Abstract
BACKGROUND
Limited data guide the prediction of weight loss success or failure following bariatric surgery according to pre-surgery factors. There is significant variation in weight change following bariatric surgery and much interest in identifying pre-operative factors that may contribute to these differences.
OBJECTIVE
This report evaluates the associations of a comprehensive set of baseline factors and three-year weight change.
SETTING
Ten hospitals in six geographically diverse clinical centers in the United States.
METHODS: PARTICIPANTS and INTERVENTIONS
Adults undergoing a first bariatric surgical procedure as part of clinical care by participating surgeons were recruited between 2006 and 2009. Participants completed research assessments utilizing standardized and detailed data collection on over 100 preoperative and operative parameters for individuals undergoing Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB). Weight was measured 3 years following surgery.
METHODS: MAIN OUTCOME MEASURES
Percent weight change for RYGB or LAGB from baseline to 3 years was analyzed as both a continuous and dichotomous outcome with cut points at 25% for RYGB and 10% for LAGB. Multivariable linear and logistic regression models were used to identify independent baseline predictors of the continuous and categorical outcomes, respectively.
RESULTS
The median weight loss 3 years following surgery for RYGB (n=1513) participants was 31.5% (IQR: 24.6%–38.4%; range, 59.2% loss to 0.9% gain) of baseline weight and 16.0% (IQR: 8.1%–23.1%; range, 56.1% loss to 12.5% gain) for LAGB (n=509) participants. The median age was 46 years for RYGB and 48 years for LAGB; 80% of RYGB participants and 75% of LAGB participants were female; and the median baseline Body Mass Index (BMI) was 46 kg/m2 for RYGB and 44 kg/m2 for LAGB. For RYGB, Black participants lost 2.7% less weight compared to Whites and participants with diabetes at baseline had 3.7% less weight loss at year 3 than those without diabetes at baseline. There were small but statistically significant differences in weight change for RYGB in those with abnormal kidney function and current or recent smoking. For LAGB participants, those with a large band had 75% greater odds of experiencing less than 10% weight loss after adjusting for BMI and sex.
CONCLUSIONS
Few baseline variables were associated with three year weight change and the effects were small. These results indicate that baseline variables have limited predictive value for an individual’s chance of a successful weight loss outcome following bariatric surgery.
TRIAL REGISTRATION
NCT00465829, ClinicalTrials.gov
Keywords: Bariatric surgery, Weight change, Predictors, LABS, Cohort study, Diabetes
INTRODUCTION
Given its cost and potential risks, there is considerable interest in identifying those individuals more or less likely to benefit from bariatric surgery. The Longitudinal Assessment of Bariatric Surgery consortium (LABS) has previously identified significant variation in weight loss in a large cohort of people undergoing Roux en Y Gastric bypass (RYGB, n=1738) or laparoscopic adjustable gastric banding (LAGB, n=610).(1) This report from LABS seeks to examine a comprehensive set of baseline variables and their association with weight change three years following surgery. Among the major priorities for LABS is to determine patient, procedure, and provider characteristics associated with weight, and with medical, surgical, and behavioral outcomes, including incidence and remission of co-morbid conditions, following bariatric surgery.(2) LABS created a detailed and comprehensive database, including a wide range of baseline factors within many different domains including demographics, health status, eating and physical activity behaviors, and many others. Variables in the database are measured in a standardized way across LABS sites.(2) An important question is whether baseline demographic or clinical characteristics predict weight loss success or failure.
Various predictors of weight change following bariatric surgery have been suggested by prior reports in several case series studies, mostly after RYGB, with variable magnitudes of effects. (3–7) These studies have identified that lower body mass index (BMI) or starting weight, larger waist circumference, younger age, White race, lower hemoglobin A1c and either lower or higher triglyceride values (in different studies) were associated with greater weight loss. Large pouch size and diabetes were also found to be independently associated with poor weight loss 12 months following RYGB.(3,6) Other studies show that White individuals, as compared to non-Whites, experience significantly greater weight loss at both 6 months and 2 years following RYGB.(8–10) A multi-institutional study examined 1168 RYGB cases and found that initial weight and sex were the only independent predictors of weight loss outcomes with an advantage for females.(11) A recent report for RYGB examined common clinical variables associated with weight loss and found 12 variables associated with weight loss at different time points after surgery.(12) Several of these confirmed associations of previously reported factors (initial BMI, age, diabetes, smoking status) and others were new (iron deficiency, liver fibrosis).(12)
For LAGB, one study of 380 people with a median of 5 years follow up showed that older age, binge eating disorder, and sweet eating behavior were predictors of lower weight loss outcomes.(13) Other studies in people undergoing LAGB have shown at 1 and 3 year time points that older individuals, males, those with higher pre-surgery BMI, and hyper-insulinemia were predictors of poorer weight loss(14,15) and those who used alcohol regularly before surgery or who have a history of substance abuse may experience more weight loss.(15,16) A systematic review and meta-analysis including 115 articles was published in 2012 that focused on preoperative psychosocial factors and their association with weight loss after bariatric surgery.(17) The authors found that mandatory preoperative weight loss may be positively associated with weight loss (7 of 14 studies); while pre-surgery body mass index (BMI) (37 of 62 studies), super obesity (BMI >50) (24 of 33 studies), and personality disorders (7 of 14 studies) had negative associations with weight change.(17) The overall conclusions from this systematic review and meta-analysis were that further studies were needed to investigate whether preoperative factors can predict a clinically meaningful difference in weight loss after bariatric surgery and that identifying predictive factors may help to improve patient selection.
