Abstract
Objectives/Hypothesis
Head and neck squamous cell carcinoma (HNSCC) is strongly associated with tobacco use. We sought to examine the relationship between self-reported tobacco use and the level of urinary tobacco carcinogen metabolites in a cohort of patients with HNSCC.
Study Design
Cross-sectional analysis.
Methods
Eighty-four cigarette smokers with head and neck cancer completed tobacco and alcohol use questionnaires, and the following urinary tobacco metabolites were quantified: 1-hydroxypyrene (1-HOP), N′-nitrosonornicotine and its glucuronides (total NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL), and cotinine. A cross-sectional analysis was performed with assessment of correlation coefficients.
Results
When analyzed based on self-reported cigarettes per day (CPD), no significant correlation with any of the studied tobacco carcinogen metabolites was found. However, urinary cotinine showed significant correlation with total NNN, total NNAL, and 1-HOP. Total NNN, total NNAL, and 1-HOP showed significant correlation with each other suggesting exposure occurs to each proportionally.
Conclusions
In smokers with HNSCC, self-reported tobacco use does not predict actual carcinogen exposure. In contrast, urinary cotinine levels significantly correlate with carcinogen levels. Therefore, urinary cotinine is the preferred value for estimating carcinogen dose in these patients. 1-HOP levels were significantly associated with total NNN and total NNAL suggesting that smokers are exposed to these carcinogens proportionally. These data indicate that utilizing conventional methods of estimating tobacco exposure (CPD) may not accurately approximate exposure to tobacco carcinogens in smokers with HNSCC. These data have implications for future studies focused on screening and epidemiology of smokers with HNSCC.
Level of Evidence
NA
Keywords: Tobacco, head and neck cancer, metabolites, biomarkers, carcinogenesis
INTRODUCTION
A significant proportion of patients with head and neck squamous cell carcinoma (HNSCC) report a history of past or current tobacco use. The role of tobacco in the development of head and neck cancer is firmly established.1 Although patients with newly diagnosed carcinoma are routinely queried regarding their past and current tobacco use, the preponderance of evidence suggests that this method of data collection may result in significant inaccuracies.2 However, clinicians continue to rely on the use of self-reported tobacco history as the primary measure of carcinogen exposure.
In the general population, self-reported quantification of cigarette smoking may potentially underestimate or overestimate the actual exposure to tobacco-related toxins.3 Examination of cancer patients adds additional complexity and considerations to this analysis for multiple reasons. Cigarette smokers who have just been diagnosed with a cancer may feel more pressure to meet perceived expectations of their physicians and thus do not accurately represent their tobacco use history. Additionally, smokers who continue to smoke during treatment commonly misrepresent their use, presumably associated with fear and guilt as they are treated for malignancy.4
Because nicotine is difficult to accurately and reliably measure given its very short half-life, measurement of cotinine has become common when studying recent nicotine exposure. Cotinine is formed from nicotine following cytochrome P450 metabolism and has a half-life of 18 to 24 hours.5 Cotinine measurements can be made in the urine, saliva, and serum.6–8 Recent data show that serum cotinine analysis provides increasingly reliable data regarding actual tobacco consumption in addition to acting as a marker of potential wound complications following head and neck surgery.9,10
The examination of tobacco carcinogen metabolites represents a broader application of this latter technique. The study of tobacco carcinogen metabolites has already yielded striking risk-related data in carcinoma occurring outside the head and neck. N′-nitrosonornicotine (NNN) is one prominent carcinogenic nitrosamine present in tobacco smoke. NNN measurements are commonly described as total urinary NNN, which includes free NNN plus NNN-N-glucuronides. In cigarette smokers, prediagnostic levels of urinary total NNN were found to be strongly associated with the risk of developing esophageal cancer in a prospective study based on a cohort of 18,244 Chinese men in Shanghai, China.11 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is another carcinogenic constituent of tobacco smoke. NNK metabolite urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronide (NNAL-gluc) act as markers of exposure to NNK. The presence of elevated levels of total NNAL (NNAL plus NNAL-gluc) has been linked to the risk of eventual lung carcinoma after controlling for cigarette intake.12–14 Last, 1-hydroxypyrene (1-HOP) is the major metabolite of pyrene, which is a polycyclic aromatic hydrocarbon (PAH). 1-HOP is always present in mixtures of PAH.15 PAH have been shown to have a potential role in the development of lung cancer. 16 Recently, preliminary evidence has been presented showing that cigarette smokers with HNSCC have higher levels of NNN and 1-HOP compared to smokers without HNSCC after controlling for cigarette intake.17
All of these data suggest that the study of tobacco carcinogen metabolites may be a useful tool in estimating actual carcinogen dose and carcinoma risk as well as examining the validity of self-reported cigarette use through analysis of easily obtained urine specimens. This approach is felt to have potential in the study of HNSCC.18 Currently, in the clinical realm, physicians most commonly rely on self-reported smoking history (cigarettes per day [CPD]) as the main measure of tobacco dose. However, it is not clear that gathering such data provides any useful information regarding actual carcinogen dose. Therefore, we sought to examine the relationship between self-reported cigarette intake among patients with HNSCC and the urinary concentration of tobacco carcinogen metabolites and markers to determine the utility of CPD as a measure of carcinogen exposure in this population.
