Abstract
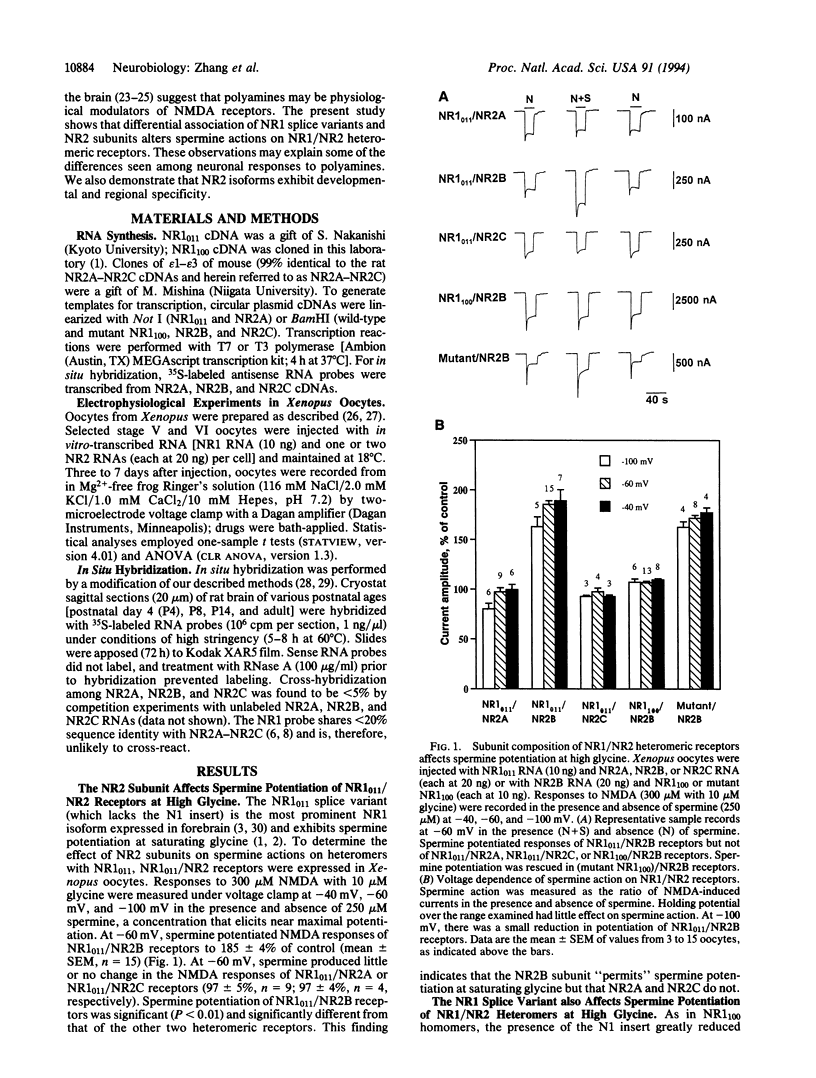
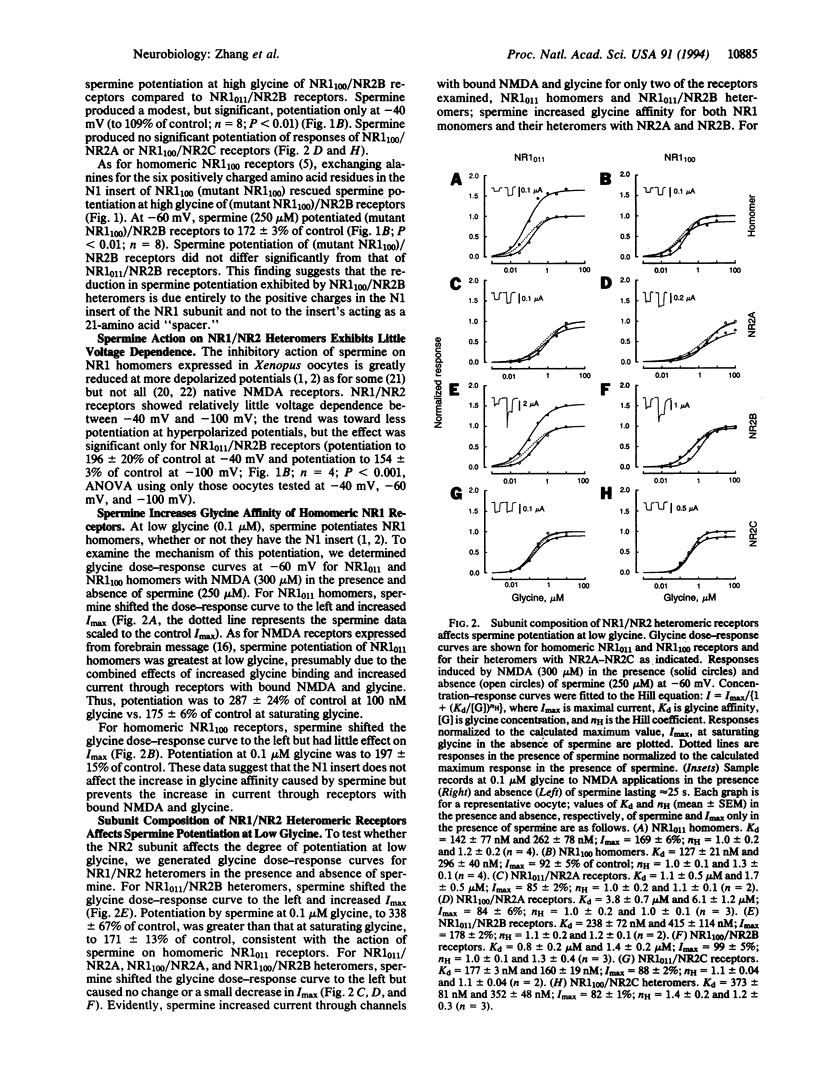
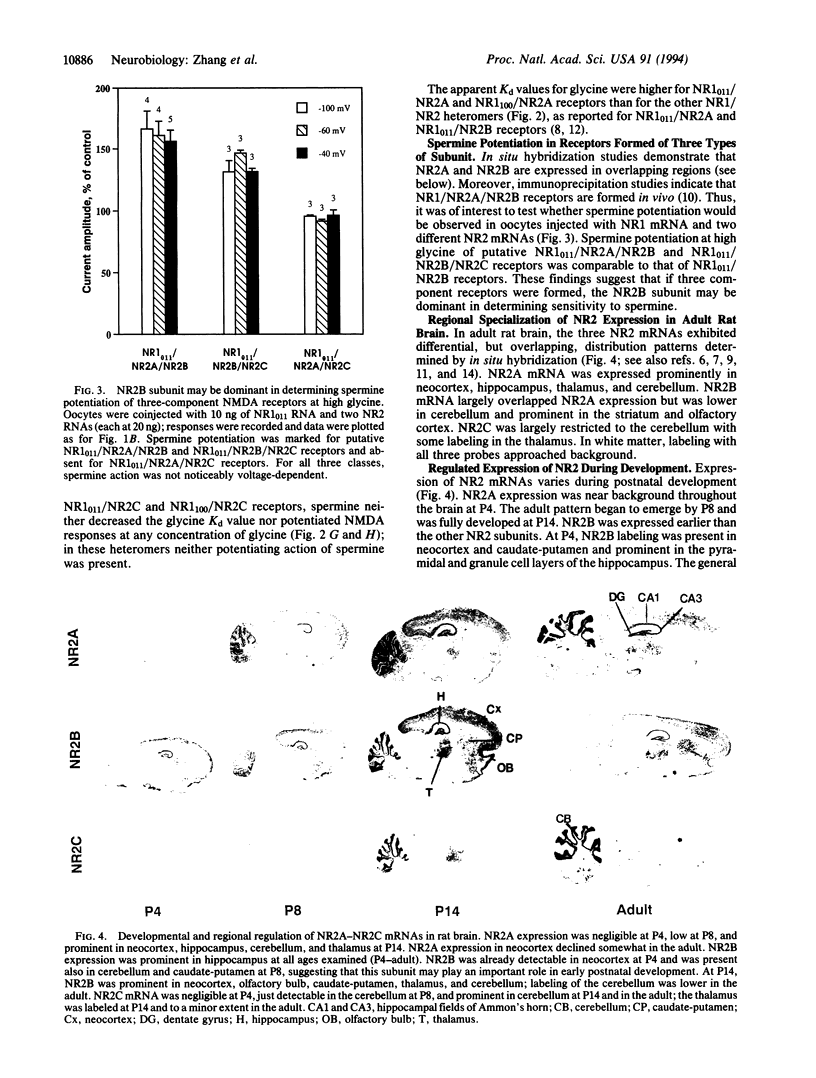
The present study shows that both the NR1 and NR2 subunits critically affect spermine potentiation of heteromeric recombinant N-methyl-D-aspartate receptors. NR1(011), the most prominent NR1 splice variant in rat forebrain, and NR1(100), prominent in midbrain, were expressed in Xenopus oocytes singly and in combination with NR2A, NR2B, and NR2C subunits. As for NR1(011) homomers, NR1(011)/NR2B receptors exhibited spermine potentiation by two mechanisms: by increasing glycine affinity and by increasing current through receptors with bound N-methyl-D-aspartate and glycine. NR1(011)/NR2A receptors exhibited only the increase in glycine affinity, and NR1(011)/NR2C receptors exhibited neither. As for NR1(100) homomers, NR1(100)/NR2B and NR1(100)/NR2A receptors exhibited spermine potentiation only by increasing the glycine affinity. Spermine produced no potentiation of NR1(100)/NR2C receptors. Thus, the NR2B subunit "permits" both forms of spermine potentiation, the NR2A subunit permits spermine potentiation only by increasing the glycine affinity, and th NR2C subunit permits neither form of potentiation. Spermine actions on NR1/NR2 showed little voltage dependence. These observations are of interest because the NR1 and NR2 subunits are differentially distributed and developmentally regulated. At early postnatal ages, NR2B subunit mRNA was more highly expressed than NR2A and NR2C mRNAs in hippocampus, neocortex, and caudate-putamen. These findings account for many of the observed differences among neurons in polyamine actions and suggest that these actions will vary in a cell-specific and age-related manner.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneda R. C., Zukin R. S., Bennett M. V. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: a whole-cell and single-channel study. Neurosci Lett. 1993 Apr 2;152(1-2):107–112. doi: 10.1016/0304-3940(93)90495-7. [DOI] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol. 1993 May;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackley P., Goodnow R., Jr, Nakanishi K., Sudan H. L., Usherwood P. N. Spermine and philanthotoxin potentiate excitatory amino acid responses of Xenopus oocytes injected with rat and chick brain RNA. Neurosci Lett. 1990 Jun 22;114(1):51–56. doi: 10.1016/0304-3940(90)90427-b. [DOI] [PubMed] [Google Scholar]
