Abstract
Background
In cardiac transplant recipients, the development of antibodies to the endothelial intermediate filament protein vimentin (anti-vimentin antibodies, AVA) has been associated with rejection and poor outcomes. However, the incidence of these antibodies prior to transplantation and their association with early rejection has not been investigated.
Methods
Pre-transplant serum was analyzed from 50 patients who underwent de novo cardiac transplant at Johns Hopkins Hospital from 2004-2012. Demographic, one year rejection, and survival data were obtained from the transplant database.
Results
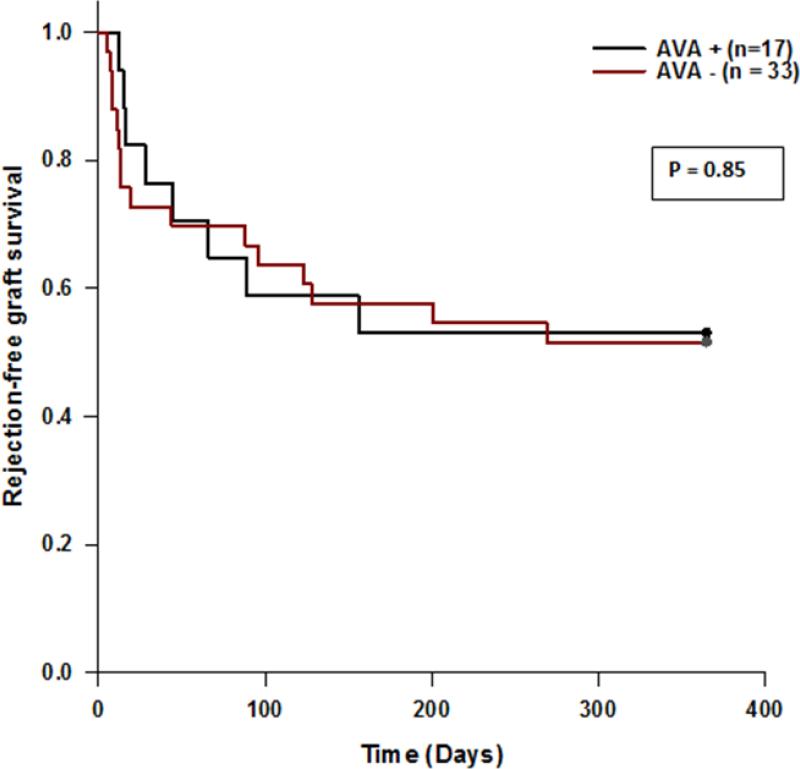
The incidence of pre-transplant AVA was 34%. AVA positive patients were younger (p=0.03) and there was an increased incidence in females (p=0.08). Demographic data were similar among both groups. AVA positivity did not predict rejection in the 1st year post-transplant. There was no difference in rejection-free graft survival (53 vs. 52%, p=0.85) at 1 year. Similarly there was no difference in graft survival at 1 year (82 vs. 88%, p=0.56) or graft survival at a median follow up of 23 and 26 months, respectively (76 vs. 85%, p=0.41).
Conclusions
AVA is common in the cardiac pre-transplant population with a higher incidence in the young. The presence of detectable AVA did not correlate with early post-transplant rejection or graft survival.
Keywords: Anti-vimentin antibodies, Pre-transplant, transplant rejection, cardiac transplantation, non-HLA antibodies
Introduction
Vimentin is an intermediate filamentous protein expressed in the cytosol of adult leukocytes, fibroblasts and endothelial cells. This protein is also expressed on the cell surface of activated and damaged cells within solid organ transplanted allografts. Antibodies to vimentin (AVA) have been shown to be an independent risk factor for the development of cardiac allograft vasculopathy (CAV) (1). In addition to its association with CAV, AVA has been shown to accelerate cardiac graft rejection in animal models and potentially increase the risk of antibody mediated rejection (AMR) in cardiac transplant patients (2-4). In solid organ transplant recipients, AVA is most commonly detected post-transplantation. However, AVA has also been found in the serum of patients with autoimmune diseases. Therefore, AVA may be present in some individuals prior to cardiac transplantation and those recipients may be at a higher risk for early graft rejection or failure. In renal transplant recipients, Bersarni et al. recently demonstrated that higher pre-transplant AVA titers (which continuously increased after transplantation) were associated with allograft fibrosis, atrophy, and rejection (5). In our study we sought to determine the incidence of AVA prior to cardiac transplantation and if the presence of pre-transplant AVA increased the risk of post-transplant rejection and/or graft failure.
Methods
After institutional review board approval, we retrospectively reviewed patients from the Johns Hopkins Hospital who underwent de novo cardiac transplantation between January 2004 to June 2012 (n=161). Patient selection was based on the availability of pre-transplant serum samples that could be tested for the presence of AVA (n=50). Demographic and outcomes data were collected from the electronic medical record.