MATERIALS AND METHODS
Study Population
Approval from our institutional review board was obtained (study #0903M62203). Enrolled subjects consisted of cigarette smokers with a new or recent diagnosis of HNSCC presenting to the otolaryngology clinic in our institution from April 2009 to April 2014. After obtaining consent, a tobacco- and alcohol-use questionnaire was administered. This questionnaire is comprehensive and queries multiple aspects of all types of tobacco (smokeless and smoked) and alcohol use including duration of use, current and previous rates of use, specific products used, attempted cessation methods, and perceived obstacles to cessation. Following completion of the questionnaire, the enrollees submitted a 10-mL urine and 10-mL blood sample. The urine samples were kept in a −20°C freezer until assays were performed. The blood samples were centrifuged to allow separation of the buffy coat and then kept at −20°C for future studies.
Experimental Assays
Total NNAL (NNAL plus NNAL-Gluc), total NNN (NNN plus NNN-N-glucuronides), and 1-HOP were determined as described previously.19 The detection limit for NNAL was 0.4 fmol, and calibration curves were linear in the range measured (R2 = 0.99). 1-HOP was determined by high performance liquid chromatography with fluorescence detection, using [D9]1-HOP as the internal standard.20,21
The analysis of total NNN was performed as described previously.22–24 The intraday precision measures of the assays for free NNN and NNN-N-gluc were 7.7% relative standard deviation (RSD) and 8.4% RSD, respectively. The corresponding interday precision measures were 10.6% and 12.8% RSD.
Urinary total cotinine (cotinine plus cotinine glucuronide) levels were assayed by gas chromatography-mass spectrometry as described previously.25 Urinary creatinine was assayed in all subjects by Fairview–University Medical Center Diagnostic Laboratories (Minneapolis, MN) with a Kodak Ektachem 500 chemistry analyzer (Kodak, Rochester, NY). Total NNAL, total 1-HOP, total NNN, and total cotinine were divided by creatinine to normalize for urinary dilution. For pairwise associations between NNAL, total 1-HOP, total NNN, total cotinine, and CPD, we used the nonparametric Spearman rank correlation coefficient. Adjustment for multiple comparisons was done via the false discovery rate method.26 Statistical analyses were performed using the R software (R Project for Statistical Computing, Vienna, Austria).
RESULTS
The study group was composed of 84 subjects, all of whom had either a new or recent diagnosis of squamous cell carcinoma of the upper aerodigestive tract at one of the following subsites: oral cavity, oropharynx, larynx, or hypopharynx. All of the enrolled subjects used conventional cigarettes as their tobacco product and were smoking at their usual rate at the time of enrollment. Subjects consisted of 69 males and 15 females; the average age was 59 years (range, 30–77 years). Tumor site was the oral cavity (n = 23), oropharynx (n = 28), larynx (n = 22) or hypopharynx (n = 5), or unknown primary (n = 3). Three subjects had missing data regarding subsite.
Creatinine-corrected levels of carcinogen metabolites were as follows: total NNAL ranged from 0.06 to 7.75 pmol/mg, with mean 1.78 pmol/mg; total NNN ranged from 0.0019 to 2.09 pmol/mg, with a mean 0.14 pmol/mg; 1-HOP ranged from 0.277 to 19.20 pmol/mg, with a mean of 1.96 pmol/mg.