The aim of this report is to present a comprehensive analysis of baseline variables collected in LABS-2 to assess their association with weight outcomes at three years.
METHODS
PARTICIPANTS
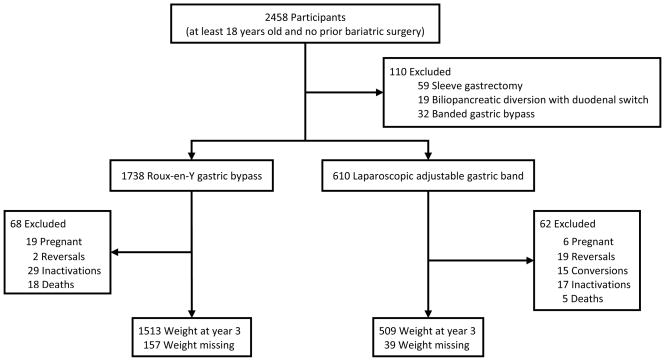
The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2), one of the studies in LABS, is an observational cohort study of 2458 adults undergoing an initial Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric band (LAGB) or other bariatric procedure at 10 centers across the United States.(2) Participants (1738 RYGB, 610 LAGB, 110 other procedure) underwent surgery between March 2006 and April 2009 (Figure 1). This report utilized data collected preoperatively and up to 3 years following surgery. Details regarding data collection, cohort characteristics, and 3 year results have been previously published.(1,18) The institutional review boards at each center approved the protocol and consent forms. LABS is registered at ClinicalTrials.gov (NCT00465829).
Figure 1.
Participants and weight measurements
aWeights of women in their second or third trimester and those up to 6-months postpartum were excluded from analyses.
WEIGHT CHANGE
Weight change is reported as the percent change from baseline and was measured within 30 days prior to surgery and 3 years following surgery. During in-person follow-up visits, weight was measured using a standard protocol on a study-purchased standard scale (Tanita Body Composition Analyzer, model TBF-310). If a protocol weight was not obtained, weight was measured by research or medical personnel on a non-study scale. If neither of these weights were available, a self-reported weight was used. Differences between measured and self-reported weights in this cohort were small, and did not systematically differ by measured BMI or degree of postoperative weight change. The average degree of underreporting by self-report was 0.7 kg for women and 1.0 kg for men.(19) The 36 participants whose initial bariatric procedure was subsequently reversed or revised to a different bariatric procedure before the 3 year weight measurement were excluded from this analysis. Women in their second or third trimester of pregnancy and those up to 6-months post-partum when weighed at year 3 were also excluded from analyses.
Three year weight change was analyzed as both a continuous and dichotomous outcome. Based on previous literature, u points for dichotomous weight change were chosen at 10% for LAGB and 25% for RYGB.(20–22)
PREDICTORS
Table 1 lists the 113 RYGB and 107 LAGB preoperative and operative potential predictive factors that were investigated. Demographics (age, gender, race, ethnicity, and marital status) were collected by self-report; as were socioeconomic variables (education, work for pay, work night or evening shifts, and annual household income). The work productivity and activity impairment questionnaire (WPAI),(23) general health version, was used to assess the impact of health problems on work productivity and regular daily activities outside of work.
Table 1.
Potential preoperative and operative predictors of weight loss at year 3
| Demographics |
| Eating Behaviors |
| Laboratory Values |
| Age |
| Gender |
| Race Ethnicity Marital status |
| Socioeconomic |
| Eat breakfast regularly |
| Eat lunch regularly |
| Eat dinner regularly |
| Eat breakfast, lunch, dinner regularly |
| Number of times eat per day |
| Number of restaurant meals per week |
| Number of fast food meals per week |
| Eat when feel full, more than once a week Eat when not hungry, more than once a week |
| Eat continuously during the day or part of the day |
| Binge eating |
| Preoperative Weight Change |
| Total cholesterol |
| Insulin |
| Proinsulin C-peptide |
| High-sensitivity C-reactive protein ALT |
| AST |
| Ghrelin Leptin |
| Comorbidities |
| Education |
| Work for pay |
| Work night or evening shifts |
| Annual household income |
| Work Productivity and Activity Impairment Percent of overall work impaired |
| Percent of daily activity impaired |
| Anthropometrics |
| Asthma |
| Abnormal kidney function Cardiovascular disease Diabetes |
| Dyslipidemia components |
| Hyperlipidemia Low HDL |
| High triglycerides |
| Hypertension |
| Musculoskeletal pain Pulmonary hypertension Severe walking limitation Sleep apnea |
| Urinary incontinence |
| Venous edema |
| WOMAC Osteoarthritis index Overall knee score |
| Overall hip score |
| Women’s Health |
| Special preoperative diet |
| Preoperative weight loss |
| Quality of Life and Interpersonal Support |
| Preoperative body mass index |
| Neck circumference Waist circumference |
| Sagittal abdominal diameter |
| Body mass index at age 18 |
| Alcohol, Smoking, and Illegal Drugs |
| Short Form 36-Item Health Survey |
| Physical component summary Mental component summary Physical functioning |
| Role-physical |
| Bodily pain General health Vitality |
| Social functioning |
| Role-emotional Mental health |
| Impact of Weight on Quality of Life – lite Overall |
| Work |
| Physical functioning Public distress |
| Sex life |
| Self-esteem |
| Interpersonal Support Evaluation List |
| Overall Appraisal Belonging Tangible |
| Importance of weight and shape on self-worth |
| Behavioral |
| Alcohol use disorder |
| Frequency of drinking alcohol Current or recent smoker Illegal drug use |
| Weight Loss Practices |
| Self-weigh at least weekly |
| See nutritionist or dietitian |
| See personal trainer or exercise specialist Kept a food diary |
| Number of dietary changes |
