Abstract
Background
Despite the emerging association between Heart Failure (HF) and inflammation, the role of T cells, major players in chronic inflammation, has only recently begun to be explored. Whether T cell recruitment to the left ventricle (LV) participates in the development of HF requires further investigation to identify novel mechanisms that may serve for the design of alternative therapeutic interventions.
Methods and Results
Real time videomicroscopy of T cells from non- ischemic HF patients or from mice with HF induced by transverse aortic constriction (TAC) revealed enhanced adhesion to activated vascular endothelial cells under flow conditions in vitro compared with T cells from healthy subjects or sham mice. T cells in the mediastinal lymph nodes and the intramyocardial endothelium were both activated in response to TAC and the kinetics of LV T cell infiltration was directly associated with the development of systolic dysfunction. In response to TAC, T cell deficient mice (TCRα−/−) had preserved LV systolic and diastolic function, reduced LV fibrosis, hypertrophy and inflammation, and improved survival compared to WT mice. Furthermore T cell depletion in WT mice after TAC prevented HF.
Conclusions
T cells are major contributors to non-ischemic HF. Their activation combined with the activation of the LV endothelium results in LV T cell infiltration negatively contributing to HF progression through mechanisms involving cytokine release and induction of cardiac fibrosis and hypertrophy. Reduction of T cell infiltration is thus identified as a novel translational target in HF.
Keywords: heart failure, T cells, cell adhesion, left ventricle, inflammation, remodeling
Heart failure (HF) is still a leading cause of morbidity and mortality, affecting more than 24 million people worldwide.1,2 It is a very complex syndrome involving the interplay of myocardial factors, inflammation, renal dysfunction, and neurohormonal activation.3, 4 Based on the observations that patients with HF have increased circulating pro-inflammatory cytokines correlating with disease stage and mortality,5-9 clinical trials were launched targeting inflammatory mediators such TNFα with infliximab and etanercept. These trials were deemed unsuccessful and were terminated prematurely due to lack of both improved survival and hospitalization rate.8, 9The failure of these particular anti-inflammatory agents despite the known activation of the immune system in HF, underscores the importance of better understanding the specific inflammatory mechanisms contributing to the development of left ventricular (LV) remodeling and HF progression.
To date, most of the immune mechanisms studied in HF have focused on the activation of the immune system in response to classical immune triggers such as infectious or autoimmune myocarditis that induce an adaptive immune response mediated by CD4+ T lymphocytes, or in response to ischemic heart injury provoked by myocardial infarction (MI).10 In humans, recent studies demonstrate a positive correlation between inflammatory cytokines potentially produced by T cells and LV dysfunction in patients with chronic ischemic HF or with idiopathic dilated cardiomyopathy.11 Whether this takes place systemically or as a result of T cell infiltration in the heart is still unclear, but, at least in the settings of infection, heart transplantation, myocardial infarction causing heart cell death and when self-tolerance to heart antigens is disrupted in autoimmunity, T cells can infiltrate the heart, which is normally devoid of T cells, and negatively affect cardiac function.10, 12-14 However, the role T cells play in the development of LV hypertrophy, remodeling, and dysfunction in response to more common pathologies, such as occurs with HF, has only recently begun to be investigated, with recent evidence that CD4+ T cells promote cardiac remodeling in HF in mice.15 These recent findings raise additional questions including the mechanisms involved in attracting T cells to the heart, the role they play once in the heart, as well as whether T cells can be a novel translational target for potential therapeutic intervention. In addition, the role of T cells in non-ischemic HF in humans has not been explored. Therefore, better insights into the kinetics of T cell recruitment during the progression of HF, the mechanisms mediating T cell recruitment and the impact they have on cardiac remodeling during HF is warranted. While systemic inflammation and the T cell mediated immune response have recently been associated with the prognosis of HF,16, 17 whether T cells respond to pressure overload (PO) and develop high affinity for the vascular endothelium as a mechanism that facilitates their infiltration in the heart remains unknown.
In our current investigation, we tested the hypothesis that T cell mediated immune responses and their recruitment into the heart influence cardiac remodeling and contribute to the pathogenesis of HF. Our study demonstrates that human and mouse T cell recruitment into the LV negatively contributes to the pathophysiology of HF. It also highlights the potential of depleting T cells from the circulation over the course of HF to ameliorate cardiac fibrosis associated with HF.
Methods
Human Subjects
Blood Samples: from non-ischemic human subjects with NYHA Class III-IV heart failure referred for cardiac catheterization as part of their evaluation for advanced heart failure therapy (n=5); From healthy, non-heart failure volunteers (males 30-45 years of age). Viable LV free wall tissue control (n=3) from the National Disease Research Interchange (NDRI), and from end stage heart failure subjects after LV assisted device (LVAD) support (n=3). The studies were approved by Tufts University Institutional Review Board and all subjects gave informed consent.
Mice
Bred and maintained under pathogen-free conditions. All protocols were approved by the Tufts Medical Center Institutional Animal Care and Use Committee. Mice were sacrificed at 10-14 wk of age for tissue collection.
Mouse model of Transverse Aortic Constriction (TAC)
Pressure overload induced HF was produced by constricting the transverse aorta as previously described.18, 19
In vivo transthoracic Echocardiography
Assessed in conscious mice as previously described.19. The analysis was performed blinded. M Mode and 2-dimensional images were obtained from the short-axis view, as described previously.18
In vivo Hemodynamics
LV function was assessed by pressure volume (PV) transducing catheter as previously described.19 Absolute volume was calibrated by the saline injection parallel conductance method as described19 and data were assessed at steady state. Data were digitized and analyzed with custom software (EMKA version 2.1.10).
Flow Cytometry was performed to analyze the immune profile present in heart failure. The data were acquired on a FACSCanto (Becton Dickinson) and analyzed using FlowJo software.
Histological analysis
Heart samples were excised and LV separated from the Right Ventricle (RV). 1/3 of LV was immediately embedded in OCT and 1/3 fixed in 10% formalin, embedded in paraffin and cut into 5μm sections. Haematoxylin and eosin or picrosirius red staining was performed as described.20 Cardiomyocyte cross sectional area was quantified by tracing the outline of 5-12 myocytes in each section.21
In vivo T cell depletion
WT C57BL/6 mice were treated i.p. with 300μg/ml of monoclonal αCD3 antibody (BioXcell, West Lebanon, NH) or isotype-matched control mAb starting at 48 hours post surgery and then every 3rd day for 4 weeks.
