Abstract
Background
Lung weight characterises severity of pulmonary oedema and predicts response to mechanical ventilation. The aim of this study was to evaluate the accuracy of quantitative analysis of thorax computed tomography (CT) for measuring lung weight in pigs with or without pulmonary oedema.
Methods
Thirty-six pigs were mechanically ventilated with different tidal volumes and positive end-expiratory pressures that did or did not induce pulmonary oedema. After 54 h, they underwent thorax CT (CTin vivo) and were then sacrificed and exsanguinated. Fourteen pigs underwent a second thorax CT (CTpost-exsang.) after exsanguination. Lungs were excised and weighed with a balance (balancepost-exsang.). Agreement between lung weights measured with the balance (considered as reference) and those estimated by quantitative analysis of CT was assessed with Bland-Altman plots.
Results
One animal unexpectedly died before CTin vivo. In 35 pigs, lung weight measured with balancepost-exsang. was 371 ± 184 g and that estimated with CTin vivo was 481 ± 189 g (p < 0.001). Bias between methods was −111 g (−35%) and limits of agreement were −176 (−63%) and −46 g (−8%). Measurement error was similar in animals with (−112 ± 45 g; n = 11) or without (−110 ± 27 g; n = 24) pulmonary oedema (p = 0.88). In 14 pigs with thorax CT after exsanguination, lung weight measured with balancepost-exsang. was 342 ± 165 g and that estimated with CTpost-exsang. was 352 ± 160 g (p = 0.02). Bias between methods was −9 g (−4%) and limits of agreement were −36 (−11%) and 17 g (3%). Measurement errors were similar in pigs with (−1 ± 26 g; n = 11) or without (−12 ± 7 g; n = 3) pulmonary oedema (p = 0.12).
Conclusions
Compared to the balance, CT obtained in vivo constantly overestimated the lung weight, as it included pulmonary blood (whereas the balance did not). By contrast, CT obtained after exsanguination provided accurate and reproducible results.
Keywords: Computed tomography, Lung weight, Ventilator-induced lung injury, Pulmonary oedema
Background
During ventilator-induced lung injury and acute respiratory distress syndrome, lung weight increases as a result of inflammatory pulmonary oedema [1,2]. Exact quantification of this phenomenon can help diagnosis, prognosis and even therapy. For instance, patients with heavy (and largely recruitable) lungs benefit the most from use of high positive end-expiratory pressure [3] or prone position [4].
One method for estimating lung weight in vivo is quantitative analysis of thorax computed tomography (CT). Lungs are selected on CT images and their total (gas and tissue) volume and physical density (that presumably reflects relative content of gas and tissue) are measured. Lung tissue volume is derived from these two parameters. Lung tissue weight is computed assuming that the density is 1 g/ml [5].
Selection of region of interest is crucial for quantitative analysis of CT. It is usually performed manually, based on individual judgement, and is prone to errors especially when contours are not linear and content is not homogeneous [6]. Inaccuracy is indeed less than 1% for phantoms with regular shape [7] but as high as 10% for objects with complex geometry, including human organs [8]. Oedema may act as an additional confounder as it deforms anatomy and increases inhomogeneity.
The aim of this study was to validate quantitative analysis of CT (against gravimetry) for measuring lung weight in pigs with or without pulmonary oedema.
Methods
This is a sub-study of our past [9,10] and present works. Experiments complied with international recommendation [11] and were approved by the Italian Ministry of Health.
