Abstract
The human immunoglobulin repertoire is vast, producing billions of unique antibodies from a limited number of germline immunoglobulin genes. The immunoglobulin heavy chain variable region (IGHV) is central to antigen binding and is comprised of 48 functional genes. Here we analyzed whether HIV-1 infected individuals who develop broadly neutralizing antibodies show a distinctive germline IGHV profile. Using both 454 and Illumina technologies we sequenced the IGHV repertoire of 28 HIV-infected South African women from the Center for the AIDS Programme of Research in South African (CAPRISA) 002 and 004 cohorts, 13 of whom developed broadly neutralizing antibodies. Of the 259 IGHV alleles identified in this study, approximately half were not found in the International Immunogenetics Database (IMGT). This included 85 entirely novel alleles and 38 alleles that matched rearranged sequences in non-IMGT databases. Analysis of the rearranged H chain V region genes of monoclonal antibodies isolated from 7 of the CAPRISA women and previously isolated broadly neutralizing antibodies from other donors provided evidence that at least 8 novel or non-IMGT alleles contributed to functional antibodies. Importantly, we found that despite a wide range in the number of IGHV alleles in each individual, including alleles used by known broadly neutralizing antibodies, there were no significant differences in germline IGHV repertoires between individuals who do and do not develop broadly neutralizing antibodies. This study reports novel IGHV repertoires and highlights the importance of a fully comprehensive immunoglobulin database for germline gene usage prediction. Furthermore, these data suggest a lack of genetic bias in broadly neutralizing antibody development in HIV-1 infection, with implications for HIV vaccine design.
Introduction
The induction of broadly neutralizing antibodies (bNAbs) is likely to be crucial for an efficacious HIV vaccine. While the majority of chronically HIV-infected individuals develop some level of cross-neutralizing activity (1), bNAbs are generally found in less than 20% of HIV-infected individuals (2). The mechanisms underlying bNAb emergence are largely unknown, but a better understanding of how these antibodies arise in natural infection would provide a blueprint for a vaccine designed to elicit them. Over the last few years a large number of potent and broad bNAbs have been isolated from selected HIV-infected donors. These bNAbs target conserved epitopes on the HIV envelope including the membrane proximal external region (MPER), V2 glycans, V3 glycans, CD4 binding site (CD4bs) and the gp120/gp41 interface (3, 4). However, most bNAbs have unusual genetic features including high levels of somatic hypermutation (SHM), long CDRH3s (the complementary determining region 3 on the heavy chain) (3, 5) and for some classes, biases in germline IGHV gene usage (3, 5–9).
The VRC01 class of antibodies to the CD4bs (including NIH45-46, 12A12, 3BNC117, VRC-PG04 and VRC-CH31 isolated from multiple donors) have been shown to preferentially use either IGHV1-2*02 or IGHV1-46*02 germline alleles (3, 10, 11). This preference is thought to be due to the electrostatic and hydrophobic contacts afforded by conserved residues in framework 1 (FR1), CDRH2 and FR3 of this allele (some of these features are conserved in IGHV1-46*02 and IGHV1-3*01 which are also used by CD4bs antibodies) (9, 10). Anti-HIV antibodies frequently use IGHV1 genes compared to healthy individuals (5, 6). In particular, IGHV1-69 is used by mAbs that target V2, the CD4 induced site (CD4i) and gp41 in HIV infection as well as other viral infections such as influenza (8, 12). The preference for this particular gene can be attributed to the interaction of hydrophobic residues in the CDRH2 with helical elements or hydrophobic β-sheets like those found on the hemagglutinin (HA) of influenza, and gp41 and gp120 of the HIV envelope (8, 13). The use of IGHV5-51*01 and IGHV5-51*03, which are underrepresented in mature antibodies, has also been reported to be favored by anti-V3 HIV antibodies (6, 7).
The human germline IGHV repertoire consists of 7 IGHV subgroups, which are described in the International Immunogenetics Database (IMGT, www.imgt.org). These IGHV1-7 subgroups include functional genes, open-reading frames (ORF) and pseudogenes with only functional genes being involved in antibody production (14). IGHV3 is the largest of the IGHV subgroups, with 21 functional genes, followed by IGHV1 and IGHV4 both with 10 and IGHV2, IGHV5, IGHV6 and IGHV7 with three or less genes (14). Most of these genes have multiple alleles, including functional and non-functional alleles, which differ by either a single nucleotide polymorphism (SNP) or by multiple SNPs, which can be either synonymous or nonsynonymous, or frameshift mutations caused by indels that contribute diversity to the immunoglobulin gene repertoire. Furthermore, whole IGHV genes have been reported to have been duplicated or deleted from the germline repertoire of some individuals resulting in varied gene copy numbers (15, 16). IGHV genes make up the majority of the heavy chain variable region (VH) of mature antibodies and are central to antigen binding. Differences in germline IGHV repertoires between different populations were recently highlighted in an extensive study of the human immunoglobulin gene locus (16), where African individuals were found to be particularly diverse.
Given the propensity of some HIV mAbs to use restricted IGHV genes, we examined whether HIV-infected individuals who develop bNAbs have unique IGHV repertoires compared to individuals who do not, in order to determine whether the ability to develop these types of antibodies is genetically restricted.