Real-time Quantitative Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from mouse heart LV tissues directly using Trizol (Invitrogen). RNA was then reverse-transcribed using the ThermoScript RT-PCR system according the manufacturer’s instructions (Invitrogen), and amplified by real-time PCR with SYBR green PCR mix (Applied Biosystems). Samples were quantified in triplicates using 40 cycles performed at 94°C for 30 sec, 60°C for 45 sec, 72°C for 45 sec using an ABI Prism® 7900 Sequence Detection System.
Endothelial cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured as described.22 Confluent HUVEC monolayers on fibronectin-coated glass coverslips were stimulated with TNF-α (25ng/ml) for 4 hours before the adhesion assays. Mouse heart endothelial cells (MHEC) were isolated from hearts of newborn C57/BL6 (WT) animals 7-9 days old as described23, and also plated on fibronectin-coated glass coverslips and stimulated with TNF-α 4h before the T cell adhesion assay.
Videomicroscopy image acquisition and analysis
T cell interactions with MHEC or HUVECs were observed by videomicroscopy under defined laminar flow conditions in a parallel plate apparatus.24, 25 T cell interactions with confluent TNF-α activated MHECs or HUVECs grown on glass coverslips observed at 20X magnification. Data was recorded and analyzed using the Nikon Elements Software (NES). Adhesion of T cells on activated endothelial cells was quantified in 6 fields of view per condition.
Statistics
Data are expressed as the mean ± SD unless otherwise indicated. Statistical analyses between two groups were done by student t test and Mann Whitney non-parametric test to adjust for non-equal Gaussian distributions among groups. Intergroup comparisons were done by 2-way ANOVA and Bonferroni post-test to adjust for the multiple comparisons. Kaplan Meier analysis with log-rank testing was used for survival analysis. Differences were considered statistically significant at p< 0.05 and are indicated with an (*). Graph Pad Prism software was used in all analysis.
Results
T cells from humans and mice with heart failure have high affinity for the activated vascular endothelium and are recruited into the heart’s left ventricle
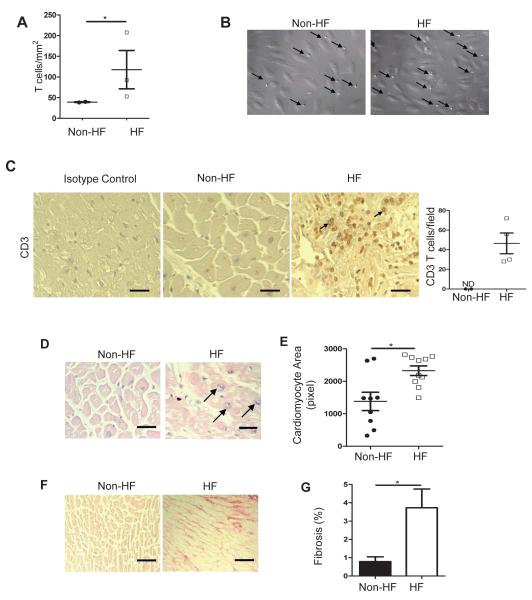
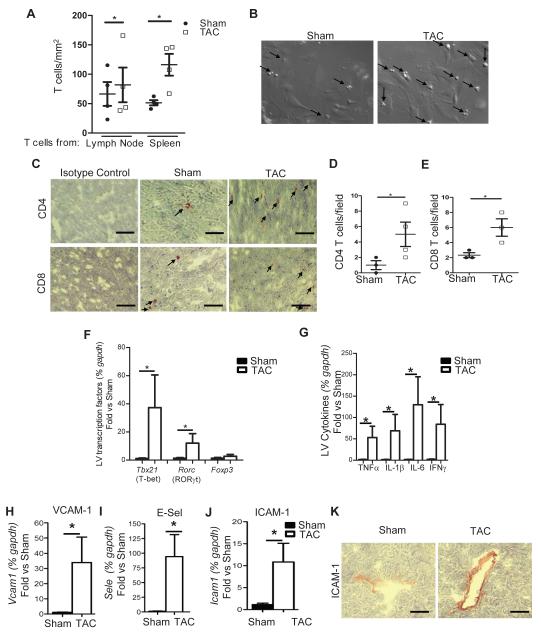
We used a real time videomicroscopy approach that mimics physiological shear flow conditions in small capillaries and venules to study the ability of CD3+ T cells from patients with Class III-IV non-ischemic HF to interact with activated vascular endothelial cells. T cells from HF patients adhered to activated endothelial cells in significantly higher numbers than T cells from non-HF volunteers (Figure 1A and 1B). CD3+ T cells also infiltrated the LV of patients with non-ischemic end stage HF (Figure 1C), and this was associated with significant cardiac hypertrophy and fibrosis, and the presence of vesiculated pyramids as indicators of pathologically hypertrophied myocytes (Figure 1D-1G). Consistent with the literature, systemic T cells were found to be elevated in patients with HF and were activated based on their ability to differentiate into various T cell subsets compared to control patients (Supplemental Figure 1A-1D). To study this phenomenon further, we evaluated whether T cells responded to PO induced HF using transverse aortic constriction (TAC) induced HF in mice. Consistent with our human data, we observed that CD4+ T cells isolated from lymph nodes and spleen 4 weeks after TAC adhered to activated mouse heart endothelial cells (MHEC) in significantly higher numbers than those from sham operated controls (Figure 2A and 2B). TAC induced LV recruitment of both CD4+ and CD8+ T cells (Figure 2C-2E), whereas Tbet and RORγT, the signature transcription factors of T helper (Th) type 1 and Th type 17 cells, respectively, were significantly upregulated in the LV, in contrast to Foxp3, the signature transcription factor for Treg cells which remained unchanged between Sham and TAC mice (Figure 2F). Furthermore, the cytokines TNFα, IL1β and IL6, and the Th1 signature cytokine IFNγ were significantly upregulated in TAC vs Sham mice (Figure 2G). In addition, TAC resulted in upregulation of RNA of the endothelial cell adhesion molecules, VCAM-1, E-selectin and ICAM-1 (Figure 2H-2J), and significantly enhanced ICAM-1 protein expression in the endothelial cells of the LV intramyocardial vessels (Figure 2K). Taken together these findings demonstrate that PO in mice and humans activates T cells which become pro-adhesive and more prone to interact with the activated vascular endothelium in vitro. Moreover, in vivo, TAC promotes upregulation of ICAM-1 and other endothelial cell adhesion molecules that can lead to T cell recruitment, and induces pro-inflammatory cytokine release that can further promote T cell survival and subsequent endothelial cell activation.
