Abstract
There is an unmet need for pharmacodynamic and predictive biomarkers for antiangiogenic agents. Recent studies have shown that soluble vascular endothelial growth factor receptor 2 (sVEGFR2), VEGF, and several other soluble factors may be modulated by VEGF pathway inhibitors. We conducted a broad profiling of cytokine and angiogenic factors (CAF) to investigate the relationship between baseline CAF levels, CAF changes during treatment, and tumor shrinkage in early-stage non–small cell lung cancer (NSCLC) patients treated with pazopanib, an oral angiogenesis inhibitor targeting VEGFR, platelet-derived growth factor receptor, and c-kit. Plasma samples were collected before treatment and on the last day of therapy from 33 patients with early-stage NSCLC participating in a single-arm phase II trial. Levels of 31 CAFs were measured by suspension bead multiplex assays or ELISA and correlated with change in tumor volume. Pazopanib therapy was associated with significant changes of eight CAFs; sVEGFR2 showed the largest decrease, whereas placental growth factor underwent the largest increase. Increases were also observed in stromal cell–derived factor-1α, IP-10, cutaneous T-cell–attracting chemokine, monokine induced by IFN-γ, tumor necrosis factor–related apoptosis-inducing ligand, and IFN-α. Posttreatment changes in plasma sVEGFR2 and interleukin (IL)-4 significantly correlated with tumor shrinkage. Baseline levels of 11 CAFs significantly correlated with tumor shrinkage, with IL-12 showing the strongest association. Using multivariate classification, a baseline CAF signature consisting of hepatocyte growth factor and IL-12 was associated with tumor response to pazopanib and identified responding patients with 81% accuracy. These data suggest that CAF profiling may be useful for identifying patients likely to benefit from pazopanib, and merit further investigation in clinical trials.
Introduction
Angiogenesis and the vascular endothelial growth factor (VEGF) pathway have been recently validated as therapeutic targets in non–small cell lung cancer (NSCLC), with bevacizumab prolonging survival when added to chemotherapy in patients with advanced disease (1). A growing number of antiangiogenic, multitargeted receptor tyrosine kinase inhibitors (TKI), with activity against the VEGF family of receptors, have shown clinical activity in NSCLC (2-7). These results raise the possibility that antiangiogenic therapy may benefit NSCLC patients at earlier stages of their disease, where new blood vessel formation is critical for tumor growth and metastatic spread, as well as in advanced disease (8).
Tumor angiogenesis is controlled by proangiogenic and antiangiogenic factors (9) produced by tumor or stromal cells, inflammatory cells, or other circulating populations. The efficacy of VEGF inhibitors is likely affected by the balance of these factors, as well as cytokines and chemokines, through complex interactions within the tumor microenvironment; VEGF pathway blockade, in turn, affects levels of many of these factors (10-13). Satisfying a growing and unmet need, baseline levels of these cytokine and angiogenic factors (CAF) could therefore be exploited to predict which patients will derive the most benefit from specific antiangiogenic agents, and the modulation of these CAFs could potentially be used to monitor drug activity, to determine the optimal antitumor dose, and to help identify possible mechanisms of resistance.
Blood-based biomarkers, including VEGF, soluble VEGF receptor 2 (sVEGFR2), and circulating endothelial cells, have been assessed for several different VEGF pathway inhibitors (12, 14-16). Recently, we and others have found that baseline circulating VEGF levels may be predictive of clinical benefit or tumor response for these agents (17-19). A limited number of factors have been analyzed in these studies, however. Multiplex technologies offer a noninvasive and convenient method of simultaneously assessing a much larger number of biologically relevant CAFs from small plasma volumes (20, 21). Recently, we and other investigators have tested these methods to investigate CAFs modulated by treatment with chemotherapy, angiogenesis inhibitors, or other targeted agents or associated with therapeutic resistance (21-23). It is unclear whether changes of any of these factors correlate with tumor response in NSCLC patients treated with TKIs. It is also unknown whether baseline levels of any single factor or combinations of factors may be predictive of response to these agents.