Materials and Methods
Samples and Ethics Statement
This study involved 28 adult (> 18 years) women of African ancestry (mostly Zulu speaking) with HIV-1 subtype C infection from the CAPRISA 002 (17) and 004 cohorts (18) who were being followed at urban and rural clinics in KwaZulu-Natal, South Africa. Of the 28 women, 13 developed broadly neutralizing anti-HIV antibodies and 13 did not develop broadly neutralizing antibodies despite chronic HIV infection and two were intermediate neutralizers (Supplemental Table 1). Throughout this paper "BCN" is used to refer to the HIV infected women who develop broadly neutralizing antibodies and "non-BCN" to women who did not develop broadly neutralizing antibodies. Additionally bNAbs refer to isolated broadly neutralizing monoclonal antibodies. BCN individuals were defined as those whose sera from 2 years post-infection were able to neutralize 33–94% (median 56%) of a panel of 18 viruses, made up of 6 subtype A, 6 subtype B and 6 subtype C viruses of which 2 viruses were isolated from the women in the cohort. Non-BCN individuals neutralized 0–11% of the 18 viruses and had the same viral loads as the BCN individuals at 6 months post-infection (to remove viral load biases associated with the development of bNAbs(19)).
This study was given Ethics Clearance from the Human Research Ethics Committee for Medical Research in Johannesburg, South Africa (Clearance number: M111104 for this particular study and M080470 for the CAPRISA parent study).
DNA Extraction
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) from each individual. Prior to extraction, PBMCs were thawed (at 37°C) and washed with 10ml RPMI with 10% FBS. Genomic DNA was extracted from the pellet from all 28 individuals using a Promega Wizard Genomic purification kit.
Primer Design and Amplicon Library Construction
Alignments were created for each IGHV subgroup using sequences obtained from ENSEMBL and IMGT. Forward primers (with the exception of IGHV1-F) were mapped to the intron in leader sequence. The reverse primers were mapped to the intron after the CDR3 region. IGHV1, IGHV3 and IGHV4 primers were designed based on previously published primer sets (20). New primers for IGHV2, IGHV5, IGHV6 and IGHV7 were designed based on related sequences for each subgroup. A total of eight primer sets were designed to amplify all seven subgroups. The binding properties of the primer sets were determined using UCSC’s BLAT and NCBI’s BLAST. Additional next-generation sequencing (NGS) specific sequences were added to the gene-specific primers to allow sequencing of the Roche 454 and Illumina MiSeq. The 454 primers included the 454 adapter sequence, key sequence and 10bp MID sequences (4 unique sequences in total) (primers listed in Supplemental Table 2). The Illumina MiSeq primers included a MiSeq index binding tag and the read 1 or read 2 tags (used for paired-end reads) (primers listed in Supplemental Table 3). Nextera XT Indexing tags, which allow pooling of samples on the MiSeq, were added during library construction rather than included into the primer.
Each IGHV subgroup was amplified three times for each individual to ensure adequate coverage of the subgroup and minimize PCR bias. The PCR conditions for all eight amplicons and both 454 and Illumina primers were the same with the exception of the annealing temperatures; IGHV1, IGHV3a and IGHV5 at 56°C, IGHV2, IGHV6 and IGHV7 at 59°C and IGHV3b and IGHV4 at 55°C. The PCR conditions were as follows: initial denaturation at 94°C for 3 minutes, 35 cycles of denaturation at 94°C for 15 seconds, annealing for 45 seconds, extension for 1 minute at 72°C, final extension at 72°C for 8 minutes and held at 4°C. Each PCR contained 17.5µl dH2O, 2.5µl Roche FastStart HiFi 10× buffer with 18mM MgCl2, 0.5µl dNTP mix (10mM each), 1µl each primer (10uM), 0.25µl of Roche FastStart HiFi Enzyme (5U/µl) and 1µl of 10ng/µl DNA. The PCR amplicon lengths ranged from ~390–440bp. All replicates of the eight amplicons from each individual were pooled to create a full IGHV repertoire per individual.
NGS Sequencing
Fifteen individuals were sequenced on the Roche 454 GS Junior, with four individuals pooled per sequencing run. All clean-up procedures and sequencing on the Roche 454 GS Junior (Titanium) was done as per manufacturers recommendations.
In order to confirm rare alleles that were only found in single individuals eleven of the individuals sequenced on the GS Junior were re-sequenced on the Illumina MiSeq along with thirteen additional individuals. A custom amplicon sequencing approach was used for the MiSeq sequencing. Nextera XT Indexing tags were added to the pooled MiSeq amplicon libraries for each individual using 5µl of each Nextera XT index (two per sample), 1µl of cleaned PCR product (amplicon library prep), 0.5µl EpiCentre FailSafe enzyme, 25µl EpiCentre FailSafe PCR PreMix and 13.5µl dH2O. Thermocycler conditions: 72°C for 3 minutes, 95°C for 30 seconds, 12 cycles of 95°C for 10 seconds, 55°C for 30 seconds and 72°C for 30 seconds followed by a final extension at 72°C for 5 minutes. All products were checked on an Agilent bioanalyser and cleaned-up using 0.75× Ampure Beads, using the manufacturers protocol. Each sample was quantified on a Qubit and diluted to 8nM. A single 8nM pooled library was then created by pooling 4µl of each diluted sample, 5µl of which was denatured using 5µl 0.2N NaOH, according to MiSeq protocol. A final concentration of 12pM denatured DNA library with 15% PhiX control was run onto the Illumina MiSeq, using the MiSeq reagent kit (version 2) with 2×250 paired-end reads.
