Abstract
Cardiac fibroblasts are a major cell population of the heart and are characterized by their capacity to produce extracellular matrix (ECM). In hearts subjected to pressure overload, excessive fibroblast accumulation is responsible for fibrosis of the myocardium, a major clinical issue. Hence, understanding mechanisms generating fibroblasts in this context has become a key question in the cardiovascular field. Recent studies now point to the activation of resident fibroblasts as the underlying cause of fibrosis. However, de novo generation of fibroblasts from endothelium and circulating hematopoietic cells has also been proposed to significantly contribute to fibrosis. Here we discuss the latest findings on fibroblast origins, with a particular emphasis on the pressure overload model, and the implication of these findings for the development of anti-fibrotic therapies that are currently lacking.
Heart failure is a major cause of mortality in the western world[1]. Heart failure of diverse etiologies is preceded by adverse remodeling of the heart, involving fibrosis, the excessive deposition of extracellular matrix (ECM) in interstitial and perivascular areas by fibroblasts. In the context of pressure overload, reactive fibrosis causes increased tissue rigidity and ischemia, leading to heart failure.
Although much has been learned about fibroblasts, notably in terms of ECM secretion, electrophysiological properties and signaling (reviewed elsewhere[2,3]), origins of these cells during fibrosis has remained controversial. During development, fibroblasts have been shown to derive from the epicardium[4–8], however, multiple alternative sources of fibroblasts, in addition to resident fibroblasts, have been reported to be involved in pathological remodeling process[9– 11]. Notably, previous reports point to the conversion of endothelium into fibroblasts, known as endothelial to mesenchymal transition (EndoMT), as well as the recruitment of circulating hematopoietic progenitors in failing hearts[9,12,13].
This review will focus on the current state of the literature on the identification and origins of fibroblasts in hypertensive heart disease, discuss controversies, notably concerning fibroblast markers, and point to future directions.
Cardiac fibroblast: definition and markers
Cardiac fibroblasts perform the essential function of synthesizing the collagen-rich ECM network which provides structural integrity, as well as a source of biomechanical and ECM related signaling[2,14]. Notably, the makeup of the ECM varies during development and into adulthood, promoting myocyte proliferation during early development and hypertrophy after birth.[15] In adult heart, excessive ECM deposition by activated fibroblasts in pathological contexts promotes adverse remodeling of the myocardium[14]. Hence, through their central role in the constitution of the ECM, fibroblasts are key mediators of cell signaling and myocardial remodeling.
Defining the relative proportions of the various cardiac cell lineages has proven challenging, and to date there is no clear consensus. Early studies defining cardiac cell populations, using rat as a model organism, established that the heart is comprised of approximately 70% myocytes and 30% non-myocytes[16]. A subsequent study using flow cytometry analysis of murine heart has shown that 45% of cells are non-myocytes, among which fibroblasts were predominant, and 55% myocytes[17]. However, accurately quantifying relative numbers of non- myocyte cell types, including fibroblasts, endothelial cells, pericytes, smooth muscle cells and resident immune cells, has proven to be more challenging. Indeed, this requires cell-type specific markers, or marker combinations. Although pan lineage-specific markers have long been identified for major cell types such as endothelium (PECAM1,VE-cadherin) and immune cells (CD45), markers for cardiac fibroblasts have remained more controversial.
In addition to “stromal-like” morphological characteristics for identifying fibroblasts, a plethora of molecular markers have been used (Table I). This has resulted from a lack of consensus over how to identify fibroblasts in the absence of more robust markers. Among commonly used markers is Discoidin Receptor 2 (DDR2), a receptor for extracellular matrix proteins, which labels fibroblasts, but not endothelium, smooth muscle or myocytes[18]. However, it is not clear that all fibroblasts are DDR2+, and obtaining a specific antibody is challenging. Thymocyte 1 (Thy1, CD90) is also commonly used to identify fibroblasts. Although expressed by cardiac fibroblasts, this receptor is also expressed by immune cells[19], lymphatic endothelium[20] and pericytes[21], making its use limited, at least for immunohistological examination of fibroblasts. The intermediate filament Vimentin has also been used commonly to identify fibroblasts, although it is expressed in many cell types including endothelial cells[22]. The transcription factor TCF21, a marker of proepicardium among other mesothelial populations, has also been used to identify fibroblasts in embryonic heart[23,24,8]. Fibroblast specific protein 1 (FSP1, S100A4) was first identified in fibroblast cell lines[25] and is currently considered a reliable fibroblast marker, but recent publications have shown that, in vivo, it labels a subset of immune cells and endothelial cells [26–28].