| Counted fat grams Decreased fat intake |
| Reduced number of calories eaten Used a very low calorie diet |
| Cut out between-meal-snacking Eaten fewer high carbohydrate foods Eaten special low calorie diet foods Eaten or drank meal replacements |
| Increased fruits and vegetables |
| Cut out sugar-sweetened beverages |
| Physical Function |
| Polycystic ovary syndrome |
| Menopause Prescription estrogen |
| Expectations |
| Acceptable percent weight loss |
| Laparoscopic Adjustable Gastric Band |
| Gastric Band size |
| Roux-en-Y Gastric Bypass |
| Laparoscopic or open procedure |
| Length of staple line Type of stapling line Banding or ring used |
| Route of alimentary limb ascension Length of biliopancreatic limb Length of alimentary limb |
| Beck Depression Inventory |
| Medication for psychiatric or emotional problems |
| Counseling for psychiatric or emotional problems Binge eating disorder |
| Loss of control eating |
| Night eating syndrome |
| Bulimia nervosa |
| Time to complete 400 meter corridor walk |
Anthropometrics (preoperative body mass index (BMI), neck circumference, and waist circumference) were assessed within 30 days of surgery by trained personnel following standardized protocols. Sagittal abdominal diameter was collected at the time of surgery by the surgeon. BMI at age 18 was collected by self-report and was only used in this analysis if the participant was at least 70% sure of their height and weight at age 18.(24)
Preoperative weight loss 3-months before surgery was self-reported as was having been on a special preoperative diet. Participants were considered to be on a special preoperative diet if they reported that they “usually” or “always” followed an advised special preoperative diet. Participants were not on a special preoperative diet if they followed the advised diet less frequently or were not advised to follow a special preoperative diet.
Frequency of self-weighing was self-reported. Participants were considered to regularly see a nutritionist/dietitian or trainer/exercise specialist if they self-reported seeing a specialist at least 6 times in the 6-months prior to surgery. Participants self-reported the frequency of different weight loss practices (e.g. count fat grams, kept a food diary). Participants were identified as engaging in the practice if they reported doing so for the last 26 weeks prior to surgery.
Quality of life was assessed using the Short Form 36-item Health Survey (SF-36) and the Impact of Weight on Quality of Life–Lite (IWQOL-lite) questionnaire. Interpersonal support was assessed using the Interpersonal Support Evaluation List (ISEL). In addition to these standardized measures, participants were also asked, “During the past 6 months, how important has your weight or shape been in how you feel about or evaluate yourself as a person – as compared to other aspects of your life, such as how you do at work, as a parent, or how you get along with other people?” Depression symptoms over the past week were measured with the Beck Depression Inventory (BDI), version 1.(25) Participants self-reported currently taking prescription medications for psychiatric or emotional problems and whether or not they attended counseling for psychiatric or emotional problems in the past 12-months. Binge eating disorder, loss of control eating, night eating syndrome, and bulimia nervosa were assessed via a self- reported questionnaire. Detailed definitions have been reported previously.(26) Alcohol use disorder, defined previously, and the frequency of alcohol use were based on self-report.(27) Current or recent smoking status (smoker in the previous year) and illegal drug use were also based on self-report.
Eating behaviors were assessed via a self-administered questionnaire. The form assessed the frequency of eating each type of meal per week (breakfast, lunch, and dinner), frequency of total snacks/meals per day, frequency of eating meals at fast food and non-fast food restaurants, frequency of continuing to eat when no longer hungry or full. Participants were identified as having regular meals if they had the meal 6 to 7 times per week. The form also asked, “During the past 6 months, have you had times when you eat continuously during the day or parts of the day without planning what and how much you would eat?”, a question designed to address grazing.(26)
As a measure of walking capacity, the time to complete the 400 meter long distance corridor walk, was recorded by trained study personnel.(28)
Definitions of asthma, urinary incontinence, severe walking limitation, venous edema, pulmonary hypertension, sleep apnea, hypertension, hyperlipidemia, diabetes, and abnormal kidney function have been published.(18) Low high-density lipoprotein cholesterol (HDL) was defined as less than 40 mg/dL and high triglycerides as fasting level of 200 mg/dL or greater. A LABS-certified clinical researcher used the best available information (patient interview or medical records) to determine the presence, absence, or history of cardiovascular disease (ischemic heart disease, congestive heart failure or stroke). Pain, stiffness and physical function related to osteoarthritis of the knee and hip was assessed using the overall scores of the Western Ontario and McMaster Universities osteoarthritis index (WOMAC).(29) Participants who self-reported having back or leg pain that was “very bothersome” or “extremely bothersome” in the past 4 weeks were identified as having musculoskeletal pain as were individuals who were taking opioids or steroids.