Sequence Data Analysis
FASTQ files were extracted from the raw GS Junior output (.sff files) or automatically generated on the MiSeq. The resulting sequences were quality trimmed using QTrim (21). Sequences with a minimum read length of 260bp and a mean quality score of 23 or higher (≥99% confidence) were considered high quality and used for downstream analyses. Paired-end MiSeq reads were merged using PEAR (22), after quality trimming. All identical sequences were collapsed to single unique reads. Unique reads compiled from 6 or more identical sequences were used for further analysis while all unique reads compiled from less than 6 sequences were discarded from further analysis. The unique sequences were compared to a database of IGHV alleles downloaded from IMGT using a custom BLAST approach with match reward score of 2, mismatch penalty of 5, a gap penalty for insertions of 16 and a gap extension for deletions with a penalty of 4. All related sequences for each IGHV gene were aligned using RAMICS (23).
Sequences with 100% matches to functional IMGT alleles were assigned as that particular IMGT allele. Sequences matching open reading frames or pseudogenes were removed from further analysis. Sequences with nonsynonymous and/or synonymous single nucleotide polymorphisms (SNPs), insertions or deletions compared to their top matched functional IMGT sequence were given the name of the top scoring IMGT match with the suffix “m” to denote a single mismatch (nonsynonymous and synonymous SNPs), e.g. IGHV1-2*5m and “mm” for multiple SNPs, insertions or deletions, e.g. IGHV1-2*5mm. Where more than one sequence with a single mismatch or multiple mismatches to the same top scoring IMGT functional sequence was observed, an additional numerical identifier was given to the name, for example: IGHV1-2*5m2 and IGHV1-2*5mm2. These sequences where compared to those listed in the Immunoglobulin Polymorphism Database (IgPdb), GenBank, NCBI dbSNP, NCBI’s IgBLAST and other published IGHV data (24–28). If 100% matches were found in any of these databases or publications these sequences are described as non-IMGT alleles. If no match was found and sequences were observed in multiple individuals or in both sequencing platforms in a single individual, we report these as novel alleles.
Broadly neutralizing antibody (bNAb) sequences were obtained from CATNAP or GenBank. The germline IGHV gene usage for these sequences were also obtained from CATNAP or the relevant publication. The bNAb sequences were compared to our IMGT, non-IMGT and novel allele sequences, using blastn from BLAST 2.2.29+ with a match reward of 1, mismatch penalty of -1, gap deletions of 5, gap extension of 2, word size of 7 and an E value threshold of 1e−10. Monoclonal antibodies (mAbs) have previously been isolated from 7 of the CAPRISA participants examined in this study using single cell sorting and PCR amplification of the heavy and light chains (N. Mkhize, E. Gray and L. Morris, unpublished observations). The majority of these mAbs were not HIV-specific. The sequences obtained from these mAbs were used to assign germline IGHV gene usage in each participant.
Statistical Analysis
Two-tailed Fisher’s exact tests with 95% confidence intervals were used to test the difference in germline gene repertoires between the BCN and non-BCN sample groups.
Results
Novel Germline IGHV Alleles in South African Women
