| ADP receptor inhibitor | 99.5% | 99.8% | 99.7% | 0.18 |
| Statin | 95.3% | 95.6% | 97.0% | 0.35 |
| ACEI/ARB | 73.4% | 75.9% | 74.5% | 0.11 |
| Beta-blocker | 92.5% | 93.6% | 94.7% | 0.14 |
| Cardiac rehab referral | 79.5% | 79.2% | 75.8% | 0.43 |
| Cardiology inpatient service | 88.7% | 88.2% | 88.7% | 0.82 |
| Provider explained reason for each med | 87.4% | 83.1% | 81.1% | <0.0001 |
| Provider explained side effect for each med | 63.8% | 55.1% | 55.0% | <0.0001 |
| F/U appointment made before discharge | 70.1% | 69.5% | 63.9% | 0.07 |
| Post-discharge care | ||||
| Physician visit by 6 weeks | 63.0% | 64.1% | 57.6% | 0.09 |
| Cardiologist visit by 6 weeks | 70.8% | 73.0% | 65.2% | 0.01 |
| No physician visit since discharge | 10.3% | 8.7% | 14.6% | 0.004 |
| Cardiac rehab participation | 31.2% | 29.9% | 26.8% | 0.23 |
| Current smoker | 12.8% | 17.5% | 24.2% | <0.0001 |
| Smoking cessation since discharge‡ | 66.6% | 57.9% | 55.0% | <0.0001 |
| Know their most recent blood pressure | 72.8% | 70.8% | 61.3% | <0.0001 |
| Day per week of exercise ≥20 minutes§ | 3 (0,5) | 3 (0,5) | 2 (0,4) | <0.0001 |
| Medication management | ||||
| Assistance from friends | 17.5% | 18.5% | 19.9% | 0.43 |
| Pillbox/calendar to keep track of meds | 64.8% | 64.0% | 62.6% | 0.65 |
| Understands what to do medications run out | 95.5% | 93.0% | 88.7% | <0.0001 |
| Missed an ADP inhibitor in last 4 weeks | 4.8% | 21.7% | 51.5% | <0.0001 |
| Never | 95.2% | 78.0% | 48.3% | |
| Once a week | 3.5% | 13.6% | 18.1% | |
| 2–3 times a week | 1.0% | 6.3% | 26.1% | |
| 4 or more times a week | 0.3% | 1.8% | 7.3% | |
| Medication expenses | ||||
| >$200/month out of pocket | 17.1% | 18.6% | 15.9% | 0.57 |
| Financial hardship with meds | 19.7% | 25.9% | 30.1% | <0.0001 |
| Insurance program helps pay for meds | 88.7 | 87.0 | 81.5 | 0.0003 |
Compared using Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Reported as median (25th and 75th percentiles). Financial hardship meds = patient reported hardship with medication costs,
Among current smokers at baseline index admission
Reported as (median, IQR)
ADP indicates adenosine diphosphate; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; cardiac rehab referral, cardiac rehabilitation referral made; F/U, follow-up
There were no significant differences in the rates of follow-up appointments arranged before discharge between adherence groups, yet patients with lower medication adherence were less likely to visit any physician within the first 6 weeks post-MI (Table 3). Compared with highly adherent patients, those with lower medication adherence were less likely to exercise regularly and to know their most recent home blood pressure measurement.
Pill boxes, calendars, or other tools to help keep track of medications were commonly used (65%) in this study population and were used similarly among the groups. Patients with lower medication adherence were less likely to know what to do when a medication ran out. Notably, when these post-PCI patients were asked about the frequency of missing an ADP receptor inhibitor dose, 8% of moderate adherence patients and 33% of low adherence patients reported missing a dose at least twice a week. The majority of patients (88%) had insurance or a prescription assistance program to help support payment of medication expenses; however, patients with lower adherence were more likely to report financial hardship paying for medications and were less likely to have prescription assistance coverage.
