Abstract
As the human body ages, the risk of heart disease and stroke greatly increases. While there is evidence that lifelong exercise is beneficial to the heart’s health, the effects of beginning exercise later in life remain unclear. This study aimed to investigate whether exercise training started later in life is beneficial to cardiac aging in Drosophila. We examined 4-week-old wild-type virgin female flies that were exposed to exercise periods of either 1.5, 2.0, or 2.5 h per day, 5 days a week for 2 weeks. Using M-mode traces to analyze cardiac function by looking at parameters including heart rate, rhythmicity, systolic and diastolic diameter, and interval and fractional shortening, we found that cardiac function declined with age, shown by an increase in the number of fibrillation events and a decrease in fractional shortening. About 2.0 and 2.5 h of exercise per day displayed a reduced incidence of fibrillation events, and only physical exercise lasting 2.5-h period increased fractional shortening and total sleep time in Drosophila. These data suggested that training exercise needs to be performed for longer duration to exert physiological benefits for the aging heart. Additionally, climbing ability to assess the exercise-induced muscle fatigue was also measured. We found that 2.0 and 2.5 h of exercise caused exercise-induced fatigue, and fatiguing exercise is beneficial for cardiac and healthy aging overall. This study provides a basis for further study in humans on the impact of beginning an exercise regimen later in life on cardiac health.
Keywords: Physical exercise, Cardiac aging, Climbing index, Locomotor activity
Introduction
Aging is characterized by impaired circadian rhythms that cause disruption of sleep-wake cycles, diminishing of hormonal rhythms, and weakening of clock gene oscillations (Valentinuzzi et al. 1997; Huang et al. 2002; Hofman and Swaab 2006; Kondratova and Kondratov 2012; Rakshit et al. 2012; Rakshit and Giebultowicz 2013). There is also a progressive decline in cardiac function including stroke volume, ejection fraction, and cardiac output (Beere et al. 1999; Fiechter et al. 2013) as well as an increase in susceptibility to arrhythmia, cardiac hypertrophy, and diastolic dysfunction (Higashi et al. 2012; Sastre et al. 2000). One of the important aspects of healthy aging is the body’s ability to withstand homeostatic insults, such as oxidative stress. A previous study reported that flies with disrupted clocks/impaired circadian rhythms showed significantly increased mortality risk after short-term oxidative challenge (24 h of 100 % hyperoxia) in the middle age and/or older age groups (Krishnan et al. 2009). The age-related recession of cardiac function is a major risk factor for the development of cardiovascular disease and heart failure that contributes to increased cardiovascular mortality and morbidity in elderly humans (Scalia et al. 2010). Endurance exercise has long been known to improve cardiac function and to benefit even patients who are already experiencing heart failure (Erbs et al. 2010; Kavanagh et al. 1996). Most of these studies on the effect of exercise training on cardiac aging have been based on epidemiological and cross-sectional studies, since longitudinal studies across the lifespan of humans are intricate. In addition, improvements to vertebrate cardiac function following endurance training have also been documented in both young and old animals (Suvorava et al. 2004; Derumeaux et al. 2008). However, studies on cardiac aging across ages in long-term and longitudinal study designs in mammals are also complicated because of their long lifespan and genetic redundancy. Therefore, the use of the Drosophila model is utilized for this study in order to circumvent these complications.
The complexity and difficulty of studying aging and longevity in humans and mammals make the Drosophila model an attractive alternative for in-depth exploration and hypothesis testing. Drosophilae has been previously shown to be effective models for the study of aging (Hughes and Reynolds 2005; Demontis et al. 2013) and adult cardiac function (Piazza and Wessells 2011), and flies also respond to endurance training by improving cardiac output and stress resistance at advanced ages (Piazza et al. 2009). This, combined with the relative shorter lifespan, simpler genome, and unparalleled genetic tools available for studies in the fly, presents an opportunity to examine the impact of exercise training on cardiac function at varying ages and varying degrees of exercise.
Lifelong exercises positively impacts cardiac functioning, which is manifested by maintaining left ventricular compliance (Bhella et al. 2014) and acquiring VO2 max, stroke volume, and heart rate regulation during exercise through aging (Carrick-Ranson et al. 2014). However, it is still uncertain whether the age-associated impairment in cardiac function may be reduced by exercise training started later in life. A better understanding of how exercise influences the healthy aging heart may support the exercise intervention and thus reduce the burden of cardiovascular disease and heart failure. In this study, we investigate whether different doses of exercise training started later in life have different impacts on cardiac function. We characterized the cardiac properties in flies by measuring changes in their dynamic parameters including heart rate, rhythmicity, systolic and diastolic diameters, and intervals and fractional shortening. We also want to explore how to assess the dose that is appropriate for exercise intervention of cardiac aging. The results of our study demonstrated that exercise within the range of 2 to 2.5 h per day is beneficial for slowing the resting heart rate in drosophilae. Exercise for only 1.5 h did not show the same effects; this suggests that there is a threshold in the amount of exercise that needs to be met by people who have been sedentary in the past in order to promote the effects of exercise on the heart rate.
