Abstract
OBJECTIVE
To investigate medical decision-making capacity (MDC) in patients with brain metastasis.
METHODS
Participants were 41 adults with brain metastases with Karnofsky Performance Status scores ≥70 were recruited from an academic medical center and 41 demographically-matched controls recruited from the community. We evaluated MDC using the Capacity to Consent to Treatment Instrument (CCTI) and its four clinically relevant consent standards (expressing a treatment choice, appreciation, reasoning, and understanding). Capacity impairment ratings (no impairment, mild/moderate impairment, and severe impairment) on the consent standards were also assigned to each participant with brain metastasis using cutoff scores derived statistically from the performance of the control group.
RESULTS
The brain metastases patient group performed significantly below controls on consent standards of understanding and reasoning. Capacity compromise was defined as performance ≤1.5 standard deviations (SD) below the control group mean. Using this definition, approximately 60% of the participants with brain metastases demonstrated capacity compromise on at least one MDC standard.
CONCLUSION
When defining capacity compromise as performance ≤1.5 SD below the control group mean, over half of patients with brain metastases have reduced capacity to make treatment decisions. This impairment is demonstrated shortly after initial diagnosis of brain metastases and highlights the importance of routine clinical assessment of MDC following diagnosis of brain metastasis. These results also indicate a need for the development and investigation of interventions to support or improve MDC in this patient population.
Keywords: treatment consent capacity, decisional capacity, malignant brain tumor, medical ethics, functional abilities, neoplasm
INTRODUCTION
Medical decision making capacity, also known as treatment consent capacity, is a higher-order functional ability that refers to a person's cognitive and emotional ability to make informed decisions related to one's treatment and care[1, 2]. It is comprised of four consent abilities or standards derived from the medical and legal literature: expressing a treatment choice, appreciating the risks/benefits of a choice, reasoning about choices, and understanding the disease and treatment options[3, 4]. Given that a central tenet in providing high quality, ethically-sound clinical care is to allow patients the maximum level of autonomy and participation in their treatment decisions but to also protect patients who are incapable or marginally capable of making these decisions[3, 5–8], the study of treatment consent capacity in different patient populations is needed.
Medical decision making capacity is a key clinical and ethical concern in the treatment of patients with serious neurological and neuropsychiatric illness[3, 9, 10]. Although only about 7% of healthy older adults have impaired medical decision making capacity4,[11] prior studies noted that nearly all individuals with Alzheimer's disease4,[11] and approximately half of older adults with mild cognitive impairment have impaired medical decision making capacity[11]. In addition, we have reported that about half of patients with malignant glioma have impaired medical decision making capacity[12].
To our knowledge, there have been no prior studies investigating medical decision making capacity in patients with brain metastases. This is surprising given that brain metastases occurs in approximately 25% of the adult cancer population[13] and that such metastases affect cognition14 and presumably decisional capacity. After diagnosis, patients with brain metastases will be faced with numerous questions pertaining to their medical care but will often be presenting with notable neurocognitive, behavioral, and functional changes[14–20]. Consequently, judgments by clinicians about the competency or decision-making capacity of patients with brain metastases will be required. Thus, research on this topic is needed to guide clinicians, to increase public awareness, and to determine what future intervention efforts are needed.
In this study, we investigated cross-sectionally medical decision making capacity in a sample of patients with brain metastases. In an earlier study, we investigated the medical decision making capacity of another sample of patients with brain cancer (i.e., malignant glioma) and found that performances on the more cognitively demanding consent standards of reasoning and understanding were impaired in comparison to demographically-matched, healthy controls. Performances on the less cognitively demanding consent standards of expressing choice and appreciation did not differ significantly between the patient and sample groups. Thus, it was hypothesized that patients with brain metastases would exhibit deficits on the complex consent standards of reasoning and understanding relative to healthy demographically-matched controls. We further hypothesized that scores on the less complex consent standards of expressing choice and appreciation would be similar between the patient and control groups.
METHODS
This study was conducted at the University of Alabama at Birmingham (UAB). The research protocol met HIPAA standards and UAB's IRB approved all procedures. Written informed consent was obtained from either the participant and the participant's legally authorized representative (if applicable) prior to data collection. In cases of suspected impaired research consent capacity, consent was obtained from the legally authorized representative and assent was obtained from the research participant.
