Abstract
Previous structural imaging studies of autistic individuals have identified gray matter abnormalities. It remains unclear, however, which abnormalities contribute to the etiology of autism and, among these abnormalities, which reflect genetic factors. Using voxel-based morphometry, we compared regional gray matter volume in 23 parents of autistic children to an age and sex-matched control sample. We identified relative local gray matter volume increases and decreases in the parent group that are consistent with previous research with autistic individuals. Further, differences were identified in regions that are functionally associated with social-cognitive and motor processes that are impaired in autism. This investigation constitutes the first whole-brain study of regional brain volume in the parents of autistic children, and suggests that a number of structural changes observed in autism may be familial.
Keywords: cerebellum, magnetic resonance imaging, medial frontal gyrus, motor cortex
Introduction
Autism is a neurodevelopmental disorder of unknown etiology characterized by social and communication deficits in conjunction with a restricted range of behaviors [1]. Many studies with autistic individuals have identified structural brain abnormalities, although findings have been inconsistent [2]. Most have used a manual-tracing technique in which analyses were limited to a priori regions of interest. Voxel-based morphometry (VBM) enables regional volume comparisons across the whole brain. Using VBM, several investigators have identified local gray matter (GM) abnormalities among autistic individuals. Abell et al. [3] identified abnormalities in the frontal cortex, peri-hippocampal cortex, fusiform gyrus, occipital–temporal junction, and the cerebellum. Waiter et al. [4] obtained similar findings with some discrepancies (i.e. precise locus, increase or decrease). McAlonan et al. [5] identified reductions in fronto-striatal regions. Boddaert et al. [6] found bilateral GM decreases in the superior temporal sulci. Salmond et al. [7] identified abnormalities in the amygdala and hippocampal region, orbital frontal cortex, the superior temporal gyri, and the cerebellum. In summary, VBM studies suggest that autism may be associated with GM differences in several frontal and temporal areas, the cingulate, the precuneus, the cerebellum, and the caudate region. Across the autism literature, however, inconsistencies remain [2]. Further, it is unclear which abnormalities reflect heritable factors.
Many behavioral studies have identified sub-clinical autism-like deficits in parent samples, providing support for a broad autism phenotype and demonstrating the potential value of studying parents in autism research [8]. Studying the brain anatomy of parent samples may provide direction for future genetically sensitive studies while reducing some of the problems associated with heterogeneity of participants (e.g. developmental status). Two anatomical studies have examined parent samples [9,10]. Rojas et al. [9] manually traced whole brain, hippocampal, and amygdala volumes in parents and probands. In both groups, the left hippocampal volume was increased relative to controls. Palmen et al. [10] compared a parent and control sample for volume differences in total brain, cortical lobes, cerebellum, and ventricles and found no group differences, replicating the Rojas et al. study for those structures. In the present study, we used VBM to perform the first whole-brain analysis of regional GM volume in parents of children with autism.
Methods
Participants
Twenty-three biological parents of children with autism (18 families, 15 mothers, eight fathers) were compared with 23 control participants (eight men) recruited from the Denver area, group-matched on age, handedness [11], and socioeconomic status [12]. For all participants, cognitive status was estimated using the Wechsler Abbreviated Scale of Intelligence [13] and screening for psychiatric disorders was assessed with the Structured Clinical Interview for DSM-IV axis I disorders, research version [14]. Inclusion in the control sample required no personal or family history of neurological or axis I psychiatric illness and ‘never mentally ill’ status according to the Research Diagnostic Criteria [15]. Except for one family with two autistic children, each parent had one child who met DSM-IV criteria for autism, as determined by the Autism Diagnosis Observation Schedule [16] in combination with either the Autism Diagnostic Interview, Revised (ADI-R) [17] or the Autism Screening Questionnaire, which includes the diagnostic questions from the ADI-R [18]. See Table 1 for details of participant characteristics. All participants provided informed consent.
Table 1.