Table 1. Fibroblast markers in healthy heart and following pressure overload.
The table includes indications regarding the specificity of the markers and references in which they were tested. Markers have been arbitrarily ranked beginning with the overall most robust fibroblast marker, PDGFRα.
| Fibroblasts labelled | Other cell types labelled | |||
|---|---|---|---|---|
| Healthy adult heart |
Hypertensive disease |
Healthy adult heart |
Hypertensive disease |
|
| PDGFRα | All (7,28) | All (7,28) | Epicardium (28) | Epicardium (28) |
| Collagen 1a1-GFP | All (7,28) | All (7,28) | Epicardium, large vessels (28) | Epicardium, large vessels (28) |
| Tcf21 | All/most (8) | A subset in interstitial and fibrotic lesions | Epicardium (8,42) | Epicardium (42) |
| DDR2 | All (18) | All (18) | Epicardium (71) | Epicardium (72) |
| Thy1 | Most/all (28, 54) | Most/all (28, 54) | Endothelium, immune cells | Endothelium, immune cells |
| αSMA | No expression | A subset in interstitial lesions (28) | Smooth muscle (38), pericytes | Smooth muscle, pericytes, endothelium (9) |
| Vimentin | All | All | Multiple cell types including endothelium (22) | Multiple cell types |
| Wt1 | - | A subset in interstitial lesions (42) | Epicardium (42, 73) | Epicardium (42) |
| Tbx18 | - | A subset in interstitial lesions (42) | Epicardium (42) | Epicardium (42) |
| Periostin (secreted) | - | Associated with areas of interstitial and perivascular fibrosis (39, 74) | Valves (75) | - |
| FSP1 | Rare (9) | A subset in perivascular lesions (28) | Immune cells (28, 27, 9) | Immune cells (28, 27, 9), endothelial cells (9) |
Using markers of ECM production provides a rational approach to identifying cardiac fibroblasts, as well as their homologues in other tissues, including portal fibroblasts in liver and osteoblasts in bone marrow. ECM is composed of numerous ECM molecules, but mainly consists of heterotrimers of Collagen type I alpha subunits[29]. Recently, a Collagen1a1-GFP reporter[30] has been used to specifically identify cardiac fibroblasts[8,28]. These cells also co-expressed platelet derived growth factor receptor alpha (PDGFRα), which is emerging as a definitive marker of cardiac fibroblasts in adult tissues, and is also strongly expressed in fibroblasts during development and in disease[7,28]. Moreover, PDGFRα is expressed by collagen-producing fibroblasts in skeletal muscle and lung, indicating that it is a robust fibroblast lineage marker[31–33]. Limited expression of this receptor has also been noted in vascular smooth muscle cells in a disease setting[34]. PDGFRα presents major advantages over other markers described above in terms of specificity and expression in fibroblasts regardless of developmental stage and disease state (see Table 1). However, it is important to note that during embryonic development, this receptor is broadly expressed in mesenchymal tissues[35]. The Collagen1a2 inducible Cre driver is another transgenic tool, complementary to the Collagen1a1-GFP reporter, targeting Cre recombinase expression to cells actively expressing collagen[36]. This potentially enables identification and subsequent genetic lineage tracing of fibroblasts with a Cre reporter or conditional gene deletion for functional studies.