Setting and Population
Forty-one adults with brain metastases were recruited from the Department of Radiation Oncology and the Department of Neurosurgery at the University of Alabama at Birmingham (UAB) between August 2011 and May 2014 (see Figure 1). All diagnoses of brain metastases were made by a board-certified radiation oncologist. Eligible patient cases were aged ≥19 years, with at least one supratentorially-located metastatic brain lesion, and English speaking. We excluded patients with a prior brain tumor or cranial radiation (i.e., recurrent disease), prior/existing neurological or psychiatric illness, substance abuse history, or serious co-existing medical illness (other than cancer) adversely affecting cognition. Additionally, participants had to have a Karnofsky Performance Status (KPS) score[21] of 70 or greater.
Figure 1.
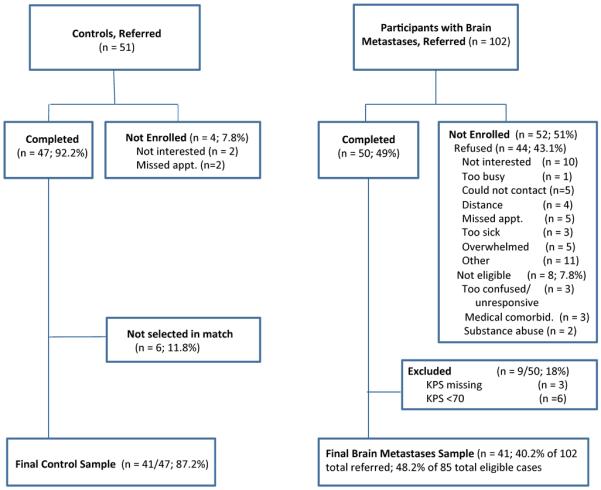
Sample for evaluation of medical decision making capacity in patients with brain metastases and controls.
Controls were 41 healthy adults who were volunteers (not relatives or friends of the patients) individually recruited from the community using advertisements. Controls were selected to match patients on age (±5 years) and education (±2 years). Controls met the same eligibility criteria as patient cases except no diagnosis of cancer or brain metastases. Controls were called over the telephone before study enrollment and asked a series of questions regarding medical and psychiatric health to screen out persons with a history of any medical or psychiatric conditions that could impair cognition. None of the controls reported any cognitive symptoms.
Data Collection
Study staff identified potentially eligible patients from physician referrals and/or discussion of cases at the weekly radiation oncology treatment rounds. Study staff obtained permission to contact patients, consented patients, and conducted testing.
All data for patients was collected after any postoperative visits (for patients having surgical resection) and either before or within a week of starting whole or focal brain radiation treatment. The following treatments for brain metastases were used: conventional surgery; single fraction radiosurgery with Gamma Knife or LINAC technology (15 Gy–24 Gy) for tumors ≤4cm; hypofractionated focal radiation with LINAC for tumors ≤3–4cm (5–6 Gy × 5 fractions for 25–30 Gy total); and whole brain radiation therapy (WBRT) (with LINAC) (30 Gy in 10 fractions to 37.5 Gy in 15 fractions). Off-study guidelines for radiosurgical treatment at UAB followed maximum tolerated doses outlined in RTOG 9005[22]. Assessment included neuropsychological testing (40 minutes), decisional capacity instrument (15 minutes), and structured self-report measures (quality of life, depression scales) (10 minutes). All data were collected at the same time. Results from these additional tests were reported in a separate paper[23]. Medical and treatment data was abstracted from medical records for the patients with brain metastases and was obtained by self-report for controls. Results from this research study were not used to guide participants' actual clinical treatment.