Laboratory values (total cholesterol, insulin, proinsulin, c-peptide, high sensitivity C reactive protein, ALT, AST, ghrelin, and leptin) were measured in a central laboratory.
Women’s health variables, polycystic ovary syndrome (PCOS) and menopause status were collected by self-report. Prescription estrogen was collected by self-report and by reviewing medical records.
Participants were asked to report their expectations about weight loss, specifically, a weight that they might not be particularly happy with, but could accept as their final weight following surgery. For analyses this expectation was converted to an acceptable percent weight loss from baseline.
Various gastric bands used in the surgical procedures were categorized as ‘large’ or ‘small’ depending on the band type and size recorded by the LABS-certified surgeon who performed the LAGB procedure. LABS-certified surgeons also reported RYGB procedure details: laparoscopic or open procedure, length of staple line as a measure of pouch size, type of stapling line, use of banding or ring, route of alimentary limb position, length of biliopancreatic limb, and length of alimentary limb.
STATISTICAL ANALYSIS
For RYGB and LAGB separately, descriptive statistics summarize three year weight change and baseline predictors. Frequencies and percentages are reported for categorical data. Medians and interquartile ranges (IQR) are reported for continuous data.
Simple linear and logistic regression models were used to investigate the relationship of each baseline predictor in Table 1 with three year weight change and dichotomous three year weight change, respectively, for each procedure using all available observations.
Multivariable linear and logistic regression models were constructed separately for each procedure to ascertain the statistical significance of independent baseline predictors of continuous and dichotomous three year weight change, respectively. Predictors that only apply to women (PCOS, menopause status, and prescription estrogen), only apply to participants who did not work night or evening shifts (night eating syndrome), and those that only applied to participants who were currently working (WPAI overall work impairment score) were included only in models restricted to those subgroups. Values for missing baseline predictors were imputed using multivariate imputation by chained equations.(30) For each procedure separately, imputation models were constructed for each predictor with missing data. Each imputation model included variables with a measure of association of 0.1 or greater with the predictor being imputed (using complete cases) or with the corresponding missing data indicator for that variable. The measures of association were Pearson’s correlation for continuous, binary, and ordinal variables, and Cramer’s V for nominal variables.
Variables that could be in the imputation model were removed from consideration if they were missing for at least 50% of those for whom imputation was needed. Three year percent weight change, site, age, sex, and baseline BMI were included in every imputation model. Surgery related variables were not used in the imputation models of non-surgery variables and only surgery related variables could be used to impute surgery variables. For each procedure, 100 completed datasets were generated.
Imputed values were inspected for implausible values by comparing the distributions of imputed and observed values. The potential impact of imputed data on the results was assessed by calculating the proportion of variation due to missing data for each unadjusted result and by comparing the unadjusted coefficient estimates of each predictor using the observed data and the observed plus imputed data.
Only participants with weight recorded at year three were included in the multivariable models. The multivariable linear regression models for three year weight change were constructed separately for each procedure by applying the Least Absolute Shrinkage and Selection Operator (LASSO),(31) specifically the group LASSO(32) to each completed dataset for each type of procedure. The LASSO algorithm relies on a penalty to set the parameters of the least important independent variables to zero. This analysis used the penalty factor that gave the model with the smallest squared prediction error with 5-fold cross-validation. Predictors with non-zero parameter estimates in each of the completed datasets were selected for the multivariable model for both RYGB and LAGB. The selected model was then fit to each completed dataset and the results combined following standard multiple imputation rules.
The same variables and model-building approach was used for multivariable logistic regression models with dichotomous dependent variables for weight change, i.e., weight change categories of at least vs. less than 25% weight loss for RYGB and at least vs. less than 10% for LAGB, using the maximum C-statistic as the criterion for the penalty factor and selecting the predictors with non-zero parameter estimates in each of the completed datasets. C-statistics range from 0 to 1, where C-statistic = 0.5 indicates that the model prediction for low weight loss is no better than chance.
The same model building strategies were used for analyses restricted to women only (including independent measures of women’s health), to employed participants (including measures of work productivity), and to day time workers (including night eating syndrome). Model diagnostics were performed to check for outliers and influential observations and to verify model assumptions. No outliers or influential observations were identified and all model assumptions were reasonable.
Analyses were conducted using R (version 3.0.2), including the mice package(30) and the SGL package.(32) All reported P-values are two-sided. P-values less than 0.05 are considered to be statistically significant.
RESULTS
Among the participants included in this analysis the observed median percent weight loss 3 years after surgery for participants who underwent RYGB was 31.5% (IQR, 24.6%-38.4%; range, 59.2% loss to 0.9% gain) of baseline weight and 16.0% (IQR, 8.1%-23.1%; range, 56.1% loss to 12.5% gain) for LAGB. The actual median 3-year weight loss was 40 kg (IQR, 31–52; range, 110 loss to 1 gain) for RYGB and 20 kg (IQR, 10–29; range, 75 loss to 20 gain) for LAGB. The median age was 46 years for RYGB and 48 years for LAGB; 80% of RYGB participants and 75% of LAGB participants were female; and the median BMI was 46 kg/m2 for RYGB and 44 kg/m2 for LAGB. As shown in Figure 1, 3 years after surgery weight was obtained for 1513 (91%) participants who underwent RYGB and 509 (93%) participants who underwent LAGB.