Demographic characteristics (mean ± standard deviation) and total GM volume by sex and group
| Parents | Controls | t Statistic (df=22) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 39.59 ± 5.99 | 38.31 ± 6.37 | 0.71, NS |
| Education | 15.83 ± 1.70 | 16.61 ± 1.95 | 1.25, NS |
| Handednessa | 0.80 ± 0.35 | 0.78 ± 0.31 | 0.17, NS |
| SESb | 49.15 ± 8.06 | 49.57 ± 6.81 | 0.19, NS |
| Full scale IQ | 115.83 ± 7.70 | 119.17 ± 9.61 | 1.35, NS |
| Verbal IQ | 114.78 ± 8.61 | 114.09 ± 9.85 | 0.25, NS |
| Performance IQ | 113.48 ± 11.52 | 119.87 ± 9.70 | 2.22, P<0.05 |
| Total GM (ml)c | |||
| Parents | Controls | ||
| Male: 767 | Male: 754 | 0.31, NS | |
| Female: 658 | Female: 666 | 0.36, NS | |
NS, non significant; GM, gray matter.
Handedness was assessed using the Annett Handedness Questionnaire [II]. Scale ranges from −1.0 (left-handed) to 1.0 (right-handed). The score was obtained by dividing total of right preference (+ 1), either hand (0), and left preference (− 1) items by 12.
Socioeconomic status (SES) was determined by Hollingshead 4-Factor Index of Social Status [12].
No group differences were observed in total GM although GM differed by sex (males > females) whether collapsed across group (P<0.0001) or separated by group (parent males > parent females, P = 0.002; control males > control females, P = 0.007).
Magnetic resonance imaging acquisition and data analysis
Magnetic resonance imaging scans were acquired on a G.E. Signa 3.0-T scanner (SPGR IR, TR = 9ms, TE = 2ms, TI = 500 ms, NEX = 1, 0.94 × 0.94 × 1.7 mm voxels). Scans were prepared for analysis using optimized VBM implemented in SPM2 (Wellcome Department of Imaging Neuroscience, London, UK), as described in Good et al. [19].
Statistical comparison
Group comparisons were conducted using the analysis of covariance model in SPM2. In a series of individual correlation analyses, all demographic characteristics (Table 1), the presence of psychopathology (in the parent group), and total GM volume were examined to identify potential interactions with regional volume. Only total GM correlated with local volume and was included as a covariate in the final analysis of covariance. Maxima that survived a false discovery rate correction [20] at 0.01 were converted into Talairach coordinates (mni2tal.m conversion) for examination in the Talairach Daemon [21].
Results
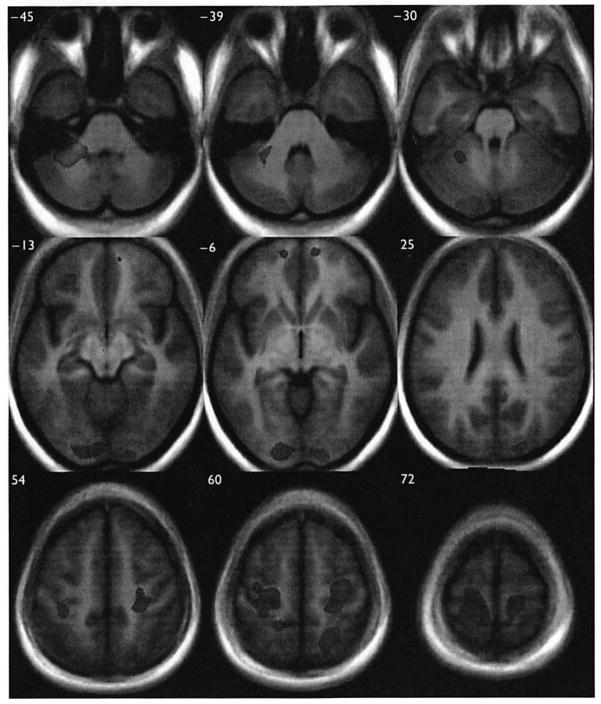
The groups did not differ on total GM, although when collapsed across group, volume differed by sex (Table 1). No evidence was found of any interactions involving sex or psychopathology (in the parent group). Many local volume group differences were identified (Table 2, Fig. 1). A single large relative decrease in the parent group occurred in the anterior portion of the left cerebellar hemisphere. Large GM increases were observed in the left postcentral gyrus, extending into the precentral gyrus, and in the right precentral gyrus. Large increases were also observed in the left posterior cerebellar hemisphere, extending anteriorly through the fusiform gyrus into the cuneus, and in the right middle occipital gyrus, extending into the lingual gyrus, and left middle occipital gyrus, the right superior parietal lobule, and bilaterally in the superior temporal gyri. Smaller GM increases were observed bilaterally in the medial frontal gyri, the inferior temporal gyri, the left inferior and middle frontal gyri, the left superior parietal lobule, the right uncus, and the anterior cingulate.