The germline IGHV gene repertoire of 28 HIV-infected women in the CAPRISA 002 and 004 cohorts based in KwaZulu-Natal, South Africa were sequenced using both the 454 and Illumina NGS platforms. Sequences with perfect matches to IMGT (29) listed alleles were given the IMGT allele name and are referred to as IMGT alleles. Sequences that did not match IMGT alleles due to synonymous or nonsynonymous mutations or indels but were matches to non-IMGT immunoglobulin gene databases (30–33) or immunoglobulin gene publications (24–28) were given the published name and referred to as non-IMGT alleles. Sequences with no matches to either IMGT or non-IMGT alleles and were observed in multiple individuals or across both technologies in one individual are hereafter referred to as novel alleles.
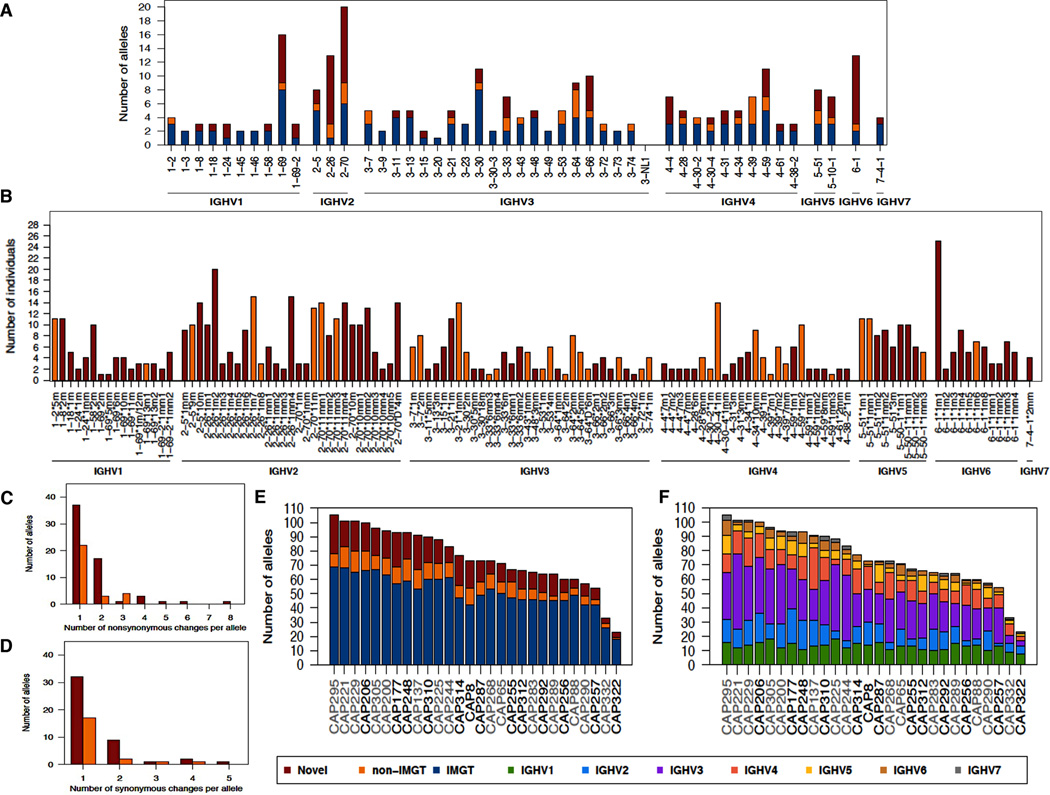
A total of 47 functional IGHV genes representing all 7 subgroups were found in our cohort (Fig. 1A). Only 1 published gene, IGHV3-NL1 first identified in Papua New Guinea (20), was not detected. There was a wide range in the number of alleles associated with each gene with 20 alleles in the IGHV2-70 gene compared to only a single allele for the IGHV3-20 gene. Of the 259 alleles identified in this population, just over a half (~52%, n=136) had an exact match to those listed in IMGT, while ~15% (n=38) were non-IMGT alleles and ~33% (n=85) were novel alleles. Eighty one of the 85 novel alleles (95%) were observed in more than one individual (Fig. 1B). The 4 novel alleles observed in single individuals were confirmed with both 454 and Illumina sequences. The most commonly observed novel alleles were IGHV6-1*1m1 found in 25 individuals (~89%) and IGHV2-26*1m2 found in 20 individuals (~71%) both of which contained a single SNP compared to the known alleles. Overall, 48% (n=60) of the novel and non-IMGT alleles identified in this study were found in at least 4 individuals indicating that many are fairly common. Furthermore this study significantly expanded the number of alleles for certain genes. For example, IGHV6-1, which previously only had 2 alleles reported in IMGT was found to have 10 novel alleles and 1 non-IMGT allele (Figs. 1A and 1B). Similarly, IGHV2-26 was found to have 13 alleles (including 10 novel alleles and 2 non-IMGT) compared to 1 recorded in IMGT. Other significant expansions were in the IGHV1-69 and IGHV2-70 genes.
FIGURE 1. Novel germline IGHV alleles in 28 South African individuals.
(A) Number of alleles observed for each IGHV gene. Shown are the numbers of novel (red), non-IMGT (orange) and IMGT (blue) alleles. (B) Prevalence of novel and non-IMGT germline IGHV alleles. (C) Number of non-synonymous changes in each allele compared with the top matched IMGT allele. (D) Number of synonymous changes in each allele compared with the top matched IMGT allele. (E) Total number of IMGT, novel and non-IMGT germline IGHV alleles. BCN individuals are highlighted in black and non-BCN individuals in grey. (F) Total number of germline alleles from each IGHV subgroup. Shown are the total numbers of alleles colored according to IGHV subgroup.