Measures
The primary outcome variable was treatment consent capacity performance as measured by Vignette B of the Capacity to Consent to Treatment Instrument (CCTI)[4]. The CCTI is a conceptually-based, reliable, and valid instrument designed to assess for medical decision-making ability (also known as treatment consent capacity) in adults[4, 24]. The CCTI comprises two clinical vignettes (Vignettes A and B). Vignette B was utilized for this study. This vignette presents a hypothetical medical problem and symptoms (i.e., cardiovascular disease) and two treatment alternatives with associated risks and benefits. Participants then answer standardized questions designed to test each of the following four core consent standards (Ss) that increase in complexity and cognitive burden from S1 to S5 [3, 4]:
S1: expressing a treatment choice (expressing choice);
S3: appreciating the personal consequences of a treatment choice (appreciation);
S4: providing rational reasons for a treatment choice (reasoning); and
S5: understanding the treatment situation, treatment choices, and respective risks/benefits (understanding).
A fifth experimental standard [S2] measures the ability to make a reasonable treatment choice but is not available for CCTI Vignette B. At present, normative data for the CCTI is not available. However, our group is developing normative data for the measure that will increase its utility at the individual patient level.
The CCTI was administered by the Principal Investigator or a trained research assistant and scored by the research assistant according to detailed, objective, and well-operationalized criteria[4].
The KPS scale was used as a measure of functional status[21]. A score of 100 indicates no complaints or evidence of disease. A score of 90 indicates minor disease symptoms and the ability to carry on normal activity. A score of 80 indicates obvious disease symptoms and the ability to engage in normal activities with effort. A score of 70 indicates obvious disease symptoms and the ability to independently care for self but lacking the ability to participate in active work. A score of 60 or below indicates that the person is no longer able to independently care for self.
Statistical Analyses
Demographic variables of patients with brain metastases and matched controls were analyzed using paired t-tests (age, education) and Pearson's chi-square tests (gender, race). Group comparisons on the CCTI consent standards were performed using paired t-tests (S4–S5), Wilcoxon signed rank test (S3), or Fisher's exact test (S1).
Assignment of psychometric cutoff scores derived from control group performance can be useful in categorizing level of decisional impairment and this approach has been successfully used in earlier capacity studies[4, 11, 25]. Each participant with brain metastases was assigned an impairment rating score based on control group performance. For S3–S5, an intact outcome was defined as a score >1.5 SD below the control mean for that standard; a mild/moderate impairment outcome was defined as a score ≤1.5 SD but >2.5 SD below the control mean; and severe impairment outcome defined as a score ≤2.5 SD below the control mean. For S1, which has a range of scores of 0–2, a no impairment rating was defined as a score of 2, mild/moderate impairment rating as a score of 1, and a severe impairment rating as a score of 0. For each standard's total score, the percentage of patients falling into the no impairment, mild/moderate impairment, and severe impairment ranges were calculated.
We had 80% power (α=0.05) to detect an effect size of 0.3 SD (small to medium effect size) between groups for the CCTI standard scores. Alpha of .01 was used for all analyses. Statistical analyses were conducted using IBM SPSS Version 22[26].
RESULTS
Sample Characteristics
As displayed in Table 1, the final sample of patients with brain metastases and healthy controls did not differ significantly in age, education, gender, or ethnicity. Table 1 also displays the clinical characteristics of the brain metastases sample. All participants in this study had KPS scores of 70 or greater, meaning they were independent with self-care activities and had minimal or no disability. Eight patients underwent surgical resection prior to testing. Thirty-one patients were within a week of starting brain radiation treatment (9 whole brain and 22 focal) at the time of the study assessment. All 10 patients included in this study that were not within a week of starting brain radiation treatment were assessed prior to beginning any radiation treatment for brain metastases. In total, 28 patients had undergone chemotherapy treatments in the past and 3 were receiving chemotherapy at the time of their study assessment. There were 12 patients treated with antiepileptic drugs and 25 patients treated with corticosteroids. All patients had supratentorial tumors.
Table 1.