To address these questions, we performed an exploratory CAF analysis in untreated, early-stage NSCLC patients treated with preoperative, single-agent pazopanib. Pazopanib is an oral angiogenesis inhibitor targeting VEGFR1, VEGFR2, VEGFR3, platelet-derived growth factor receptor (PDGFR), and c-kit, which has recently received approval by the U.S. Food and Drug Administration for treatment of advanced renal cell carcinoma and has previously shown clinical activity against multiple other tumor types, including ovarian cancer and sarcoma (24-26).
Materials and Methods
Patients, study design, and overall objectives
Thirty-five patients participated in this nonrandomized, open-label, single-arm phase II trial (NCT00367679; VEG105290) conducted in eight sites in the United States, Spain, and Israel. Patients were to receive oral pazopanib at 800 mg daily for 2 to 6 wk before scheduled surgery followed by a washout period of 7 d before surgery. No pazopanib dose modifications were planned. Patients with histologically or cytologically confirmed, resectable, stage I to II (to T2) NSCLC were included. No prior approved or investigational anticancer therapy within 6 mo before the start of therapy was permitted. Baseline measurements included tumor assessment by high-resolution computed tomography (HRCT), tumor biopsy, and plasma sampling for CAF analyses. Tumor volume assessments were conducted by a central reviewer blinded to scan sequence. Volumetric instead of unidimensional measurements were used for biomarker correlations because of the high degree of reproducibility compared with investigator-assessed unidimensional measurements (27-29) and because recent data suggest that volumetric measurements could detect treatment-induced tumor changes earlier than unidimensional measurements in lung cancer patients (29).
Patients consented to enroll in the trial and for plasma and tumor sample collection before and after surgery for translational studies.
The primary objective of this study was to evaluate tumor volume reduction assessed by HRCT after preoperative treatment with pazopanib. Secondary objectives included evaluation of safety and tolerability of pazopanib (800 mg once-daily monotherapy). The analyses of CAF profiles in plasma and transcriptional profiles in tumor tissue were explorative objectives of this study.
Plasma preparation and measurements
Plasma samples with EDTA were collected at baseline and on the last day of pazopanib dosing. Samples were centrifuged at 1,100 × g (relative centrifugal force) for 15 min at 4°C for separation of plasma and mononuclear cell layers. Plasma was stored at −70°C. Before analysis, samples were thawed overnight at 4°C and centrifuged at 1,500 × g to remove debris.
CAFs were analyzed with commercially available multiplexed bead suspension arrays (MBA). MBA kits were used as per the manufacturer’s instructions using a BioPlex 200 machine (Bio-Rad) in the Thoracic Blood-Based Biomarkers Laboratory at the M.D. Anderson Cancer Center. The human group I, 27-plex cytokine panel (Bio-Rad) and human group II, 23-plex cytokine panel (Bio-Rad) were used. Placental growth factor (PlGF), VEGF, and sVEGFR2 concentrations were determined by ELISA (R&D Systems). Analytes for which >25% of patients had nondetectable levels were not included in the subsequent analyses.
Statistical methods
CAF analytes that were significantly changed from baseline following treatment were determined using the nonparametric Wilcoxon signed-rank test. Spearman’s rank correlation test was used to determine CAF analytes that were significantly correlated with volumetric shrinkage. This test was also used to determine an association at baseline between CAF analytes and tumor shrinkage. Hierarchical clustering of the CAF change data was done using Ward’s algorithm (implemented in Spotfire v8.2.1). CAF log ratios (posttreatment divided by pretreatment) were mean centered and unit variance scaled. To understand whether the patient clustering was related to tumor response, volumetric shrinkage was added as an extra variable in the clustering analysis. Euclidean distance was used as similarity measure for clustering, and profiles were ordered by average value. Multivariate classification was used to distinguish “responders” from “nonresponders” using baseline CAF profiles. To maximize the information from this small data set, patients were divided in two equally sized groups and responders were defined as patients whose volumetric tumor shrinkage was greater than the median shrinkage of 17% over all patients. The CAF data were mean centered and unit variance scaled. Univariate feature selection was done using t test, requiring at least 1.5-fold change between the two groups. Class prediction was performed using the nearest centroid method. To avoid overfitting of the data, leave-one-out cross-validation was used to determine the smallest number of CAF profiles that maximized overall prediction accuracy. Feature selection and class prediction were properly embedded within the cross-validation loop to avoid any selection bias. Class label permutations were done to assess classifier significance.