Independent Factors Associated with Nonadherence
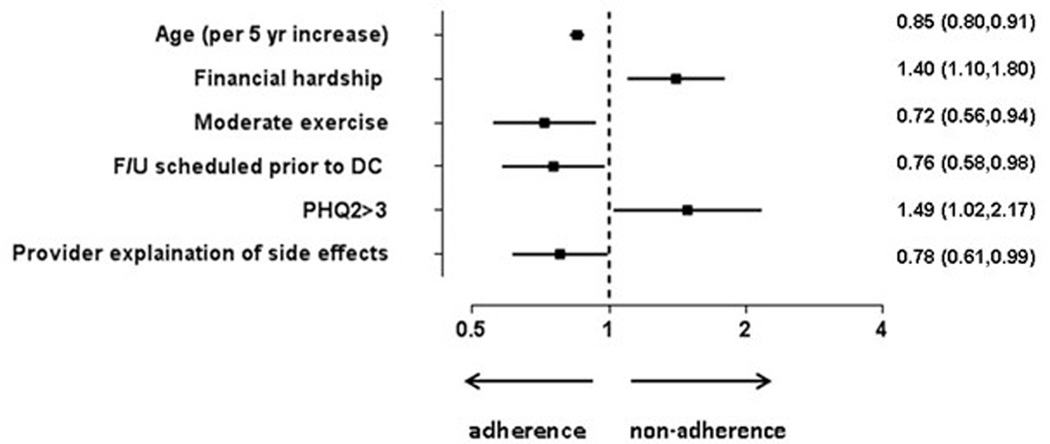
After multivariable modeling, baseline factors significantly associated with medication nonadherence at 6 weeks are presented in the Figure 1. We found that patient-reported financial hardship due to medication expenses and depression were associated with a higher likelihood of medication nonadherence. Patients who had follow-up appointments made prior to discharge and those who reported having a provider explain potential side effects of their medications were more likely to be adherent at 6 weeks. Interestingly, while increasing age was associated with greater medication adherence, we found that other demographic variables such as race and ethnicity were not. Those who engaged in regular exercise post-discharge were more likely to be adherent; however, cardiac rehabilitation participation was not independently associated with medication adherence.
Figure 1. Forest Plot.
This figure displays significant factors associated with medication nonadherence. Other variables included in the model: gender, non-Hispanic, black race, smoker, cardiac rehab referral, EuroQol-5 Dimensions score, married, ≥high school graduation, employed, written discharge medication list/instructions, insurance coverage, assistance program to pay for medications, cardiac rehab participation. Moderate exercise= at least 1 day a week of ≥20 minutes of exercise.
OR listed with 95% confidence intervals.
OR, odds ratio
Longitudinal Outcomes
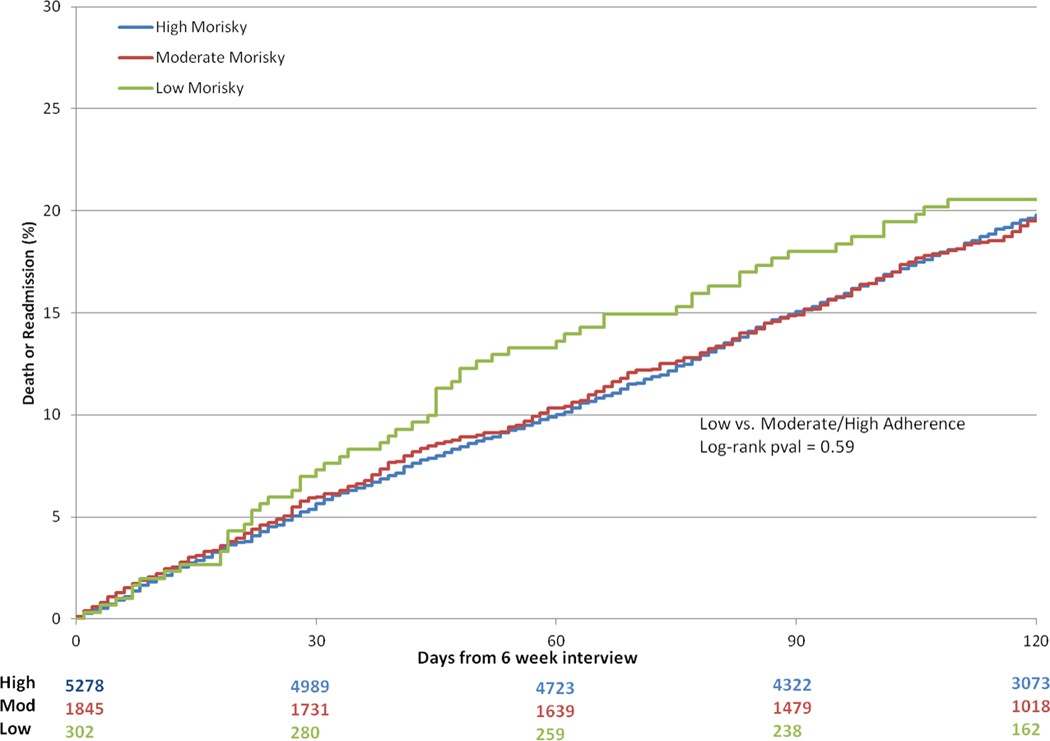
The median duration of follow-up post-discharge was 164 days (25th and 75th percentiles, 156–175). Between the 6-week interview and 6-month follow-up, there were an additional 66 deaths and 1,573 readmissions. Unadjusted event curves for patients with moderate and high medication adherence tracked closely throughout the entire 6-month post-MI period (Figure 2). Event curves diverged early for patients with low medication adherence (Figure 2). At 60 days, the risk of death or readmission was higher among patents with low medication adherence, but did not reach statistical significance in unadjusted (log-rank p-value 