The majority (~97%, n=119) of novel and non-IMGT alleles had single or multiple SNPs (synonymous and nonsynonymous), with single nonsynonymous SNPs being the most common (Figs. 1C and 1D). One novel allele (IGHV2-26*1mm4, observed in 15 individuals) had 8 nonsynonymous SNPs (Figs. 1B and 1C). Four novel alleles had indels relative to their most closely matched IMGT allele and three of the four had additional SNPs. IGHV3-11*5mm, observed in two individuals, had a full codon insertion, while frameshifts as a result of single nucleotide deletions were found in IGHV4-59*8mm and IGHV4-61*2mm, each observed in two individuals, and IGHV6-1*1m8 that was found in six individuals. It is likely that these three alleles with the frameshift mutations are not functional but rather novel pseudogenes.
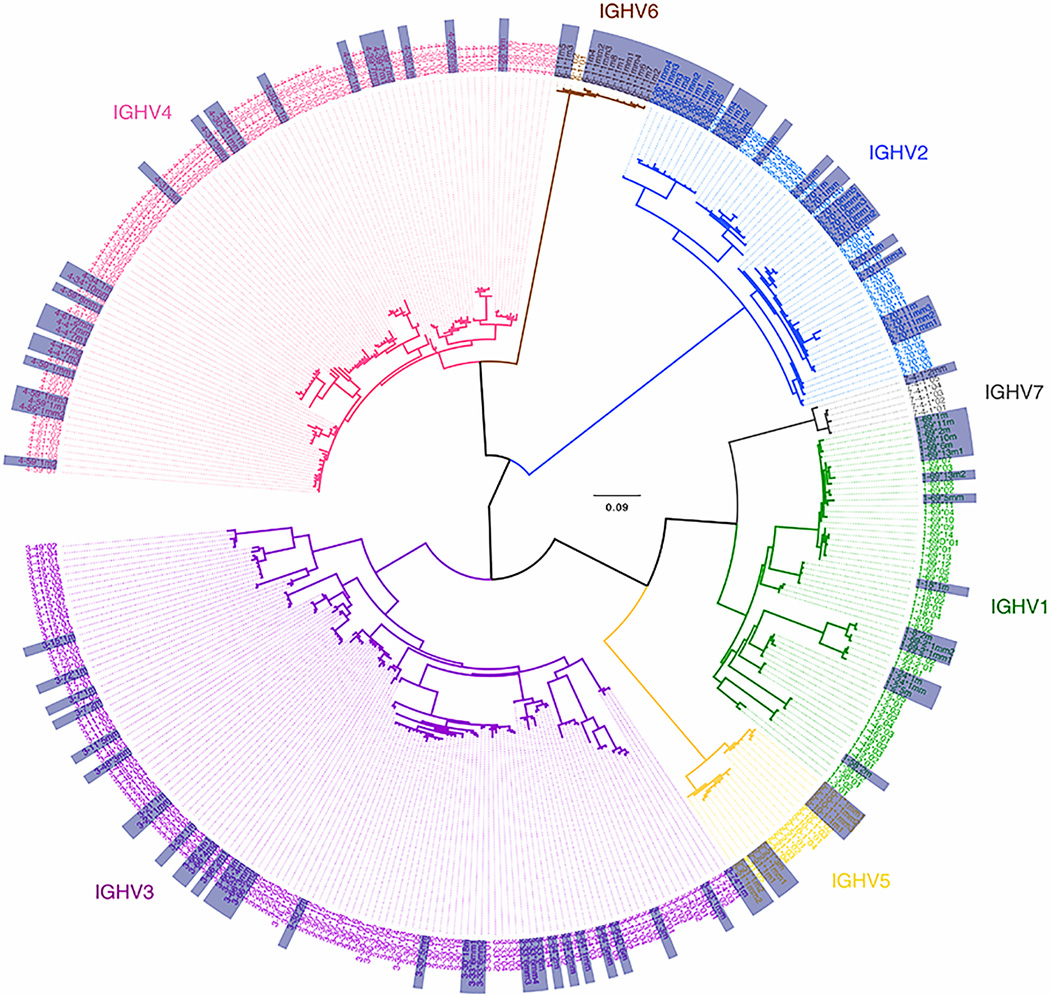
When the repertoires for each of the 28 individuals were analyzed separately, it was apparent that there was a wide range in the number of alleles per individual (Figs. 1E and 1F). CAP295 had the largest number of alleles with 105 while CAP322 had the fewest at 23 alleles. Each individual’s repertoire included IMGT, non-IMGT and novel alleles. There was variation in the number of alleles per IGHV family as some individuals lacked alleles for IGHV6 or IGHV7 while others had smaller numbers of IGHV2, IGHV4 or IGHV5 alleles. A phylogenetic tree of all 259 alleles, including IMGT, non-IMGT and novel alleles, sequenced in this study (Fig. 2) revealed separate clustering of each of the IGHV subgroups, with all of the novel and non-IMGT alleles clustering with their respective IMGT genes.
FIGURE 2. Phylogenetic tree of all germline IGHV alleles observed in 28 South African individuals.
Shown are all IMGT, non-IMGT and novel alleles observed in this study. Alleles are colored according to IGHV subgroup and the non-IMGT and novel alleles are highlighted in the grey boxes.
Novel Germline IGHV Allele Usage in bNAbs
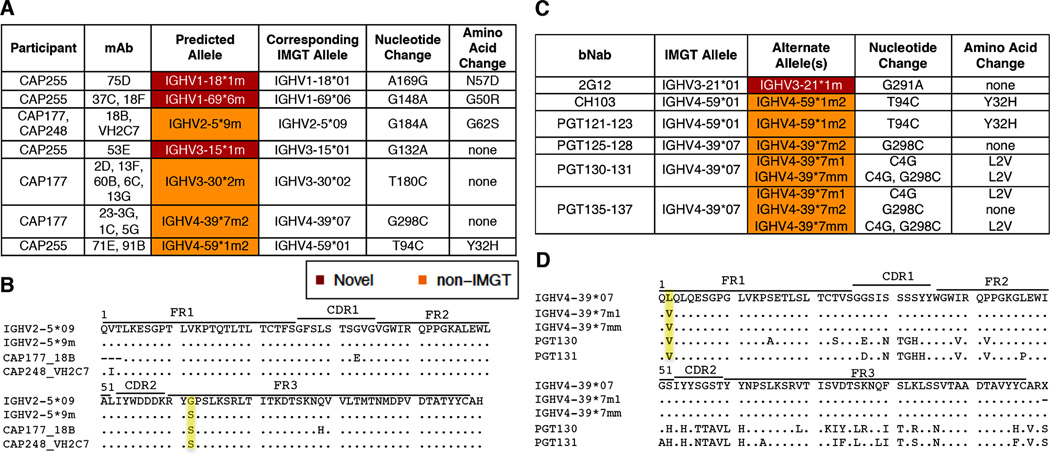
In order to determine whether these novel and non-IMGT germline alleles are being used by the human immune system to generate functional antibodies, we analyzed the germline gene usage of monoclonal antibodies (mAbs) isolated from 7 of the CAPRISA participants this study. We identified 15 mAbs that made use of 7 novel or non-IMGT alleles identified from NGS sequencing of the 28 individuals (Fig. 3A). Of these, 2 clonally related mAbs were HIV-1 specific (CAP255-37C and CAP255-18F). An example of one of the new non-IMGT alleles used by two mAbs isolated from two different individuals (CAP177 and CAP248) is shown in Fig. 3B. Based on the IGHV repertoires from CAP177 and CAP248 the germline IGHV gene predicted to be used by these 2 mAbs is IGHV2-5*9m which has a Serine (S) at position 62 rather than a Glycine (G) present in the closest related IMGT allele IGHV2-5*09. All other SNPs found in these two mAbs, compared to the germline allele are likely the results of SHM. Of the remaining 7 novel and non-IMGT alleles used by these mAbs, 4 (57%) had a better match at the amino acid level and 3 (43%) at the nucleotide level compared to the closest matching IMGT allele (Fig. 3A). We also analyzed the IGHV gene usage of 57 well known HIV-1 bNAbs and found 14 instances where the novel or non-IMGT alleles, identified in this study, provided the same or a better match than their currently predicted IMGT allele (Fig. 3C). Two of the predicted alleles (IGHV4-39*7m2 and IGHV4-59*1m2) were also observed among the mAbs isolated from the CAPRISA participants. An example of alternate IGHV allele usage for the bNAbs is shown in Fig. 3D, where PGT130 and PGT131 are more likely to be using IGHV4-39*7m1 or IGHV4-39*7mm, which like the mAbs have a Valine (V) at the second amino acid, rather than a Leucine (L) as seen in IGHV4-39*07.