Results
Patients
Details of the clinical trial results and analysis of paired tumor specimens are described in a separate article (30).5 The average duration of treatment was 16 days (ranging from 3 to 29 days). Thirty-three of 35 early-stage NSCLC patients had pretreatment and posttreatment plasma samples available for CAF analysis. The median age was 65 years (range, 51–79), of which 62% were female. Histologies included adenocarcinoma (63%), undifferentiated adenocarcinoma (3%), undifferentiated carcinoma (6%), squamous cell carcinoma (11%), and other NSCLCs (17%). Stage IA included 19 patients, stage IB included 14 patients, stage IIA included 1 patient, and stage IIB included 1 patient. Thirty patients (86%) had a reduction in tumor volume after pazopanib treatment. Tumor changes ranged from −86% to +17% (Fig. 1). Three patients had a partial response according to Response Evaluation Criteria in Solid Tumors criteria.
Figure 1.
Tumor volume changes after treatment with pazopanib. Thirty patients (85.7%) achieved a reduction in tumor volume after pazopanib treatment. Tumor volume changes ranged from −86% to +17%.
Baseline CAF levels as predictors of tumor response
We hypothesized that a broad profile of circulating factors known to affect angiogenesis, tumor-associated inflammatory cells, or other aspects of the tumor microenvironment (16, 19, 22, 23, 31-34) would be useful for identifying candidate markers of activity and baseline (pretreatment) markers of response and resistance. A total of 31 analytes were used in the final analysis as summarized in Table 1. These included proangiogenic [e.g., VEGF, hepatocyte growth factor (HGF), PDGF, PlGF, and stromal cell–derived factor-1α (SDF-1α)] and antiangiogenic factors (e.g., IFN-α2 and IFN-γ), interleukins (IL), chemokines involved in myelomonocytic cell mobilization or recruitment [e.g., granulocyte macrophage colony-stimulating factor (GM-CSF), MIP-1β, and GRO-α], and markers of endothelial function or damage (sVEGFR2). Note that several known angiogenic factors, such as basic fibroblast growth factor (bFGF), were assessed but were not detectable in >25% of patients and were therefore excluded from the analysis.
Table 1.
CAFs analyzed in this study with baseline levels and posttreatment/pretreatment change in plasma CAFs in response to pazopanib
| CAF | Median baseline value (pg/mL) |
IQR at baseline |
Median fold change (P)* |
|---|---|---|---|
| Proangiogenic/antiangiogenic factors | |||
| VEGF† | 77.6 | 74.8 | −1.01 (0.326) |
| HGF | 530.3 | 381.1 | — |
| PDGF-BB | 195.6 | 492.1 | — |
| IFN-α2 | 73.5 | 18.7 | 1.04 (0.021) |
| IFN-γ | 51.5 | 58.8 | — |
| PlGF‡ | 1.1 | 2.2 | 18.04 (<0.0001) |
| ILs | |||
| IL-2 | 3.5 | 9.1 | — |
| IL-2Rα | 107.1 | 56.5 | — |
| IL-1Rα | 94.5 | 194.6 | — |
| IL-3 | 58.0 | 41.4 | — |
| IL-4 | 0.8 | 2.1 | — |
| IL-9 | 11.4 | 16.5 | — |
| IL-12 | 407.9 | 341.0 | — |
| IL-16 | 81.8 | 38.8 | — |
| IL-18 | 22.3 | 14.3 | — |
| Chemokines/monokines | |||
| MCP-3 | 32.9 | 27.2 | — |
| MIP-1β | 14.6 | 10.2 | — |
| MIG | 186.0 | 162.0 | 1.33 (0.007) |
| Eotaxin | 32.3 | 26.9 | — |
| IP-10 | 416.5 | 399.3 | 1.60 (0.00024) |
| CTACK | 248.9 | 69.4 | 1.12 (0.0029) |
| MIF | 268.4 | 347.4 | — |
| GRO-α | 57.5 | 23.6 | — |
| Hematopoietic growth factors | |||
| GM-CSF | 13.8 | 26.7 | — |
| G-CSF | 14.5 | 25.0 | — |
| M-CSF | 21.2 | 10.9 | — |
| SCF | 29.5 | 17.0 | — |
| SCGF-β | 4,881.7 | 4,984.3 | — |
| SDF-1α | 397.9 | 196.7 | 1.08 (0.0062) |
| EC function/damage | |||
| sVEGFR2 | 12,577.3 | 3,215.2 | −1.35 (<0.0001) |
| Apoptosis mediator | |||
| TRAIL | 145.8 | 55.8 | 1.05 (0.0093) |
Abbreviations: IQR, interquartile range; MCP-3, chemokine C-C motif ligand 7; MIP-1β, chemokine C-C motif ligand 5; IP-10, IFN-inducible cytokine IP-10; MIF, macrophage migration inhibitory factor; GRO-α, GRO1 oncogene (melanoma growth-stimulating activity, α); SCF, stem cell factor; SCGF-β, stem cell growth factor-β.