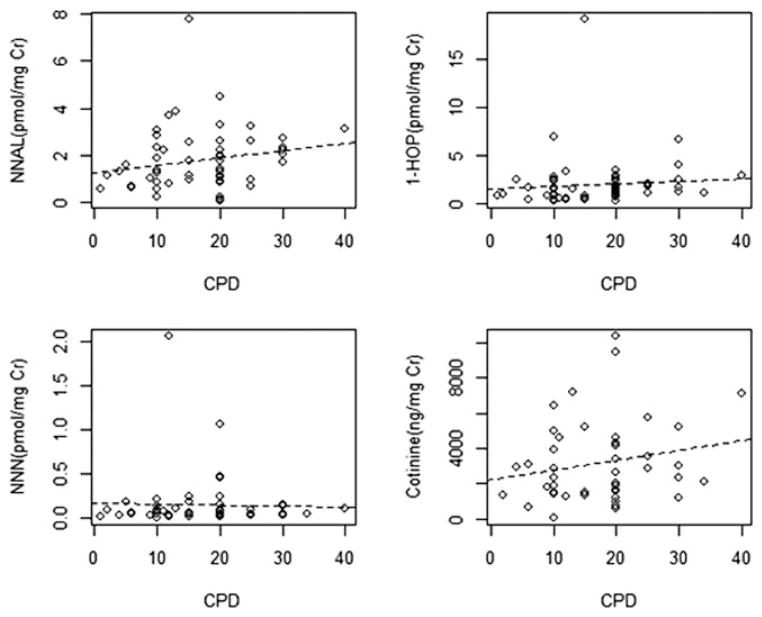
Our first analysis was performed based on self-reported CPD. As shown in Table I, CPD was not significantly correlated with any of the urinary metabolites studied. Correlation coefficients for CPD with total NNN, total NNAL, and 1-HOP were all greater than zero. The scatterplots (Fig. 1A–C) show a modest upward trend for NNAL and 1-HOP, whereas the plot for NNN is nearly flat. CPD did not significantly correlate with urinary cotinine levels.
TABLE I.
Spearman Correlation Coefficients for Cases.
| CPD | Length | NNAL | NNN | 1-HOP | Cotinine | |
|---|---|---|---|---|---|---|
| CPD | 1 | 0.149 (P = .24) | 0.252 (P = .103) | 0.022 (P = .873) | 0.258 (P = .103) | 0.197 (P = .243) |
| Length | 1 | −0.300 (P = .103) | −0.130 (P = .426) | −0.279 (P = .103) | −0.246 (P = .200) | |
| NNAL | 1 | 0.426 (P = .006) | 0.359 (P = .025) | 0.601 (P = .0002) | ||
| NNN | 1 | 0.363 (P = .018) | 0.531 (P = .0005) | |||
| 1-HOP | 1 | 0.564 (P<.001) | ||||
| Cotinine | 1 |
1-HOP = 1-hydroxypyrene; CPD = cigarettes per day; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)−1-butanol; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN = N′-nitrosonornicotine.
Fig. 1.
Scatterplots of CPD with (A) NNAL, (B) 1-HOP, (C) NNN, and (D) cotinine. The dashed line in each of the panels represents the least-square fit. 1-HOP = 1-hydroxypyrene; CPD = cigarettes per day; Cr = creatinine; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)−1-butanone; NNN = N′-nitrosonornicotine.
Next, a separate analysis was performed based on urinary total cotinine levels (Table I). This revealed significant positive correlation coefficients between urinary cotinine and total NNN, total NNAL. and 1-HOP (Table I). The corresponding scatterplots (Fig. 2A–C) show an upward linear relationship between urinary cotinine and each carcinogen exposure marker. Total NNN, total NNAL, and 1-HOP showed statistically significant correlation with each other. Analysis of duration of smoking did not reveal any statistically significant association with cotinine, total NNN, total NNAL, or 1-HOP (data not shown).
Fig. 2.
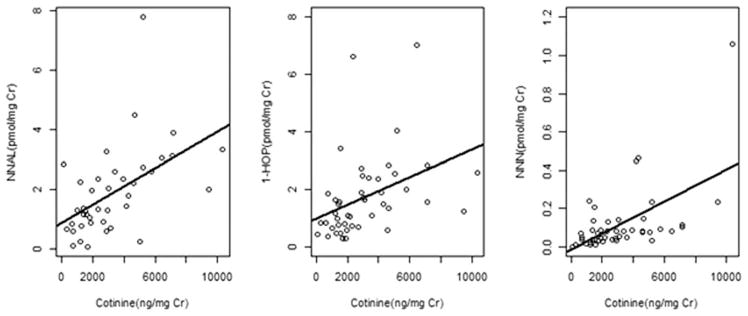
Scatterplots of urinary cotinine with (A) NNAL, (B) 1-HOP, and (C) NNN. The solid line in each of the panels represents the least-square fit. 1-HOP = 1-hydroxypyrene; Cr = creatinine; NNAL = 4-(methylnitrosamino) −1-(3-pyridyl)−1-butanol; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN = N′-nitrosonornicotine.