Figure 1. T cell activation, adhesion to activated vascular endothelial cells and infiltration into the LV in humans.
(A, B) CD3+ T cells isolated from healthy volunteers or from patients with NYHA-III-IV HF were drawn in a flow chamber across TNFα (25ng/ml) activated HUVEC at a shear stress of 0.8 dynes/cm2. Data represents mean ± SD of 2 independent experiments using 2 different control and 3 different heart failure samples. Representative images from the videos are shown and arrows point at T cells arrested on the vascular endothelium (B). (C) Non-heart failure (Non-HF) and end stage HF LV tissues were obtained from NDRI and LVAD placements, respectively, and sections were stained for CD3 or isotype control. The arrows point at two representative infiltrated CD3+ T cells and the quantitative analysis is shown (right). (D, E) Representative H&E staining (D) and cardiac myocyte size quantification (E) of LV sections from non-HF and end stage HF. Arrows point to vesiculated pyramids, standard histological markers of hypertrophic myocytes. (F, G) Representative photomicrographs (F) and quantification (G) of myocardial fibrosis evaluated by picrosirius red staining of LV sections. Data represented as mean ±SEM; *p<0.05. Scale bars, 500μm (C, D and F).
Figure 2. TAC induces T cell adhesion to mouse heart endothelial cells in vitro and endothelial and T cell activation and recruitment to the heart in vivo.
(A, B) CD4+ T cells from lymph nodes and spleen isolated from 4 weeks TAC mice or sham surgery were drawn across TNFα (100ng/ml) activated mouse heart endothelial cells at a shear stress of 0.8 dynes/cm2. Data represents mean ± SD of 4 independent adhesion experiments, each with a pool of T cells from n=3 sham and n=3-5 TAC, and 4 independent MHEC preparations. Data represents the average of 3-6 different fields of view for each condition in the different experiments (B). Representative frames from videos with arrows pointing at T cells arrested on MHEC. (C-E) LV tissue sections from 4 week sham or TAC mice were stained for CD4+ and CD8+ T cells. (C) Representative immunohistochemistry and (D, E) quantitative analysis of CD4 and CD8 staining. (F-J) RNA expression for T cell subset transcription factors (F), inflammatory cytokines (G) and adhesion molecules (H-J) in the LV of 4 week TAC WT mice. n=4-6 shams and n=6-10 TAC per group. (K) Representative immunohistochemistry staining of ICAM-1 in the LV of 4 weeks sham and TAC WT mice. Data represented as mean ±SD; *p<0.05. Scale bars, 500μm (C, K).
T cell recruitment into the LV is associated with the progression of HF in response to TAC
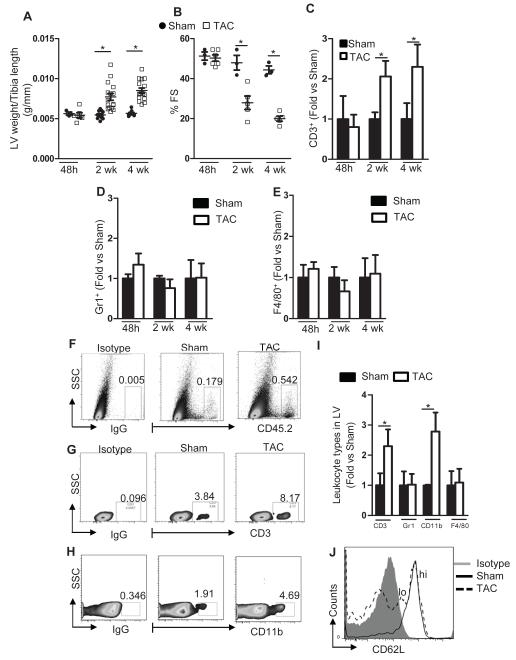
We next investigated the time course of LV T cell infiltration after TAC using quantitative flow cytometry to determine whether it was directly associated with the progression of HF. Forty eight hours after TAC, we did not detect significant T cell infiltration in the LV, and at this time point, LV weight and LV systolic function remained similar between TAC and sham controls. Infiltration of T cells to the LV occurred during the development of cardiac remodeling and systolic dysfunction 2 and 4 weeks after TAC (Figure 3A-3C), peaking at 4 weeks. Further analysis revealed that Gr1+ neutrophils and F4/80+ macrophages did not follow the same dynamics as T cells (Figure 3D, 3E and 3I). 4 weeks after TAC there was notable increase in infiltrated CD45.2+ leukocytes (Figure 3F), and these included T cells and CD11b+ myeloid cells (Figure 3G, 3H and 3I). From our immunohistochemistry studies these T cells included CD4+ and CD8+, possibly IFNγ producers given the increased LV levels of IFNγ in response to TAC observed in Figure 2. Moreover, we isolated the mediastinal lymph nodes of both Sham and TAC mice and found that it not only was significantly enlarged in TAC mice as compared to Sham (data not shown), but it indeed had more activated T cells that had therefore lost the expression of L-selectin (Figure 3J). Importantly, we were able to determine the levels of circulating T cells and Th cell subsets and found that these were increased in response to TAC (Supplemental Figure 2), similarly to what we observed in patients with HF (Supplemental Figure 1). Taken together, these data support the idea that T cells respond to cardiac specific damage in the mediastinal lymph nodes, become activated and are recruited to the LV. This recruitment correlates with the development of pathological cardiac remodeling and worsening of systolic function.
Figure 3. Time course of T cell recruitment into the LV as cardiac remodeling and systolic function develop in PO induced heart failure.
WT mice were subjected to sham (n=3-11) or TAC (n= 5-16) surgery and sacrificed at the indicated times. (A) LV weight normalized to tibia length. (B) Systolic function measured by echocardiography. (C-E) Quantification of LV T cell (C), neutrophil (D) and F4/80+ macrophage (E) infiltration by FACS during the time course of TAC. (F-H) Representative FACS dot plot for total leukocytes (F) CD3 T cells (G) and CD11b+ myeloid cells (H) at 4 weeks post TAC. Numbers represent the percent positive in the indicated gate. (I) Leukocyte expression of CD3, Gr1, CD11b and F4/80 positive cells in the LV 4 weeks after sham or TAC mice. (J) Representative FACS histogram of CD62L expression in the mediastinal lymph nodes in sham and TAC mice 4 weeks post TAC surgery. Data represent mean ± SD; *p<0.05.