Material and methods
Drosophila stocks and culture
Wild-type w1118 virgin female flies were collected within 8 h after eclosion. All flies were raised on the following foods: 6 L distilled water, 51.4 g agar, 369.6 g sucrose, 369.6 g maltose, 148.8 g yeast, 504.0 g corn flour, 120.5 g soybean meal, 20 mL propionic acid, and 20 g para-hydroxybenzene melt in 200 mL absolute ethyl alcohol. Flies were kept at 25 °C and 65 % humidity, on a 12-h light/dark cycle, and transferred to fresh food once every 2 days. Two- and 6-week-old flies were used as controls (referred to as 2wC and 6wC, respectively).
Exercise training device and protocols
In constructing the exercise device, we took advantage of the flies’ natural negative geotaxis behavior to induce upward walking. Vials with diet housing 20 flies each were loaded horizontally into a steel tube that was rotated about its horizontal axis by an electric motor with a gear regulating its shaft speed. Thus, with the accompanying rotating steel tube, each vial was rotated along its long axis, which stimulated the flies to climb. Most flies continued to respond by climbing throughout the exercise period. The few that failed to climb were actively walking at the inner wall of the vial. Three groups of 4-week-old flies were exercised in vials of 2.8-cm inner diameter while rotating 0.24 rev/s for 1.5, 2, and 2.5 h, respectively (referred to as 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE). This occurred for five sessions a week, lasting 2 weeks.
The exercise periods were performed at 1400–1700 hours each day, after which cardiac dynamic parameters, climbing index, and locomotor activity were assayed (Fig. 1).
Fig. 1.
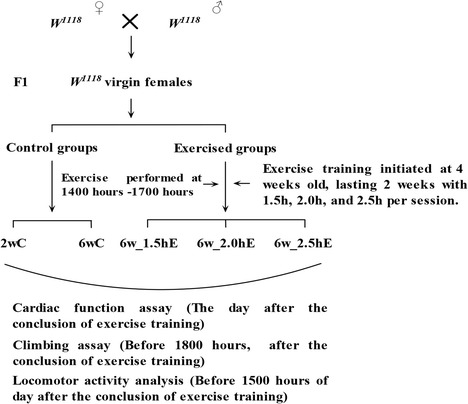
Schematic representation of exercise schedule of flies
Cardiac function
The cardiac function of the flies was tested the day after 2 weeks of training. Flies were anesthetized with FlyNap™ (Carolina Biological, Burlington, NC, USA) for about 5 min. The heart tubes were exposed by removing the heads, ventral thoraces, and ventral abdominal cuticles. All internal organs and abdominal fat were carefully removed by microsurgery forceps, leaving the heart and associated cardiac tissues (Ocorr et al. 2009). Dissections were performed under oxygenated artificial hemolymph containing 108 mM NaCl, 5 mK KCl, 2 mM CaCl2, 8 mM MgCl2, 10 mM sucrose, 5 mM trehalose, 5 mM HEPES (pH 7.1), 1 mM NaH2PO4, and 4 mM NaHCO3 (Singleton and Woodruff 1994) at room temperature (24 °C). High-speed digital movies of beating hearts were taken using a Hamamatsu EMCCD 9300 camera (Hamamatsu, Inc.; 100–140 frames per second) and SimplePCI image capture software (Hamamatsu, Inc., PA, USA). The heart physiology of the flies was assessed using a semi-automated optical heartbeat analysis program (Fink et al. 2009; Ocorr et al. 2009) (kindly gifted by Ocorr and Bodmer) that quantifies heartbeat parameters including diastolic and systolic diameters, heart period and rate, heartbeat regularity, and fractional shortening (Ocorr et al. 2007). Moreover, the arrhythmia index, a measure of heartbeat regularity, was calculated from high-speed digital movies using the mean standard deviation of the heart period normalized to the median heart period. M-modes, qualitative records showing heart edge movement over time, were also produced using the optical heartbeat analysis program (Fink et al. 2009). Furthermore, automated quantification of fibrillation was manifested by prolonged systoles (>0.5 s or twice the average systolic interval) and long systolic intervals (SIs) that were interrupted by very short diastolic intervals (<0.06 s) (Fink et al. 2009) in 20-s M-mode traces.
Climbing assay
Flies were tested through climbing assay on the last day (before 1800 hours) of the 2 weeks of training. The climbing apparatus consisted of an 18-cm-long glass tube with an inner diameter of 2.8 cm. Due to the instinctive negative geotaxis displayed by drosophilae, the flies climbed up the sides of the apparatus after being tapped down at the bottom. Flies were allowed 10 s to climb after being tapped down, and the climbing heights reached by the flies were calculated. Sponges were placed in the ends of the tube to prevent escape yet allowing air exchange. Ninety–120 flies, 20 flies per tube, were measured for each group. The flies were given five timed trials before being tested.
Locomotor activity analysis
The flies were put into the glass tube the day (before 1500 hours) after the 2 weeks of training. The flies were entrained in a 12-h light/dark cycle (light turned on at 0700 hours, light turned off at 1900 hours. 0700 hours referred to zeitgeber time 0, and 1900 hours referred to zeitgeber time 12) at 25 °C, and their locomotor activity was continuously monitored and recorded in 1-min bins by placing individual flies in glass tubes and monitoring their activity using the Drosophila Activity Monitoring System (DAMS) and Data Acquisition System (TriKinetics, Waltham, MA). Generally, flies subjected to 12-h light/dark cycles in DAMS were given a day for adaptation, and data from the second day in the glass tubes were used for analysis. Activity was measured using the infrared DAMS. As the flies moved back and forth in the tube, they interrupted an infrared beam at the center of the tube. These interruptions were counted and stored per minute. The number of activity counts per waking minute was defined as waking activity. Fly sleep in these devices was defined as any period of inactivity longer than 5 min, as reported previously (Shaw et al. 2000; Huber et al. 2004). The average total sleep, the average sleep episode duration, and the sleep bout number were calculated based on the sleep definition as a period of five or more minutes of behavioral immobility (Andretic and Shaw 2005). Waking activity, sleep episode duration, and bout number were divided into daytime/nighttime waking activity, daytime/nighttime sleep episode duration, and daytime/nighttime bout number. For each group, 27–31 flies were measured.