Fibroblast markers in the context of fibrosis
Following pressure overload, fibroblasts can adopt an “active” state known as “myofibroblast”. This transition is associated with the expression of a number of markers not expressed by fibroblasts in healthy myocardium. The key marker originally used for identification of myofibroblasts in multiple tissues and pathological contexts is αSMA[37,38,9]. However, this marker is not expressed in all fibroblasts associated with fibrosis, notably following pressure overload[9,28]. The ECM component periostin has also been shown to be secreted by fibroblasts in a pathological context.[39,40] Another marker is fibroblast activation protein-α (FAP), an integral membrane serine protease discovered in the context of epithelial carcinomas[41].
Interestingly, differential expression of fibroblast markers between perivascular and interstitial fibrotic cells of hearts subjected to pressure overload or ischemia/reperfusion (I/R) has been described. TCF21, WT1 and TBX18 are transcription factors expressed in embryonic fibroblast progenitors that are induced in adult cardiac fibrosis. TCF21 is broadly associated with perivascular fibrosis and interstitial fibrosis resulting from pressure overload or ischemic injury. In contrast WT1 and TBX18 are not prevalent in perivascular fibrosis associated with hypertensive disease, but are expressed in interstitial fibroblasts following I/R or aortic banding[42]. Notably, TCF21, WT1 and TBX18 are not expressed in infiltrating immune cells identified by CD45. FSP1 expression is specifically upregulated in perivascular fibroblasts following pressure overload but also can mark infiltrating immune cells and endothelial cells [28]. Finally, stem cell antigen 1 (Sca1) expression has been associated with perivascular (or adventitial) fibroblasts, both in healthy and fibrotic hearts.[43]
Hence, expression of a number of markers is associated with an activated state in fibroblasts. With the exception of TCF21, these markers are not fibroblast-specific. Rather, in combination with fibroblast specific markers including PDGFRα, they provide complementary information on the signaling and properties of specific subsets of fibroblasts within the failing heart.
Developmental origins of cardiac fibroblasts
Epicardium
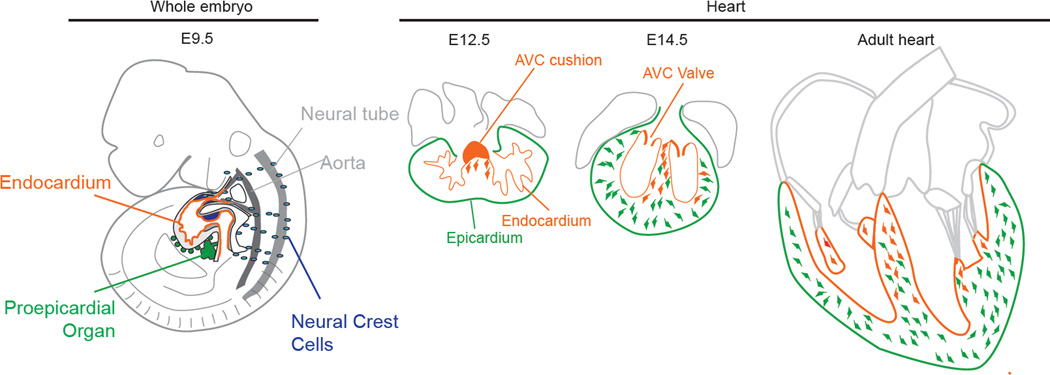
Until recently, studies focusing on the developmental origin(s) of fibroblasts have been lacking. Several reports showed that cardiac fibroblasts derive from the epicardium, the coelomic mesothelium that covers the heart[4–8]. Epicardium is derived from the proepicardial organ (PEO) that develops in association with the septum transversum at the venous pole of the heart, and is itself a derivative of the splanchnic mesoderm (see Figure 1). It has been shown that the epicardium gives rise to multiple cardiac lineages, including fibroblasts, pericytes, smooth muscle cells and, perhaps, myocytes and endothelium[44,4,6,45,5].
Figure 1.