T cells are major contributors to the pathogenesis of HF
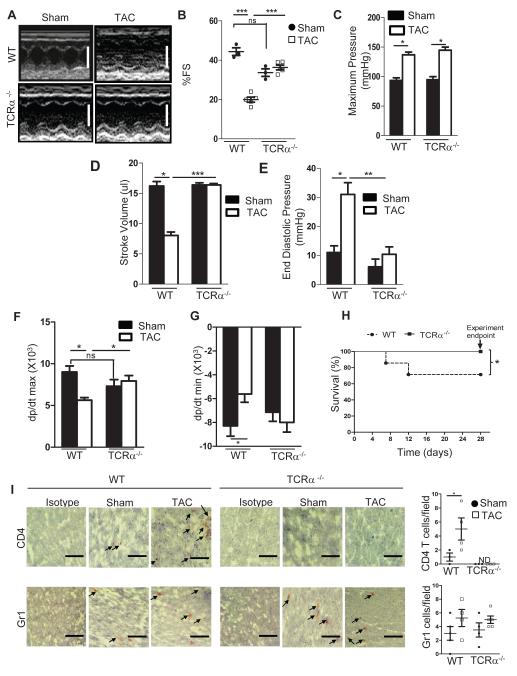
Based on our findings that T cell recruitment into the LV correlates with adverse remodeling, we next assessed the functional necessity of T cells to promote HF. We specifically tested the response to LV PO in mice deficient in T cells harboring a genetic deletion of the alpha chain of the T cell receptor (TCRα−/−). Echocardiography analysis 4 weeks post TAC showed that in contrast to WT mice, TCRα−/− mice had normal LV dimensions (Figure 4A), preserved systolic function, measured by fractional shortening (FS) (Figure 4B), and preserved LV end diastolic diameter (LVEDD) (Table). This was consistent with invasive hemodynamic measurements revealing that despite similar aortic pressure gradients generated in response to 4 weeks TAC in WT and TCRα−/− mice (Figure 4C), the stroke volume, end diastolic pressure, and systolic and diastolic functions were all preserved in TCRα−/− mice (Figure 4D-4G and Table). Intriguingly, of all the mice that underwent TAC surgery, thirty percent of the WT TAC mice died by 4 weeks, whereas no deaths occurred in TCRα−/− mice (Figure 4H). As expected, the LV of TCRα−/− mice was free of recruited T cells. Moreover, Gr1+ neutrophils were found recruited in the LV in similar numbers in sham and TAC mice in both WT and TCRα−/− mice (Figure 4I). In addition, TAC did not induce significant upregulation of endothelial cell adhesion molecules, including ICAM-1, or upregulation of pro-inflammatory cytokines in TCRα−/− mice (Supplemental Figure 3). RORγT and Tbet were also significantly decreased as compared to WT, suggesting that most of the Tbet expression observed in WT mice (Figure 2 and Supplemental Figure 3I, 3J) may be attributed to Th1 cells. Taken together, our results support a role for T cell recruitment to the LV being critical for the observed cardiac dysfunction in response to TAC. The absence of T cells results in a suppressed pro-inflammatory state within the LV, and as such, the deleterious actions of cytokines TNFα and IFNγ on cardiac resident cells and endothelial cell activation are prevented. Thus, subsequent immune cell recruitment is avoided and LV function is protected from any negative impact mediated by T cells. This could potentially explain why TCRα−/− do not develop HF.
Figure 4. Preserved cardiac function and survival to PO induced heart failure in TCRα−/− mice.
(A, B) Representative echocardiograms, white scale bars, 4mm (A) and quantification of fractional shortening (% FS) (B) 4 weeks after sham or TAC surgery in WT and TCRα−/− mice (n=3 Sham, and n=5 TAC). (C-G) Invasive hemodynamic measurements in WT and TCRα−/− mice measured 4 weeks after sham and TAC surgery (n=3 sham, and n=7 TAC). (H) Kaplan-Meier survival curves in WT (n=19) and TCRα−/− (n=10) mice after TAC. (I) Representative immunohistochemical staining of 4 week TAC LV sections for T cells (CD4+) and neutrophils (Gr1+). Quantification (right) of positive cells/field for n=3-5 mice. Data represent mean ±SD; *p<0.05; **p<0.005; ***p<0.0005, ns, not significant Scale bars, 500μm (I).
Table.
Characterization of Heart Failure in Wild-type and TCRα−/− mice
| Parameters | Sham | TAC (4 weeks) |
|---|---|---|
| Total Body Weight, g/Tibia length, mm | ||
| WT (n=Sham 11, TAC 16) |
1.457 ± 0.022 | 1.437 ± 0.021 |
| TCRα−/− (n=Sham 5, TAC 8) |
1.455 ± 0.032 | 1.491 ± 0.044 |
| Total Lung Weight, mg/Tibia length, mm | ||
| WT (n=Sham 11, TAC 16) |
0.009 ± 0.000 | 0.01230 ± 0.001* |
| TCRα−/− (n=Sham 5, TAC 8) |
0.009 ± 0.000 | 0.009 ± 0.000† |
| Heart Rate, bpm | ||
| WT (n=Sham 3, TAC 7) |
535± 45 | 553 ± 40* |
| TCRα−/− (n=Sham 3, TAC 7) |
494.7 ± 49.30 | 525.2 ± 42.18 |
| LV end-systolic pressure, mmHg | ||
| WT (n=Sham 3, TAC 7) |
114 ± 12 | 130 ± 18* |
| TCRα−/− (n=Sham 3, TAC 7) |
99.95± 8.79 | 142.3 ± 9.29* |
| LV end-diastolic diameter, mm | ||
| WT (n=Sham 3, TAC 5) |
3.674 ± 0.121 | 4.007 ± 0.115* |
| TCRα−/− (n=Sham 3, TAC 5) |
3.912 ± 0.048 | 3.753 ± 0.277† |
| Posterior Wall Thickness, mm | ||
| WT (n=Sham 3, TAC 5) |
0.9780 ± 0.151 | 1.4 ± 0.3* |
| TCRα−/− (n=Sham 3, TAC 5) |
1.091 ± 0.149 | 0.952 ± 0.133† |
| Maximum Pressure, mmHg | ||
| WT (n=Sham 3, TAC 7) |
93.46± 7.689 | 136.912± 8.003* |
| TCRα−/− (n=Sham 3, TAC 7) |
94.90 ± 8.698 | 144.8 ± 9.063* |
TAC indicates thoracic aortic constriction; WT, wild-type; and LV, Left ventricle
Values are means ± SD,
P<0.05, 4 weeks of TAC versus Sham.
