Abstract
Over a decade and a half of intensive study has shown that the Transient Receptor Potential family ion channels TRPV1 and TRPM8 are the primary sensors of heat and cold temperatures in the peripheral nervous system. TRPV homologues and TRPA1 are also implicated, but recent genetic evidence has diminished their significance in thermosensation and suggests that a number of newly identified thermosensitive channels, including TRPM3, two-pore potassium channels, and the chloride channel Ano1, require further consideration. In addition to novel thermostransducers, recent genetic and pharmacological approaches have begun to elucidate the afferent neurocircuits underlying temperature sensation, continuing the rapid expansion in our understanding of the cellular and molecular basis of thermosensation that began with the discovery of TRPV1 and TRPM8.
INTRODUCTION
The ability to detect temperature change enables mammals to find suitable thermal climes, maintain core body temperature, and perceive painful (nociceptive) stimuli. This important process is initiated in peripheral terminals of dorsal root (DRG) or trigeminal ganglia (TG) neurons where the intensity and quality of these stimuli are converted into neural activity and conveyed to the CNS. Thermosensory afferents fall into four subtypes based on their temperature response range; from innocuous warm (30°C–43°C) to noxious heat (>43°C), innocuous cool (15°C – 30°C) to painful cold (< 15°C). Over the last decade and a half, ion channels of the TRP family have been recognized as the principle detectors of thermal stimuli in the peripheral nervous system [1], yet recent genetic analyses in mice finds that some of these channels’ roles in acute thermosensation are limited at best, suggesting that other molecular mechanisms underlying thermosensation are yet to be identified. Here, we discuss these recent results, as well as describe several newly identified candidate thermostransducers and their putative roles in thermotransduction. In spite of these new observations, a select few channels relevant to thermosensation have been used as molecular gateways to study the cellular basis of thermosensation, with recent evidence supporting the presence of distinct afferent labeled lines for heat and cold sensation, a topic discussed herein.
Are TRPV channels required to heat sensation?
The landmark discovery of the heat-activated channel TRPV1 as the receptor for capsaicin, the pungent ingredient in chili peppers that produces a sensation of heat, provided the first insights into the molecular basis for thermotransduction [2]. TRPV1 expression, its temperature threshold of 43°C, gating mechanisms [3], and other functional properties clearly show its major role in noxious heat perception (for review see [4]). However, mice lacking TRPV1 channels (Trpv1−/−), while less-sensitive to extreme heat, still retain moderate heat sensitivity, with the most profound phenotype being a total absence of inflammatory thermal hyperalgesia [5,6].
What then underlies TRPV1-independent heat sensation? TRPV1 homologues, such as TRPV2, TRPV3, and TRPV4 are also heat-sensitive when expressed heterologously, with TRPV2 activated above 52°C [7], and both TRPV3 (which is expressed in keratinocytes) and TRPV4 responsive to warmth (~26–34°C) [8–10]. However, cellular and behavioral heat responses are normal in Trpv2−/− mice, and no additional thermosensory deficits occur when both TRPV1 and TRPV2 are absent [11*]. Warm preference and acute heat responses were initially reported to be impaired in Trpv3−/− and Trpv4−/− mice [12,13]. However, preliminary knockout characterizations were performed on mixed strain mice, which can strongly influence animal behaviors. Indeed, no deficiencies were recently observed in Trpv3−/− and Trpv4−/− animals on pure genetic backgrounds, nor any differences in mice lacking both channels [14*]. These findings suggest that previous reports of thermosensory deficits were strain-dependent, and what underlies the differences in heat evoked behaviors between different mouse strains remains to be determined.
Are there other heat receptors?
The diminishing the significance of TRPV2–4 in heat sensation suggest the existence of additional thermosensors, leading many to test the thermosensitivity of other TRP channels. However, only TRPM3 (melastatin), which is expressed in ~80% of DRG and TG neurons, has thermosensory properties in vitro and in vivo [15**]. Warmth (30–35°C) evokes TRPM3 currents and potentiates agonist-evoked (pregnenolone sulfate; PS) responses in vitro. PS induces nociceptive behaviors in wildtype mice and Trpm3−/− animals are less sensitive to noxious heat and deficient in inflammatory thermal hyperalgesia, a phenotype remarkably similar to Trpv1−/− mice [5,6,15]. TRPM3 is an intriguing channel requiring further study due to its many alternatively-spliced isoforms, role in modulating insulin release and the secretion of inflammatory cytokines, and a novel, agonist-dependent dual permeation pathway [16].