Lineages giving rise to cardiac fibroblasts during embryonic development. Illustration of an E9.5 embryo showing the myocardium with its inner endocardium, the proepicardial organ and migrating neural crest cells. Fibroblasts derived from the AVC cushion (orange) first invade the septum at E12.5. Subsequently, epicardial EMT generates fibroblasts (green) that invade the free walls by E14.5. In adult heart, endothelially derived fibroblasts are found most abundantly in the septum, whereas epicardially derived fibroblasts populate the free walls.
Epicardial cells express a number of markers, including WT1[45], TBX18[6] and TCF21[24]. Heterogeneity in expression of these markers among epicardial cells has been linked to their specification into fibroblast and pericyte/vascular smooth muscle cell lineages. Notably, expression of TCF21 (Epicardin/Pod1/Capsulin) in a subset of epicardial cells is required for specification of epicardially-derived cardiac fibroblasts[8].
Endocardium
The endocardium is a specialized endothelial layer that lines the heart. During development, at least some endocardial progenitors diverge from myocardial lineages at or prior to gastrulation[44,46]. During heart development, subsets of endocardial cells undergo EndoMT to give rise to cushion mesenchyme of atrioventricular canal and outflow tract (see Figure 1) that acts as primitive valves during early development, and will be remodeled to contribute to cardiac valves[47]. Recently, it was shown that fibroblasts generated during this process also invade proximal myocardium, in particular the interventricular septum, a region found to lack substantial numbers of epicardially-derived fibroblasts[28] (see Figure 1). Another study has reported that epicardially-derived valve interstitial fibroblasts are prominent in the parietal (mural) rather than septal leaflets of atrioventricular valves[48]. Hence, mesenchyme associated with the septum, including fibroblasts and valve interstitial cells, includes a large proportion of endocardial cushion derived cells.
Neural Crest
The neural crest is a heterogeneous population of cells that originates from the dorsal aspect of the neural tube. These cells arise from the dorsal aspect of the neural tube and undergo endothelial to mesenchymal transition (EMT), generating various cell types including neurons, glial cells, melanocytes and mesenchymal cells[49]. The cardiac neural crest, a specific subpopulation, plays a key role in morphogenesis of the outflow region of the heart (Figure 1)[50,51]. Genetic lineage tracing in mouse models has confirmed that cardiac neural crest cells first populate the aorticopulmonary septum and conotruncal cushions before septation and contribute to remodeling, notably by giving rise to valve mesenchyme[52]. Interestingly, this study also demonstrated some neural crest derivatives in mature semilunar valves. Another study has shown persistence of significant number of neural crest derivatives in semilunar and atrioventricular valves, but these cells were melanocytes rather than valve interstitial cells[53]. Recently, a minor subset of neural-crest derived fibroblasts were found within myocardium, mainly residing in right atrium[54].
Origins of fibroblasts during fibrosis associated with pressure overload
Resident fibroblast lineages
Organ fibrosis is a major health issue, entailing multiple studies addressing origins of fibroblasts that accumulate in various pathological contexts. Intense proliferation of resident fibroblasts occurs in the context of pressure overload and heart failure[28,40,54,55]. Lineage studies with several endothelial or epicardial Cres in TAC models demonstrated that developmentally derived endogenous fibroblast populations contribute to fibroblasts responsible for fibrosis. Endothelially derived fibroblasts contributed approximately 15–20% of fibrotic fibroblasts in ventricles, and epicardially derived fibroblasts contributed the remaining 80–85% of the total fibroblast pool in failing hearts, these ratios being comparable to those observed at baseline[28,54]. Consistent with these findings, the two fibroblast populations respond similarly to pressure overload in terms of proliferation and gene expression[28,54]. The foregoing observations demonstrated that fibroblast accumulation results from proliferation of developmentally-derived fibroblast lineages [28,54] (see Figure 2).
Figure 2.
Interstitial and perivascular fibrosis results from the proliferation of resident fibroblast lineages. Following pressure overload, local signaling from myocytes and non-myocytes, including vascular cells and immune cells, promotes PDGFRα+;Collagen1a1-GFP+ fibroblast proliferation throughout the myocardium. Several markers have specifically been associated with fibroblasts in interstitial and perivascular fibrotic lesions, but are not expressed by all fibroblasts in these areas (+/−).