The unadjusted effects of each baseline measure shown in Table 1 are reported in eTables 1 and 2 for RYGB and LAGB, respectively. Unadjusted results for RYGB identified age, sex, race, BMI, neck circumference, self-weighing frequency, current or recent smoking within the last year, eating breakfast or all 3 daily meals regularly, frequently eating when feeling full, diabetes, hyperlipidemia, abnormal kidney function, and having higher weight loss expectations were significantly associated with weight change with differences in weight change ranging from 3.2% more weight loss among smokers to 4.4% less weight loss for those participants with diabetes prior to surgery. Most of the other unadjusted effects were small. For example, those with higher expectations for an acceptable post-surgery weight change lost 1.2% more weight than those with lower expectations and those who weighed themselves more often (weekly) lost 1.7% less weight, on average, than those who did so less frequently. Older RYGB participants, males, and Blacks lost less weight. Similarly, for LAGB participants the unadjusted effects were significant for sex, ethnicity, alcohol problems, weight loss expectations, and band size.
The multivariable linear regression model for RYGB participants presented in Table 2. Abnormal kidney function was associated with 2.3% more weight loss and current or recent smoking was associated with 2.6% greater weight loss. Black participants lost 2.7% less weight when compared to Whites. Participants with diabetes at baseline had 3.7% less weight loss at year 3 compared to participants without diabetes at baseline, on average. The use of meal replacements as a weight loss practice in the year leading up to surgery was associated with 3.0% more weight loss. Other factors that were statistically significant such as neck circumference, age, eating when full, self-weighing, weight loss expectations, and emotional counseling ranged in magnitude from 0.2% less weight loss for each centimeter larger neck circumference and 0.6% less weight loss (per 10 years of age) to 1.6% more weight loss (eating beyond feeling full). Of note, then model explains only 14% of the variability in 3 year weight loss R2=0.14.
Table 2.
RYGB baseline multivariable prediction models of three year weight change
| Difference in Percent Weight Change at Year 3a
|
|||
|---|---|---|---|
| Difference | 95% CI | P-value | |
| Age, per 10 years | 0.55 | (0.06, 1.03) | 0.03 |
| Race | 0.004 | ||
| White | (reference) | ||
| Black | 2.68 | (1.09, 4.27) | |
| Other | 0.18 | (−2.41, 2.77) | |
| Baseline BMI, per 5 kg/m2 | −0.32 | (−0.80, 0.15) | 0.18 |
| Neck circumference, per 1 cm | 0.19 | (0.06, 0.31) | 0.003 |
| Abnormal kidney function | −2.31 | (−4.27, −0.35) | 0.02 |
| Diabetes | 3.68 | (2.58, 4.78) | <0.001 |
| AST, per 10 IU/L | −0.31 | (−0.59, −0.02) | 0.04 |
| Leptin, per 5 ng/mL | −0.14 | (−0.28, 0.00) | 0.04 |
| Counseling for psychiatric or emotional problems | 1.51 | (0.32, 2.70) | 0.01 |
| Current or recent smoker | −2.63 | (−4.05, −1.21) | <0.001 |
| Eat breakfast regularly | 0.88 | (−0.17, 1.92) | 0.10 |
| Eat when feel full, more than once a week | −1.62 | (−2.74, −0.50) | 0.005 |
| Number of times eat per day | 0.052 | ||
| 1 – 2 | (reference) | ||
| 3 – 4 | −0.08 | (−2.15, 1.98) | |
| 5 – 6 | 1.06 | (−1.01, 3.13) | |
| 7 or more | 2.08 | (−0.36, 4.52) | |
| Eaten or drank meal replacements | 2.97 | (0.10, 5.84) | 0.04 |
| Self-weigh at least weekly | 1.28 | (0.26, 2.30) | 0.01 |
| Acceptable percent weight loss, per 5% | −0.82 | (−1.20, −0.45) | <0.001 |
|
Odds Ratio of less than 25% Weight Loss at Yearb
|
|||
| Odds Ratio | 95% CI | P-value | |
|
| |||
| Diabetes | 2.58 | (2.03, 3.28) | <0.001 |
| Acceptable percent weight loss, per 5% | 0.83 | (0.77, 0.90) | <0.001 |
Negative numbers indicate that the group, on average, achieved greater weight loss while positive numbers indicate relatively less weight loss.
Odds ratios greater than 1 indicate that the group is more likely to not lose at least 25% of baseline weight, on average.
For the dichotomous outcome the selected model had a c-statistic of 0.65 (Table 2) indicating that the dichotomous model does a poor job discriminating between those with and without less weight loss. For those participants with diabetes at baseline, the odds ratio for losing less than 25% of baseline weight was 2.58 (2.03, 3.28 95%CI, p< 0.001).
In the linear model for those undergoing LAGB there were no predictors that were consistently important in every completed dataset and the only predictor always identified in the dichotomous model was band size. Participants with a large band had 90% greater odds of losing less than 10% of their initial weight. After adjusting for age and baseline BMI, participants with a large band had 75% greater odds of losing less than 10% of their baseline weight (C-statistic 0.58, for both models).
DISCUSSION
The most consistent baseline variables associated with weight change were diabetes among those who underwent RYGB and band size for those undergoing LAGB. Despite the relatively large sample size in LABS-2, and the breadth of baseline factor data collected, a predictive model to adequately explain variability in weight change did not emerge.