TRP-independent candidate thermosensors have recently emerged. For example, the Ca2+-activated Cl− channel Anoctamin 1 (ANO1) is activated by noxious heat (>44°C), even in the absence of intracellular Ca2+, and acute and pathological heat-evoked behaviors are reduced in mice with sensory neuron-specific deletion of Ano1, or when the channel is blocked pharmacologically [17**,18*]. In addition, Orai channels cluster with stromal interacting molecule 1 (STIM1) upon depletion of intracellular Ca2+ stores, thereby leading to channel gating and store-operated Ca2+ currents. Surprisingly, heat (>35°C) also induces STIM1 clustering which leads to Orai channel activation when temperatures reduce [19], yet it remains to be elucidated if such a mechanism leads to changes in somatosensory responses. When taken as a whole, the molecular basis for heat sensation is diverse and future studies into the role of these newly described thermosensors, and others yet to be found, should expand our understanding of mammalian thermosensation (Figure 1).
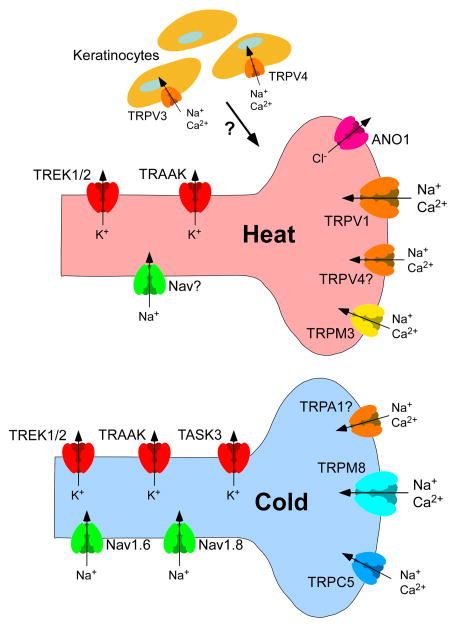
Figure 1. Molecules mediating thermosensation.
The sensations of heat and cold are transduced by distinct subsets of sensory neurons characterized by their expression of TRPV1 and TRPM8, respectively. Ion channels like TRPM3 and ANO1 also contribute to heat detection. Similarly, TRPM8-independent molecular mechanisms of cold-sensitivity include cold-activated channels like TRPC5. Even though the role of the TRPA1 channel as a cold sensor is controversial, it contributes to cold hypersensitivity in pathological conditions. Furthermore, the differential expression of Na+ and K+ channels, like Nav1.6, Nav1.8, TREK1/2, TRAAK and TASK3 modulates the temperature thresholds of TRPV1- and TRPM8-expressing sensory afferents. In addition to neurons, keratinocytes expressing the temperature-sensitive TRPV3 and TRPV4 channels may contribute, but recent evidence questions their role in thermosensation.
Molecular basis of cold transduction
The number of molecules linked to cold reception is small compared to heat, but as with the chili pepper, the first insights were obtained via a naturally occurring plant product, menthol, the cooling mimetic of mint. TRPM8 was cloned based on its sensitivity to menthol and is activated at temperatures below 26°C, with currents increasing in a largely linear manner into the noxious range (<15°C) [20,21]. Trpm8−/− mice are insensitive to innocuous cool temperatures and partially deficient in response to noxious cold, but retain some cold sensitivity [22–25**].
TRPA1 (ankyrin), a channel expressed in a subset of TRPV1+ neurons, was initially observed to be cold-activated (~17°C threshold) [26]. However, the role of TRPA1 as a noxious cold-sensor is controversial as others fail to observe cold activation, and the channel is robustly activated by a plethora of agonists that are not perceived as cold (for review see [4]). Furthermore, agonist-evoked currents are ~20-fold larger than cold currents [1], and TRPA1 channels have distinct thermosensory functions in different species [4]. Studies of Trpa1−/− mice are inconclusive as well [27–30], and the preponderance of evidence suggests TRPA1 plays no role in acute cold sensation. However, endogenous and exogenous TRPA1 agonists induce cold hypersensitivity, and cold potentiates agonist-evoked TRPA1 currents [30], suggesting that TRPA1 channels play an important role in cold pain after injury (Figure 2).