AVA levels were measured using a solid phase multiplexed bead immunoassay performed on a Luminex® fluoroanalyzer, which was designed and validated by parallel testing with a commercially available ELISA(6). ELISA testing was also performed in a subset of patients (n=20). For continuous variables, data are presented as mean ± standard deviation if normally distributed; otherwise as median [interquartile range]. Comparison of continuous variables was performed by Student's t-test or rank sum test as appropriate; comparison of categorical variables by chi squared or Fisher's exact test. Survival analysis was performed by Kaplan-Meier and log rank testing. Cell-mediated rejection was defined by the 2004 International Society for Heart and Lung Transplantation (ISHLT) grading system of 2R or greater. Antibody mediated rejection was defined as positive immunofluorescence or immunoperoxidase staining for peri-capillary deposition of immunoglobulins and /or complement (C4d, C3d). Discrete AMR episodes required either a negative biopsy between episodes or prior cessation of AMR treatment that was restarted after a subsequent biopsy at least one month later.
Results
Seventeen of 50 patients tested positive for the presence of AVA prior to transplantation (34%). The AVA positive group was younger (27 vs. 41 years; p=.03), and trended toward female predominance (p=0.08); other demographic data were similar among the two groups (Table). AVA positivity did not predict rejection in the first year post-transplant, including time to first episode, compared to AVA negative patients. There was no difference in rejection-free graft survival (53 vs. 52%, p=0.85) at 1 year. Similarly there was no difference in graft survival at 1 year (82 vs. 88%, p=0.56) or graft survival at a median follow up of 23 and 26 months, respectively (76 vs. 85%, p=0.41) (Figure). In a subset of 20 patients who also underwent ELISA testing, the incidence of pre-transplant AVA was 45%. Eleven of the pre-transplant AVA positive patients lost their positivity within the first year after transplant (n=11).
| Pre-Transplant AVA+ (17) | Pre-Transplant AVA− (33) | ||
|---|---|---|---|
| Demographics | |||
| Ischemic Cardiomyopathy | 1 (6) | 3 (9) | 1.0‡ |
| Non-Ischemic Cardiomyopathy | 16 (94) | 30 (91) | 1.0‡ |
| Congenital Heart Disease | 2 (12) | 6 (18) | 0.70‡ |
| Age at transplant (years) | 27 ± 21 | 41 ± 21 | 0.03* |
| Female Gender | 13 (76) | 16 (48) | 0.08‡ |
| Race | |||
| White | 5 (29) | 17 (52) | 0.19‡ |
| Black | 10 (59) | 13 (39) | |
| Hispanic | 1 (6) | 0 (0) | |
| Asian | 1 (6) | 3 (9) | |
| Prior LVAD/BiVAD implantation | 8 (47) | 14 (42) | 0.99† |
| Pregnancy within 2 years of transplant | 1 (6) | 2 (12) | 1.0‡ |
| Presence of non-self HLA antibodies | 11 (65) | 16 (48) | 0.43† |
| 3rd Party | 11 (65) | 16 (48) | 0.43† |
| Donor specific | 4 (24) | 5 (15) | 0.47‡ |
| Outcomes | |||
| Total number of biopsies | 144 | 356 | - |
| Biopsies per patient | 6 [5-12.5] | 12 [10-13] | 0.12 |
| Time to first rejection episode (days) | |||
| CMR or AMR | 31 [14-89] | 13 [8-103] | 0.41 |
| CMR | 45 [18-111] | 54 [12-127] | 0.81 |
| AMR | 14 [12-15] | 8 [7-12] | 0.27 |
| Pt with at least one rejection episode | |||
| CMR or AMR | 6 (35) | 14 (42) | 0.86† |
| CMR | 5 (29) | 11 (33) | 0.97† |
| AMR | 2 (12) | 6 (18) | 0.70‡ |
| Total biopsies positive for rejection | |||
| CMR or AMR | 15 (10) | 37 (10) | 0.87† |
| CMR | 12 (8) | 16 (4) | 0.14† |
| AMR | 4 (3) | 21 (6) | 0.22‡ |
| # of discrete rejection episodes/pt | |||
| CMR or AMR | 0.65 ± 1.1 | 0.64 ± 0.93 | 0.91 |
| CMR | 0.53 ± 0.97 | 0.46 ± 0.75 | 0.85 |
| AMR | 0.12 ± 0.33 | 0.18 ± 0.39 | 0.57 |
| Rejection-free graft survival at 1 year | 9 (53) | 17 (52) | 0.85¥ |
| Graft survival at 1 year | 14 (82) | 29 (88) | 0.56¥ |
| Graft survival | 13 (76) | 28 (85) | 0.41¥ |
| Median length of follow up (months) | 23 [12.5-34.5] | 26 [18.5-49] | 0.26 |
For continuous variables, mean ± SD if normally distributed; otherwise median [interquartile range], Comparison of continuous variables by student t-test or rank sum test as appropriate*; Comparison of categorical variables by Chi Square† or Fisher Exact test‡ as appropriate. Survival analysis by Kaplan-Meier¥. Percentages in parenthesis. [] = interquartile range. CMR = cellular mediated rejection; AMR = antibody mediated rejection; Pt = patient
FIGURE 1.