Nevertheless, there is much to learn from these results. The importance of initial weight or BMI as a predictive factor, as shown in prior studies, was not confirmed in the final multivariable model.(4,6,7,12) The impact of older age found in other studies was confirmed in the final model for RYGB but the effect was small (less than 1% weight difference for a difference of 10 years) while the lack of a difference in weight change by sex was contrary to prior studies.(4,6,7) As in prior studies White race was confirmed to be associated with more weight loss compared to Blacks for those undergoing RYGB but the effect was small (2.7%).(4,8–10) There were some comorbid effects for those undergoing RYGB with diabetes (less weight loss) and abnormal kidney function (more weight loss). The association of diabetes with less weight loss for RYGB was in the same direction as in prior studies but the magnitude, overall, was smaller(3,5,7,12) and it is possible that the enhanced weight loss with kidney disease is perhaps a function of diuresis or other medication use in those individuals. There was not a significant association of any measured technical aspect of the RYGB on predicted weight loss, contrary to prior single reports indicating such effects.(3,33) Band size was found to be significant for LAGB with those having large bands more likely to lose less weight. Smoking was associated with more weight loss in the RYGB model and alcohol use disorder was associated with more weight loss for the LAGB in unadjusted analysis. Though these behaviors may act in some way to contribute to weight loss or be associated with unmeasured factors that are, they cannot be condoned as their negative health effects likely far outweigh any positive effects on weight loss, which are small. Such behaviors may occur after bariatric surgery, and clinicians must be vigilant for such behaviors in the post operative period.(16,27,34)
The negative findings of this study are of clinical importance. Mandated preoperative weight loss prior to bariatric surgery has been advocated in many publications and guidelines(35–40) but was not associated with 3 year weight change for either procedure. According to these results, that practice should be abandoned if it is required because of its presumed relation to post- operative weight loss. Mental health factors, quality of life, interpersonal support measures, and physical functioning did not predict weight change in these analyses, which raises questions concerning the relevance of routine mental health screening prior to bariatric surgery, again if it is considered to be necessary for optimum weight loss after surgery.(41–43) Weight loss practices, eating behaviors, and weight loss expectations prior to surgery were not found to be associated with meaningful changes in weight loss post-surgery either. For example, more frequent self-weighing before operation was associated with less weight loss afterwards, in contrast to well- characterized non-surgical weight loss advice and guidelines.(44–47) It is possible that there were changes in these variables (mental health, quality of life, behaviors) after surgery that were related to weight loss, but identifying such factors is beyond the scope of this investigation which attempts to identify factors prior to surgery that can help predict who will be more successful with respect to post-operative weight loss.
A limitation of the LABS cohort is that it is non-randomized and surgeon selection bias may exist. For example, surgeons may select for patients with a behavioral predisposition towards greater weight loss based upon prior successful preoperative weight loss efforts or other ill-defined factors which may confound the results of this study. However, this is likely to be the case in all surgical practices, and not unique to LABS. In that sense, the results from this investigation should be relevant in clinical practice. A strength of LABS is the large well characterized, multi-center cohort that has been carefully studied and characterized in a standardized way with data collected at baseline on a broad range of potential predictor variables.
In conclusion, we report a thorough analysis of a comprehensive set of potential baseline predictive factors on three year weight change and find few parameters that might be useful as predictors of weight loss success or failure following RYGB or LAGB. However, a combination of several of these small, independent effects together in an individual might result in a larger and more clinically important difference in weight change. For example, the relatively small subset of individuals undergoing a RYGB who are Black, non-smokers, with pre-operative diabetes may be at higher risk for weight loss failure. In addition, baseline clinical characteristics, as a group, are poor predictors of weight loss after bariatric surgery that are not particularly useful to select or deny surgical care for patients based on anticipated weight loss.
The small effect sizes of the few variables that do predict greater or less weight loss may identify small cohorts of individuals that have substantially different weight change than the “typical” patient. However, post operative behaviors and events may be more predictive and are the next targets for investigation. Identifying pre-operative factors for surgical weight loss may be helpful to enhance clinical decision-making for individuals considering bariatric surgery, but this analysis of LABS-2 baseline parameters demonstrates this is an elusive goal.
Supplementary Material
Acknowledgments
Funding/Support: LABS-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants U01 DK066557 (University of Pittsburgh, Data Coordinating Center); U01-DK66667 and UL1-RR024996 (Columbia- Presbyterian in collaboration with Cornell University Medical Center Clinical and Translational Research Center [CTRC]); U01-DK66568 and M01RR-00037 (University of Washington in collaboration with Cornell University Medical Center CTRC); U01-DK66471 (Neuropsychiatric Research Institute); U01-DK66526 (East Carolina University); U01-DK66585 and UL1- RR024153 (University of Pittsburgh Medical Center in collaboration with Cornell University Medical Center CTRC); and U01-DK66555 (Oregon Health & Science University).
Role of the Sponsor: The NIDDK scientists contributed to the design and conduct of the study, which included collection, and management of data. The project scientist from the NIDDK served as a member of the Steering Committee, along with the Principal Investigator from each clinical site and the Data Coordinating Center. The Data Coordinating Center housed all data during the study and performed data analyses according to a plan developed by the Data Coordinating Center biostatistician and approved by the Steering Committee. The decision to publish was made by the Longitudinal Assessment of Bariatric Surgery-2 Steering Committee, with no restrictions imposed by the sponsor. As a coauthor, an NIDDK scientist contributed to the interpretation of the data and preparation, review, and approval of the manuscript.