0.049) and multivariable adjusted comparisons (adjusted hazard ratio [HR] = 1.35, 95% confidence interval [CI] = 0.98–1.87) than patients with moderate/high adherence. The curves converged later such that 6-month post-MI event rates were similar between all groups (Figure 2, unadjusted log rank p-value = 0.59, adjusted HR 1.02, 95% CI 0.80–1.30).
Figure 2. Rates of Death/Readmission.
Kaplan Meier curves for rates of death/readmission within 120 days according to Morisky score. Variables included in the model: age, gender, race, ethnicity, insurance status, marital status, educational level, prior MI, prior PCI, prior CABG surgery, prior stroke or transient ischemic attack, peripheral arterial disease, prior heart failure, prior atrial fibrillation or flutter, diabetes, hypertension, dyslipidemia, dialysis, smoking status, chronic lung disease, recent gastrointestinal or genitourinary bleeding in the 6 months prior to index MI admission, STEMI presentation, cardiac arrest within 24 hours of admission, cardiogenic shock within 24 hours of admission, heart failure signs or symptoms within two weeks before admission, body mass index, admission heart rate, admission systolic blood pressure, pre-procedure hemoglobin, pre-procedure creatinine clearance, multivessel disease on angiography, multivessel PCI, bifurcating culprit lesion, culprit lesion involving a CABG graft, drug-eluting stent implantation, culprit lesion successfully dilated, left ventricular ejection fraction, transfer-in status, number of hospitalizations between index hospital discharge and the 6-week interview, and hospital referral region.
CABG, coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction
Discussion
In this large, multicenter study of MI patients treated with PCI, approximately 30% of patients reported suboptimal adherence to prescribed cardiovascular medications in the first 6 weeks after their MI hospitalization. Non-adherent patient behavior may be associated with early mortality and readmission risks. While some non-modifiable socioeconomic and clinical factors are associated with nonadherence, our results suggest several opportunities for provider intervention.