Demographic Characteristics and Functional Status of Participants
| Range | Controls n = 41 | Patients with Brain Metastases n = 41 | P † | |
|---|---|---|---|---|
| Age | 31–84 | 57.78 (9.41) | 59.61 (12.37) | 453 |
| Gender, n (%) | .651 | |||
| Female | 24 (58.5) | 26 (63.4) | ||
| Male | 17 (41.5) | 15 (36.6) | ||
| Race, n (%) | .391 | |||
| African American | 9 (22.0) | 5 (12.2) | ||
| Caucasian | 32 (78.0) | 35 (85.4) | ||
| Asian-American | 0 (0) | 1 (2.2) | ||
| Education | 9–20 | 14.44(1.73) | 13.65 (2.74) | .128 |
| Karnofsky Performance Status, median | 70–100 | N/A | 80 | N/A |
| 100 | 2 (4.9) | |||
| 90 | 15 (36.6) | |||
| 80 | 15 (36.6) | |||
| 70 | 9 (22.0) | |||
| Primary Cancer Location | N/A | N/A | ||
| Lung* | 18 (43.8) | |||
| Melanoma | 8 (19.5) | |||
| Breast | 8 (19.5) | |||
| Colon | 2 (4.9) | |||
| Gynecological | 2 (4.9) | |||
| Head and Neck | 1 (2.4) | |||
| Renal | 1 (2.4) | |||
| Esophageal | 1 (2.4) | |||
| Number of Brain Metastases | N/A | N/A | ||
| 1 | 17 (41.5) | |||
| 2 | 6 (14.6) | |||
| 3 or more | 18 (43.9) | |||
| Tumor Location/Hemisphere | N/A | N/A | ||
| Right | 9 (22.0) | |||
| Left | 12 (29.2) | |||
| Both | 20 (48.8) | |||
| Assessed prior to treatment | N/A | 10 (24.4) | N/A | |
| Surgery | N/A | 8 (19.5) | N/A | |
| Focal Radiation | N/A | 22 (53.7) | N/A | |
| Whole Brain Radiation | N/A | 9 (22.0) | N/A | |
| Past chemotherapy use | N/A | 28 (68.3) | N/A | |
| Current chemotherapy use | N/A | 3 (7.3) | N/A | |
| AEDuse | N/A | 12 (29.3) | N/A | |
| Corticosteriod use | N/A | 25 (61.0) | N/A | |
| Extracranial Mets | N/A | 26 (63.4) | N/A |
Values are mean (SD) for age and education.
p value for test of group differences (age, education) or Pearson's chi square test (gender, race). Race analyzed white compared to other using Pearson's chi square test.
14 non-small cell, 3 small cell, 1 mixed small cell and large cell lung cancer.
CCTI
Performance of controls and patients with brain metastases on the CCTI standards can be found in Table 2. As expected, significant differences were observed between controls and patients with brain metastases on understanding (S5) and reasoning (S4), but not on appreciation (S3) or expressing choice (S1).
Table 2.
Comparisons between Controls and Patients with Brain Metastases on CCTI Consent Standards (S)
| Measures | Range | Controls n = 41 | Patients with Brain Metastases n = 41 | t , z, or χ2 | df | p † |
|---|---|---|---|---|---|---|
| S1, expressing choice | 0–2 | 1.95 (0.22) | 1.98 (0.16) | .346 | 1 | .500 |
| S3, appreciation | 0–4 | 3.66 (0.73) | 3.34 (1.02) | −1.668 | N/A | .095 |
| S4, reasoning | 0–8 | 4.76(1.26) | 3.32 (1.88) | 4.632 | 70.0 | <.001 |
| S5, understanding | 0–41 | 33.61 (5.33) | 26.76 (9.05) | 5.308 | 64.8 | <.001 |
Values are mean (SD).
p value for t test of group differences (S4 and S5), Wilcoxon test (S3), or Fisher's exact test (S1).
Capacity outcomes are displayed in Table 3. Capacity compromise was defined as performance ≤1.5 standard deviations (SD) below the control group mean, with mild/moderate impairment defined as a score ≤1.5 SD but >2.5 SD below the control mean and severe impairment defined as a score ≤2.5 SD below the control mean. When applying these definitions, 25 (61%) of all brain metastases patients exhibited some type of consent capacity deficit. In total, 1 (2.4%) patient had impairment in expressing choice, 17% were impaired in appreciation, 39% were impaired in reasoning, and 46% were impaired in understanding. The CCTI is designed to increase in complexity from S1–S5, with reasoning and understanding being the two most complex standards, which was reflected by patient performance. As complexity of the consent standard increased, so did the number of patients exhibiting compromised capacity.
Table 3.