Kaplan Meier Curve of Rejection-Free Graft Survival.
Of the 50 patients, 39 (11 AVA positive, 28 AVA negative) underwent evaluation of coronary arteries (median time of evaluation: 12 months [IQR 12-25]). One AVA positive patient (69 months post-transplant – ISHLT grade CAV 3) and 4 AVA negative patients (1 month – CAV 3, 12 months – CAV 1, 25 months – CAV 1, and 64 months – CAV 1) had evidence of CAV 1 or greater (p=0.44).
Discussion
Despite the paucity of large-population based data evaluating the incidence of AVA in healthy individuals, small studies have shown that the presence of AVA in healthy controls is virtually absent. In our study, we found the incidence of AVA prior to transplant to be relatively high. It is possible that antibody formation could develop as a consequence of vimentin expression in damaged native hearts or in patients with a predisposition to autoimmunity. We did observe pre-transplant AVA was more common in the young and trended toward female predominance, a subgroup more customarily afflicted with autoimmune disease and immunogenicity. In a recent analysis of kidney transplant recipients, pre-transplant AVA levels were no different between disease subgroups or when considering the duration of pre-transplant dialysis (5). Similarly, we did not detect a difference in the levels of AVA prior to transplant between heart failure etiologies and also found no difference between those who did and did not require a left ventricular assist device prior to transplant. Furthermore, the Luminex® immunoassay, the primary instrument used for antibody detection in our study has a higher sensitivity for AVA than traditional ELISA. Yet, the incidence of pre-transplant AVA was also high (45%) in the ELISA subset making it less likely that the increased sensitivity of the detection assay accounted for our results. Overall, the factors which led to AVA formation pre-transplant remain uncertain.
Although the presence of post-transplant AVA and its associated deleterious effects on cardiac allografts have been well documented in the literature (1-4), we did not find any such association with pre-transplant AVA. Its presence did not predict cardiac allograft rejection (cellular or AMR), graft failure or survival at 1 year. Thus, it remains possible that AVA is simply an associated bystander of rejection or CAV and does not have a pathogenic role. Because of our relatively small sample size we cannot exclude the possibility that type II error contributed to our negative findings. Most patients in this study did not yet have long term follow-up (i.e. 5-10 years) in which observations of CAV would be expected. Therefore, the presence of pre-transplant AVA may still increase the risk of CAV, and this requires additional longitudinal study. Interestingly, we also found that more than half the patients who tested positive for AVA prior to transplant lost their positivity within the first year after transplant. We believe future studies should be aimed at understanding: 1) what factors contribute to the high incidence of AVA antibody pre-transplant; 2) if the persistent presence or disappearance and reappearance of pre-transplant AVA lead to worse outcomes in cardiac transplant recipients; 3) how AVA titers vary with the level and type of immunosuppression. In renal allografts, increasing titers of AVA after transplant were associated with kidney dysfunction (5), perhaps suggesting multiple and longitudinal measures may be more predictive of outcomes.
In summary, we report 1) a high incidence of pre-transplant AVA, and 2) pre-transplant AVA was not associated with rejection or poor outcomes in the first year post-transplant. These findings suggest that further study is necessary before routine clinical testing for AVA can be recommended and before the presence of this antibody guides clinical decision making.
Acknowledgments
Funding Sources: The authors wish to acknowledge funding from the National Heart, Lung, and Blood Institute [L30 HL110304].
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi:10.1111/ctr.12567
Disclosures: The authors have no conflict of interests to report.
REFERENCES
- 1.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Mahesh B, Leong HS, Sarathchandra P, et al. Antivimentin Antibodies Mediate Acute and Chronic Damage in MHC-matched Allografts. J Heart Lung Transpl. 2007;26:S221. [Google Scholar]
- 3.Nath D, Basha H, Tiriveedhi V, et al. Characterization of Immune Responses to Cardiac Self-antigens Myosin and Vimentin in Human Cardiac Allograft Recipients with Antibody-mediated Rejection and Cardiac Allograft Vasculopathy . J Heart Lung Transpl. 2010;29:1277–1285. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose M. Role of anti-vimentin antibodies in allograft rejection. Hum Immunol. 2013;74:1459–1462. doi: 10.1016/j.humimm.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bersarni D, Cerundolo L, Fuggle SV, et al. Role of Anti-Vimentin Antibodies in Renal Transplantation. Transplantation. 2014;98:72–78. doi: 10.1097/01.TP.0000443224.66960.37. [DOI] [PubMed] [Google Scholar]
- 6.Dale B, Lucas P, Leffell M, Zachary A. Design Of A Specific, Sensitive Assay For The Detection Of Anti-Vimentin Antibody Using Luminex® Bead-Based Technology. Hum Immunol. 2013;74:132. [Google Scholar]