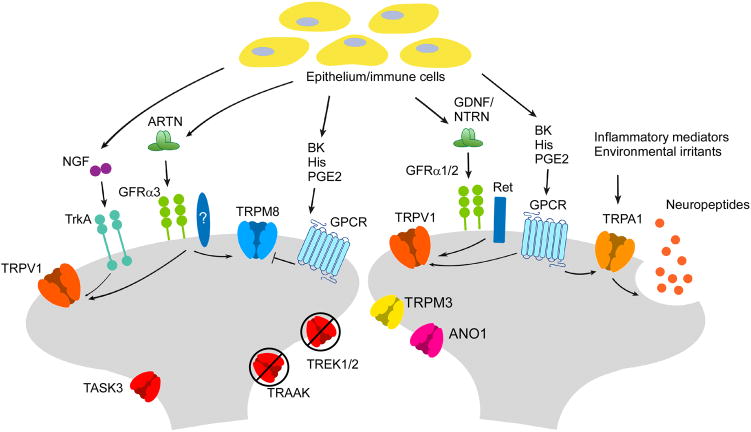
Figure 2. Mechanisms of thermal hypersensitivity.
Proalgesic agents, released from epithelium or immune cells or from nerve terminals neurogenically, modulate neuron activity by binding to their receptors, which then influence neuron activity through downstream effects. TRPV1 mediates heat hyperalgesia by becoming sensitized downstream of a large number of inflammatory mediators. TRPA1 is involved in heat, cold and mechanical hyperalgesia after injury, both by being activated by channel agonists and by perpetuating neurogenic inflammation. TRPM8/TRPV1 co-expressing neurons are sensitized to cold by NGF and artemin, while non-TRPV1 expressing TRPM8 neurons (involved in sensing innocuous cool and cold-mediated analgesia) are inhibited by certain inflammatory mediators. Cold hyperalgesia also occurs as a result of the downregulation of “excitability breaks” such as the K2P class of K+ channels.
TRPM8 is also involved in both inflammatory and neuropathic pain, but is unique in that it’s both pro- and anti-nociceptive. Cooling and menthol are known analgesics, and require TRPM8 channels and neurons [22,25,31]. Paradoxically, injury-induced cold sensitization is reduced when TRPM8 is blocked or absent [24,25,32,33], and the signal transduction pathways leading to cold hypersensitivity have been poorly described [24,25,34,35]. Many proalgesics work via G-protein coupled receptors which inhibit TRPM8 gating by direct binding of the Gαq subunit (Figure 2), a process that is proposed to diminish cooling analgesia [36*]. To date, only artemin, a glial cell-line derived neurotrophic factor-like ligand, has been found to induce TRPM8-dependent cold hypersensitivity [37*]. Of note, the artemin-specific receptor GFRα3 is expressed in the subset of TRPM8 neurons expressing TRPV1 which, when considered in the context of the inhibitory actions of other proalgesic receptors, suggest the presence of distinct TRPM8-neural circuits that are either proalgesic or analgesic.
Are there other cold sensors? TRPC5 is reportedly cold-sensitive between 37°C and 25°C, but neuronal cold-activated TRPC5 currents have yet to be recorded [38]. Moreover, Trpc5−/− mice show no deficits in cold sensation, although there is a decrease in cold-sensitive DRG neurons in these animals, a phenotype that is likely due to a reduction in TRPM8 expression. It is also worth noting that while heat induces STIM1/Orai1 clustering, subsequent cooling is required for channel gating [19]. Thus, it remains to be determined if either of these newly identified temperature sensitive molecules serve any appreciable role in cold transduction.
Reception or transduction?
Thermosensation is also modulated by channels that establish the threshold membrane voltage for action potential firing. For example, the two-pore K+ (K2P) channels TREK1, TREK2, and TRAAK are moderately sensitive to warmth and modulate sensitivity over largely non-overlapping temperatures. Trek1−/− and Traak−/− mice display heat hyperalgesia from 46–50°C, whereas mice lacking both channels are sensitized to cold from 20–10°C [39,40]. Conversely, Trek2−/− mice show normal sensitivity to extreme heat or cold, but are sensitized to moderate temperatures (warmth: 40–46°C; cooling 25–20°C) [40*]. Mice lacking all three K2P channels show broad thermal hypersensitivity, suggesting that these channels work to dampen excitability in different temperature ranges. Furthermore, they are down-regulated in a model of chemotherapeutic-induced neuropathic pain that displays cold hypersensitivity, a phenotype also observed in mice lacking the related K2P channel TASK-3 [34,41].
Sodium conductances are also linked to control of cold perception. Cold typically inhibits neuronal excitability, partly by increasing voltage-dependent inactivation of voltage-gated sodium (Nav) channels. However, inactivation of the tetrodotoxin (TTX)-resistant channel Nav1.8 is resistant to cold, and Nav1.8−/− mice display reduced cold sensitivity, properties suggesting Nav1.8 is the primary impulse generator in the cold [42]. Similarly, neuropathic cold hypersensitivity is attenuated by Nav1.6 antagonists [43]. Taken together, these studies show that, as with any biological system, the molecular mechanisms underlying thermotransduction are complex and likely utilize many functionally overlapping or redundant molecules.