Validation of CT performed in living animals (CTin vivo) for measuring lung weight
Thirty-six consecutive healthy female pigs (23 ± 3 kg) were mechanically ventilated with different tidal volumes and positive end-expiratory pressures under general anaesthesia (propofol [5 to 10 mg/kg/h IV] and medetomidine [2.5 to 10.0 μg/kg/h IV]) and paralysis (pancuronium bromide [0.3 to 0.5 mg/kg/h IV]). After 54 h, thorax CT (CTin vivo) was obtained. Animals were then sacrificed (KCl 40 mEq IV) and exsanguinated through an incision in the inferior vena cava. Lungs were excised en bloc, dissected from the trachea, main bronchi and hilar lymph nodes, and weighted with a balance (balancepost-exsang.) within 30 to 60 min from CTin vivo. The balance (Bizerba Maxima Super Elox, Bizerba, Milan, Italy) underwent periodical tests with calibrated weights. Percentage error (see below) was always <1%. Ventilator-induced pulmonary oedema was diagnosed if balancepost-exsang. lung weight exceeded 400 g, the upper limit for healthy pigs of similar sex and weight [1,9,10].
One animal unexpectedly died before we could obtain thorax CTin vivo. It was immediately exsanguinated and included in the second part of the study (see below).
Validation of CT performed after exsanguination (CTpost-exsang.) for measuring lung weight
Fourteen (out of 36) animals underwent a second thorax CT (CTpost-exsang.) once exsanguinated. The volume of blood removed was always quantified, whereas density (g/ml) was measured in three cases. Lungs were then excised and weighted with the balance (and pulmonary oedema was eventually diagnosed) as above.
Quantitative analysis of CT
CTs were obtained at 0 cmH2O of airway pressure with the following settings: collimation, 5 mm; interval, 5 mm; bed speed, 15 mm/s; voltage, 140 kV; and current 240 mA (Lightspeed QXi, GE Healthcare, Madison, WI, USA). Quality controls were performed every month using standard phantoms.
Experienced operators manually countered the lung profile excluding proximal airways, large vessels and lymph nodes, mediastinum, muscles and bones and pleural effusions (Maluna 3.15, University Hospital of Goettingen, Germany).
For each voxel of interest, tissue weight was
Voxel density was expressed in Hounsfield units (HU), with values of −1,000, 0 and +1,000 HU assigned to air, lung tissue (including parenchyma, blood and water) and bone, respectively. Voxel volume was 1.8 mm3.
Lung tissue weight was the sum of the weight of all selected voxels [5].
Repeatability and reproducibility of quantitative analysis of thorax CT
Thorax CTsin vivo of one animal with and one animal without pulmonary oedema were analysed thrice by the same operator (repeatability) and once by three different operators (reproducibility).
Statistical analysis
Normality of data distribution was verified with the Shapiro-Wilk test. Results are reported as means ± standard deviations. Difference between groups was assessed with Student's t test or Mann-Whitney rank sum test. Agreement between reference (balance; gravimetry) and test (quantitative analysis of CT) methods was studied with Bland-Altman plots and analysis of correlation [12]. Percentage error was the ratio between the limits of agreement and reference measurement. Repeatability and reproducibility were expressed as coefficients of variation, the ratio between standard deviations and means. Statistical significance was defined as p < 0.05 (SigmaPlot 11.0, Jandel Scientific Software, San Jose, CA, USA).
Results
Validation of CT performed in living animals (CTin vivo) for measuring lung weight
In 35 pigs (11 with pulmonary oedema), lung weight was 371 ± 184 g when measured with balancepost-exsang. and 481 ± 189 g (p < 0.001) when estimated with quantitative analysis of CTin vivo. Bias was −111 g (−35%) and limits of agreement were −176 (−63%) and −46 g (−8%) (Figure 1a). Percentage error was 35% and coefficient of correlation was 0.97 (p < 0.0001) (Figure 1b).
Figure 1.