Glossary
- ALT
alanine transaminase
- AST
aspartate transaminase
- BDI
Beck Depression Inventory
- BMI
Body Mass Index
- GED
General Equivalency Diploma
- HDL
high-density lipoprotein cholesterol
- HS
high school
- ISEL
Interpersonal Support Evaluation List
- IQR
interquartile ranges
- IWQOL-lite
Impact of Weight on Quality of Life-Lite
- LABS
Longitudinal Assessment of Bariatric Surgery
- LAGB
laparoscopic adjustable gastric banding
- LASSO
Least Absolute Shrinkage and Selection Operator
- PCOS
polycystic ovary syndrome
- RYGB
Roux-en-Y gastric bypass
- SE
standard error
- SF-36
Short Form 36-item Health Survey
- WOMAC
Western Ontario and McMaster Universities osteoarthritis index
- WPAI
work productivity and activity impairment questionnaire
Footnotes
Conflict of Interest Disclosures: Dr. Courcoulas has received research grants from the NIH/NIDDK, EndoGastric Solutions, Nutrisystem®, is a project consultant for Apollo Endosurgery, and was a project consultant for Ethicon J & J Healthcare.
Declaration of transparency: The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Data sharing: no additional data available
Authors Contributions: Drs. Courcoulas and Christian had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Courcoulas, Christian, Belle, Mitchell, Pomp, Pories
Acquisition of data: Courcoulas, Mitchell, Pomp, Pories
Analysis and interpretation of data: Christian, Belle, O’Rourke, Kalarchian, Mitchell, Pories, Spaniolas
Drafting of the manuscript: Courcoulas, Christian
Critical revision of the manuscript for important intellectual content: Courcoulas, Christian, Belle, O’Rourke, Dakin, Dellinger, Flum, Kalarchian, Mitchell, Patterson, Wolfe, Pomp, Pories, Spaniolas, Steffen
Statistical analysis: Christian, Belle
Obtained funding: Courcoulas, Belle, Mitchell, Pomp, Pories
Study supervision: Courcoulas, Mitchell, Pomp, Pories
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anita P. Courcoulas, University of Pittsburgh Medical Center, Department of Surgery.
Nicholas J. Christian, University of Pittsburgh Graduate School of Public Health, Department of Epidemiology.
Robert W. O’Rourke, University of Michigan, Department of Surgery.
Greg Dakin, Weill Cornell Medical College.
E. Patchen Dellinger, University of Washington.
David Reed Flum, University of Washington.
Melissa Kalarchian, Duquesne University.
James E. Mitchell, Neuropsychiatric Research Institute.
Emma Patterson, Legacy Good Samaritan Hospital.
Alfons Pomp, Weill Cornell Medical College.
Walter J. Pories, East Carolina University.
Konstantinos Spaniolas, East Carolina University.
Kristine Steffen, North Dakota State University.
Bruce M. Wolfe, Oregon Health and Science University.
Steven H. Belle, University of Pittsburgh Graduate School of Public Health, Department of Epidemiology.
References
- 1.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–25. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Rel Dis. 2007;3:116–26. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos GM, Rabl C, Mulligan K, et al. Factors associated with weight loss after gastric bypass. Arch of Surg. 2008;143:877–83. doi: 10.1001/archsurg.143.9.877. discussion 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin AM, O’Connor EA, Genaw JA, Kawar S. Preoperative weight loss is not a predictor of postoperative weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Obes Rel Dis. 2008;4:481–5. doi: 10.1016/j.soard.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Lee WJ, Lee TS, et al. Prediction of successful weight reduction after bariatric surgery by data mining technologies. Obes Surg. 2007;17:1235–41. doi: 10.1007/s11695-007-9322-9. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;16:1227–31. doi: 10.1381/096089206778392284. [DOI] [PubMed] [Google Scholar]
- 7.Ortega E, Morinigo R, Flores L, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012;26:1744–50. doi: 10.1007/s00464-011-2104-4. [DOI] [PubMed] [Google Scholar]
- 8.Bayham BE, Bellanger DE, Hargroder AG, Johnson WD, Greenway FL. Racial differences in weight loss, payment method, and complications following Roux-en-Y gastric bypass and sleeve gastrectomy. Adv Ther. 2012;29:970–8. doi: 10.1007/s12325-012-0062-4. [DOI] [PubMed] [Google Scholar]
- 9.Cheung LK, Lal LS, Chow DS, Sherman V. Racial Disparity in Short-Term Outcomes after Gastric Bypass Surgery. Obes Surg. 2013;23:2096–103. doi: 10.1007/s11695-013-1034-8. [DOI] [PubMed] [Google Scholar]
- 10.Harvin G, DeLegge M, Garrow DA. The impact of race on weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2008;18:39–42. doi: 10.1007/s11695-007-9278-9. [DOI] [PubMed] [Google Scholar]
- 11.Dallal RM, Quebbemann BB, Hunt LH, Braitman LE. Analysis of weight loss after bariatric surgery using mixed-effects linear modeling. Obes Surg. 2009;19:732–7. doi: 10.1007/s11695-009-9816-8. [DOI] [PubMed] [Google Scholar]
- 12.Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity. 2014;22:888–94. doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolnerhanssen BK, Peters T, Kern B, et al. Predictors of outcome in treatment of morbid obesity by laparoscopic adjustable gastric banding: results of a prospective study of 380 patients. Surg Obes Rel Dis. 2008;4:500–06. doi: 10.1016/j.soard.2008.03.252. [DOI] [PubMed] [Google Scholar]