Cellular basis for thermosensation
The identification of thermosensory molecules has provided a genetic inroad that enables the dissection of the cellular circuitry of temperature. For example, genetic ablation of Nav1.8+ neurons, which account for 75% of DRGs, resulted in reduced inflammatory heat hyperalgesia, but strikingly no deficits in acute heat-evoked behaviors [44]. Moreover, these mice were deficient in noxious cold-evoked behaviors, but responded normally to innocuous cool temperatures. Conversely, a phenotype similar to Trpv1−/− mice was found when these cells were synaptically-silenced by conditional deletion of the glutamate transporter VGlut2 [45,46]. Thus, Nav1.8+ afferents appear to serve a partial role in mammalian thermosensation.
In contrast, when similar approaches targeted TRPV1+ neurons directly, heat sensation was largely abolished. Mice in which TRPV1-lineage neurons are genetically ablated are thermally insensitive, a phenotype significantly more severe than Trpv1−/− mice [47]. Embryonically, TRPV1 is expressed in ~90% of all afferents, including those fated to express TRPM8 and TRPA1, suggesting these animals to be a general model of de-afferentation and not specifically relevant to TRPV1-neuron function in the adult. This is consistent with a total absence of heat-evoked behaviors, but normal cold responses, in mice in which TRPV1+ afferents were ablated genetically or pharmacologically in adulthood [47–49**]. Similarly, electrically silencing TRPV1+ afferents by channel-specific uptake of a cell-impermeant Nav channel blocker also abolished heat responses [50], suggesting that TRPV1+ afferents account for the cellular basis for heat thermosensation.
Genetic ablation of TRPM8-neurons also found robust deficits in cold sensation beyond that of Trpm8−/− mice, a phenotype particularly evident at noxious cold temperatures [25]. Moreover, mice lacking both TRPV1 and TRPM8 neurons are largely thermally-insensitive [49], results showing that these neurons constitute the cellular neurocircuit underlying temperature, at least at the level of the afferent neuron. Of note the latter study did observe minor behaviors at temperature extremes, responses absent when neurons expressing the Mas-related GPCR Mrgprd were ablated with both TRPM8 and TRPV1 fibers [49]. Ablation of the Mrgprd cohort alone had no effect on thermal responses [48,49], suggesting an even more complex coordination of thermosensory and non-thermosensory afferents in mouse behaviors.
CONCLUSION
The discovery of additional temperature-activated channels expands our understanding of the basis for thermosensitivity in mammals. Particularly for heat, it is clear that other TRPV channels cannot account for the residual heat sensitivity observed in Trpv1−/− mice, and additional heat sensors must complement TRPV1’s function. Nevertheless, the more profound phenotype observed in TRPV1 or TRPM8 cell-ablated mice compared to channel-only deletion confirms that these are the cell populations responsible for thermosensitivity and that within these populations there are additional molecules mediating thermosensation. Furthermore, temperature-sensitive K+ and Na+ channels provide a mechanism for fine-tuning the activity of the primary thermosensors. Thus, while the cellular basis of thermosensation is founded on two neural populations, its mechanism at the molecular level is mediated by an array of ion channels that, in combination with the primary sensors TRPV1 and TRPM8, determine the net response. Elucidating the details of how these additional channels behave in relation to each other will provide crucial insight into the molecular and cellular basis of thermosensation.
Highlights.