- Durand G. M., Bennett M. V., Zukin R. S. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand G. M., Gregor P., Zheng X., Bennett M. V., Uhl G. R., Zukin R. S. Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L. K., Pellegrini-Giampietro D. E., Sperber E. F., Bennett M. V., Moshé S. L., Zukin R. S. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci. 1994 May;14(5 Pt 1):2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., Boulter J., Maron C., Beasley L., Sullivan J., Pecht G., Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993 May;10(5):943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Kushner L., Lerma J., Zukin R. S., Bennett M. V. Coexpression of N-methyl-D-aspartate and phencyclidine receptors in Xenopus oocytes injected with rat brain mRNA. Proc Natl Acad Sci U S A. 1988 May;85(9):3250–3254. doi: 10.1073/pnas.85.9.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Seeburg P. H. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994 May;14(5 Pt 2):3180–3194. doi: 10.1523/JNEUROSCI.14-05-03180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J. Spermine regulates N-methyl-D-aspartate receptor desensitization. Neuron. 1992 Feb;8(2):343–352. doi: 10.1016/0896-6273(92)90300-3. [DOI] [PubMed] [Google Scholar]
- McGurk J. F., Bennett M. V., Zukin R. S. Polyamines potentiate responses of N-methyl-D-aspartate receptors expressed in xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9971–9974. doi: 10.1073/pnas.87.24.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D. J., Sakmann B., Seeburg P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994 Mar;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mori H., Yamakura T., Masaki H., Mishina M. Involvement of the carboxyl-terminal region in modulation by TPA of the NMDA receptor channel. Neuroreport. 