FIGURE 3. Novel IGHV alleles used by isolated mAbs and some bNAbs.
(A) Germline IGHV allele usage for isolated mAbs from study participants. Showing the germline IGHV alleles used, their corresponding IMGT allele, nucleotide and amino acid substitutions between the two alleles. (B) Alignment of germline IGHV allele usage for isolated mAbs from two study participants. Highlighted in yellow are the differences in amino acids at position 62, G (Glycine) and S (Serine). Amino acid changes not highlighted in the figure are likely a result of somatic hypermutation during antibody maturation. (C) Germline IGHV allele usage for selected bNAbs. Showing the published IMGT predicted allele and alternate allele(s) predicted from IGHV alleles in 28 South African individuals. (D) Alignment of predicted germline IGHV allele usage for PGT130 and PGT131. Highlighted in yellow are the differences at the second amino acid V (Valine) and L (Leucine). Amino acid changes not highlighted in the figure are likely a result of somatic hypermutation during antibody maturation.
Comparison of Germline IGHV Repertoires in BCN and non-BCN Individuals
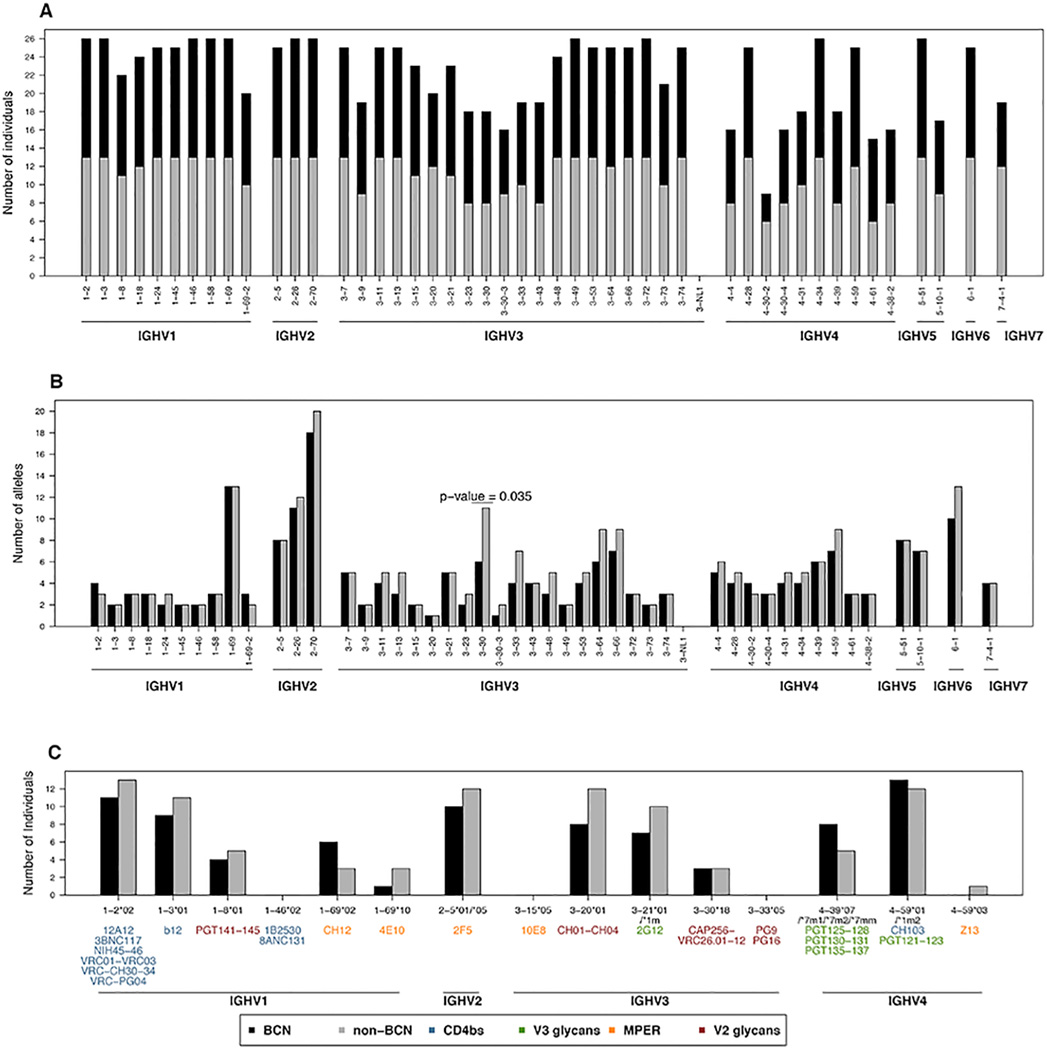
Given that some HIV broadly neutralizing mAbs show biased variable heavy chain gene usage, we analyzed the germline IGHV repertoires of BCN and non-BCN individuals to determine whether the ability to develop bNAbs was associated with a particular genotypic profile. Among the 28 CAPRISA women, 13 developed bNAbs in their plasma after 2–4 years of HIV infection, 13 women did not develop bNAbs, despite matching viral loads, and two were intermediate neutralizers (Supplementary Table 1, (19) and N. Mkhize, E. Gray and L. Morris, unpublished observations). Comparison of the BCN and non-BCN individuals revealed that both groups had similar profiles of IGHV genes (Fig. 4A), approximately the same number of alleles for each of those genes (Fig. 4B) and the same number of potential alleles (Fig. 1E and F). The only gene that showed statistical significance between the two groups was IGHV3-30 (p-value=0.035, although significance was lost following Bonferroni multiple testing correction); where non-BCN individuals had five more alleles (n=11) compared to the BCN individuals (n=6) (Fig. 4B).