DISCUSSION
Relationship of CPD to Carcinogen Metabolites
This report demonstrates that self-reported tobacco use correlates poorly with actual levels of carcinogen markers among cigarette smokers with squamous cell carcinoma of the upper aerodigestive tract. The examination of tobacco carcinogen metabolites provides an opportunity for a more objective analysis of actual tobacco exposure and carcinogen dose. This approach has only recently been applied to patients with head and neck cancer.17 As a result, this study is the first of its kind to focus on smokers with HNSCC, and there are no other studies in the literature with which to compare our results. This line of research has, however, been pursued extensively in cancer-free tobacco users.11,13 Thus, our current work provides an important opportunity to compare smokers with HNSCC to prior studies investigating smokers without cancer using existing data in the literature regarding the latter.
Joseph et al. examined tobacco carcinogen exposure markers in a cohort of cancer-free smokers that included both heavy and light smokers.3 Their findings both compare and contrast to our current study. When considering the relationship between CPD and tobacco carcinogen metabolites, the plot of total NNAL versus CPD (Fig. 1A) is very similar in both studies with regard to both absolute value and slope. Xia et al. utilized the National Health and Nutrition Examination Survey to study NNAL in smokers.27 Their study found positive correlation between CPD and NNAL at lower smoking rates, but a nonsignificant association between these two variables when examined through regression. Thus, the data from Joseph et al. and Xia et al. in cancer-free smokers differ somewhat from our findings in smokers with HNSCC.
The relationship between CPD and 1-HOP in our study demonstrated a modest upward slope with nonsignificant correlation, whereas it is relatively flat in cancer-free smokers (Joseph et al.). Although the number of subjects in our study of cancer patients is naturally smaller, the finding of increased 1-HOP values and slope (when compared to CPD) in our data point to a potential difference identified between smokers with carcinoma and those without carcinoma. A similar phenomenon was identified in a previous report that also showed 1-HOP is elevated in HNSCC compared to noncancer patients.17 In addition, our analysis of smokers with cancer reveals that Spearman coefficients between CPD and carcinogen metabolites are smaller than that seen in cancer-free smokers studied by Joseph et al. Further examination of the literature reveals that self-reported CPD has been shown to roughly correlate with cotinine, NNAL, and PAH among white, but not African American, smokers.28 We feel this suggests a complex relationship between self-reported CPD and tobacco carcinogen metabolites that requires consideration of multiple variables and appears to exist in both smokers with and without carcinoma.
Although data regarding tar yield of the cigarettes smoked in our study subject were not collected, prior work has shown that variations in tar yield can result in carcinogen exposure that is highly variable. Mendes et al. noted variability in NNAL per cigarette to be 64% to 92%.29 Thus, depending on the type of cigarette smoked, the amount of NNAL could vary dramatically and could partially explain the lack of correlation with CPD. Furthermore, genetics may certainly play a role in interindividual differences in tobacco carcinogen exposure. One investigation of genes encoding the subunits of a nicotinic acetylcholine receptor (CHRNA3 and CHRNA5) found that smokers who carry two specific genetic variants extract a greater amount of nicotine per cigarette and are exposed to a higher internal dose of an NNK per cigarette compared to noncarriers.30 Future examination of this locus, and others, in our cohort is felt to be worthwhile and potentially illuminating.
Examination of Cotinine and Relationships of NNAL, NNN, and 1-HOP
A second analysis in this study focused on cotinine values as a means to investigate tobacco exposure and carcinogen levels. Nicotine is not an overt carcinogen but has been shown to act as a promoter of proliferation and survival in non–small cell lung cancer cells.31 Humans metabolize nicotine to cotinine, 3′-hydroxycotinine, and their glucuronides, and these metabolites are excreted in urine.7 Cotinine is the most specific and widely used marker of nicotine exposure.32 As a result, cotinine values are commonly used as a means to bypass any inaccuracies associated with CPD and gain a more precise assessment of actual tobacco product consumption. Cotinine values may then be compared to carcinogen levels (i.e., nitrosamines) to obtain unbiased correlations between tobacco dose and carcinogen exposure. Among the patients in this study, this analysis reveals a modest increase in cotinine with increasing CPD. This demonstrates that, in smokers with head and neck cancer, dramatic increases in self-reported cigarette use were accompanied by only negligible increases in urinary cotinine. This is further evidence that CPD does not provide carcinogen dose information in this population.