Capacity Outcomes for Patients with Brain Metastases on CCTI Consent Standards
| Standards | No Impairment | Mild/Moderate Impairment | Severe Impairment |
|---|---|---|---|
| S1, expressing choice | 40 (97.6) | 1 (2.4) | 0 (0) |
| S3, appreciation | 34 (82.9) | 4 (9.8) | 3 (7.3) |
| S4, reasoning | 25 (61.0) | 7 (17.1) | 9 (22.0) |
| S5, understanding | 22 (53.7) | 11 (26.8) | 8 (19.5) |
Note. Values are n (%).
Brain metastases patient group sample size was 41.
CONCLUSIONS
Consistent with a priori expectations, medical decision making capacity compromise was high in this cohort of patients with brain metastases and increased in accordance with decisional complexity. Although participants with brain metastases did not differ from healthy controls on the simple consent standard of expressing choice, performance on the more complex standards of reasoning and understanding was impaired relative to demographically-matched controls. In relation to the control group, over half of the patients with brain metastases examined in this study exhibited some level of medical decision making capacity impairment (i.e., performance ≤1.5 SD below control group mean). These results, which are similar to those noted in an earlier study of patients with malignant glioma[12], suggest that capacity compromise is a typical occurs frequently in patients with malignant brain tumors. Implications of these findings are discussed below.
As noted, participants with brain metastases performed below controls on the more complex consent standards of understanding and reasoning. Thus, around the time that brain metastasis is first detected and treatment options are presented, many of these patients will already have impaired capacity to make treatment decisions. This finding parallels the broader neuropsychological literature on cognitive impairment in patients with brain metastases, which has found neurocognitive impairment in up to 90% of patients at the time brain metastasis is first detected[14].
A similar pattern of results emerged with respect to capacity impairment ratings. When capacity compromise was defined as performance ≤1.5 standard deviations (SD) below the control group mean, patients with brain metastases demonstrated high proportions of capacity compromise (combined mild/moderate and severe impairment ratings) on the standards overall. Although nearly all of the patients were able to express a treatment choice, 20% had difficulty appreciating the emotional and practical consequences of a treatment choice and nearly half of the sample had impaired ability to understand and reason about treatment choices. At the same time, our study demonstrated heterogeneity in the medical decision making capacity of patients with brain metastases. Even on the most stringent standard (understanding), approximately half of patients still possessed an ability to comprehend information about treatment, and by inference likely continued to possess MDC. This variability in performance of individual patients on the CCTI underscores the importance of careful evaluation of treatment consent capacity in these patients on a case-by-case basis. Impairment in individual cases may relate to a number of factors, including brain tumor related effects, treatment side-effects, comorbid medical and psychiatric symptoms, stress of the diagnosis itself, and patient variables (e.g., age and education)[14–20]. Additional investigation is needed to better delineate the different characteristics of patients who have impaired MDC, and we are pursuing this topic in a follow-up paper.
Understanding in a medical context is the most cognitively demanding consent standard[3, 4] and requires a patient to encode, consolidate, and recall information. This standard is factually intensive and successful performance relies heavily on short-term verbal memory[4, 12, 28], which is a common cognitive impairment among patients with brain metastasis[14, 23, 27]. Because patients with brain metastases have been shown to exhibit cognitive deficits characteristic of striatofrontal dysfunction, verbal memory seems to be a particular area of vulnerability[23]. As such, it is not surprising that half of the patients with brain metastases were impaired on this standard.
Reasoning is a fairly stringent consent standard that requires a person to reason and compare relative risks and benefits of various treatment alternatives, and to use that information to arrive at a treatment decision[3, 4]. However, generating coherent reasons for a treatment choice appears to be a very challenging task for many patients with brain metastases, as nearly 40% of our patient sample exhibited compromised capacity on this standard. This finding is logical given that verbal memory and semantic knowledge have been noted as being associated with reasoning in patients with brain cancer[12] and also found to be primary cognitive impairments in patients with brain metastases[23].
An interesting finding of this study was that our patient cohort performed very similar to another group of patients with brain cancer (i.e., malignant glioma) described in a prior study[12]. In both studies, approximately half of patients with brain cancer demonstrated impaired understanding relative to demographically-matched controls. In addition, the two groups of patients with brain cancer performed almost identically on reasoning and very similarly on appreciation. Taken together, the similarities between these two samples of patients with brain cancer suggests that consent capacity needs to be carefully assessed in patients with intracranial brain tumors regardless of primary versus secondary status.