Genetic evidence questions the importance of many thermally-sensitive ion channels
Candidate thermosensors provide novel molecular mechanisms for thermosensation
TRPV1 and TRPM8 neurons constitute the cellular basis for thermosensation
Acknowledgments
We thank Bo Yeon Kim for the design of the figures presented in this review. We also thank the various researchers whose work was referenced here, as well as acknowledge the many other significant scientific contributions to the field of thermosensation that could not be discussed herein due to space limitations. This work was supported by NINDS of the National Institutes of Health under awards number NS078530 and NS087542.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 6.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 8.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 9.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- *11.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. A comprehensive analysis of the phenotype of Trpv2−/− mice that found no evidence that the channel serves as a heat sensor in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. As in Park et al. 2011; this study found that previous reports of thermal deficiencies in Trpv3−/− and Trpv4−/− mice were strain-dependent and provided evidence that neither channel contributes significantly to thermosensation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. This paper identified an additional TRP channel involved in pain and heat sensation. In vitro data showed the channel is activated by heat, while behavior studies of Trpm3−/− mice to show that TRPM3 is required for noxious heat sensation and plays a role in inflammation-induced heat, but not cold, hyperalgesia. [DOI] [PubMed] [Google Scholar]
- 16.Vriens J, Held K, Janssens A, Toth BI, Kerselaers S, Nilius B, Vennekens R, Voets T. Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat Chem Biol. 2014;10:188–195. doi: 10.1038/nchembio.1428. [DOI] [PubMed] [Google Scholar]
- **17.Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat Neurosci. 2012;15:1015–1021. doi: 10.1038/nn.3111. This study, along with Lee et al. (2014) provides evidence of a substantial role for a chloride channel, ANO1, in thermal hypersensitivity. In-depth investigations into various pain models show that mice in which the channel has been functionally ablated in sensory ganglia are deficient in injury-induced nociception. [DOI] [PubMed] [Google Scholar]
- *18.Lee B, Cho H, Jung J, Yang YD, Yang DJ, Oh U. Anoctamin 1 contributes to inflammatory and nerve-injury induced hypersensitivity. Mol Pain. 2014;10:5. doi: 10.1186/1744-8069-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca(2)+ influx and modulates gene expression. Nat Chem Biol. 2011;7:351–358. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 21.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 22.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 24.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- **25.Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A Sensory-Labeled Line for Cold: TRPM8-Expressing Sensory Neurons Define the Cellular Basis for Cold, Cold Pain, and Cooling-Mediated Analgesia. J Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. The first study to establish that TRPM8 marks a cellular pathway specific to cold-sensation. Using a genetic ablation strategy to eliminate TRPM8-expressing neurons in mice, the authors showed that TRPM8-neurons are responsible for multiple aspects of cold-sensation including the detection of innocuous and noxious cold and cooling-mediated pain-relief. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 27.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Knowlton WM, Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia Mediated by the TRPM8 Cold Receptor in Chronic Neuropathic Pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 32.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological Blockade of TRPM8 Ion Channels Alters Cold and Cold Pain Responses in Mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauchan P, Andoh T, Kato A, Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA. Direct inhibition of the cold-activated TRPM8 ion channel by Galphaq. Nat Cell Biol. 2012;14:851–858. doi: 10.1038/ncb2529. This study provides evidence for a novel mechanism of TRPM8 inhibition by bradykinin and histamine, which involves direct inhibition of the channel by the G-protein subunit Gαq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a Glial Cell Line-Derived Neurotrophic Factor Family Member, Induces TRPM8-Dependent Cold Pain. J Neurosci. 2013;33:12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. First study to identify an individual factor, artemin, capable of inducing cold hypersensitivity, a process that requires TRPM8. While NGF is able to slightly sensitize cold responses, artemin is so far the only molecule that has been shown to be sufficient to induce cold hypersensitivity on a level comparable to that seen in pain models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci U S A. 2011;108:18114–18119. doi: 10.1073/pnas.1115387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. Embo J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Pereira V, Busserolles J, Christin M, Devilliers M, Poupon L, Legha W, Alloui A, Aissouni Y, Bourinet E, Lesage F, et al. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain. 2014;155:2534–2544. doi: 10.1016/j.pain.2014.09.013. In addition to TREK1 and TRAAK, TREK2 has been shown to have a degree of thermosensitivity. This study confirms that the channel regulates innocuous warmth and cool as well as certain types of pain. Strikingly, it also shows that these three K+ channels contribute to the detection complementary temperature ranges, based on results from single-, double- and triple-knockout mice. [DOI] [PubMed] [Google Scholar]
- 41.Morenilla-Palao C, Luis E, Fernandez-Pena C, Quintero E, Weaver JL, Bayliss DA, Viana F. Ion channel profile of TRPM8 cold receptors reveals a role of TASK-3 potassium channels in thermosensation. Cell Rep. 2014;8:1571–1582. doi: 10.1016/j.celrep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1. 8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- 43.Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, Vetter I. An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1. 6 in peripheral pain pathways. Pain. 2013;154:1749–1757. doi: 10.1016/j.pain.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. Embo J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **49.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. As in Knowlton et al., this study utilized a genetic ablation strategy to observe the contribution of different subsets of sensory neurons to thermosensation. An important conclusion from these experiments was that TRPM8 and TRPV1 neurons are responsible for detecting all thermal input from the periphery between 0°C to 50°C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreiros N, Strominger A, Cobos EJ, et al. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J Neurosci. 2013;33:315–326. doi: 10.1523/JNEUROSCI.2804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]