Statistical analyses
Independent-samples t tests were used to assess differences between the 2wC and 6wC group. One-way analysis of variance (ANOVA) with least significant difference (LSD) tests was used to identify differences among the 6wC, 6w_1.5hE, 6w_1.5hE, and 6w_1.5hE groups. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 16.0 for Windows (SPSS Inc. Chicago, USA), with statistical significance set at P < 0.05.
Results
Aging affects Drosophila cardiac function
To study heart function, we dissected adult flies in artificial hemolymph to record cardiac contractions with a high-speed digital video camera. M-mode traces illustrate the rhythmicity and the dynamics of heart contractions (Ocorr et al. 2007). Effects of aging and exercise in different durations on cardiac rhythm of Drosophila-represented M-mode traces (20 s) from high-speed movies for semi-intact flies that are 2 and 6 weeks old illustrated qualitative differences in heart function parameters: The M-mode of the flies’ hearts showed regular rhythmic contractions (Fig. 2a, 2-week-old flies), which become progressively irregular with age (Fig. 2a, 6-week-old flies), and moreover exhibited a significantly reduced heart rate that corresponded to an increased heart period (HP, defined as diastolic plus systolic interval) at 6 weeks of age compared to 2-week-old flies (Fig. 2b, c), which is due to prolonged systolic interval (Fig. 2e). The variability in the heart periodicity can be quantified using the heart period standard deviation as an “arrhythmicity index” (AI). The flies showed a low value for this AI at 2 weeks, and this value increased in the 6-week-old flies (Fig. 2f).
Fig. 2.
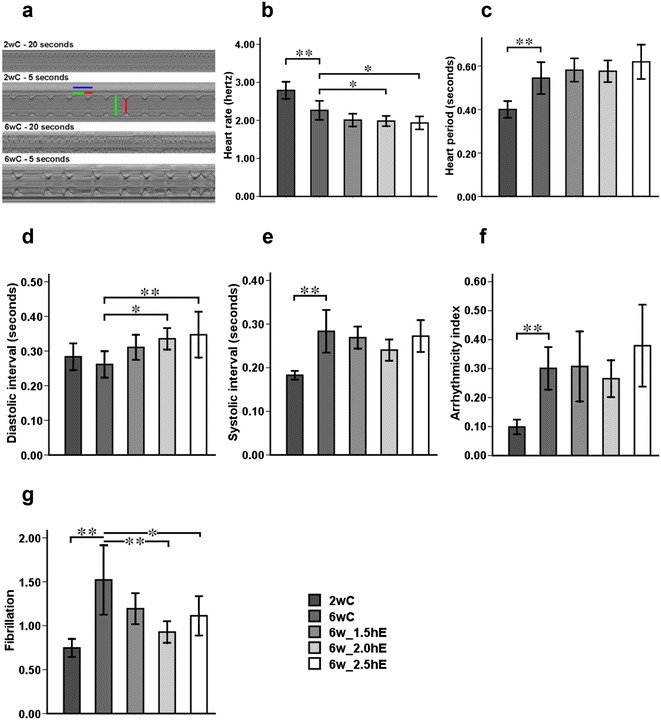
Effects of aging and exercise of different durations on cardiac rhythm of Drosophila. a Representative M-mode traces (20 s) from high-speed movies for semi-intact flies that are 2 and 6 weeks old, illustrating qualitative differences in heart function parameters: heart period (HP, horizontal blue line), diastolic interval (DI, horizontal green line), diastolic diameter (vertical green line), systolic interval (SI, horizontal red line), and systolic diameter (vertical red line). b Heart rate. c Heart period. d Diastolic interval. e Systolic interval. f Arrhythmicity index, defined as standard deviation of the heart period. g Automated analysis of fibrillation obtained from M-mode traces. *P < 0.05; **P < 0.01 using an independent-samples t test between 2wC and 6wC and using a one-way analysis of variance (ANOVA) followed by an LSD test among 6wC, 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE. Sample size was 20 to 30 flies per group. Data are displayed as mean ± SEM
The incidence of fibrillation events was automatically quantified as the number of unusually long systolic interval (>0.5 s or twice the average systolic interval) and long systolic interval that were interrupted by very short diastolic intervals (<0.06 s). The highly rhythmic beating pattern deteriorates as flies age by evidence of fibrillation events significantly increasing in 6-week-old flies compared to 2-week-old flies (Fig. 2g).
Our image analysis of heart contractions also provided cardiac chamber parameters, including diastolic and systolic diameters (Fig. 3a–j) and effects of aging and exercise in different durations on the cardiac contraction of Drosophila. Additionally, the proportional decrease in heart wall diameter during contraction, referred to as the fractional shortening, provided an indication of the cardiac output, and this value reduced with age due to decrease in the diastolic diameter. The systolic diameter of 6-week-old flies did not change much when compared with 2-week-old flies (Fig. 3k–m).