These findings have other clinical and research implications. Clinically, our finding that nearly half of the patients with brain metastases had compromised capacity ratings shortly after diagnosis suggests that MDC should be carefully considered in all patients with brain metastases Although rapid medical intervention is critical for increasing survival in patients with brain metastases[29], there are also potential side-effects associated with treatments that need to be considered during the medical decision making process[30]. Our findings suggest that many patients with brain metastases may lack the comprehension and reasoning skills necessary to make informed treatment decisions. Accordingly, clinicians treating these patients are encouraged to give careful attention to the informed consent process while working with this patient population. Clinical recommendations include simplifying the language used in the verbal dialogue and in any written consent forms[31], reducing information load by presenting information in manageable segments[32], asking patients to explain the information presented to them[33], questioning participants to verify adequate comprehension of the treatment information presented[32], using multiple modalities (auditory and visual) to convey treatment information, or administering formal capacity measures like the CCTI. Using these approaches can help ensure that an obtained consent to treatment is valid and supports the ability of patients with milder consent impairments in continuing to make informed treatment decisions. In regards to research, a large proportion of primary brain cancer patients show impairments in research consent capacity[34]. Although this study did not specifically assess the related but distinct consent ability of research consent capacity, there is preliminary support that the strategies to enhance treatment consent capacity can also be beneficial in research settings[33].
In clinical practice, it is common for patients to attend advanced cancer care appointments with a family member or friend. Given the high rates of decisional impairment demonstrated in this sample, persons diagnosed with brain metastases should be encouraged to bring someone else with them to their medical appointments. In addition to providing emotional support, family and friends can provide cognitive support by helping the patient remember and comprehend the treatment information. They can take notes, ask questions, and review written materials to educate themselves about the various treatment options. This information would then be available for discussion after the session has ended. In addition, family members can help the patient remember future appointments and medical care instructions. Although this topic needs additional investigation, having adequate social support might improve clinical decision making and lead to better outcomes for adults with diminished capacity. Additional guidance is needed in terms of what to do with patients lacking adequate social support or with patients who prefer to let others make their medical decisions.
There are several limitations to be considered. First, the CCTI exhibits scientific value by providing a standardized means of evaluating treatment consent capacity across various patient groups. The CCTI also exhibits clinical value by providing clinicians a means to objectively evaluate different consent abilities, thus, allowing for better informed judgments regarding treatment consent capacity to be made. However, performance on the CCTI is not, by itself, representative of a patient's actual clinical competency status and, therefore, not designed to replace or supersede judgments made by clinicians about a patient's treatment consent capacity. Further, state, province, and country law dictate and define legal standards of treatment consent capacity. Thus, performance on the CCTI is not representative of a patient's actual legal competency status and should not be used by clinicians in this regard. Second, while our study population was comparable to other brain metastases patient populations, participants who agree to be in research studies may not fully reflect the general population. In addition, the sample utilized in the current study was comprised of a somewhat heterogeneous group of patients with brain metastasis. However, the size of the sample included in this study limited our ability to evaluate for the effects of treatment (i.e., radiation, chemotherapy, resection) on medical decision making capacity. Third, we excluded participants with poor performance status, so it is likely that the rates of impairment in the general brain metastases patient population are even higher than that reported in our study. Fourth, our approach to assessing consent capacity was based on performance on clinical vignettes. However, a single instrument cannot account for all of the medical, legal, ethical, and other factors that inform a competency decision; therefore, objective measures such as the CCTI can assist, but do not replace the clinician[35]. In addition, patients may respond differently to hypothetical vignettes than to actual medical situations. For example, the emotional reaction associated with real-life medical decisions may not triggered by hypothetical vignettes[4, 36]. However, the use of standardized vignette-based measures allows better comparison across different patients and patient groups, and this is especially important in diseases such as brain metastases where there are often multiple options for treatment. Finally, the current study utilized the CCTI to evaluate for treatment consent capacity. However, different capacity instruments are constructed differently and, therefore, the findings of the current study should be replicated using different capacity measures.