Medication Adherence Remains Suboptimal Early After MI
As early as 6 weeks after a hospitalization for MI, we found that approximately 30% of contemporary MI patients are suboptimally adherent to prescribed cardiovascular medications. This rate has not changed significantly compared with earlier studies.6,23,24 In 2008, Jackevicius et al. found that nearly 25% of patients do not fill prescriptions for cardiac medications early after an MI discharge with little improvement 4 months later.6 Prior studies have also noted steady declines in beta blocker and statin use over time to <50% use by 12 months after a cardiac procedure.25 Post-PCI, optimal adherence to dual antiplatelet therapy is critical, as its premature discontinuation increases the risk of stent thrombosis, MI, and death.2,3,10,26 In our study, medication adherence was self-reported using the MMAS and may, in fact, underestimate true adherence given pitfalls such as recall bias. Of concern, one-third of patients in the low adherence group reported missing doses of prescribed ADP receptor inhibitor at least twice a week. This highlights the continuing challenge and opportunity for improvement in ensuring the optimal medical management of patients after MI and PCI.
Implications of Low Medication Adherence
Medication nonadherence is a widespread problem across healthcare and may reflect discrepancies between provider and patient perception of treatment benefits. For instance, asymptomatic conditions such as hypertension and hyperlipidemia have been associated with suboptimal medication adherence in part because patients believe they do not feel any different on or off medications.27–30 Even after an acute MI, patients who do not perceive a beneficial effect of taking medications are less likely to be adherent to secondary prevention medications.31 Therapies with noticeable (even if minor) side effects (e.g., easy bruising with antiplatelet therapy) or those with less apparent health impact (e.g., statin therapy) are particularly vulnerable to nonadherence.29 Our results show that a substantial proportion of low adherence patients reported self-discontinuation of medications if they believed it made them feel poorly or if they perceived that their cardiac condition had improved. Although one might hypothesize that patients with a first-time MI might have more difficulty with adherence compared with patients previously hospitalized for a similar event, our results actually show a higher prevalence of prior MI among those with lower medication adherence.
Lack of adherence to secondary preventative care contributes to a greater likelihood of disease recurrence and treatment complications,32 and may be a driver of increased healthcare costs.33 Previous studies have linked poor medication adherence after MI with higher risks of readmission and mortality.34,35 In our study, event curves appeared to initially diverge, but then converged later such that 6-month death/readmission risks were similar between groups. Potential explanations for this finding include: 1) a smaller number of low adherence patients limits the power to show significant differences between groups; and 2) adherent behavior may change over time with more patients crossing over to lower adherence after 6 months.
Potential Solutions
A major strength of our study was the ability to examine a breadth of potential patient and provider factors associated with adherence. These factors can broadly be categorized into motivational (i.e., exercise, tobacco use, home blood pressure monitoring), communication-related (i.e., written instructions, provider explanation of side effects, health literacy), and socioeconomic factors (i.e., cost).29 As the reasons for medication nonadherence are multifactorial, effective and sustainable solutions must be multimodal and simultaneously address these various facets of nonadherence.36–38 For example, patient tools such as pillboxes and written discharge medication lists have been shown to be highly effective in improving medication adherence.39,40 In our study, these tools were commonly used but, in and of themselves, did not predict high medication adherence. In a recent qualitative assessment, MI patients most frequently cited poor communication and education on the need or importance of specific therapies as reasons for premature medication cessation.41 This finding is consistent with our results in that patients who had low adherence cited poor communication of the need and reasons for each of the discharge medications, as well as the possible side effects that they may encounter. Provider explanation of potential side effects of the medications prescribed at discharge was independently associated with better short-term adherence. Our study also shows the importance of assessing health literacy when disseminating written patient instructions and educational materials.