Fig. 3.
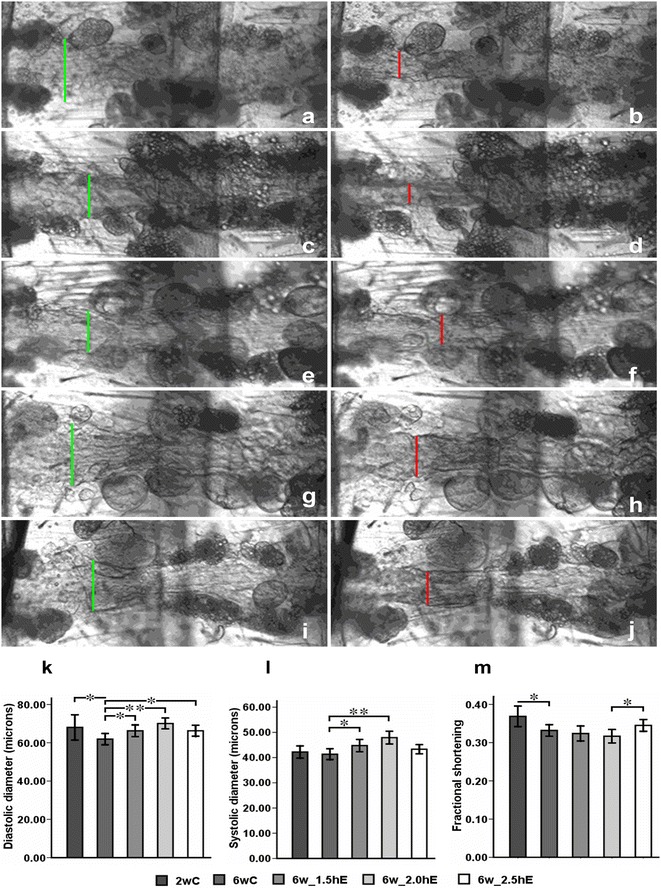
Effects of aging and exercise of different durations on the cardiac contraction of Drosophila. a–j Representative diastolic and systolic heart tubes from high-speed movies for semi-intact flies: diastolic diameter (vertical green line) and systolic diameter (vertical red line). a, b Diastolic and systolic heart tube for 2wC. c, d Diastolic and systolic heart tube for 6wC. e, f, g, h,i,j Diastolic and systolic heart tube for 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE, respectively. k Systolic diameter. l Diastolic diameter. m Cardiac output, quantified as fractional shortening. *P < 0.05; **P < 0.01 using an independent-samples t test between 2wC and 6wC and using a one-way analysis of variance (ANOVA) followed by an LSD test among 6wC, 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE. Sample size was 20 to 30 flies per group. Data are displayed as mean ± SEM
Exercise initiated later in life affected cardiac function of Drosophila
Heart rate was reduced in 6-week-old flies that performed 2.0 or 2.5 h of exercise per day compared to age-matched controls (Fig. 2b). The decreased heart rate was mainly due to a longer diastolic interval (relaxation period; Fig. 2d). Arrhythmicity index showed no significant difference between exercised and non-exercised old flies (Fig. 2f). The incidence of fibrillation events was reduced in flies exercised 2.0 or 2.5 h per day, especially in flies exercised 2.0 h per day. There was no significant decrease of fibrillation events in the flies when they were exposed to 1.5 h exercise per day compared to age-matched controls (Fig. 2g). Exercised old flies had a significantly wider diastolic diameter, and 1.5 and 2.0 h of exercise increased systolic diameter (Fig. 3a–j). Only 2.5 h of exercise per day increased fractional shortening compare to 2.0 h of exercised flies (Fig. 3k–m).
Exercise training with different doses initiated later in life exerted different effects on the climbing height of drosophilae
Climbing height was measured 1–1.5 h after the conclusion of exercise training. Effects of exercise with different durations started later in life on climbing height of Drosophila was measured using an independent-samples t test between 2wC and 6wC and using a one-way ANOVA followed by an LSD test among 6wC, 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE. Sample size was 90 to 120 flies per group. The climbing height reduced significantly with age; 1.5 h physical exercised flies remained unchanged compared to age-matched controls. However, 2.0- and 2.5-h exercised flies displayed apparent decrease in climbing height when compared with 1.5 h exercised flies and age-matched controls (Fig. 4).
Fig. 4.
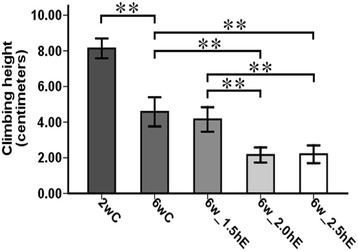
Effects of exercise with different durations started later in life on climbing height of Drosophila. *P < 0.05; **P < 0.01 using an independent-samples t test between 2wC and 6wC and using a one-way analysis of variance (ANOVA) followed by an LSD test among 6wC, 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE. Sample size was 90 to 120 flies per group. Data are displayed as mean ± SEM
Aging affects Drosophila sleep-wake behavior at nighttime
The 24-h profiles of locomotor activity for the 2- and 6-week-old flies are shown in Fig. 5a and Table 1. The evening activity was significantly decreased in 6-week-old flies, compared to 2-week-old flies (Fig. 5b). Similar to sleep patterns in humans, sleep patterns in flies changed with age; young flies slept more (Koh et al. 2006; Shaw et al. 2000). In this study, both sleep bout number and sleep episode duration at nighttime were significantly reduced in 6-week-old flies, compared to 2-week-old flies (Fig. 5e, g), which meant that older flies had defects in sleep maintenance and sleep initiation. In addition, the daily sleep data of these 2- and 6-week-old flies is depicted in Fig. 5f, h, that suggests that there were no significant differences between young and old flies.