P<0.05, TAC TCRα−/− versus TAC WT.
T cells regulate cardiac hypertrophy and fibrosis in response to pressure overload
To study the T cell dependent mechanisms leading to improved cardiac function and survival in TCRα−/− mice, we initially evaluated cardiac hypertrophy in WT and TCRα−/− mice in response to TAC. As expected, the gross LV weight normalized to tibia length was increased in WT mice in response to 4 weeks TAC. However, the LV weight and size of TCRα−/− mice was similar between sham and TAC mice, and significantly decreased in TCRα−/− mice vs WT mice in response to TAC (Figure 5A and 5B). We further analyzed by H&E the cardiomyocyte cross sectional area (CSA) and found that while cardiac myocytes from WT mice have increased CSA in response to TAC, those from TCRα−/− mice remain similar in area to the cardiac myocytes from TCRα−/− sham controls (Figure 5C and 5D). Several studies have demonstrated that atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are elevated in serum of HF patients; these two molecules are considered serum markers of HF in response to pressure and volume overload, and their increased transcription in LV tissue represents a phenotype of pathologic, rather than physiologic, hypertrophy.25 Therefore we evaluated the levels of the natriuretic peptides by qRT-PCR using LV tissues and revealed that both BNP and ANP are elevated in WT but not in TCRα−/− in response to TAC (Figure 5E and 5F). We also investigated whether TCRα−/− mice developed cardiac fibrosis, which is intimately associated with LV systolic dysfunction and HF pathogenesis. TAC induced an expected increase in LV fibrosis determined by collagen deposition detected by histology in WT mice. However, LV fibrosis was completely absent in TCRα−/− mice (Figure 6A and 6B). Markers of fibrosis such as TGF-β, Collagen type I and alpha smooth muscle actin (αSMA) were also elevated in WT mice in response to TAC, but not in TCRα−/− mice (Figure 6C-6E).Taken together, these data directly associate the absence of T cells with the absence of cardiac hypertrophy and fibrosis and positions T cells as regulators of these processes.
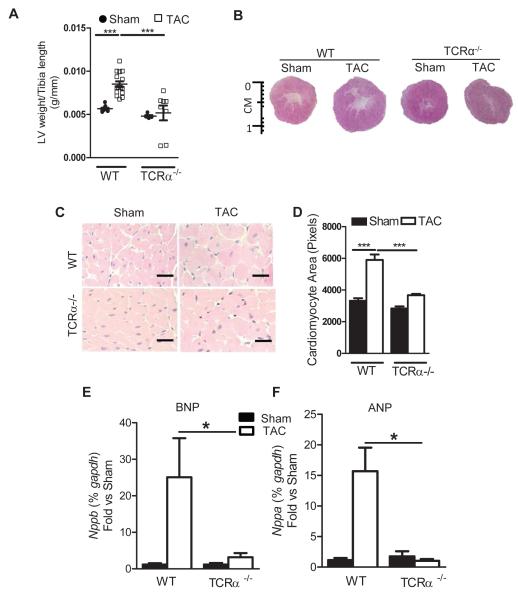
Figure 5. Absence of LV hypertrophy in response to PO induced heart failure in TCRα−/− mice.
(A) LV weight, normalized to tibia length in 4 week sham or TAC mice (WT n=6-11 mice for sham and 11-16 TAC mice; TCRα−/− n=5 sham and n=8 TAC). (B) Representative pictures from LV cross sections of WT and TCRα−/− mice 4 weeks post sham or TAC surgery. (C) Representative H&E staining of LV sections from mice 4 weeks after sham or TAC surgery (D) Quantification of cardiac myocyte area (WT mice n=4 Sham and n=6 TAC; TCRα−/− mice n=4 Sham and n=7 TAC). (E, F) qPCR for hypertrophy markers BNP and ANP. n=5 sham and n=7 TAC mice. Data represented as mean ± SD. *=p<0.01, ***=p<0.0001. Scale bars, 500μm (C).
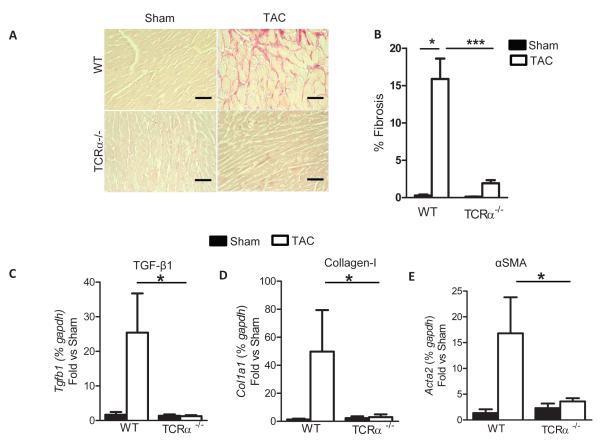
Figure 6. Absence of LV fibrosis in response to PO induced heart failure in TCRα−/− mice.
(A) Representative photomicrographs and (B) quantification of myocardial fibrosis evaluated by picosirius red staining of LV sections from 4 week sham and TAC mice (WT: n=3-5 sham and 6 mice TAC; TCRα−/− n=4 sham and n=8 TAC). (C-E) qPCR for fibrotic markers TGFβ, Collagen I, and SMAα. Data represent mean ±SD; *p<0.05; ***p<0.005. Scale bars, 500μm (A).