We found follow-up appointment arrangement prior to discharge to be strongly associated with adherence. Lack of contact represents a lost opportunity not only for reinforcing continued treatment goals, but also for assessing medication intolerance and patient knowledge gaps that might contribute to nonadherence. We found that patients with low adherence were also less likely to engage in healthy behaviors such as home blood pressure monitoring or routine exercise, and were more often smokers compared with medium or high adherence patients. Among the patient characteristics included in our multivariate model, we found that those who exercised on a routine basis were nearly 30% more likely to be adherent on short-term follow-up. These results affirm the “healthy adherer effect” hypothesis, which suggests that the lower risk of adverse outcomes in patients with high adherence may be a surrogate for overall healthier behavior.19,20 Creative multimodality solutions bridging the gap between hospital discharge and outpatient follow-up that engage a patient’s interest in his or her own healthcare may represent the greatest opportunity for success. Interventions that foster continued consistent communications between patients and the healthcare system, such as telemonitoring42 or pharmacist interactions,43 have been recommended for conditions beyond acute MI.
We found that nonadherent patients were more likely to report financial hardship due to medication costs. In the Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) trial, Chaudry et al. found that reducing patient copayments was associated with improved medication use.44 Strategies such as this, which are referred to as value-based insurance design, hope to improve long-term adherence. In our study, highly adherent patients more often reported coverage of medication costs by their insurance. Notably, improvements in adherence in MI FREEE were modest with copayment reduction alone, suggesting that additional measures are needed to improve medication-taking behavior and outcomes.
Our study had several limitations. First, medication adherence was patient-reported and, therefore, was not verified by pill count. Nevertheless, the MMAS is a validated questionnaire that has demonstrated good correlation to quantified monitoring methods and provides a more feasible alternative to assess adherence in real-world practice. Also, adherence was assessed only at one timepoint and, consequently, may not be reflective of downstream adherence. Similarly, patients who died prior to the 6-week follow-up interview were not included in the final study population; therefore, very early nonadherence could be under-reported. Second, while this study goes beyond typical databases in collecting potential patient-reported barriers to adherence, recall bias is a limitation especially when evaluating patient-reported engagement in exercise or recollection of provider instructions. It is also possible that participants in a research study may be more likely to maintain adherent behaviors than individuals in the general population. Third, even after adjustment for baseline differences, unmeasured confounding must be considered when interpreting these results. Additionally, a causal relationship cannot be inferred between adherence and risk of long-term outcomes such as death and re-hospitalization in this observational analysis. Finally, the study population consisted of MI patients entered from sites participating in TRANSLATE-ACS, so all patients underwent PCI. Early adherence rates may differ in other patients and settings.
In conclusion, adherence to prescribed therapies remains suboptimal for contemporary MI patients, and may be related to worse long-term outcomes. While certain socioeconomic and clinical factors are particularly associated with medication nonadherence, our study highlights several potentially modifiable factors that may provide opportunities for intervention. Tailored patient education and pre-discharge planning, as well as the availability of continued patient interactions with the health system early after hospital discharge, may represent key actionable opportunities to optimize patient adherence and improve outcomes.
Supplementary Material
Acknowledgements
The authors would like to thank Erin Hanley, MS for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Sources of Funding
The TRANSLATE-ACS study is sponsored by Daiichi Sankyo and Lilly USA. The Duke Clinical Research Institute is the coordinating center for this study, which represents a collaborative effort with the American College of Cardiology.
Robin Mathews is supported by grant number KM1CA156687 from the National Institute of Health/National Cancer Institute.
Footnotes
Conflict of Interest Disclosures
R Mathews: Dr. Mathews has no relevant disclosures to report.
ED Peterson: Dr. Peterson reports research funding for the American College of Cardiology, American Heart Association, Eli Lilly & Company, Janssen Pharmaceuticals, and Society of Thoracic Surgeons (all significant); consulting (including CME) for Merck & Co. (modest), Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, and Sanofi-Aventis (all significant).