Adult endothelial-to-mesenchymal origin
A previous study utilizing FSP1 as a fibroblast marker and a constitutive endothelial/hematopoietic restricted Tie1Cre resulted in the observation of increased numbers of FSP1 positive cells, interpreted to be fibroblasts, that were lineage traced by Tie1Cre, in the setting of pressure overload[9]. These findings were interpreted as evidence that EndoMT from coronary vasculature was a major contributor to cardiac fibroblasts, in this case contributing up to a third of the total fibroblast pool. The recent findings that FSP1 marks substantial numbers of immune cells[26–28], together with the fact that Tie1Cre labels immune cells as well as endothelium[56], suggested that it was important to revisit the occurrence of EndoMT in the setting of pressure overload, utilizing more specific markers of fibroblasts. Toward this end, one study was performed where inducible VE-cadherin CreERT2 was used to lineage label adult endothelium prior to thoracic aortic banding (TAC), with Collagen1a-GFP and PDGFRα as fibroblast markers[28]. Another study relied on Tie2Cre[57] labelling of endothelium in combination with Thy1+CD45−CD31− utilized as a specific signature for cardiac fibroblasts[54]. Results of these studies demonstrated that adult endothelial cells did not contribute to cardiac fibroblasts.
Finally, a recent paper utilizing Col1a2-CreERT2, and DDR2 and Collagen1a1-GFP as markers of cardiac fibroblasts, found that upon cardiac I/R injury, a subset of fibroblasts rapidly up-regulates endothelial markers and undergoes mesenchymal to endothelial transition (MET)[58], adding a novel twist to the nature of the role played by cardiac fibroblast during fibrosis and their relationship to endothelium. Indeed, this effect is beneficial, and the authors suggest that a subset of fibroblasts act as endothelial progenitors.
Fibroblasts of hematopoietic/circulating origin
In the context of pressure overload, the immune response involves multiple immune cell populations that infiltrate the heart in distinct phases[59]. Since the observation of the presence of circulating “fibrocytes” with mesenchymal-like features such as collagen production[60], several studies have reported a contribution of blood-borne fibroblasts in cardiac fibrosis in various disease models, including pressure overload[13,61,10,62,9]. However, lineage studies with the hematopoietic specific Vav-Cre[28], or with genetically labeled bone marrow transplants[54] and specific markers of fibroblasts demonstrated that circulating hematopoietic cells do not contribute to cardiac fibroblasts in hypertrophic hearts.
Pericytes
Pericytes also represent a potential fibroblast progenitor pool in the context of fibrosis. Pericytes are vascular support cells found associated with smaller vessels that are developmentally close to smooth muscle cells and fibroblasts[4,63] and express markers such as PDGFRβ and NG2[64]. Notably, in other organ systems such as the liver and kidney, pericytes have been shown to give rise to collagen-producing fibroblast[65–67]. Although recent work has suggested this may not the case in heart[68], this questions needs to be directly addressed by lineage tracing.
Conclusion
In conclusion, novel markers for cardiac fibroblasts have enabled recent advances in our understanding of cardiac fibroblast origins during development and in fibrosis. Notably, PDGFRα and the Collagen1a1-GFP reporter appear to be robust cardiac fibroblast markers. Although these seem to label a majority of cells responsible for fibrosis, there appears to be considerable heterogeneity among activated fibroblasts, and future studies may reveal more comprehensive markers, and/or markers specific of a particular disease context.
Lineage tracing using these and other markers has shown that fibroblast heterogeneity is initially acquired during development as a result of two main lineages deriving from epicardium and endothelium. These lineages respond similarly to pressure overload, and proliferate extensively in the regions they initially populate, leading to fibrosis. Interestingly, differential expression of a number of markers, particularly following pressure overload, distinguishes fibroblasts involved in interstitial versus perivascular fibrosis. Furthermore, αSMA clearly labels a subset of “myofibroblasts” following pressure overload, but whether all myofibroblasts are direct descendants of quiescent fibroblasts, or other lineages such as pericytes, remains unclear. Finally, definition of the specific cellular and signaling contributions of immune cell populations should considerably help understand fibrotic processes. Notably, establishing whether any functional overlap exists between fibroblasts and hematopoietic-derived lineages, currently and area of intense debate, is a key issue.