Only significant values are presented.
Changes in VEGF were not statistically significant.
Thirteen of 33 PlGF baseline values were below the lowerlimit of detection of the assay and, therefore, were assigned a nominal value equal to half the lower detected values in the assay.
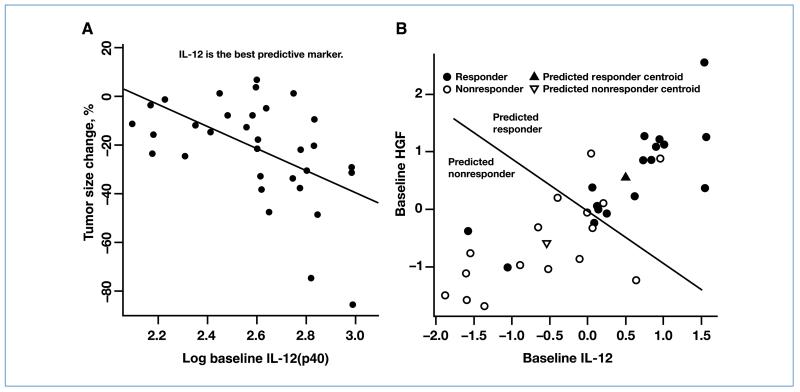
Baseline levels of 11 CAFs were statistically significantly correlated with tumor response. These included IL-12, HGF, IL-16, IP-10, SDF-1α, IL-2Rα, IL-3, IFN-α2, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), M-CSF, and PlGF (Table 2). Higher levels of these factors correlated with better tumor response. Baseline levels of VEGF were not associated with tumor response. The strongest association was observed with baseline IL-12 and tumor response (Fig. 2A). IL-12 levels did not seem to be modulated by treatment.
Table 2.
Correlation of baseline levels of plasma CAFs with tumor shrinkage
| CAF | Correlation P | Correlation r |
|---|---|---|
| IL-12 | 0.00065 | −0.57 |
| HGF | 0.001 | −0.55 |
| IL-16 | 0.003 | −0.51 |
| IP-10 | 0.0055 | −0.48 |
| SDF-1α | 0.0085 | −0.45 |
| IL-2Rα | 0.01 | −0.45 |
| IL-3 | 0.011 | −0.44 |
| IFN-α2 | 0.013 | −0.43 |
| TRAIL | 0.015 | −0.42 |
| M-CSF | 0.024 | −0.39 |
| PlGF | 0.047 | −0.35 |
NOTE: Reproduced from Nikolinakos and colleagues.
Figure 2.
A, baseline plasma IL-12 levels were significantly correlated to tumor response and were the best individual predictive marker. B, multivariate classification applied to baseline CAF levels developed a compact response signature with 81% accuracy using two analytes.
CAF signature associated with response
To determine whether combining markers added value to the predictive ability of individual markers, we analyzed the baseline CAF levels using multivariate classification techniques. The optimal number of CAF analytes was determined to be two via cross-validation, and class label permutations showed this model to be significant (P = 0.003 in 1,000 permutations). Cross-validation results revealed the ability of the model to predict 13 of 16 responders and 14 of 17 nonresponders according to the rule described earlier, and the model was able to classify patients into responders and nonresponders with an accuracy of 81% (27 of 33 correct predictions). In a final model training step, all the available data (without leaving out any patients) were used to build the classification model and select the top 2 analytes: IL-12 and HGF. Classification results using these two analytes and all the available data are shown in Fig. 2B.