E Honeycutt: Ms. Honeycutt has no relevant disclosures to report.
CT Chin: Dr. Chin has no relevant disclosures to report.
MB Effron: Dr. Effron reports being an employee of Eli Lilly & Company; shareholder of Lilly, USA.
M Zettler: Dr. Zettler reports being an employee of Eli Lilly & Company.
GC Fonarow: Dr. Fonarow reports being a consultant to Novartis (significant) and Janssen (modest).
TD Henry: Dr. Henry has no relevant disclosures to report.
TY Wang: Dr. Wang reports research funding from AstraZeneca, Gilead, Lilly, The Medicines Company, and Canyon Pharmaceuticals (all significant); educational activities or lectures (generates money for Duke) for AstraZeneca (modest); consulting (including CME) for Medco (modest) and American College of Cardiology (significant).
References
- 1.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Division of Vital Statistics. Deaths: Final Data for 2009. [Updated December 29, 2011. Accessed October 3, 2013];National Vital Statistics Reports, Volume 60, Number 3. Centers for Disease Control and Prevention web site. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf. [PubMed]
- 2.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Zidar JP. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:e215–e367. doi: 10.1016/j.jacc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 4.Somma KA, Bhatt DL, Fonarow GC, Cannon CP, Cox M, Laskey W, Peacock WF, Hernandez AF, Peterson ED, Schwamm L, Saxon LA. Guideline adherence after ST-segment elevation versus non-ST segment elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:654–661. doi: 10.1161/CIRCOUTCOMES.111.963959. [DOI] [PubMed] [Google Scholar]
- 5.Hospital Compare. [Accessed June 3, 2013];Medicare.gov: The Official U.S. Government Site for Medicare web site. http://www.medicare.gov/hospitalcompare/?AspxAutoDetectCookieSupport=1.
- 6.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 7.Cherry SB, Benner JS, Hussein MA, Tang SSK, Nichol MB. The clinical and economic burden of nonadherence with antihypertensive and lipid-lowering therapy in hypertensive patients. Value Health. 2009;12:489–497. doi: 10.1111/j.1524-4733.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 8.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, Krumholz HM, Cohen DJ. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 11.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38:1363–1368. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 14.Chin CT, Wang TY, Anstrom KJ, Zhu B, Maa JF, Messenger JC, Ryan KA, Davidson-Ray L, Zettler M, Effron MB, Mark DB, Peterson ED. Treatment with adenosine diphosphate receptor inhibitors-longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) study design: expanding the paradigm of longitudinal observational research. Am Heart J. 2011;162:844–851. doi: 10.1016/j.ahj.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Validation of the EQ-5D in patients with a history of acute coronary syndrome. Curr Med Res Opin. 2005;21:1209–1216. doi: 10.1185/030079905X56349. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 17.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 18.Powers BJ, Trinh JV, Bosworth HB. Can this patient understand written health information? JAMA. 2010;304:76–84. doi: 10.1001/jama.2010.896. [DOI] [PubMed] [Google Scholar]
- 19.Canner PL, Forman SA, Prud’homme GJ, Berge KG, Stamler J. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303:1038–1041. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 20.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin CT, Chen AY, Wang TY, Alexander KP, Mathews R, Rumsfeld JS, Cannon CP, Fonarow GC, Peterson ED, Roe MT. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: the Acute Coronary Treatment and Intervention Outcomes network (ACTION) registry-Get With The Guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113–22. doi: 10.1016/j.ahj.2010.10.004. e2. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub WS, Grau-Sepulveda MV, Weiss JM, Delong ER, Peterson ED, O'Brien SM, Kolm P, Klein LW, Shaw RE, McKay C, Ritzenthaler LL, Popma JJ, Messenger JC, Shahian DM, Grover FL, Mayer JE, Garratt KN, Moussa ID, Edwards FH, Dangas GD. Prediction of long-term mortality after percutaneous coronary intervention in older adults: results from the National Cardiovascular Data Registry. Circulation. 2012;125:1501–1510. doi: 10.1161/CIRCULATIONAHA.111.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Bushnell CD, Olson DM, Zhao X, Pan W, Zimmer LO, Goldstein LB, Alberts MJ, Fagan SC, Fonarow GC, Johnston SC, Kidwell C, Labresh KA, Ovbiagele B, Schwamm L, Peterson ED. AVAIL Investigators. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011;77:1182–1190. doi: 10.1212/WNL.0b013e31822f0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newby LK, LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 26.Ho PM, Tsai TT, Maddox TM, Powers JD, Carroll NM, Jackevicius C, Go AS, Margolis KL, DeFor TA, Rumsfeld JS, Magid DJ. Delays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of care. Circ Cardiovasc Qual Outcomes. 2010;3:261–266. doi: 10.1161/CIRCOUTCOMES.109.902031. [DOI] [PubMed] [Google Scholar]
- 27.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 28.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 29.Baroletti S, Dell'Orfano H. Medication adherence in cardiovascular disease. Circulation. 2010;121:1455–1458. doi: 10.1161/CIRCULATIONAHA.109.904003. [DOI] [PubMed] [Google Scholar]
- 30.Marshall IJ, Wolfe CD, McKevitt C. Lay perspectives on hypertension and drug adherence: systematic review of qualitative research. BMJ. 2012;345:e3953. doi: 10.1136/bmj.e3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen LaPointe NM, Ou FS, Calvert SB, Melloni C, Stafford JA, Harding T, Peterson ED, Alexander KP. Association between patient beliefs and medication adherence following hospitalization for acute coronary syndrome. Am Heart J. 2011;161:855–863. doi: 10.1016/j.ahj.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Miedema MD, Cohn JN, Garberich RF, Knickelbine T, Graham KJ, Henry TD. Underuse of cardiovascular preventive pharmacotherapy in patients presenting with ST-elevation myocardial infarction. Am Heart J. 2012;164:259–267. doi: 10.1016/j.ahj.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 34.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88:229–233. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 36.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 37.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 38.Cutler DM, Evertt W. Thinking outside the pillbox-medication adherence as a priority for health care reform. N Engl J Med. 2010;362:1553–1155. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 39.Morello CM, Chynoweth M, Kim H, Singh RF, Hirsch JD. Strategies to improve medication adherence reported by diabetes patients and caregivers: results of a Taking Control of Your Diabetes Survey (February) Ann Pharmacother. 2011;45:145–153. doi: 10.1345/aph.1P322. [DOI] [PubMed] [Google Scholar]
- 40.Metlay JP, Hennessy S, Localio AR, Han X, Yang W, Cohen A, Leonard CE, Haynes K, Kimmel SE, Feldman HI, Strom BL. Patient reported receipt of medication instructions for warfarin is associated with reduced risk of serious bleeding events. J Gen Intern Med. 2008;23:1589–1594. doi: 10.1007/s11606-008-0708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garavalia L, Ho PM, Garavalia B, Foody JM, Kruse H, Spertus JA, Decker C. Clinician-patient discord: exploring differences in perspectives for discontinuing clopidogrel. Eur J Cardiovasc Nurs. 2011;10:50–55. doi: 10.1016/j.ejcnurse.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Veldhuisen DJ, Maass AH. Telemonitoring of outpatients with heart failure: a search for the holy grail? Circulation. 2012;125:2965–2967. doi: 10.1161/CIRCULATIONAHA.112.118141. [DOI] [PubMed] [Google Scholar]
- 43.Walker PC, Bernstein SJ, Jones JN, Piersma J, Kim HW, Regal RE, Kuhn L, Flanders SA. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009;169:2003–2010. doi: 10.1001/archinternmed.2009.398. [DOI] [PubMed] [Google Scholar]
- 44.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH. Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.