FIGURE 4. Comparison of germline IGHV repertoires between BCN and non-BCN individuals.
(A) Number of BCN and non-BCN individuals with each of the functional IGHV genes in their IGHV repertoire. (B) Number of alleles observed for each of the functional IGHV genes in BCN and non-BCN individuals. (C) Number of BCN and non-BCN individuals with the same IGHV alleles in their repertoire as those used by monoclonal bNAbs.
We also compared the frequency of the specific germline IGHV alleles used by broadly neutralizing mAbs between the non-BCN and BCN groups. Of the 57 monoclonal bNAbs targeting one of 4 different epitopes on the HIV envelope glycoprotein, most made use of IGHV1, IGHV3 and IGHV4 germline genes (Fig. 4C) and as reported above some may use novel/non-IMGT alleles (Fig. 3C). This included IGHV1-46*02, used by some CD4bs antibodies, however there were two other alleles for this gene, including IGHV1-46*01, that were found in all individuals. Similarly, IGHV3-15*05 (used by bNAb 10E8) and IGHV3-33*05 (used by bNAbs PG9 and PG16) were not observed in any of the individuals in this study, but other alleles were more commonly observed including some novel/non-IMGT alleles. The IGHV1-2*02 allele which is also preferentially used by CD4bs antibodies (shown in blue in Fig. 4C) occurred at high frequency in both the non-BCN and BCN groups. The most prevalent IGHV alleles in both groups were IGHV4-59*01 (used by the CD4bs bNAb CH103 and the V3/glycan bNAbs PGT121–123), IGHV1-2*02 (used by CD4bs mAbs), IGHV1-3*01 (used by the CD4bs bNAb IgG1b12) and IGHV2-5*01/05 (used by bNAb 2F5 that targets the MPER). Importantly, there was no difference in the frequency of any of these alleles between the 13 BCN and 13 non-BCN individuals. Thus, the inability of all HIV-infected individuals to develop bNAbs is not due to a paucity of the relevant alleles in their IGHV germline gene repertoires.
Discussion
We analyzed the germline immunoglobulin heavy chain variable gene repertoire encoded in the genomic DNA of individuals from KwaZulu-Natal, South Africa and noted that ~48% of the alleles seen in this population are not reported in IMGT. Some of these alleles (non-IMGT alleles) were described in published studies of re-arranged antibodies, although most alleles were novel and are described here for the first time, to our knowledge. Further analysis of these IGHV repertoires revealed there to be no differences between those individuals who developed bNAbs to HIV compared to those who did not, despite equivalent antigenic load. Since the induction of these types of antibodies is considered essential for an effective HIV vaccine, these data suggest that the ability to develop bNAbs is not restricted by the IGHV repertoire.
Previous studies have reported differences in the frequencies of IGHV genes between different populations, with Africans showing particularly unique profiles (16). The presence of IGHV3-64D, IGHV5-10-1 and IGHV7-4-1 has been reported to be lower in African (Luyha, Maasai and Yoruba) populations compared to Asian and European groups (16). We found a similarly low frequency of IGHV3-64D (14%) in this Zulu-speaking South African population. However, IGHV5-10-1 was observed at a higher prevalence (64%) compared to the studied African, Asian and European groups (0.03–16%, 20–21% and 34–48%, respectively) (16), while IGHV7-4-1 frequencies were similar (75%) to those seen in Asian (~78%) and European (54–75%) groups (16). IGHV1-69-2, IGHV3-43D and IGHV4-38-2 genes have been reported to be common in African populations (16) which we corroborated in our study for IGHV1-69-2 and IGHV4-38-2 (75% and 61%, respectively). However, we observed IGHV3-43D*01 in only 29% (8/28) of individuals studied which is lower than the prevalence reported in other African groups (45–65%, (16)).
We have shown that both novel and non-IMGT alleles are being used by mAbs isolated from individuals in this study as well as some well-characterized anti-HIV bNAbs. This included CH103 and 12 antibodies in the PGT121-137 series all of which were isolated from African donors (34–36). Given that 3 of the novel and non-IMGT alleles (IGHV3-21*1m, IGHV4-39*1m2 and IGHV4-39*7m2) potentially being used by these bNAbs were fairly common in the South African individuals (39%, 36% and 21%, respectively), it is perhaps not surprising, that these have been found to contribute to functional antibodies in other African individuals. IGHV4-39*7m1 and IGHV4-39*7mm were less common in our study group, at 4% and 11% respectively. The other novel and non-IMGT alleles used by mAbs isolated from the study participants were also fairly commonly observed ranging from 14-36%. Only two of the 15 mAbs isolated from the study participants were able to bind HIV, highlighting that the use of novel and non-IMGT alleles by functional antibodies is not HIV specific and thus could play a role in immune responses to other diseases or infections.