T cell depletion after TAC prevents cardiac fibrosis and LV dysfunction
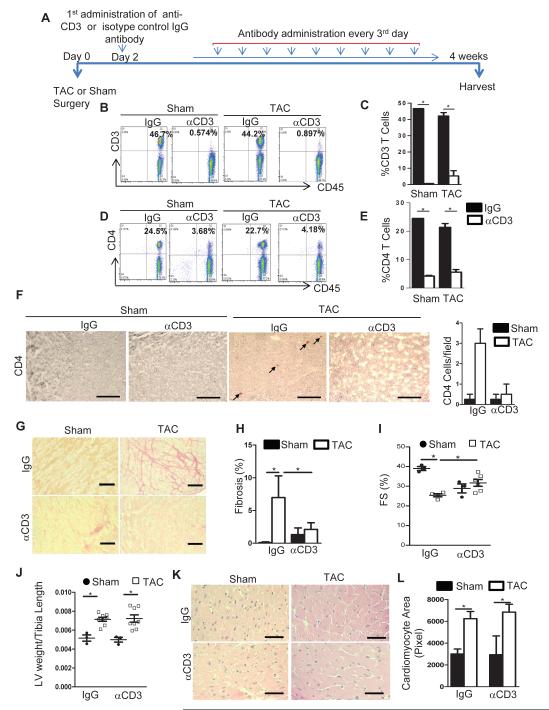
Because of the above observations that genetic depletion of T cells inhibits pathologic remodeling and the HF phenotype, we hypothesized that depletion of T cells in the onset of LV PO prevents pathological cardiac remodeling and may represent a potential novel therapeutic target. We tested whether administration of anti-CD3 antibody could effectively reduce T cells in the setting of LV PO. We therefore depleted WT mice of T cells beginning 48 hours post TAC surgery, when we demonstrate that the mice are responding to TAC but T cells have not yet infiltrated the LV (as shown in Figure 3) (Figure 7A). Approximately 95% of CD3+ T cells were depleted compared to the isotype control IgG treated animals in both sham and TAC mice after 4 weeks (Figure 7B and 7C). Likewise, CD4+ T cells were also depleted using the anti-CD3 antibody compared to the control treated mice (Figure 7D and 7E). While IgG antibody control treated mice had CD4+ T cells infiltrated in the heart in response to TAC, anti-CD3 treatment resulted in absent LV T cell infiltration (Figure 7F). TAC treated mice depleted of CD3+ T cells also demonstrated absence of cardiac fibrosis, compared to isotype control IgG treated mice (Figure 7G and 7H). Furthermore, TAC anti-CD3 treated mice had increased % fractional shortening in contrast to isotype antibody control TAC treated mice (Figure 7I). While the fibrosis and echocardiography data mimicked the phenotype observed for TCRα−/− mice, WT mice depleted of T cells still developed cardiac hypertrophy (Figure 7J-7L). These data demonstrate the feasibility of reducing T cells in the setting of TAC and suggest a robust anti-fibrotic effect of T cell depletion in the LV that results in improved cardiac function despite the increase in LV hypertrophy.
Figure 7. T cell depletion in WT mice post TAC surgery results in preserved cardiac fibrosis and improved systolic function.
(A) Diagram of experimental protocol for T cell depletion with αCD3 Ab in WT sham and TAC mice. IgG Ab was used as control. (B, D) Representative FACS dot plots (C, E) and quantification of CD3+ T cells (B,C) and CD4+ T cells (D, E) in lymph nodes of 4 week WT sham or TAC mice treated as in A. (F) Representative immunohistochemical staining of CD4+ T cells and quantification (right) of LV T cells in the indicated groups 4 week post TAC. (G) Representative photomicrographs and (H) quantification of LV fibrosis evaluated by picrosirius red staining of LV sections in the indicated groups. (I) Echocardiography analysis in Sham and TAC WT mice treated with control IgG Ab or depleted of T cells with αCD3 Ab after sham or TAC surgery. (J) LV weight, normalized to tibia length. (K) Representative H&E staining and (L) quantification of cardiac myocyte area of LV sections among the different groups. Data represent mean ±SD; *=p<0.05. Scale bars, 500μm (n=3-4 IgG and n=4-6 αCD3).
Discussion
Our findings in the present study reveal several aspects identifying T cell recruitment into the LV as a negative contributor to pathological cardiac remodeling in HF. Our observations using human T cells and LV tissue from non-ischemic end-stage HF patients indicate systemic T cell activation, enhanced T cell adhesion to activated vascular endothelial cells under physiological flow conditions in vitro, and T cell LV infiltration, directly correlating with LV myocyte hypertrophy and fibrosis. We obtained similar results using WT mice undergoing PO induced HF, established for the first time the kinetics of LV T cell infiltration, and further characterized T cell activation, LV endothelial cell activation and LV T cell infiltration. Our studies using TCRα−/− mice together with the successful pharmacological depletion of T cells in the setting of LV PO are in support of a role for T cells in PO induced HF as both T cell depletion approaches resulted in significantly attenuated LV fibrosis and improved LV function and HF. Our findings support a model in which LV recruitment of activated T cells in the setting of PO contributes to myocardial dysfunction, remodeling, and ultimately HF. Our findings also suggest that pharmacologic down-regulation of T cell recruitment in the LV may represent a novel strategy for the prevention and potential treatment of HF in humans.
Combination of T cell and intramyocardial endothelial cell activation in response to PO lead to LV T cell recruitment and HF
There is very recent growing awareness that humans with non- ischemic and non- infectious induced HF present chronic systemic inflammation potentially mediated by T cells26, 27. T cells are also found infiltrated in the heart in both humans and experimental mouse models in response to classical triggers of the T cell mediated immune response28. Intriguingly the mechanisms of action of the T cells and whether they play a role systemically, in the heart, or in both is poorly understood to date. We demonstrate for the first time that human T cells from non-ischemic HF are activated and predisposed to interact with activated vascular endothelial cells in vitro, and infiltrate the human LV in vivo. Our studies in WT mice responding to PO support this concept because naïve non-activated T cells do not have the properly modified adhesion ligands to roll and arrest on the heart vascular endothelium, as we and others have previously described23, 29,30. Furthermore, we demonstrate that T cell activation occurs in the mediastinal lymph nodes in response to cardiac antigens, as the lymph node size was significantly increased in response to TAC (data not shown) and a high percent of the T cells present had lost the expression of L-selectin. Moreover, both mice and humans with non ischemic HF had higher numbers of blood circulating T cells and effector T cell subsets, although the nature of the antigens inducing this T cell response remains unknown. The possibility that peptide antigens resulting from cardiac cell death trigger this response as it has been suggested in response to myocardial infarction31 is unlikely, given that TAC does not induce cardiac apoptosis significantly until 8 weeks post banding32, and T cells infiltrate the heart as early as 2 weeks post TAC. We speculate that PO disrupts the immune cell tolerance taking place in the heart allowing self-cardiac antigens to induce a T cell immune response, as it has been described in autoimmune myocarditis.33 These antigens may include alpha-myosin, which is not expressed in the thymus and therefore can impact immune tolerance two ways: not being negatively selected against by clonal deletion34 and not being able to positively select alpha- myosin specific natural T regulatory cells. However, this requires further investigation.