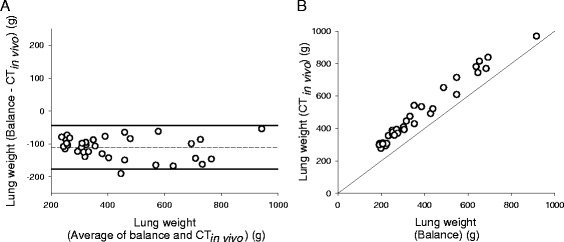
Agreement and association between balance (balance post-exsang. ) and CT performed in living animals (CT in vivo ) for measuring lung weight. (A) Plot of individual differences between lung weights measured with balancepost-exsang. (reference) and CTin vivo against their means. Dashed line refers to overall mean difference (bias), and solid lines correspond to 95% limits of agreement (mean difference ± 1.96 standard deviation). (B) Individual lung weights measured with balancepost-exsang. and CTin vivo, with line of identity [CTin vivo lung weight = 106 + 1.01 × (balancepost-exsang. lung weight)].
On average, balancepost-exsang. lung weight was 606 ± 140 g in animals with pulmonary oedema (n = 11) and 263 ± 55 g in those without pulmonary oedema (n = 24). Absolute measurement errors were not associated with (mean) lung weight (r = 0.16) (p = 0.36) and did not differ between animals with (−112 ± 45 g) or without (−110 ± 27 g) pulmonary oedema (p = 0.88). Diversely, relative measurement errors were smaller in animals with (−19%; limits of agreement −36% and −3%) than in those without (−43%; limits of agreement −61% and −24%) pulmonary oedema (p < 0.01).
Validation of CT performed after exsanguination (CTpost-exsang.) for measuring lung weight
In 14 pigs (three with pulmonary oedema), lung weight was 342 ± 165 g when measured with balancepost-exsang. and 352 ± 160 g when estimated with quantitative analysis of CTpost-exsang. (p = 0.02). Bias was −9 g (−4%) and limits of agreement were −36 (−11%) and 17 g (3%) (Figure 2a). Percentage error was 15% and coefficient of correlation was 0.99 (p < 0.0001) (Figure 2b).
Figure 2.
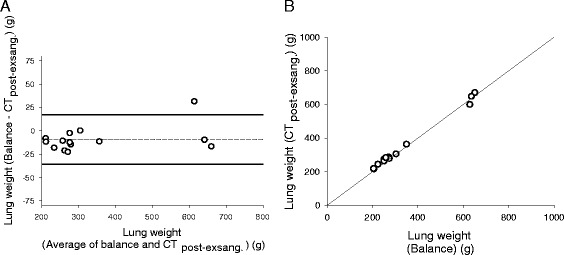
Agreement and association between balance (balance post-exsang. ) and CT performed after exsanguination (CT post-exsang. ) for measuring lung weight. (A) Plot of individual differences between lung weights measured with balancepost-exsang. (reference) and CTpost-exsang. against their means. Dashed line refers to overall mean difference (bias), and solid lines correspond to 95% limits of agreement (mean difference ± 1.96 standard deviation). (B) Individual lung weights measured with balancepost-exsang. and CTpost-exsang., with line of identity [CTpost-exsang. lung weight = 22 + 0.96 × (balancepost-exsang. lung weight)].
On average, balancepost-exsang. lung weight was 640 ± 11 g in animals with pulmonary oedema (n = 3) and 261 ± 42 g in those without pulmonary oedema (n = 11). Absolute measurement errors were not associated with (mean) lung weight (r = 0.41) (p = 0.15) and did not differ between animals with (−1 ± 26 g) or without (−12 ± 7 g) pulmonary oedema (p = 0.12).
The volume of blood aspirated during exsanguination was 1,049 ± 283 ml. The density of blood (measured in three animals) was 1.01 g/ml.
In 13 animals that underwent both CTs, CTin vivo overestimated lung weight by 105 ± 32 g compared to CTpost-exsang. and by 117 ± 32 g compared to the balance (p = 0.23).
Repeatability and reproducibility of quantitative analysis of thorax CT
Coefficients of intra- and inter-subject variability were 2.4% and 4.2%, respectively, for healthy lungs and 2.2% and 0.3%, respectively, for oedematous lungs.