CAFs modulated during treatment with pazopanib
Analysis of CAF levels after treatment revealed eight analytes that were changed significantly during treatment. These included a decrease in sVEGFR2 and an increase in PlGF, IP-10 (CXCL10), cutaneous T-cell–attracting chemokine (CTACK), SDF-1α, monokine induced by IFN-γ (MIG; CXCL9), TRAIL, and IFN-α, all with a Wilcoxon P value of <0.05 (Table 1; Fig. 3A and B). Changes in VEGF levels were not statistically significant (Table 1). PlGF, on the other hand, showed both the maximal and the most significant increase in response to treatment with pazopanib (Fig. 3B).
Figure 3.
Posttreatment changes in plasma sVEGFR2 (A) and PlGF (B) for individual patients. C, hierarchical clustering of posttreatment changes in CAFs (larger increases in red and lesser increases or decreases in green; see Materials and Methods for details). Note that some individuals experienced greater changes across virtually all CAFs and others had consistent lesser changes.
Modulated CAFs as markers of tumor response
sVEGFR2 and PlGF both showed pronounced changes after pazopanib treatment (Fig. 3A and B). However, analysis of CAF changes in relation to tumor shrinkage identified only sVEGFR2 as a potentially useful marker of tumor response. That is, greater decreases in sVEGFR2 correlated with larger tumor shrinkages as shown in Fig. 4A. Increase in IL-4 levels also correlated with tumor response (Fig. 4B). VEGF changes from baseline were insignificant and did not correlate with tumor volume changes. Interestingly, although PlGF had the largest modulation with treatment, those changes did not correlate with tumor shrinkage, suggesting that it may be a pharmacodynamic marker but not a marker of tumor response.
Figure 4.
Changes in plasma levels of (A) sVEGFR2 and (B) IL-4 (represented by the log of posttreatment to pretreatment ratio) are correlated with tumor shrinkage. Increase in sVEGFR2 (r = 0.41, P = 0.017) and decrease in IL-4 (r = −0.37, P = 0.033) are associated with greater tumor shrinkage.
Hierarchical clustering analysis of CAF changes
Hierarchical clustering analysis results are shown as a heat map in Fig. 3C. Each row represents a patient, and each column a CAF analyte or volumetric shrinkage. Red color indicates the largest changes in CAF levels after treatment and green color denotes smallest changes. For example, in the case of a CAF analyte that increased for every patient following treatment, the smallest increases will be green and the largest increases will be red. Clustering analysis revealed three major patient groups: those in the top few rows of the heat map who experienced mostly large changes (in red) in multiple analytes, those in the bottom few rows of the heat map who experienced mostly smaller changes (in green), and patients in the middle that experienced more variable changes in baseline CAF levels. Because the tumor volume change variable in the heat map does not follow the pattern described above, the arrangement of patients in the heat map does not seem to be related to response. We explored other patient characteristics (age, gender, race, and initial tumor volume). However, none was significantly correlated with the order of patients in the heat map.
Discussion
In cancer patients treated with antiangiogenic TKIs, the ability to noninvasively measure the baseline and modulated levels of multiple circulating CAFs could help satisfy an unmet need for biomarkers of drug activity and efficacy. Prior clinical studies have focused on only single or few markers, and multiple CAF measurements have mostly been explored with non–TKI-based therapies (20, 21). Recent preclinical and clinical studies, however, have suggested that multiple circulating factors are modulated by VEGF inhibitors given as single agents or in combination with chemotherapy and could potentially serve as markers of activity (13, 16, 22, 23). The aim of this study was to explore which factors and to what extent they are modulated by the novel antiangiogenic TKI, pazopanib, and to identify possible predictive and early response markers. The primary findings of this study are that, first, baseline levels of 11 different markers associated with tumor response were identified. Second, we show that combining the baseline levels of two factors (HGF and IL-12) identified patients likely to benefit from preoperative pazopanib treatment. Third, pazopanib therapy results in the modulation of multiple plasma CAFs, with PlGF showing a striking rise from baseline. Finally, plasma sVEGFR2 not only decreased during therapy but also correlated with tumor shrinkage. This study identifies several plasma markers associated with tumor shrinkage and indicates that combinations of these may have greater predictive value.