In terms of future directions, cross-validation of the findings in other samples of patients with brain metastases would further establish external validity of the study findings. Second, investigations of clinical predictors of MDC in this patient population are needed. Third, studies comparing consent capacity in patients with cancer with and without brain tumors would help further differentiate the effect of CNS versus non-CNS disease on MDC. Fourth, longitudinal studies are needed to determine how decisional capacity may change over time in this patient population. Finally, there is a need for research examining the feasibility and utility of interventions to support or improve medical decision making in patients with intracranial brain tumors.
Acknowledgements
This research was supported by a NIH/NCATS (KL2 TR000166; Triebel) and by funds from the UAB Department of Neurology. The authors thank the UAB research staff and the Radiation Oncology Residents for their assistance with recruitment and data collection. Statistical analyses were carried out by Dr. Triebel and were reviewed by Dr. Cutter, UAB, Birmingham, AL.
This study was funded by a grant from the NIH/NCATS (KL2 TR000166) (Primary Investigator: Kristen L. Triebel).
Footnotes
Disclosures: A portion of the study results were presented at the Association for Clinical and Translational Science Annual Conference in Washington D.C. on April 11, 2014.
REFERENCES
- 1.Grisso T. Evaluating Competencies: Forensic Assessments and Instruments. Plenum Press; New York: 1986. [Google Scholar]
- 2.Tepper A, Elwork A. Competency to consent to treatment as a psychological construct. Law and Human Behavior. 1984;8:205–223. doi: 10.1007/BF01044693. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum P, Grisso T. Assessing patients' capacities to consent to treatment. N Engl J Med. 1988;319:1635–1638. doi: 10.1056/NEJM198812223192504. [DOI] [PubMed] [Google Scholar]
- 4.Marson DC, Ingram K, Cody H, Harrell L. Assessing the competency of patients with Alzheimer's disease under different legal standards. A prototype instrument. Arch Neurol. 1995;52(10):949–54. doi: 10.1001/archneur.1995.00540340029010. [DOI] [PubMed] [Google Scholar]
- 5.Berg JW, Appelbaum PS, Lidz CW, Parker LS. Informed consent: legal theory and clinical practice. 2nd edn Oxford University Press; New York: 2001. [Google Scholar]
- 6.Appelbaum P, Roth L. Clinical issues in the assessment of competence. American Journal of Psychiatry. 1981;138:1462–1467. doi: 10.1176/ajp.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum P, Gutheil T. Clinical Handbook of Psychiatry and the Law. 2nd edn Williams & Wilkins; Baltimore, MD: 1991. [Google Scholar]
- 8.Grisso T, Appelbaum P. Assessing competence to consent to treatment: a guide for physicians and other health professionals. Oxford University Press; New York: 1998. [Google Scholar]
- 9.Palmer BW, Savla GN. The association of specific neuropsychological deficits with capacity to consent to research or treatment. J Int Neuropsychol Soc. 2007;13(6):1047–59. doi: 10.1017/S1355617707071299. [DOI] [PubMed] [Google Scholar]
- 10.Marson DC, Harrell L. Executive dysfunction and loss of capacity to consent to medical treatment in patients with Alzheimer's disease. Semin Clin Neuropsychiatry. 1999;4(1):41–49. doi: 10.1053/SCNP00400041. [DOI] [PubMed] [Google Scholar]
- 11.Okonkwo OC, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69(15):1528–35. doi: 10.1212/01.wnl.0000277639.90611.d9. [DOI] [PubMed] [Google Scholar]
- 12.Triebel KL, Martin RC, Nabors LB, Marson DC. Medical decision-making capacity in patients with malignant glioma. Neurology. 2009;73(24):2086–92. doi: 10.1212/WNL.0b013e3181c67bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posner JB. Neurologic complication of cancer. F.A. Davis Company; Philadelphia, PA: 1995. [Google Scholar]
- 14.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–65. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 15.Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60(2):277–83. doi: 10.1227/01.NEU.0000249272.64439.B1. [DOI] [PubMed] [Google Scholar]