- 14.Busetto L, Segato G, De Marchi F, et al. Outcome predictors in morbidly obese recipients of an adjustable gastric band. Obes Surg. 2002;12:83–92. doi: 10.1381/096089202321144649. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JB, Dixon ME, O’Brien PE. Pre-operative predictors of weight loss at 1-year after Lap-Band surgery. Obes Surg. 2001;11:200–07. doi: 10.1381/096089201321577884. [DOI] [PubMed] [Google Scholar]
- 16.Heinberg LJ, Ashton K. History of substance abuse relates to improved postbariatric body mass index outcomes. Surg Obes Rel Dis. 2010;6:417–21. doi: 10.1016/j.soard.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22:70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 18.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Rel Dis. 2013;9:926–35. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christian NJKW, Yanovski S, Bessler M, et al. Validity of Self-Reported Weights Following Bariatric Surgery in the LABS-2 Cohort. JAMA. 2013 doi: 10.1001/jama.2013.281043. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benotti PN, Forse RA. The role of gastric surgery in the multidisciplinary management of severe obesity. Am J Surg. 1995;169:361–7. doi: 10.1016/s0002-9610(99)80177-9. [DOI] [PubMed] [Google Scholar]
- 21.Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD, Josbeno D. Optimizing long-term weight control after bariatric surgery: a pilot study. Surg Obes Rel Dis. 2012;8:710–5. doi: 10.1016/j.soard.2011.04.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–59. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 23.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132:1098–104. doi: 10.1542/peds.2013-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gel Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JE, King WC, Courcoulas A, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Dis. 2014 doi: 10.1002/eat.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–25. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King WC, Engel SG, Elder KA, et al. Walking capacity of bariatric surgery candidates. Surg Obes Rel Dis. 2012;8:48–59. doi: 10.1016/j.soard.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana JM, Escobar A, Arostegui I, et al. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Intern Med. 2006;166:220–6. doi: 10.1001/archinte.166.2.220. [DOI] [PubMed] [Google Scholar]
- 30.Van Buuren S. Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 31.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2. New York: Springer; 2009. [Google Scholar]
- 32.Simon N, Friedman J, Hastie T, Tibshirani R. A Sparse-Group Lasso. Journal of Computational and Graphical Statistics. 2013;22:231–45. [Google Scholar]
- 33.Brolin RE. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2007;246:163–4. doi: 10.1097/SLA.0b013e318070cb43. author reply 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148:145–50. doi: 10.1001/2013.jamasurg.265. [DOI] [PubMed] [Google Scholar]
- 35.Blackburn GL, Hu FB, Hutter MM. Updated evidence-based recommendations for best practices in weight loss surgery. Obesity. 2009;17:839–41. doi: 10.1038/oby.2008.572. [DOI] [PubMed] [Google Scholar]
- 36.Jamal MK, DeMaria EJ, Johnson JM, et al. Insurance-mandated preoperative dietary counseling does not improve outcome and increases dropout rates in patients considering gastric bypass surgery for morbid obesity. Surg Obes Rel Dis. 2006;2:122–127. doi: 10.1016/j.soard.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Lehman Center Weight Loss Surgery Expert P. Commonwealth of Massachusetts Betsy Lehman Center for Patient Safety andMedical Error Reduction Expert Panel on Weight Loss Surgery: executive report. Obesity Res. 2005;13:205–26. doi: 10.1038/oby.2005.30. [DOI] [PubMed] [Google Scholar]
- 38.Alami RS, Morton JM, Schuster R, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Rel Dis. 2007;3:141–145. doi: 10.1016/j.soard.2006.11.006. discussion 145–6. [DOI] [PubMed] [Google Scholar]
- 39.Solomon H, Liu GY, Alami R, Morton J, Curet MJ. Benefits to patients choosing preoperative weight loss in gastric bypass surgery: new results of a randomized trial. J Am Coll Surg. 2009;208:241–5. doi: 10.1016/j.jamcollsurg.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Tarnoff M, Kaplan LM, Shikora S. An evidenced-based assessment of preoperative weight loss in bariatric surgery. Obesity Surg. 2008;18:1059–61. doi: 10.1007/s11695-008-9603-y. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell JE, Steffen KJ, de Zwaan M, Ertelt TW, Marino JM, Mueller A. Congruence between clinical and research-based psychiatric assessment in bariatric surgical candidates. Surg Obes Rel Dis. 2010;6:628–34. doi: 10.1016/j.soard.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neff KJ, Olbers T, le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med. 2013;11:8. doi: 10.1186/1741-7015-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saltzman E, Anderson W, Apovian CM, et al. Criteria for patient selection and multidisciplinary evaluation and treatment of the weight loss surgery patient. Obesity Res. 2005;13:234–43. doi: 10.1038/oby.2005.32. [DOI] [PubMed] [Google Scholar]
- 44.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of obesity: Clinical applications. Marcel Dekker; New York: 2004. pp. 147–67. [Google Scholar]
- 45.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82(1 Suppl):230S–5S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 46.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.