When considering total NNN, total NNAL, and 1-HOP, statistically significant positive correlation was seen between cotinine and these metabolites. These data suggest that cotinine, not CPD, is the best marker of actual carcinogen dose in smokers with HNSCC. In addition, each of the carcinogen metabolites were significantly correlated with each other, suggesting that exposure to each occurs proportionally. Given that NNN has been associated with risk of esophageal cancer11 whereas NNAL and 1-HOP have been associated with risk of lung cancer,13,16 our future work in this area will endeavor to find the relative importance of each carcinogen in the genesis of HNSCC.
This finding has implications for the potential future use of this line of research in screening for risk of HNSCC in smokers. Instead of using CPD to gauge a smoker’s eventual risk of carcinoma, the level of urinary cotinine (and perhaps levels of the carcinogen metabolites [i.e., NNN, NNAL, 1-HOP] themselves) may ultimately prove to be more useful in identifying those smokers at highest risk, who would benefit from aggressive cessation techniques and screening. Compelling data from Yuan et al. have shown that cotinine and NNAL are independently related to lung cancer risk, and those with high levels of both have a 8.5-times increased risk of developing lung cancer.13 Yuan et al.’s study also showed that r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene, which like 1-HOP is a PAH, is higher in smokers that ultimately go on to develop lung cancer. Furthermore, NNN levels have been shown to be predictive for those most at risk to later develop esophageal carcinoma.11
Clinical Implications
The lack of correlation between CPD and urinary carcinogen levels within a cohort of cancer patients could be the result of inaccurate reporting, variability in puffs per cigarette, and/or depth of inhalation or a combination of these variables. With regard to reporting, it is well accepted that newly diagnosed cancer patients often feel shame and embarrassment regarding their past tobacco use. As they struggle to adjust to the fact that their lifestyle choices may have resulted in the diagnosis of cancer, guilt may lead them to be less than forthright in describing the extent of their prior tobacco use to their treatment team.33,34 Therefore, it may be that we did not see significant levels of correlation between CPD and carcinogen metabolites because the self-reported data are subject to greater risk for inaccuracies. Our data demonstrate that cotinine shows strong correlation with the biomarkers of carcinogen exposure. Although a laboratory test for cotinine is certainly more expensive than obtaining CPD, cotinine level could be used when assessment of true carcinogen exposure is critical.
Study Limitations
The data in this study were collected in a cross-sectional manner at the time of cancer diagnosis and, therefore, are subject to some limitations. First, the subjects analyzed had varying degrees of medical problems such as heart disease and/or diabetes. We do not currently have a good understanding as to whether these medical conditions can impact the pharmacokinetics of the tobacco carcinogen exposure markers studied here. In addition, although this is the largest series of smokers with HNSCC studied for their tobacco carcinogen metabolites thus far, a larger series would help to solidify our findings. Last, racial differences in tobacco carcinogen processing do exist.28 Although our population is overwhelmingly white, the data may have been somewhat influenced by the presence of a small number of black subjects in this study.
CONCLUSION
The data presented here demonstrate poor correlation between self-reported tobacco use and tobacco carcinogen exposure among HNSCC patients. In contrast, urinary cotinine was significantly associated with carcinogen levels in HNSCC patients. Thus, attempts to assess the risk to patients based solely on cigarettes per day without measuring cotinine or actual carcinogen levels may underestimate the ultimate risk for cancer. We plan to continue this line of investigation to learn more about the interface of risk, exposure, and carcinogenesis in tobacco-induced head and neck cancer.
Acknowledgments
This project was supported by grant number K23 DE023572 (PI Khariwala) from the National Institute of Dental and Craniofacial Research and National Institutes of Health.