- 16.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 17.Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72(5):1311–8. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Newton HB. Symptom management and supportive care of the patient with brain metastases. In: Raizer JJ, Abrey LE, editors. In Brain Metastases. Springer; New York, NY: 2007. pp. 53–74. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71(1):64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 20.Platta CS, Khuntia D, Mehta MP, Suh JH. Current treatment strategies for brain metastasis and complications from therapeutic techniques: a review of current literature. Am J Clin Oncol. 2010;33(4):398–407. doi: 10.1097/COC.0b013e318194f744. [DOI] [PubMed] [Google Scholar]
- 21.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod C, editor. In Evaluation of Chemotherapeutic Agents. Columbia University Press; New York, NY: 1949. pp. 199–205. [Google Scholar]
- 22.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–8. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 23.Dymek MP, Marson DC, Harrell L. Factor structure of capacity to consent to medical treatment in patients with Alzheimer's disease: An exploratory study. Journal of Forensic Neuropsychology. 1999;1:27–48. [Google Scholar]
- 24.Triebel KL, Martin RC, Novack TA, et al. Treatment consent capacity in patients with traumatic brain injury across a range of injury severity. Neurology. 2012;78(19):1472–8. doi: 10.1212/WNL.0b013e3182553c38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IBM SPSS Statistics for Windows. Version 22.0 IBM Corp; Armonk, NY: 2013. [Google Scholar]
- 26.Herman MA, Tremont-Lukats I, Meyers CA, et al. Neurocognitive and functional assessment of patients with brain metastases: a pilot study. Am J Clin Oncol. 2003;26(3):273–9. doi: 10.1097/01.COC.0000020585.85901.7C. [DOI] [PubMed] [Google Scholar]
- 27.Gerstenecker A, Nabors LB, Meneses K, et al. Cognition in patients with newly diagnosed brain metastasis: profiles and implications. J Neurooncol. 2014 doi: 10.1007/s11060-014-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okonkwo OC, Griffith HR, Beleu K, et al. Cognitive models of medical decision-making capacity in patients with mild cognitive impairment. J Int Neuropsychol Soc. 2008;14(2):297–308. doi: 10.1017/S1355617708080338. [DOI] [PubMed] [Google Scholar]
- 29.Wen PY, Black PM, Loeffler PM. Metastatic brain cancer. In: Devita V, Hellman S, Rosenberg SA, editors. In Cancer: Principles and Practice of Oncology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2655–2670. [Google Scholar]
- 30.Kleinberg LR. Brain metastases: A Multidisciplinarym Approach. Demos Medical Publishing; New York: 2009. [Google Scholar]
- 31.Grisso T, Appelbaum P. Mentally ill and non-mentally ill patients' abilities to understand informed consent disclosure for medication. Law and Human Behavior. 1991;15:377–388. doi: 10.1007/BF02074077. [DOI] [PubMed] [Google Scholar]
- 32.Taub H, Kline G, Baker M. The elderly and informed consent: effects of vocabulary level and corrected feedback. Experimental Aging Research. 1991;7:137–146. doi: 10.1080/03610738108259796. [DOI] [PubMed] [Google Scholar]
- 33.Mittal D, Palmer BW, Dunn LB, et al. Comparison of two enhanced consent procedures for patients with mild Alzheimer disease or mild cognitive impairment. Am J Geriatr Psychiatry. 2007;15(2):163–7. doi: 10.1097/JGP.0b013e31802dd379. [DOI] [PubMed] [Google Scholar]
- 34.Marson DC, Martin RC, Triebel KL, Nabors LB. Capacity to consent to research participation in adults with malignant glioma. J Clin Oncol. 2010;28(24):3844–50. doi: 10.1200/JCO.2009.27.9091. [DOI] [PubMed] [Google Scholar]
- 35.Marson DC, Schmitt FA, Ingram KK, Harrell LE. Determining the competency of Alzheimer patients to consent to treatment and research. Alzheimer Dis Assoc Disord. 1994;8(Suppl 4):5–18. [PubMed] [Google Scholar]
- 36.Fitten LJ, Waite MS. Impact of medical hospitalization on treatment decision-making capacity in the elderly. Archives of Internal Medicine. 1990;150:1717–1721. [PubMed] [Google Scholar]