Fig. 5.
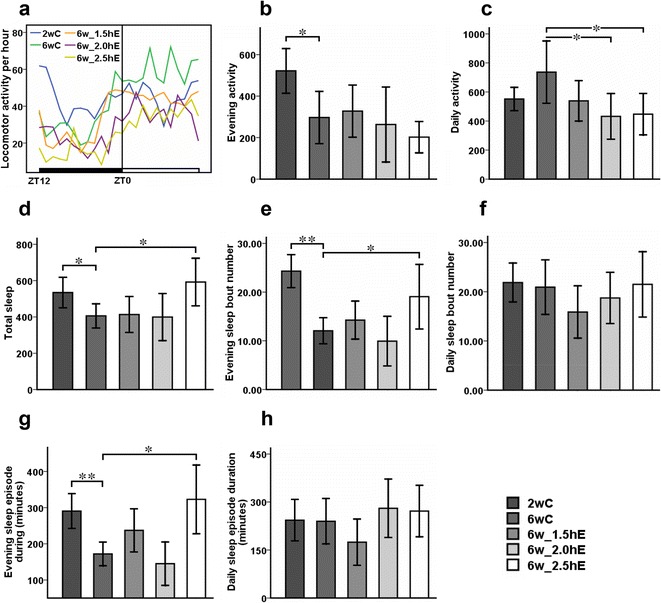
Effects of aging on sleep-wake behavior of Drosophila. Locomotor activity was recorded as the number of times a fly broke the path of an infrared beam at the midpoint of the tube. Periods of 5 min without beam crossing were regarded as a single period of sleep. a Averaged locomotor activity every hour of the 24-h cycle for flies. ZT zeitgeber time. The light bar represents lights on, and the dark bar represents lights off. ZT0 and ZT12 represent the start and end of the photoperiod, respectively. Total locomotor activity at b nighttime and c daytime. d Total sleep time. Sleep bout number at e nighttime and f daytime. Sleep episode duration at g nighttime and h daytime. *P < 0.05; **P < 0.01 using an independent-samples t test between 2wC and 6wC and using a one-way analysis of variance (ANOVA) followed by an LSD test among 6wC, 6w_1.5hE, 6w_2.0hE, and 6w_2.5hE. Sample size was 28 to 31 flies per group. Data are displayed as mean ± SEM
Table 1.
Sleep-wake behavior of Drosophila
| Group | Number | Evening activity | Daily activity | Total sleep (minutes) | Evening sleep bout number | Daily sleep bout number | Evening sleep episode duration (minutes) | Daily sleep episode duration (minutes) |
|---|---|---|---|---|---|---|---|---|
| 2wC | 31 | 521.73 ± 53.88 | 551.40 ± 40.29 | 534.31 ± 41.84 | 24.31 ± 1.69 | 21.88 ± 1.97 | 290.56 ± 24.00 | 243.25 ± 32.41 |
| 6wC | 29 | 297.00 ± 63.04 | 736.76 ± 107.06 | 406.06 ± 33.35 | 12.06 ± 1.33 | 20.94 ± 2.77 | 172.00 ± 16.45 | 239.81 ± 35.46 |
| 6w_1.5hE | 29 | 328.00 ± 62.86 | 538.60 ± 69.75 | 413.50 ± 49.33 | 14.25 ± 1.95 | 15.88 ± 2.65 | 237.20 ± 29.79 | 174.47 ± 36.05 |
| 6w_2.0hE | 28 | 263.20 ± 90.54 | 431.93 ± 78.54 | 399.50 ± 64.95 | 9.93 ± 2.54 | 18.73 ± 2.60 | 145.07 ± 30.07 | 280.29 ± 45.67 |
| 6w_2.5hE | 29 | 202.36 ± 37.84 | 447.07 ± 71.38 | 592.25 ± 65.43 | 19.06 ± 3.31 | 21.50 ± 3.31 | 323.00 ± 47.51 | 271.67 ± 40.14 |
Exercise initiated later in life affected Drosophila daily activity and evening sleep architecture
The 24-h patterns of locomotor activity for the 6-week-old flies with different doses of exercise are displayed in Fig. 5a. No apparent differences of evening activity and total daily sleep were detected between exercised and non-exercised 6-week-old flies (Fig. 5b). Flies which were exposed to 2 and 2.5 h of exercise per day incurred exercise-induced fatigue as evidenced by a significant reduction in daily activity (Fig. 5c), which has been reported in humans (Friedberg 2002; Wenzel et al. 2013) and other robust model studies (Tadano et al. 2003). Additionally, both sleep bout number and sleep episode duration at nighttime were obviously increased in flies that were exercised 2.5 h per day, which contributed to the statistically significant increase in total sleep (Fig. 5e, g).