In patients receiving pazopanib, we observed a decrease in sVEGFR2 and an increase in PlGF, IP-10 (CXCL10), CTACK, SDF-1α, MIG (CXCL9), TRAIL, and IFN-α. This is consistent with previous preclinical findings that show a decrease in sVEGFR2 and elevation in PlGF and SDF-1α following treatment with another VEGFR/PDGFR inhibitor, sunitinib (13). These changes were reported in clinical studies to reverse with discontinuation of sunitinib when used in an intermittent dosing schedule (12). Interestingly, the levels did not seem to change as a result of tumor hypoxic changes as previously thought but were mostly systemically derived (10). This may be particularly relevant for our clinical study where the tumors were localized and small in size. The mechanism for the decrease in sVEGFR2 has not been established, but preclinical studies suggest that VEGF-A stimulates shedding of VEGFR2 from endothelium by VEGFR2-dependent activation of the metalloproteinase ADAM-17 (35). The correlation between larger decreases in sVEGFR2 and a greater degree of tumor shrinkage may therefore reflect that more effective VEGFR2 inhibition results in greater inhibition of receptor shedding. The mechanism(s) underlying the weaker association between shrinkage and IL-4 changes is less clear because a greater degree of tumor shrinkage was associated with smaller changes in IL-4 (Fig. 4B) and IL-4 has been shown to have both proangiogenic and antiangiogenic effects under different conditions (36-38).
In the clinical setting, a rise in VEGF and a decrease in sVEGFR2 have been reported in multiple tumor types and with several antiangiogenic TKIs (12, 23, 39-42). The majority of these studies investigated VEGF and sVEGFR2 as potential biomarkers. We have also conducted a similar CAF analysis to the one presented here, with the use of vandetanib monotherapy and/or chemotherapy in patients with advanced NSCLC (23), which showed a decrease in sVEGFR2 and an increase in VEGF as well as IL-8 and IL-17 in the vandetanib monotherapy arm. In all these studies, a consistent pattern of VEGF elevation and sVEGFR2 decrease was observed, suggesting a class effect for antiangiogenic TKI therapies. As discussed below, the molecular mechanisms of these VEGF and sVEGFR2 changes are currently under investigation (13). Our study extends these earlier findings in identifying several new potential pharmacodynamic markers and in showing that changes in several, but not all, of these markers correlate with tumor shrinkage.
The marker that changed most dramatically during pazopanib treatment was PlGF. PlGF initiates endothelial proliferation, migration, and mobilization of angiocompetent bone marrow progenitors. Plasma PlGF levels have been shown to increase with the use of antiangiogenic treatments (16, 31, 40, 43). Specifically, patients with recurrent glioblastoma treated with cediranib had rises in PlGF plasma levels (as well as increases in VEGF and decreases in sVEGFR2; ref. 16). Among patients who experienced tumor progression during treatment, increases in tumor volume were associated with decreases in PlGF levels (as well as increases in SDF-1α, bFGF, and sVEGFR). Because of its potential role in mediating resistance to antiangiogenic therapies, anti-PlGF therapy is now being pursued as a novel antiangiogenic agent (44).
Unlike PlGF or VEGF, a decrease of soluble levels of VEGFR2 was found to correlate with tumor volume decrease. Previously, in nontumor-bearing mice treated with sunitinib, sVEGFR2 decreased and correlated with the optimal dose for antitumor response (13). Consistent with the findings in the results of the present study, a recent phase I study of the VEGFR TKI AMG706 showed a rise in PlGF and a decrease in sVEGFR2 during treatment; in that study, however, both changes correlated with a decrease in tumor size (43). Taken together, our data and these earlier studies suggest that sVEGFR2 may be a distinct marker for drug exposure and early tumor response. It is worth noting that all patients in the study had early-stage disease, and the duration of treatment was short (mean, 16 days). It is not possible to determine whether sVEGFR2 changes would continue to be associated with tumor response in the setting of advanced disease or after prolonged treatment. These issues merit further investigation in subsequent studies.