Our data indicate that in addition to T cells, CD11b+ myeloid cells infiltrate the LV after 4 weeks of TAC. This is in line with other models of PO that demonstrate macrophage infiltration in the heart35, 36, however, the similar infiltration of F480+ macrophages observed in Sham and TAC in our studies suggest these macrophages are not activated. Because endothelial ICAM-1 is significantly upregulated in response to PO, it is possible that T cells and CD11b+ cells use this pathway to infiltrate the LV. How endothelial ICAM-1 becomes upregulated in response to PO remains under investigation, but one likely possibility is that PO induces cytokine production by cardiac resident cells and these activate the endothelial cells and promote subsequent T cell recruitment7, 37, 38. We interestingly identified significant upregulation of the T helper type 1 (Th1) signature cytokine, IFNγ, and transcription factor, Tbet. Although these are not exclusively expressed on Th1 cells39, the fact that these are not upregulated in T cell deficient mice (TCRα−/−) suggests that Th1 cells are a major subset of T cells infiltrated in the LV and can alter cardiac myocyte and fibroblast function via IFNγ40. Further studies will be needed to establish a definitive role for specific T cell subsets in the LV and whether once infiltrated in the LV can further activate heart endothelial cells in vivo, similarly to what we previously described in vitro41, 42. Collectively our results support a mechanism in which PO acts two ways: by exposing self-cardiac antigens that result in T cell activation, and by inducing cytokine release in cardiac resident cells that lead to endothelial activation. These are both required for optimal T cell recruitment to the LV, where T cell cytokine secretion of TNFα and IFNγ could contribute to cardiac dysfunction.
Functional necessity of T cells in cardiac remodeling and HF
Whether such a small number of activated T cells infiltrated in the LV can have an impact in cardiac function was evaluated and confirmed in our studies with TCRα−/− mice, which showed preserved adverse cardiac remodeling, including cardiac hypertrophy, cardiac fibrosis and cardiac function in response to TAC. Our data are in agreement with recent data published during the preparation of our manuscript using a different genetic depletion of T cells approach that included the use of recombination activating gene 2 (Rag2−/− ) mice and the major histocompatibility complex II (MHCII−/−) mice, both showing blunted adverse cardiac remodeling and preserved ventricular function in response to 6 week TAC. The authors demonstrated that systemic activation of CD4+ T cells in the draining mediastinal lymph nodes is critical for the T cell immune response observed in response to TAC15. Our present study using a different strain of T cell deficient mice, TCRα−/−, and 4 weeks TAC, not only confirms a role for T cells in adverse remodeling and HF, but demonstrate for the first time that T cell infiltration specifically in the left ventricle (LV) of the heart occurs in WT mice as early as 2 weeks post TAC, suggesting a much earlier activation of T cells contributing to the progression of HF. Unlike the previous study, we have also investigated invasive LV hemodynamics in TCRα−/−mice and importantly implicated T cells in diastolic HF, a condition for which there are no current treatments available. Moreover, the protective systolic and diastolic HF phenotype resulted in 100% survival in TCRα. Together with the lack of an LV pro-inflammatory environment observed in these mice, these studies support a mechanism in which cytokine production by infiltrated activated T cells have a negative impact in LV function and survival to TAC.
Genetic vs pharmacological T cell depletion effects on cardiac fibrosis and hypertrophy
Genetic depletion of T cells using different approaches coincide with the better outcome for HF induced by PO therefore placing T cells as central regulators of pathological cardiac remodeling. It is noteworthy that genetic depletion of T cells can somehow affect other immune subsets during development, as T cells are required by other cells in the immune system to perform their functions effectively43. Also, in these genetically modified mice strains, T cell depletion occurs before the onset of PO and resultant ventricular dysfunction, and is thus less directly therapeutically applicable to patients with preexisting heart failure. To circumvent these limitations, we, for the first time, successfully achieved T cell depletion selectively in the setting of TAC. Our data strongly supports a role for T cells in cardiac fibrosis and function induced by TAC that is independent of any whole body genetic modification. Furthermore, it strengthens the idea that targeting T cells after the stimulus for HF has been induced is beneficial in HF prognosis potentially by preventing T cell LV infiltration. While using the genetic and pharmacological depletion approach we recapitulate the finding that T cells can regulate fibrosis in a variety of tissues, more intriguing is the striking observation that the lack of T cells in TCRα−/− abrogates the hypertrophic response. This phenomenon has been observed in other deficient mice44-46 including T cell deficient mice15, but it is not observed when we pharmacologically deplete T cells in the onset of HF. One could speculate that the remaining T cells in WT mice after depletion can secrete pro-hypertrophic mediators such as Ang II47 and possibly mediate physiological hypertrophy mechanisms. These mechanisms require further investigation.
Conclusions and limitations
Our combined observations in humans and mice provide evidence that T cells and their recruitment to the LV are critical regulators of pathological cardiac remodeling in HF in the setting of PO. Our observations are in line with the recently proposed changing paradigm suggesting that certain types of HF occur in novel cell compartments such as the intramyocardial vascular endothelium and the recruited immune cells48. One limitation of the human studies is that most non ischemic HF patients take β-adrenergic signal blockers which can affect T cell activation,49 also affected by hypertension50. However, our mouse data corroborates the human data, and the TCRα−/− mice have similar basal systolic pressure as WT, as assessed by aortic blood pressures in sham mice. Collectively, our data contribute to a broader understanding of the novel role T cells play in cardiac remodeling and highlight that pharmacological T cells depletion prevents HF and may serve as a potential strategy to improve the structural, functional and molecular deficits of the failing heart.
Supplementary Material
Acknowledgements
We would like to acknowledge Dr. Andrew Lichtman from the Brigham and Women’s Hospital, Boston, MA, for his careful and detailed review of the data and helpful suggestions and discussions of this manuscript, Dr. Francois Benoist from Harvard Medical School for providing the TCRα−/− mice, Tanya Kershaw from the MCRI mouse physiology core for her technical support in the mouse studies, and the Tufts CTSI for support with statistical analysis.
Sources of Funding
These studies were supported by NIH K99-HL097406, NIH-RO1-HL123658 and an American Heart Association Grant in Aid- AHA GIA 13GRNT 14560068 (P. Alcaide), NIH 1KL2TR001063-01 (R. M. Blanton) and NIH K08 HL094909 (N. K. Kapur). T. Nevers was supported by NIH T32 HL 69770. UL1TR001064 (Tufts CTSI).
Footnotes
Disclosures
None.