Discussion
A semi-intact fly preparation and an optical heartbeat analysis program have been developed by Ocorr et al. (2007), which permit researchers to precisely quantify the inherent myogenic heartbeat parameters without having confounding influences from neuronal input (Johnson et al. 2000; Dulcis and Levine 2005; Dulcis et al. 2005). Moreover, a protocol for long-term endurance training has been devised for Drosophila, which relies on the instinct for negative geotaxis that causes flies to involuntarily run upwards when knocked to the bottom of a container (Piazza et al. 2009). These effective techniques, alone with the fly’s short lifespan, makeDrosophila an excellent model for studying the effects of exercise intervention on cardiac aging.
Lifelong physical training preserves left ventricular compliance, which is beneficial to left ventricular dynamic diastolic function. This has been manifested by master athletes (Bhella et al. 2014). Moreover, lifetime exercise training also improves cardiovascular function by increasing stroke volume, cardiac output, and heart rate regulation during exercise (Carrick-Ranson et al. 2014). In older healthy subjects, it has been demonstrated that physical training ameliorates age-related deterioration of cardiac function in terms of enhanced left ventricular systolic function and increased diastolic filling (Spina et al. 1998). One year of endurance exercise training led to an increase in exercise cardiac output due mainly to increase in stroke volume (Fujimoto et al. 2010). However, it is still uncertain whether the age-associated impairment in diastolic function may be reduced by physical exercise, since endurance exercise training had only minimal effects on left ventricular diastolic function by Doppler measures in healthy elderly individuals (Prasad et al. 2007; Fujimoto et al. 2010; Vigorito and Giallauria 2014). An increased aging population, due to the extension of life expectancy, contributes to the notion of global population aging, with cardiovascular disease remaining the primary contributor to mortality (Kelly et al. 2012). It is important to investigate what are the benefits of exercise training started later in life on cardiac aging.
In this study, consistent with other divergent genetic background flies (Nishimura et al. 2011), several aspects of cardiac function have been shown to decline progressively with age in a robust fashion that is detectable in wild-type w1118 flies. Heart rate underwent a steady decline between 2 and 6 weeks of age, and heart period increased significantly with age. This increase in heart period was largely a function of lengthening systolic intervals. Moreover, fractional shortening, which is an indicator of contractive function decreased with age, and arrhythmicity index as well as incidence of fibrillation events (which was automatically detected by quantifying systolic interval >0.5 s or diastolic interval <0.06 s in flies) (Ocorr et al. 2007) occurred in older flies at an increase that was statistically significant. These changing profiles of cardiac function during aging have been exhibited in older humans. For example, the aged exhibit an increase in diastolic end-filling, resulting in an increase of contraction duration (Lakatta et al. 1975; Rodeheffer et al. 1984). This increased contraction duration, in turn, leads to a reduction in resting heart rate and an even more pronounced reduction in maximal heart rate during exercise. The decrease in maximal heart rate may also be a consequence of reduced number of functional atrial pacemaker cells, which contributes to loss of rhythmic homeostasis, as reflected in the age-related increase in atrial fibrillation events (Cheitlin 2003). After that, we explored the effect of distinct doses of physical exercise training initiated in 4-week-old flies on the cardiac dynamic aspect. We found that 2 or 2.5 h of exercise training per day for five weekly endurance exercise sessions over 2 weeks reduced the resting heart rate, which was manifested in an “athlete’s heart,” also named exercise bradycardia, which provides more cardiac reserve (Lewis et al. 1980; Azevedo et al. 2014; Heinonen et al. 2014) when the individual is suffering from cardiac stress. The decreased heart rate was due to the increasing diastolic interval, which may be beneficial to heart filling. However, the decreased heart rate was not detected in flies that were exercised 1.5 h per day for 2 weeks. The flies that were exercised for 1.5 or 2.0 h of exercise enlarged both the diastolic diameter and systolic diameter without enhancing fractional shortening that is an indicative of contracting function during exercise. On the other hand, the 2.5-h exercise training increased fractional shortening due to enlarged diastolic diameter and reserved systolic diameter. Interestingly, 2 and 2.5 h exercise training reduced the fibrillation events compared to age-matched controls; however, a discernible effect was not observed in 1.5 h exercise training flies. These findings suggested that exercise duration only lasts long enough to result in significant reduction of fibrillation events, and in order to make fractional shortening increase adaptively, exercises need to last longer than is needed for reducing the incident of fibrillation in previously sedentary seniors. We also tested the effect of exercise training started later in life on the wake-sleep cycle by detecting locomotor activity using a drosophila activity monitor. Consistent with a previous study (Rakshit et al. 2013), there was no significant difference in the locomotor activity profile in 4-week-old flies that underwent 1.5 h of exercise per day lasting two consecutive weeks compared to age-matched controls. Flies that were exposed to 2.0 h per day of exercise training displayed no apparent difference in activity at nighttime and total sleep, except that daily activity counts significantly reduced. Besides a decrease in daily activity, exercising 2.5 h per day can increase total sleep, which is due to increasing evening sleep bout number, as well as evening sleep episode duration, which indicated that 2.5 h of exercise can increase the quantity and quality of sleep, as well as the extending of sleep episode duration, which suggests that the sleep consolidation caused by senescence is alleviated by exercises and exercises enhance the sleep efficiency.