Discussion
Compared to gravimetry, quantitative analysis of thorax CT accurately measures the weight of lungs of exsanguinated animals (CTpost-exsang.: bias −4%; percentage error 15%). However, it largely overestimates the weight of lungs of living animals (CTin vivo: bias −35%; percentage error 35%) as it includes pulmonary blood.
Other authors have shown that CT properly quantifies the weight of inanimate objects [8], artificial lung models [13], exsanguinated [14] and frozen [15] lungs and surgically excised pulmonary lobes [16]. Results acquired in vivo can be less accurate. For instance, in previous studies, the weight of the right hepatic lobes differed by 20% to 35% when measured pre-operatively with CT (in vivo) or intra-operatively with a balance (ex vivo). One plausible explanation for this discrepancy is blood volume: measurements taken before surgery included blood, whereas those obtained after graft procurement did not [17,18]. In our study, lung weight measured with CTin vivo (before exsanguination) exceeded by around 100 g that obtained with CTpost-exsang. or the balance (after exsanguination). Pulmonary blood is around 5 ml/kg of body weight in pigs [19] and represents 10% to 15% of the total blood volume in mammals [20] (normal values in humans: 220 to 270 ml/m2 of body surface area [21]). Therefore, it likely approximated 100 ml in 20-kg pigs that exsanguinated, on average, 1 l of blood. Since blood density was 1 g/ml, pulmonary blood weight of animals included in our experiments was probably around 100 g and thus explains the bias reported above. Of note, measurement error was the same even in animals with (inflammatory) pulmonary oedema as if changes in lung weight induced by mechanical ventilation mainly reflected changes in extravascular water content [22]. As a consequence, relative measurement error was as low as 19% in animals with pulmonary oedema.
In line with the model described so far, differences between computed tomography and gravimetry virtually disappeared when they both measured blood-free lung weights (CTpost-exsang. vs. balancepost-exsang.). To further corroborate this finding, two other animals underwent thorax CTin vivo and were then sacrificed but not exsanguinated to allow intravascular blood clotting. Two hours later, lungs were excised with minimal blood loss. As expected, results of quantitative analysis of thorax CTin vivo differed by only 24 g (5%) and 11 g (3%) from those obtained with the balance as both methods measured the weight of blood-filled lungs.
Some limitations of the study deserve a comment. First, sample size was limited due to inconstant availability of CT, some unexpected early deaths and completion of research projects to which animals were originally assigned. Accuracy of CTpost-exsang. was evaluated only in three animals with pulmonary oedema, and therefore, results should be interpreted with caution. Second, the volume of blood withdrawn from animals was quite variable, possibly because heparin was not used to prevent post-mortem thrombosis. This may be the reason why percentage error of lung weight estimated with CTin vivo was as high as 35%. Third, the use of thorax CT in clinical practice requires transfer of patients to radiology [23] and exposure to ionising radiations [24]. In addition, quantitative analysis is complex and time consuming. However, low-dose exams [25] and simplified analysis [26,27] may limit risks and expense. Moreover, quantitative analysis of thorax CT does not only measure lung weight but also characterises patients with acute respiratory failure [28], assesses distribution of ventilation [29], quantifies pulmonary inhomogeneities [30] and predicts response to therapy [31].
Conclusions
When performed on living subjects, quantitative analysis of thorax CT scan constantly overestimated lung weight as it included pulmonary blood (whereas the balance did not). In bloodless conditions, it provided accurate, reproducible and repeatable results.
Acknowledgements
We are indebted to Luciano Lombardi (Dipartimento di Radiologia, Fondazione IRCCS Ca' Granda - Ospedale Maggiore Policlinico, Milan, Italy) and Dr. Maurizio Rozza (Dipartimento di Fisica Sanitaria, Fondazione IRCCS Ca' Granda - Ospedale Maggiore Policlinico, Milan, Italy) for their precious technical support. Preliminary results were presented at the 24th Simposio Mostra Anestesia Rianimazione Terapia intensiva (SMART) in Milan, May 2013.