Table 2.
Significant differences in modulated GM volume between groups
| Brain region | Voxels | t | Talairach coordinates | ||
|---|---|---|---|---|---|
| Parents > controls | |||||
| Frontal | |||||
| Left inferior frontal gyrus, BA 47 | 50 | 3.65 | −50 | 37 | −13 |
| Left medial frontal gyrus, BA 10 | 249 | 3.89 | −12 | 60 | −7 |
| Right medial frontal gyrus, BA 10 | 562 | 3.93 | 13 | 62 | −7 |
| Left middle frontal gyrus, BA 11 | 544 | 4.24 | −28 | 41 | −11 |
| Right precentral gyrus, BA 4, 6 | 7620 | 5.36 | 32 | −13 | 59 |
| Occipital | |||||
| Left middle occipital gyrus, BA 19 | 2156 | 4.41 | −55 | −73 | −11 |
| Right middle occipital gyrus, BA 18 | 8562 | 5.71 | 26 | −86 | 20 |
| Parietal | |||||
| Left postcentral gyrus, BA 3 | 13 941 | 5.72 | −9 | −31 | 70 |
| Left superior parietal lobule, BA 7 | 436 | 3.96 | −35 | −62 | 53 |
| Right superior parietal lobule, BA 7 | 3279 | 4.64 | 20 | −51 | 62 |
| Temporal | |||||
| Left inferior temporal gyrus, BA 20 | 367 | 4.04 | −63 | −35 | −23 |
| Right inferior temporal gyrus, BA 20 | 52 | 3.71 | 62 | −57 | −13 |
| Left superior temporal gyrus, BA 40 | 4230 | 4.84 | −23 | 8 | −40 |
| Left superior temporal gyrus, BA 38 | 837 | 4.49 | −43 | 22 | −26 |
| Right superior temporal gyrus, BA 38 | 1899 | 5.37 | 38 | 27 | −24 |
| Right uncus, BA 38 | 106 | 3.75 | 19 | 8 | −34 |
| Sublobar | |||||
| Right anterior cingulate, BA 24 | 533 | 4.24 | 2 | 19 | 22 |
| Cerebellum | |||||
| Left cerebellum (Crus II) | 11 888 | 4.64 | −17 | −86 | −23 |
| Parents < controls | |||||
| Cerebellum | |||||
| Left cerebellum (vermis XIII, IX) | 4661 | 6.92 | −22 | −41 | −37 |
GM, gray matter; BA, Brodmann area. Only GM clusters that survived a false discovery rate at P = 0.01 are reported Local maxima for each cluster are given in Talairach coordinates (http://www.mrc.cbu.cam.ac.uk/lmaging/Common/mnispace.shtml). Within the left superior temporal gyrus, there were two discrete clusters of increased GM that are listed separately.
Fig. 1.
Gray matter volume differences between groups, overlaid onto TI image from study. The red-orange scale indicates relative decreases (parent < control) while the blue scale represents relative increases. For both contrasts, regions of difference shown reflect a false discovery rate threshold of 0.01. Corresponding Talairach and Tournoux Z coordinates are provided in the upper left-hand corner of each axial slice. Images are in neurological convention (i.e. left hemisphere on the left).
Discussion
Using VBM, we identified regional GM volume differences between parents of children with autism and a control group. The VBM technique enabled an analysis of structures such as the motor cortex that have not been studied in parent samples. We identified abnormalities in the parent group that are consistent with previous proband studies (e.g. inferior and medial frontal gyri [4] and the cerebellum [7]). Large GM increases were observed in structures (e.g. motor and somatosensory cortices, left inferior frontal gyrus, and superior parietal lobules) that form part of the ‘mirror neuron system’ that has been associated with the development of social cognition [22]. In a recent proband structural study, abnormality in these regions characterized individuals with autism [23]. Increases were also observed in the medial frontal gyri, which has been associated with the capacity to consider the mental states of others. In a functional imaging study of empathy and forgiveness, Farrow et al. [24] identified activation in the medial frontal cortex (Brodmann’s areas 8, 9, and 10).