In order to identify which exercise duration flies should perform to achieve beneficial physiological adaptation for cardiac function and sleep quality with age, we evaluated muscle fatigue at 1–1.5 h after the conclusion of the 2-week exercise training scheme by assessing climbing ability. Muscle fatigue is an exercise-induced decline in maximal voluntary muscle force or power, and it develops soon after the onset of sustained physical activity (Enoka and Duchateau 2008). It has been reported that exercise performed to fatigue results in an enhanced sensitivity of myofibrillar protein synthesis to protein feeding during exercise recovery (Burd et al. 2011) and glycogen depletion. However, glycogen returns to above basal levels (super compensation) after cessation of exercise (Bergström and Hultman 1996), which is beneficial to exercise performance. The development of muscle fatigue during exercise is progressive (Gandevia 2001) and depends on the duration and intensity of the exercise bout (Enoka and Stuart 1992). In our study, exercise training initiated at middle age exerted different effects on the climbing ability of Drosophila. Climbing height showed no apparent difference in flies that exercised 1.5 h per day compared to controls. In contrast, a significant decrease in climbing height was detected in flies exposed to 2.0 and 2.5 h of exercise training. These data suggest that physical exercise lasting longer than 2.0 and 2.5 h may result in exercise-induced muscle fatigue. Interestingly, the diverse change in climbing ability after various volumes of physical exercise was in accordance with the change in cardiac function. Only flies that performed 2.0 and 2.5 h of exercise training could decrease the incidence of fibrillation, suggesting that the intensity and period of physical exercise should reach the intensity of physical fatigue to promote better adaptive change of cardiac function, such as reduction of incidence of fibrillation, and to delay the deterioration of fractional shortening. Although the effects of exercise training are variable on different organs and systems, exercise-induced fatigue can occur in the whole body, and the physiological benefits from fatiguing exercise may also be for the whole body. Thus, we assessed the sleep quality by locomotor activity system. Flies performing 2.5 h exercise could improve sleep quality due to prolonged sleep time and sleep consolidation.
The key new findings from the present study included the following: (1) 4-week-old flies exposed to 2.0 and 2.5 h of exercise per day lasting 2 weeks showed a reduced incident of fibrillation events. Moreover, the exercise training of 2.5 h per day increased fractional shortening and total sleep in Drosophila. However, this discernible effect was not detected in flies that underwent 1.5 h exercise per day, providing evidence that physical training needs to be performed long enough to exert physiological benefits for cardiac aging. (2) In order to evaluate which exercise duration was suitable, climbing ability using negative geotaxis was applied to assess the exercise-induced muscle fatigue. The occurrence of muscle fatigue after different durations was consistent to the variation of cardiac function and sleep quality. Thus, climbing ability seems to be an effective means of assessing the fatigue exercise schedule for cardiac aging.
Acknowledgments
The authors want to thank all the research staff for their participation in this study. This project was supported by the National Natural Science Foundation of China (Grant No. 31071039) and the Natural Science Foundation of Hunan Province (Grant No. 2015JJ2102).
Conflict of interest
The authors declare that they have no competing interests.
References
- Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- Azevedo LF, Perlingeiro PS, Hachul DT, Gomes-Santos IL, Brum PC, Allison TG, Negrão CE, De Matos LD. Sport modality affects bradycardia level and its mechanisms of control in professional athletes. Int J Sports Med. 2014;35:954–959. doi: 10.1055/s-0033-1364024. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.CIR.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1996;210:309–310. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B, Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64:1257–1266. doi: 10.1016/j.jacc.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol. 2014;116:736–745. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheitlin MD. Cardiovascular physiology—changes with aging. Am J Geriatr Cardiol. 2003;12:9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- Demontis F, Piccirillo R, Al Goldberg, Perrimon N. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis Model Mech. 2013;6:1339–1352. doi: 10.1242/dmm.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derumeaux G, Ichinose F, Raher MJ, Morgan JG, Coman T, Lee C, Cuesta JM, Thibault H, Bloch KD, Picard MH, Scherrer-Crosbie M. Myocardial alterations in senescent mice and effect of exercise training: a strain rate imaging study. Circ Cardiovasc Imaging. 2008;1:227–234. doi: 10.1161/CIRCIMAGING.107.745919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Levine RB. Glutamatergic innervation of the heart initiates retrograde contractions in adult Drosophila melanogaster. J Neurosci. 2005;25:271–280. doi: 10.1523/JNEUROSCI.2906-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Levine RB, Ewer J. Role of the neuropeptide CCAP in Drosophila cardiac function. J Neurobiol. 2005;64:259–274. doi: 10.1002/neu.20136. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586:11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1063/1.351680. [DOI] [PubMed] [Google Scholar]