Much of our current knowledge of fibroblast origins comes from genetic lineage tracing. The use of constitutive Cre drivers targeting progenitor/cell type specific markers provides a means by which to extensively label progenitor populations and their descendants. However, the expression of a number of genes associated with a specific cell type/developmental stage can be more dynamic than expected, in particular in the context of disease, leading to Cre activation and labelling of cells of an uncertain origin [42]. This limitation can be overcome in complementary studies using inducible Cre drivers, in which the timing of Cre expression is under control, but efficiency of excision may be lower.
Thus it appears that targeting resident fibroblast activation/proliferation following pressure overload, rather than EndoMT or circulating fibroblast progenitor recruitment, is a key issue for alleviating fibrosis. This may involve selectively targeting perivascular versus interstitial fibrosis to some extent, as differences in gene expression in fibroblasts in these areas may result from distinct pro-fibrotic signaling. Furthermore, promoting fibroblast clearance in a context where fibrosis is established, for instance by inducing fibroblast apoptosis as occurs in liver fibrosis[69], could provide a means of reversing pathological remodeling in hearts where fibrosis is established.[70]
ACKNOWLEDGEMENTS
T. Moore-Morris is funded by WHRI-COFUND, the Leducq Foundation, and the Lefoulon- Delalande foundation. KE Yutzey is funded by NIH/NHLBI P01HL069779. M. Puceat would like to acknowledge funding from the Leducq Foundation. SM. Evans is funded by grants from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Annals of the New York Academy of Sciences. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 3.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology & therapeutics. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental biology. 1996;174(2):221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 5.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation research. 1998;82(10):1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 6.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-tomesenchymal transition and fate specification require PDGF receptor signaling. Circulation research. 2011;108(12):e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development (Cambridge, England) 2012;139(12):2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 10.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. The Journal of pathology. 2008;214(3):377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 11.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circulation research. 2010;107(11):1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121(22):2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 13.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(48):18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovascular research. 2005;65(1):40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Developmental cell. 2009;16(2):233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28(109):41–61. [PubMed] [Google Scholar]
- 17.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American journal of physiology Heart and circulatory physiology. 2007;293(3):H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;230(4):787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 19.Raff MC. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplantation reviews. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 20.Jurisic G, Iolyeva M, Proulx ST, Halin C, Detmar M. Thymus cell antigen 1 (Thy1, CD90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Experimental cell research. 2010;316(17):2982–2992. doi: 10.1016/j.yexcr.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Lane EB, Hogan BL, Kurkinen M, Garrels JI. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature. 1983;303(5919):701–704. doi: 10.1038/303701a0. [DOI] [PubMed] [Google Scholar]
- 23.Robb L, Mifsud L, Hartley L, Biben C, Copeland NG, Gilbert DJ, Jenkins NA, Harvey RP. epicardin: A novel basic helix-loop-helix transcription factor gene expressed in epicardium, branchial arch myoblasts, and mesenchyme of developing lung, gut, kidney, and gonads. Developmental dynamics : an official publication of the American Association of Anatomists. 1998;213(1):105–113. doi: 10.1002/(SICI)1097-0177(199809)213:1<105::AID-AJA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Richardson JA, Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mechanisms of development. 1998;73(1):23–32. doi: 10.1016/s0925-4773(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 25.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. The Journal of cell biology. 1995;130(2):393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of Fibroblast Specific Protein (FSP)1 in cardiac remodeling and fibrosis. American journal of physiology Heart and circulatory physiology. 2013 doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. The Journal of clinical investigation. 2014;124(7):2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Molecular genetics and metabolism. 2000;71(1–2):418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 30.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology (Baltimore, Md) 2003;37(2):267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 31.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature medicine. 2012;18(8):1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. American journal of respiratory cell and molecular biology. 2012;47(4):517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. Journal of cell science. 2011;124(Pt 21):3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 34.Chong JJ, Reinecke H, Iwata M, Torok-Storb B, Stempien-Otero A, Murry CE. Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart. Stem cells and development. 2013;22(13):1932–1943. doi: 10.1089/scd.2012.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orr-Urtreger A, Lonai P. Platelet-derived growth factor-A and its receptor are expressed in separate, but adjacent cell layers of the mouse embryo. Development (Cambridge, England) 1992;115(4):1045–1058. doi: 10.1242/dev.115.4.1045. [DOI] [PubMed] [Google Scholar]