References
- (1).Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Grobbee DE. The epidemiology of heart failure. Eur Heart J. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- (2).Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;11:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- (3).Ahmad T, Fiuzat M, Felker GM, O’Connor C. Novel biomarkers in chronic heart failure. Nat Rev Cardiol. 2012;9:347–59. doi: 10.1038/nrcardio.2012.37. [DOI] [PubMed] [Google Scholar]
- (4).Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- (5).Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–82. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- (6).Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- (7).Mann DL, Young JB. Basic mechanisms in congestive heart failure. Recognizing the role of proinflammatory cytokines. Chest. 1994;105:897–904. doi: 10.1378/chest.105.3.897. [DOI] [PubMed] [Google Scholar]
- (8).Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- (9).Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- (10).Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Leone AM, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation. 2004;110:46–50. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
- (11).Fukunaga T, Soejima H, Irie A, Sugamura K, Oe Y, Tanaka T, Nagayoshi Y, Kaikita K, Sugiyama S, Yoshimura M, Nishimura Y, Ogawa H. Relation between CD4+ T-cell activation and severity of chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;100:483–8. doi: 10.1016/j.amjcard.2007.03.052. [DOI] [PubMed] [Google Scholar]
- (12).Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shim SH, Kim DS, Cho W, Nam JH. Coxsackievirus B3 regulates T-cell infiltration into the heart by lymphocyte function-associated antigen-1 activation via the cAMP/Rap1 axis. J Gen Virol. 2014;95:2010–8. doi: 10.1099/vir.0.065755-0. [DOI] [PubMed] [Google Scholar]
- (14).Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141–7. [PubMed] [Google Scholar]
- (15).Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–24. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- (16).Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev. 2006;11:83–92. doi: 10.1007/s10741-006-9196-2. [DOI] [PubMed] [Google Scholar]
- (17).Yndestad A, Holm AM, Muller F, Simonsen S, Froland SS, Gullestad L, Aukrust P. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res. 2003;60:141–6. doi: 10.1016/s0008-6363(03)00362-6. [DOI] [PubMed] [Google Scholar]
- (18).Patten RD, Pourati I, Aronovitz MJ, Alsheikh-Ali A, Eder S, Force T, Mendelsohn ME, Karas RH. 17 Beta-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail. 2008;14:245–53. doi: 10.1016/j.cardfail.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Blanton RM, Takimoto E, Lane AM, Aronovitz M, Piotrowski R, Karas RH, Kass DA, Mendelsohn ME. Protein kinase g ialpha inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1:e003731. doi: 10.1161/JAHA.112.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bozkurt B, Kribbs SB, Clubb FJ, Jr., Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–91. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- (21).Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL, Reddy AK, Coombes KR, Daher IN, Pati S, Patel SS, Pocius JS, Taffet GE, Buja LM, Entman ML, Khakoo AY. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest. 2010;120:472–84. doi: 10.1172/JCI39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation. Am J Physiol Cell Physiol. 2012;303:C385–C395. doi: 10.1152/ajpcell.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX, Luscinskas FW, Lichtman AH. Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J Immunol. 2012;188:1421–30. doi: 10.4049/jimmunol.1101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr., Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol. 2003;162:1591–601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–62. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- (26).Yu Q, Watson RR, Marchalonis JJ, Larson DF. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol. 2005;289:H643–H651. doi: 10.1152/ajpheart.00073.2005. [DOI] [PubMed] [Google Scholar]
- (27).Li N, Bian H, Zhang J, Li X, Ji X, Zhang Y. The Th17/Treg imbalance exists in patients with heart failure with normal ejection fraction and heart failure with reduced ejection fraction. Clin Chim Acta. 2010;411:1963–8. doi: 10.1016/j.cca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- (28).Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun. 2013;45:90–6. doi: 10.1016/j.jaut.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- (30).Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. J Exp Med. 1999;189:1765–76. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–63. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- (32).Li XM, Ma YT, Yang YN, Liu F, Chen BD, Han W, Zhang JF, Gao XM. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin Exp Pharmacol Physiol. 2009;36:1054–61. doi: 10.1111/j.1440-1681.2009.05243.x. [DOI] [PubMed] [Google Scholar]
- (33).Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188:4876–84. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121:1561–73. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005;111:420–7. doi: 10.1161/01.CIR.0000153800.09920.40. [DOI] [PubMed] [Google Scholar]
- (36).Kuwahara F, Kai H, Tokuda K, Niiyama H, Tahara N, Kusaba K, Takemiya K, Jalalidin A, Koga M, Nagata T, Shibata R, Imaizumi T. Roles of intercellular adhesion molecule-1 in hypertensive cardiac remodeling. Hypertension. 2003;41:819–23. doi: 10.1161/01.HYP.0000056108.73219.0A. [DOI] [PubMed] [Google Scholar]
- (37).Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol. 2013;191:4838–48. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Suzuki S, Shishido T, Funayama A, Netsu S, Ishino M, Kitahara T, Sasaki T, Katoh S, Otaki Y, Watanabe T, Shibata Y, Mantovani A, Takeishi Y, Kubota I. Long pentraxin PTX3 exacerbates pressure overload-induced left ventricular dysfunction. PLoS One. 2013;8:e53133. doi: 10.1371/journal.pone.0053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Alcaide P, Jones TG, Lord GM, Glimcher LH, Hallgren J, Arinobu Y, Akashi K, Paterson AM, Gurish MA, Luscinskas FW. Dendritic cell expression of the transcription factor T-bet regulates mast cell progenitor homing to mucosal tissue. J Exp Med. 2007;204:431–9. doi: 10.1084/jem.20060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2012;60:1430–6. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- (41).Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, Lichtman AH. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–99. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Maganto-Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK, Croce KJ, Luscinskas FW, Lichtman AH, Grabie N. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011;187:3521–9. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–47. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- (44).Moc C, Taylor AE, Chesini GP, Zambrano CM, Barlow MS, Zhang X, Gustafsson AB, Purcell NH. Physiological activation of Akt by PHLPP1 deletion protects against pathological hypertrophy. Cardiovasc Res. 2015;105:160–70. doi: 10.1093/cvr/cvu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Rifki OF, Bodemann BO, Battiprolu PK, White MA, Hill JA. RalGDS-dependent cardiomyocyte autophagy is required for load-induced ventricular hypertrophy. J Mol Cell Cardiol. 2013;59:128–38. doi: 10.1016/j.yjmcc.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–7. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- (49).Shaw SM, Coppinger T, Waywell C, Dunne L, Archer LD, Critchley WR, Yonan N, Fildes JE, Williams SG. The effect of beta-blockers on the adaptive immune system in chronic heart failure. Cardiovasc Ther. 2009;27:181–6. doi: 10.1111/j.1755-5922.2009.00089.x. [DOI] [PubMed] [Google Scholar]
- (50).Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.