1993 May;4(5):519–522. doi: 10.1097/00001756-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E., Bennett M. V., Zukin R. S. Differential expression of three glutamate receptor genes in developing rat brain: an in situ hybridization study. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. M., MacDonald R. L. Spermine and related polyamines produce a voltage-dependent reduction of N-methyl-D-aspartate receptor single-channel conductance. Mol Pharmacol. 1992 Jul;42(1):157–164. [PubMed] [Google Scholar]
- Rock D. M., Macdonald R. L. The polyamine spermine has multiple actions on N-methyl-D-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol. 1992 Jan;41(1):83–88. [PubMed] [Google Scholar]
- Seiler N., Schmidt-Glenewinkel T. Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J Neurochem. 1975 Apr;24(4):791–795. [PubMed] [Google Scholar]
- Shaw G. G., Pateman A. J. The regional distribution of the polyamines spermidine and spermine in brain. J Neurochem. 1973 Apr;20(4):1225–1230. doi: 10.1111/j.1471-4159.1973.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Sheng M., Cummings J., Roldan L. A., Jan Y. N., Jan L. Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994 Mar 10;368(6467):144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Sprosen T. S., Woodruff G. N. Polyamines potentiate NMDA induced whole-cell currents in cultured striatal neurons. Eur J Pharmacol. 1990 Apr 25;179(3):477–478. doi: 10.1016/0014-2999(90)90193-a. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Moriyoshi K., Ishii T., Masu M., Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992 Jun 30;185(3):826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Inoue Y., Sakimura K., Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992 Dec;3(12):1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Williams K., Zappia A. M., Pritchett D. B., Shen Y. M., Molinoff P. B. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994 May;45(5):803–809. [PubMed] [Google Scholar]
- Zheng X., Zhang L., Durand G. M., Bennett M. V., Zukin R. S. Mutagenesis rescues spermine and Zn2+ potentiation of recombinant NMDA receptors. Neuron. 1994 Apr;12(4):811–818. doi: 10.1016/0896-6273(94)90334-4. [DOI] [PubMed] [Google Scholar]