Footnotes
This article was approved as a thesis submission for the Triological Society (#2015-20).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 38. Lyon, France: International Agency for Research on Cancer; 1986. Tobacco Smoking. [Google Scholar]
- 2.Tunstall-Pedoe H, Woodward M, Brown CA. The drinking, passive smoking, smoking deception and serum cotinine in the Scottish Heart Health Study. J Clin Epidemiol. 1991;44:1411–1414. doi: 10.1016/0895-4356(91)90102-f. [DOI] [PubMed] [Google Scholar]
- 3.Joseph AM, Hecht SS, Murphy SE, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14:2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 4.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benowitz NL, Kuyt F, Jacob P, III, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL. The use of biologic fluid samples in assessing tobacco smoke consumption. NIDA Res Monogr. 1983;48:6–26. [PubMed] [Google Scholar]
- 7.Benowitz NL, Jacob P., III Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53:316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- 8.Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78:699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hald J, Overgaard J, Grau C. Evaluation of objective measures of smoking status—a prospective clinical study in a group of head and neck cancer patients treated with radiotherapy. Acta Oncologica. 2003;42:154–159. doi: 10.1080/02841860310005020. [DOI] [PubMed] [Google Scholar]
- 10.Marin VP, Pytynia KB, Langstein HN, Dahlstrom KR, Wei Q, Sturgis EM. Serum cotinine concentration and wound complications in head and neck reconstruction. Plast Reconstr Surg. 2008;121:451–457. doi: 10.1097/01.prs.0000297833.53794.27. [DOI] [PubMed] [Google Scholar]
- 11.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church TR, Anderson KE, Caporaso NE, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan JM, Gao YT, Murphy SE, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan JM, Koh WP, Murphy SE, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2001;45:3–13. [PubMed] [Google Scholar]
- 16.Yuan JM, Butler LM, Gao YT, et al. Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis. 2014;35:339–345. doi: 10.1093/carcin/bgt352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khariwala SS, Carmella SG, Stepanov I, et al. Elevated levels of 1-hydroxypyrene and N′-nitrosonornicotine in smokers with head and neck cancer: a matched control study. Head Neck. 2013;35:1096–1100. doi: 10.1002/hed.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khariwala SS, Hatsukami D, Hecht SS. Tobacco carcinogen metabolites and DNA adducts as biomarkers in Head and Neck cancer: potential screening tools and prognostic indicators. Head Neck. 2012;34:441–447. doi: 10.1002/hed.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol. 2013;26:1209–1217. doi: 10.1021/tx400121n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmella SG, Le K-A, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol Biomarkers Prev. 2004;13:1261–1264. [PubMed] [Google Scholar]
- 21.Hochalter JB, Zhong Y, Han S, Carmella SG, Hecht SS. Quantitation of a minor enantiomer of phenanthrene tetraol in human urine: correlations with levels of overall phenanthrene tetraol, benzo[a]pyrene tetraol, and 1-hydroxypyrene. Chem Res Toxicol. 2011;24:262–268. doi: 10.1021/tx100391z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stepanov I, Carmella SG, Briggs A, et al. Presence of the carcinogen N′-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69:8236–8240. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porubin D, Hecht SS, Li Z-z, Gonta M, Stepanov I. Endogenous formation of N′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J Agric Food Chem. 2007;55:7199–7204. doi: 10.1021/jf0712191. [DOI] [PubMed] [Google Scholar]
- 24.Stepanov I, Hecht SS. Detection and quantitation of N′-nitrosonornicotine in human toenails by liquid chromatography-electrospray ionization-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17:945–948. doi: 10.1158/1055-9965.EPI-07-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht SS, Carmella SG, Chen M, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;1:289–300. [Google Scholar]
- 27.Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)−1-(3-pyridyl)−1-butanol (NNAL) in smokers in the United States: NHANES 2007–2008. Biomarkers. 2011;16:112–119. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13:772–783. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes P, Liang Q, Frost-Pineda K, Munjal S, Walk RA, Roethig HJ. The relationship between smoking machine derived tar yields and biomarkers of exposure in adult cigarette smokers in the US. Regul Toxicol Pharmacol. 2009;55:17–27. doi: 10.1016/j.yrtph.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Le Marchand L, Derby KS, Murphy SE, et al. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsurutani J, Castillo S, Brognard J, et al. Tobacco components stimulate Akt-dependent proliferation and NFkB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–1995. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 32.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 33.Bronheim H, Strain JJ, Biller HF. Psychiatric aspects of head and neck surgery. Part I: New surgical techniques and psychiatric consequences. Gen Hosp Psychiatry. 1991;13:165–176. doi: 10.1016/0163-8343(91)90139-n. [DOI] [PubMed] [Google Scholar]
- 34.Espie CA, Freedlander E, Campsie LM, Soutar DS, Robertson AG. Psychological distress at follow-up after major surgery for intra-oral cancer. J Psychosom Res. 1989;33:441–448. doi: 10.1016/0022-3999(89)90005-6. [DOI] [PubMed] [Google Scholar]