We separately investigated baseline levels of CAFs in relation to tumor shrinkage and found 11 factors that could serve as potential predictive markers, with higher levels of these factors correlating with better tumor response. Baseline levels of IL-12 followed by HGF had the best correlation with tumor response and could potentially serve as an important predictive marker in future studies. IL-12 is a master regulator of TH1 immune responses and a known angiogenesis inhibitor. To our knowledge, this is the first report of circulating IL-12 as a predictor of response in patients receiving an antiangiogenic TKI. HGF and its receptor, the MET tyrosine kinase, mediate neoplastic invasive growth and have been the cause of many other biological events, including cell proliferation, migration, and angiogenesis (45). By multivariate classification, we derived a two-factor signature that may identify patients likely to benefit from pazopanib treatment.
As a final step in our analysis, we performed an exploratory hierarchical clustering analysis of CAF level changes before and after treatment for individual patients. This analysis showed that, for a given patient, changes in many of the factors were highly correlated with one another. Although the absolute magnitude of these changes varied between the CAFs, it suggests that groups of these factors undergo coordinated changes, suggesting that a common mechanism or pathway may be regulating them. Three major clusters of patients were identified, with one group having relatively large changes across most CAFs, another having smaller changes, and the third with mixed results. The factors regulating these changes are under investigation, but preclinical studies suggest that they are affected by both the tumor and the host (13).
In brief, our exploratory analysis revealed multiple CAFs modulated with preoperative pazopanib treatment. PlGF showed a striking rise from baseline and is a potential pharmacodynamic marker. sVEGFR2 decrease and IL-4 increase from baseline were associated with tumor response, and multiple other potentially predictive markers were identified, several of which have not been previously reported. Lastly, our finding that a combination of baseline levels of plasma factors was associated with tumor shrinkage shows the utility and feasibility of such an approach and merits further investigation in future clinical trials.
Acknowledgments
We thank Donald Norwood and Monique Nilsson for editorial assistance, Yuan Liu for contributions to the evaluation of this data set, and ProEd Communications, Inc. for editing the figures and tables for publication.
Grant Support
GlaxoSmithKline. J.V. Heymach is a Damon Runyon-Lilly Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI 24-04) and the Physician Scientist Program at the M.D. Anderson Cancer Center.
Footnotes
N. Altorki et al. J Clin Oncol. In press 2010.
Note: These data were previously presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30 to June 3, 2008, Chicago, IL (46) and at the 33rd European Society for Medical Oncology Congress, September 12–16, 2008, Stockholm, Sweden (47).
Clinical Trials Registration Number: NCT00367679.
Disclosure of Potential Conflicts of Interest
J.V. Heymach: commercial research grant and consultant/advisory board. The other authors disclosed no potential conflicts of interest.
References
- 1.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 2.Lee D, Heymach JV. Emerging antiangiogenic agents in lung cancer. Clin Lung Cancer. 2006;7:304–8. doi: 10.3816/CLC.2006.n.010. [DOI] [PubMed] [Google Scholar]
- 3.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5407–15. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 4.Natale RB, Bodkin D, Govindan R, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase II study. J Clin Oncol. 2009;27:2523–9. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–6. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenschein GR, Jr., Gatzemeier U, Fossella F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:4274–80. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 7.Schiller J, Lee J, Hanna N, Traynor A, Carbone D. A randomized discontinuation phase II study of sorafenib versus placebo in patients with non-small cell lung cancer who have failed at least two prior chemotherapy regimens: E2501 [abstract 8014] J Clin Oncol. 2008;26:8014. [Google Scholar]
- 8.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 10.Ebos JM, Lee CR, Bogdanovic E, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–9. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- 11.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–50. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 13.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zurita AJ, Jonasch E, Wu HK, Tran HT, Heymach JV. Circulating biomarkers for vascular endothelial growth factor inhibitors in renal cell carcinoma. Cancer. 2009;115:2346–54. doi: 10.1002/cncr.24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolini F, Mancuso P, Shaked Y, Kerbel RS. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discov Today. 2007;12:806–12. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanrahan EO, Ryan AJ, Mann H, et al. Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res. 2009;15:3600–9. doi: 10.1158/1078-0432.CCR-08-2568. [DOI] [PubMed] [Google Scholar]