Abbreviations
- CT
computed tomography
- Post-exsang.
following exsanguination
- HU
Hounsfield unit
- IV
Intravenous
- KCl
Potassium chloride
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AP conceived the study and wrote the draft of the manuscript. GEI conceived the study, wrote the draft of the manuscript and participated in the data acquisition and analysis. MM, VM, PP and BC participated in the data acquisition and analysis. MC and LG participated in the study design and revised the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Alessandro Protti, Email: alessandro.protti@policlinico.mi.it.
Giacomo E Iapichino, Email: giacomo.iapichino@gmail.com.
Marta Milesi, Email: marta.mil@hotmail.it.
Valentina Melis, Email: valentina.melis1@studenti.unimi.it.
Paola Pugni, Email: paola.pugni@studenti.unimi.it.
Beatrice Comini, Email: bcomini@hotmail.com.
Massimo Cressoni, Email: mcressoni@hotmail.com.
Luciano Gattinoni, Email: gattinon@policlinico.mi.it.
References
- 1.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med. 2011;183:1354–1362. doi: 10.1164/rccm.201010-1757OC. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with acute respiratory distress syndrome. N Engl J Med. 2006;354:1776–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 4.Protti A, Chiumello D, Cressoni M, Carlesso E, Mietto C, Berto V, Lazzerini M, Quintel M, Gattinoni L. Relationship between gas exchange response to prone position and lung recruitability during acute respiratory failure. Intensive Care Med. 2009;35:1011–1017. doi: 10.1007/s00134-009-1411-x. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Pesenti A, Bombino M, Baglioni S, Rivolta M, Rossi F, Rossi GP, Fumagalli R, Marcolin R, Mascheroni M, Torresin A. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology. 1988;69:824–832. doi: 10.1097/00000542-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wegener OH, Koeppe P, Oeser H. Measurement of lung density by computed tomography. J Comput Assist Tomogr. 1978;2:263–273. doi: 10.1097/00004728-197807000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Mull RT. Mass estimates by computed tomography: physical density from CT numbers. Am J Roentgenol. 1984;143:1101–1104. doi: 10.2214/ajr.143.5.1101. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DE, Whitley NO, Houk TL, Aisner J, Wiernik P, Whitley J. Volume determinations in computed tomography. JAMA. 1982;247:1299–1302. doi: 10.1001/jama.1982.03320340053035. [DOI] [PubMed] [Google Scholar]
- 9.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, Masson S, Cressoni M, Gattinoni L. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013;41:1046–1055. doi: 10.1097/CCM.0b013e31827417a6. [DOI] [PubMed] [Google Scholar]
- 10.Protti A, Andreis DT, Iapichino GE, Monti M, Comini B, Milesi M, Zani L, Gatti S, Lombardi L, Gattinoni L. High positive end-expiratory pressure: only a dam against oedema formation? Crit Care. 2013;17:R131. doi: 10.1186/cc12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council . Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996. [Google Scholar]
- 12.Bland JM, Altman DG. Statistical method for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 13.Denison DM, Morgan MD, Millar AB. Estimation of regional gas and tissue volumes of the lung in supine man using computed tomography. Thorax. 1986;41:620–628. doi: 10.1136/thx.41.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henne E, Anderson JC, Lowe N, Kesten S. Comparison of human lung tissue mass measurements from ex vivo lungs and high resolution CT software analysis. BMC Pulm Med. 2012;12:18. doi: 10.1186/1471-2466-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyde RW, Wandtke JC, Fahey PJ, Utell MJ, Plewes DB, Goske M. Lung weight in vivo measured with computed tomography and rebreathing of soluble gases. J Appl Physiol. 1989;67:166–173. doi: 10.1152/jappl.1989.67.1.166. [DOI] [PubMed] [Google Scholar]