In both previous parent studies [9,10], as in this study, total brain volume did not discriminate the groups. Using manual tracing, Rojas et al. [9] reported larger hippocampal and smaller amygdala volumes in parents, and we did not replicate that finding. Methodological differences (VBM vs. manual tracing, larger sample in this study) exist between the two studies. It should be noted that in the proband literature on hippocampal volume, however, there have been roughly equal numbers of papers published reporting larger volumes, smaller volumes, and no differences between individuals with autism and controls (see [2]). In addition, a finding of partial convergence with the proband literature would be consistent with behavioral evidence that a subset of parents display subclinical aspects of the phenotype. The lack of behavioral measures for assessing the broad autism phenotype [8] is a limitation of this study and the previous parent magnetic resonance imaging studies [9,10]. Although we identified structural abnormalities in regions associated with social–cognitive processing, we can only speculate about functional consequences. This is an important direction for future research. A second limitation concerns the presence of mood and anxiety disorders in the parent group (13 women and two men). Although this finding is consistent with previous epidemiological studies [25], it raises questions that cannot be entirely resolved in this study. We found no evidence that interactions with sex or psychopathology contributed to group differences. A larger study, however, may be required to examine conclusively the effects of comorbidity (and possible interactions with sex). Of course, given that the core clinical features of autism have not been elucidated at the neural level, it is impossible to determine whether one or more symptoms of autism and a given comorbidity reflect a common mechanism.
Conclusion
This study suggests that GM abnormalities are present in the non-affected parents of children with autism. Further, abnormalities were identified in brain regions that are functionally associated with deficits that characterize the autism spectrum.
Acknowledgments
Sponsorship: This research was supported by a grant from the Cure Autism Now Foundation entitled, ‘Planum temporale and language in parents of children with autism’.
References
- 1.Rutter M. Genetic studies of autism: from the 1970s into the millennium. J Abnorm Child Psychol. 2000;28:3–14. doi: 10.1023/a:1005113900068. [DOI] [PubMed] [Google Scholar]
- 2.Palmen SJ, van Engeland H. Review on structural neuroimaging findings in autism. J Neural Transm. 2004;111:903–929. doi: 10.1007/s00702-003-0068-9. [DOI] [PubMed] [Google Scholar]
- 3.Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- 4.Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 5.McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128 (Pt 2):268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- 6.Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Salmond CH, de Haan M, Friston KJ, Gadian DG, Vargha-Khadem F. Investigating individual differences in brain abnormalities in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:405–413. doi: 10.1098/rstb.2002.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: the phenotype in relatives. J Autism Dev Disord. 1998;28:369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- 9.Rojas DC, Smith J, Benkers TL, Camou SL, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. Am J Psychiatry. 2004;161:2038–2044. doi: 10.1176/appi.ajp.161.11.2038. [DOI] [PubMed] [Google Scholar]
- 10.Palmen SJ, Pol HE, Kemner C, Schnack HG, Sitskoorn MM, Kahn RS, et al. Brain anatomy in non-affected parents of autistic probands: an MRI study. Psychol Med. 2005;35:1–10. doi: 10.1017/S0033291705005015. [DOI] [PubMed] [Google Scholar]
- 11.Annett M. Handedness and cerebral dominance: the right shift theory. J Neuropsychiatry Clin Neurosci. 1998;10:459–469. doi: 10.1176/jnp.10.4.459. [DOI] [PubMed] [Google Scholar]
- 12.Hollingshead A. Four-factor index of social status. Department of Sociology, Yale University; New Haven, Connecticut, USA: 1975. Privately published. [Google Scholar]
- 13.The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, Texas: Harcourt Brace and Company; 1999. [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-R-TR Axis I Disorders. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- 15.Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 16.Lord C, Risi S, Lambrecht L, Cook EH, Jnr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule–Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 17.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 18.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 19.Good CD, Johnsrude IS, Ashbumer J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14 (Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 20.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston K, Evans A. A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzoff AN, Decety J. What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Philos Trans R Soc Lond B Biol Sci. 2003;358:491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj069. Epub ahead of print: 23 November 2005. [DOI] [PubMed] [Google Scholar]
- 24.Farrow TF, Zheng Y, Wilkinson ID, Spence SA, William Deaken JF, Tarrier N, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- 25.Micali N, Chakrabarti S, Fombonne E. The broad autism phenotype. Autism. 2004;8:21–37. doi: 10.1177/1362361304040636. [DOI] [PubMed] [Google Scholar]