- 18.Burstein HJ, Chen YH, Parker LM, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res. 2008;14:7871–7. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]
- 19.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14:1407–12. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 20.Yurkovetsky ZR, Kirkwood JM, Edington HD, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-α2b. Clin Cancer Res. 2007;13:2422–8. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 21.Allen C, Duffy S, Teknos T, et al. Nuclear factor-κB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182–90. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 22.Kopetz S, Hoff PM, Wolff RA, et al. Phase II trial of infusional 5-fluorouracil, irinotecan and bevacizumab (FOLFIRI+B) for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–9. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of change in plasma cytokine and angiogenic factors and markers of benefit for vandetanib and/or chemotherapy in non-small cell lung cancer patients. J Clin Oncol. 2010;28:193–201. doi: 10.1200/JCO.2009.22.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer-Soft Tissue And Bone Sarcoma Group (EORTC study 62043) J Clin Oncol. 2009;27:3126–32. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg C, Davis I, Mardiak J, et al. Pazopanib in locally advanced and/or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.23.9764. In press. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–7. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B, James LP, Moskowitz CS, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–72. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostis WJ, Yankelevitz DF, Reeves AP, Fluture SC, Henschke CI. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology. 2004;231:446–52. doi: 10.1148/radiol.2312030553. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B, Schwartz LH, Moskowitz CS, Ginsberg MS, Rizvi NA, Kris MG. Lung cancer: computerized quantification of tumor response—initial results. Radiology. 2006;241:892–8. doi: 10.1148/radiol.2413051887. [DOI] [PubMed] [Google Scholar]
- 30.Altorki N, Gurarino M, Lee P, et al. Preoperative treatment with pazopanib (GW786034), a multikinase angiogenesis inhibitor in early-stage non-small cell lung cancer (NSCLC): a proof-of-concept phase II study [abstract 7557] J Clin Oncol. 2008;26:7557. [Google Scholar]
- 31.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for anti-angiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–9. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 32.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 35.Swendeman S, Mendelson K, Weskamp G, et al. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–8. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaji-Kegan K, Su Q, Angelini DJ, Johns RA. IL-4 is proangiogenic in the lung under hypoxic conditions. J Immunol. 2009;182:5469–76. doi: 10.4049/jimmunol.0713347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong KH, Cho ML, Min SY, et al. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin Exp Immunol. 2007;147:573–9. doi: 10.1111/j.1365-2249.2006.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volpert OV, Fong T, Koch AE, et al. Inhibition of angiogenesis by interleukin 4. J Exp Med. 1998;188:1039–46. doi: 10.1084/jem.188.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, et al. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol. 2005;16:558–65. doi: 10.1093/annonc/mdi118. [DOI] [PubMed] [Google Scholar]
- 40.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–54. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 42.van Heeckeren WJ, Ortiz J, Cooney MM, Remick SC. Hypertension, proteinuria, and antagonism of vascular endothelial growth factor signaling: clinical toxicity, therapeutic target, or novel biomarker? J Clin Oncol. 2007;25:2993–5. doi: 10.1200/JCO.2007.11.5113. [DOI] [PubMed] [Google Scholar]
- 43.Rosen LS, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:2369–76. doi: 10.1200/JCO.2006.07.8170. [DOI] [PubMed] [Google Scholar]
- 44.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 46.Nikolinakos P, Altorki N, Guarino M, et al. Analyses of plasma cytokine/angiogenic factors (C/AFs) profile during preoperative treatment with pazopanib (GW786034) in early-stage non-small cell lung cancer [abstract 7568] J Clin Oncol. 2008;26(Suppl):411s. [Google Scholar]
- 47.Altorki N, Heymach J, Guarino M, et al. Phase II study of pazopanib (GW786034) given preoperatively in stage I-II non-small cell lung cancer (NSCLC): a proof-of-concept study [abstract 225O] Ann Oncol. 2008;19(Suppl 8):viii89. [Google Scholar]