- Erbs S, Höllriegel R, Linke A, Beck EB, Adams V, Gielen S, Möbius-Winkler S, Sandri M, Kränkel N, Hambrecht R, Schuler G. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left-ventricular function. Circ Heart Fail. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- Fiechter M, Fuchs TA, Gebhard C, Stehli J, Klaeser B, Stähli BE, Manka R, Manes C, Tanner FC, Gaemperli O, Kaufmann PA. Age-related normal structural and functional ventricular values in cardiac function assessed by magnetic resonance. BMC Med Imaging. 2013;13:6. doi: 10.1186/1471-2342-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Callol-Massot C, Chu A, Ruiz-Lozano P, Izpisua Belmonte JC, Giles W, Bodmer R, Ocorr K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg F. Does graded activity increase activity? A case study of chronic fatigue syndrome. J Behav Ther Exp Psychiatry. 2002;33:203–215. doi: 10.1016/S0005-7916(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122:1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Kudomi N, Kemppainen J, Kiviniemi A, Noponen T, Luotolahti M, Luoto P, Oikonen V, Sipilä HT, Kopra J, Mononen I, Duncker DJ, Knuuti J, Kalliokoski KK. Myocardial blood flow and its transit time, oxygen utilization, and efficiency of highly endurance-trained human heart. Basic Res Cardiol. 2014;109:413. doi: 10.1007/s00395-014-0413-1. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, Zhou JN. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76:597–603. doi: 10.1016/S0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27:628–639. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annu Rev Entomol. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Johnson E, Ringo J, Dowse H. Native and heterologous neuropeptides are cardioactive in Drosophila melanogaster. J Insect Physiol. 2000;46:1229–1236. doi: 10.1016/S0022-1910(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Kavanagh T, Myers MG, Baigrie RS, Mertens DJ, Sawyer P, Shephard RJ. Quality of life and cardiorespiratory function in chronic heart failure: effects of 12 months’ aerobic training. Heart. 1996;76:42–49. doi: 10.1136/hrt.76.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BB, Narula J, Fuster V. Recognizing global burden of cardiovascular disease and related chronic diseases. Mt Sinai J Med. 2012;79:632–640. doi: 10.1002/msj.21345. [DOI] [PubMed] [Google Scholar]
- Koh K, Evans JM, Hendricks JC, Sehgal A. From the cover: a Drosophila model for age-associated changes in sleep: wake cycles. Proc Natl Acad Sci U S A. 2006;103:13,843–13,847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz JM. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging. 2009;1:937–948. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Gerstenblith G, Angell CS, Shock NW, Weisfeldt ML. Prolonged contraction duration in aged myocardium. J Clin Invest. 1975;55:61–68. doi: 10.1172/JCI107918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SF, Nylander E, Gad P, Areskog NH. Non-autonomic component in bradycardia of endurance trained men at rest and during exercise. Acta Physiol Scand. 1980;109:297–305. doi: 10.1111/j.1748-1716.1980.tb06600.x. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Ocorr K, Bodmer R, Cartry J. Drosophila as a model to study cardiac aging. Exp Gerontol. 2011;46:326–330. doi: 10.1016/j.exger.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Reeves N, Wessells R, Fink M, Chen HSV, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony J, Bodmer R. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci U S A. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Fink M, Cammarato A, Bernstein S, Bodmer R (2009) Semi-automated Optical Heartbeat Analysis of small hearts. J Vis Exp (31). [DOI] [PMC free article] [PubMed]
- Piazza N, Wessells RJ. Drosophila models of cardiac disease. Prog Mol Biol Transl Sci. 2011;100:155–210. doi: 10.1016/B978-0-12-384878-9.00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza N, Gosangi B, Devilla S, Arking R, Wessells R (2009) Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One 4:e5886. [DOI] [PMC free article] [PubMed]
- Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Giebultowicz JM. Cryptochrome restores dampened circadian rhythms and promotes healthspan in aging Drosophila. Aging Cell. 2013;12:752–762. doi: 10.1111/acel.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Krishnan N, Guzik EM, Pyza E, Giebultowicz JM. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int. 2012;29:5–14. doi: 10.3109/07420528.2011.635237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Wambua R, Giebultowicz TM, Giebultowicz JM. Effects of exercise on circadian rhythms and mobility in aging Drosophila melanogaster. Exp Gerontol. 2013;48:1260–1265. doi: 10.1016/j.exger.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–213. doi: 10.1161/01.CIR.69.2.203. [DOI] [PubMed] [Google Scholar]
- Sastre J, Pallardo FV, Vina J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49:427–435. doi: 10.1080/152165400410281. [DOI] [PubMed] [Google Scholar]
- Scalia GM, Khoo SK, O’Neill S, LAW Study Group Age-related changes in heart function by serial echocardiography in women aged 40–80 years. J Women's Health (Larchmt) 2010;19:1741–1745. doi: 10.1089/jwh.2009.1752. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Singleton K, Woodruff RI. The osmolarity of adult Drosophila hemolymph and its effect on oocyte-nurse cell electrical polarity. Dev Biol. 1994;161:154–167. doi: 10.1006/dbio.1994.1017. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Turner MJ, Ehsani AA. Beta-adrenergic-mediated improvement in left ventricular function by exercise training in older men. Am J Physiol. 1998;274:H397–H404. doi: 10.1152/ajpheart.1998.274.2.H397. [DOI] [PubMed] [Google Scholar]
- Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol. 2004;44:1320–1327. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Tadano T, Nakagawasai O, Niijima F, Tan-no K, Hanawa MA, Sakata Y, Sutoo D, Nemoto Y, Ida Y, Endo Y. Effect of nutritive and tonic crude drugs on physical fatigue-induced stress models in mice. Pharmacol Res. 2003;47:195–199. doi: 10.1016/S1043-6618(02)00318-3. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Vigorito C, Giallauria F. Effects of exercise on cardiovascular performance in the elderly. Front Physiol. 2014;5:51. doi: 10.3389/fphys.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JA, Griffith KA, Shang J, Thompson CB, Hedlin H, Stewart KJ, DeWeese T, Mock V. Impact of a home-based walking intervention on outcomes of sleep quality, emotional distress, and fatigue in patients undergoing treatment for solid tumors. Oncologist. 2013;18:476–484. doi: 10.1634/theoncologist.2012-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]