- 16.Sverzellati N, Kuhnigk JM, Furia S, Diciotti S, Scanagatta P, Marchianò A, Molinari F, Stoecker C, Pastorino U. CT-based weight assessment of lung lobes: comparison with ex vivo measurements. Diagn Interv Radiol. 2013;19:355–359. doi: 10.5152/dir.2013.149. [DOI] [PubMed] [Google Scholar]
- 17.Hwang S, Lee SG, Kim KH, Park KM, Ahn CS, Moon DB, Chu CW, Lee YJ, Min PC. Correlation of blood-free graft weight and volumetric graft volume by an analysis of blood content in living donor liver grafts. Transplant Proc. 2002;34:3293–3294. doi: 10.1016/S0041-1345(02)03603-5. [DOI] [PubMed] [Google Scholar]
- 18.Lemke AJ, Brinkmann MJ, Schott T, Niehues SM, Settmacher U, Neuhaus P, Felix R. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology. 2006;240:736–742. doi: 10.1148/radiol.2403042062. [DOI] [PubMed] [Google Scholar]
- 19.Kuno Y. On the amount of blood in the lungs. J Physiol. 1917;51:154–158. doi: 10.1113/jphysiol.1917.sp001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugander M, Kanski M, Engblom H, Goetberg M, Olivecrona GK, Erlinge D, Heiberg E, Arheden H. Pulmonary blood volume variation decreases after myocardial infarction in pigs: a quantitative and noninvasive MR imaging measure of heart failure. Radiology. 2010;256:415–423. doi: 10.1148/radiol.10090292. [DOI] [PubMed] [Google Scholar]
- 21.Dock DS, Kraus WL, McGuire LB, Hyland JW, Haynes FW, Dexter L. The pulmonary blood volume in man. J Clin Invest. 1961;40:317–328. doi: 10.1172/JCI104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressure produces pulmonary microvascular injury in rats. Am Rev Respir Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 23.Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intra-hospital transfer of critically ill patients. Intensive Care Med. 2004;30:1579–1585. doi: 10.1007/s00134-004-2177-9. [DOI] [PubMed] [Google Scholar]
- 24.Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 25.Chiumello D, Langer T, Vecchi V, Luoni S, Colombo A, Brioni M, Froio S, Cigada I, Coppola S, Protti A, Lazzerini M, Gattinoni L. Low-dose chest computed tomography for quantitative and visual anatomical analysis in patients with acute respiratory distress syndrome. Intensive Care Med. 2014;40:691–699. doi: 10.1007/s00134-014-3264-1. [DOI] [PubMed] [Google Scholar]
- 26.Reske AW, Reske AP, Gast HA, Seiwerts M, Beda A, Gottschaldt U, Josten C, Schreiter D, Heller N, Wrigge H, Amato MB. Extrapolation from ten sections can make CT-based quantification of lung aeration more practicable. Intensive Care Med. 2010;36:1836–1844. doi: 10.1007/s00134-010-2014-2. [DOI] [PubMed] [Google Scholar]
- 27.Chiumello D, Marino A, Brioni M, Menga F, Cigada I, Lazzerini M, Andrisani MC, Biondetti P, Cesana B, Gattinoni L. Visual anatomical lung CT scan assessment of lung recruitability. Intensive Care Med. 2013;39:66–73. doi: 10.1007/s00134-012-2707-9. [DOI] [PubMed] [Google Scholar]
- 28.Goodman LR, Fumagalli R, Tagliabue P, Tagliabue M, Ferrario M, Gattinoni L, Pesenti A. Adult respiratory distress syndrome due to pulmonary and extrapulmonary causes: CT, clinical, and functional correlations. Radiology. 1999;213:545–552. doi: 10.1148/radiology.213.2.r99nv42545. [DOI] [PubMed] [Google Scholar]
- 29.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 30.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, Bugedo G, Gattinoni L. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:149–158. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 31.Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo SG, Cornejo R, Bugedo G, Carlesso E, Russo R, Caspani L, Gattinoni L. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2010;181:578–586. doi: 10.1164/rccm.200905-0787OC. [DOI] [PubMed] [Google Scholar]