- 36.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts : a potentially powerful technique for investigating gene function in fibrosis. The American journal of pathology. 2002;160(5):1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 38.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. The Journal of cell biology. 1986;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circulation research. 2007;101(3):313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. The Journal of clinical investigation. 2010;120(10):3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(9):3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. Journal of molecular and cellular cardiology. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ieronimakis N, Hays AL, Janebodin K, Mahoney WM, Jr, Duffield JS, Majesky MW, Reyes M. Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFbeta1 signaling in the mdx mouse model of Duchenne muscular dystrophy. Journal of molecular and cellular cardiology. 2013;63:122–134. doi: 10.1016/j.yjmcc.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(20):9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzahor E, Evans SM. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovascular research. 2011;91(2):196–202. doi: 10.1093/cvr/cvr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circulation research. 2009;105(5):408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Developmental biology. 2012;366(2):111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Douarin NM, Dupin E. The neural crest in vertebrate evolution. Current opinion in genetics & development. 2012;22(4):381–389. doi: 10.1016/j.gde.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science (New York, NY) 1983;220(4601):1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 51.Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Developmental biology. 1998;196(2):129–144. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development (Cambridge, England) 2000;127(8):1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circulation research. 2006;98(12):1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- 54.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, et al. Developmental Heterogeneity of Cardiac Fibroblasts Does Not Predict Pathological Proliferation and Activation. Circulation research. 2014 doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 55.Weber KT. Fibrosis and hypertensive heart disease. Current opinion in cardiology. 2000;15(4):264–272. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. Journal of cell science. 2001;114(Pt 4):671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 57.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 58.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014 doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisheit C, Zhang Y, Faron A, Kopke O, Weisheit G, Steinstrasser A, Frede S, Meyer R, Boehm O, Hoeft A, et al. Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PloS one. 2014;9(11):e112710. doi: 10.1371/journal.pone.0112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Molecular medicine (Cambridge, Mass) 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 61.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovascular research. 2006;71(4):661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, Wang Y. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. American journal of physiology Heart and circulatory physiology. 2011;301(2):H538–H547. doi: 10.1152/ajpheart.01114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science (New York, NY) 1997;277(5323):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 65.Milani S, Herbst H, Schuppan D, Kim KY, Riecken EO, Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990;98(1):175–184. doi: 10.1016/0016-5085(90)91307-r. [DOI] [PubMed] [Google Scholar]
- 66.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. The American journal of pathology. 2008;173(6):1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. The Journal of clinical investigation. 1990;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem cell research & therapy. 2014;5(6):122. doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. The Journal of clinical investigation. 1998;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupp H, Maisch B. Control of apoptosis of cardiovascular fibroblasts: a novel drug target. Herz. 1999;24(3):225–231. doi: 10.1007/BF03044965. [DOI] [PubMed] [Google Scholar]
- 71.Morales MO, Price RL, Goldsmith EC. Expression of Discoidin Domain Receptor 2 (DDR2) in the developing heart. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2005;11(3):260–267. doi: 10.1017/S1431927605050518. [DOI] [PubMed] [Google Scholar]
- 72.Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. The EMBO journal. 2012;31(2):429–442. doi: 10.1038/emboj.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. The Journal of clinical investigation. 2011;121(5):1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. The Journal of clinical investigation. 2010;120(1):254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mechanisms of development. 2001;103(1–2):183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]