Despite the wide range in the number of IGHV alleles present in each individual there were no differences in the overall germline IGHV repertoires between BCN and non-BCN individuals. This extended to a sub-analysis of the genes and alleles used by known broadly neutralizing mAbs whose frequency differed within the cohort but not between BCN and non-BCN groups. The only gene that showed a difference between the two groups was IGHV3-30, where non-BCN individuals had more alleles than BCN individuals. IGHV3-30 is used by the CAP256-VRC26 family of broad and potent V1V2 antibodies, which were isolated from CAPRISA donor CAP256, a participant in this study (37). We also noted that alleles IGHV1-46*02, IGHV3-15*05 and IGHV3-33*05 used by CD4bs, MPER and V2 glycan bNAbs, respectively, were not found in this South African cohort. Other alleles of these genes were however observed in this cohort, as well as allelic variants used by other bNAbs targeting the same epitopes. Thus, although the specific alleles used by these bNAbs were not present, it did not preclude these individuals from generating antibodies to these epitopes. In addition, CH103 and PGT121-123 which both use IGHV4-59*01 (or the non-IMGT allele IGHV4-59*1m2), target different epitopes, (CD4bs and V3 glycans, respectively), demonstrating that a single germline allele can be used by antibodies against numerous HIV Env targets.
The germline IGHV gene makes the greatest contribution to the unmutated common ancestor (UCA) antibody encoding the entire CDRH1, CDRH2 and FR1, FR2 and FR3 regions. Elegant studies on the UCAs of the VRC01 class of antibodies, which use the VH1-2*02 allele, have shown how two glycans in the V5 region of the HIV envelope obstruct binding of the UCA (9, 38). Such studies guide the design of suitable envelope immunogens able to trigger the VRC01-class of antibodies (9). However, other studies aimed at identifying UCA-binding envelopes for PG9 and other bNAb lineages have been less successful, primarily because the UCA approximations used in such studies were predicted from highly mutated antibodies and using incomplete germline databases. The public availability of a comprehensive database of germline immunoglobulin genes, including data from this study, will significantly enhance the accuracy with which UCAs can be inferred.
By studying an under-represented population group in southern Africa, this investigation has greatly expanded the repertoire of germline IGHV genes. We further hypothesize that the IGHD and IGHJ germline genes, which make up the remainder of the VH region of functional antibodies, are likely to be highly variable within this population as well, and thus warrant investigation. Furthermore, we have shown that some of these novel and non-IMGT alleles are functionally active in both HIV and non-HIV antibodies. This knowledge will contribute to a better understanding of antibody response to HIV as well as other infections, immunizations and B cell pathologies. Importantly, we have also shown that the development of bNAbs against HIV are not restricted by the germline IGHV repertoire, which is significant for vaccine development, as it suggests that everyone has the potential to make antibodies capable of neutralizing all strains and subtypes of HIV.
Supplementary Material
Acknowledgements
We would like to thank the participants of the CAPRISA 002 and 004 studies from whom these samples were obtained and the staff involved in all the sample collection and processing. We also thank Dr Mashudu Madzivhandila and Tandile Hermanus for generating the neutralization data and Dr Nono Mkhize and Dr Elin Gray for generating mAbs from some of these participants.
Footnotes
This project was supported by the Poliomyelitis Research Foundation, University of the Witwatersrand Health Sciences Faculty Research Council and the National Research Foundation. Cathrine Scheepers was supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP) through the Fogarty International Center, National Institutes of Health (grant # 5 D43 TW000231). CAPRISA is funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes for Health (NIH), and U.S. Department of Health and Human Services (grant: AI51794). SANBI received funding from the South African Department of Science and Technology, South African National Research Foundation DAAD Study Bursary, Atlantic Philanthropies and the South African Medical Research Council. Penny L. Moore is a Wellcome Trust Intermediate Fellow in Public Health and Tropical Medicine (Grant 089933/Z/09/Z).
References
- 1.Hraber P, Seaman M, Bailer R, Mascola J, Montefiori D, Korber B. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nature Medicine. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 3.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scharf L, Scheid J, Lee J, West A, Chen C, Gao H, Gnanapragasam P, Mares R, Seaman M, Ward A, Nussenzweig M, Bjorkman P. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Reports. 2014;7:785–795. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breden F, Lepik C, Longo NS, Montero M, Lipsky PE, Scott JK. Comparison of Antibody Repertoires Produced by HIV-1 Infection, Other Chronic and Acute Infections, and Systemic Autoimmune Disease. PLoS ONE. 2011;6:e16857+. doi: 10.1371/journal.pone.0016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorny MK, Wang XH, Williams C, Volsky B, Revesz K, Witover B, Burda S, Urbanski M, Nyambi P, Krachmarov C, Pinter A, Zolla-Pazner S, Nadas A. Preferential use of the VH5-51 gene segment by the human immune response to code for antibodies against the V3 domain of HIV-1. Mol Immunol. 2009;46:917–926. doi: 10.1016/j.molimm.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorny MK, Sampson J, Li H, Jiang X, Totrov M, Wang XH, Williams C, O'Neal T, Volsky B, Li L, Cardozo T, Nyambi P, Zolla-Pazner S, Kong XP. Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH5-51/VL lambda genes define a conserved antigenic structure. PLoS One. 2011;6:e27780. doi: 10.1371/journal.pone.0027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorny MK, Pan R, Williams C, Wang XH, Volsky B, O'Neal T, Spurrier B, Sampson JM, Li L, Seaman MS, Kong XP, Zolla-Pazner S. Functional and immunochemical cross-reactivity of V2-specific monoclonal antibodies from HIV-1-infected individuals. Virology. 2012;427:198–207. doi: 10.1016/j.virol.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jardine J, Julien J-P, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang P-S, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward A, Burton D, Stamatatos L, Nemazee D, Wilson I, Schief W. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheid J, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira T, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton D, Pereyra F, Ho D, Walker B, Seaman M, Bjorkman P, Chait B, Nussenzweig M. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West AP, Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proceedings of the National Academy of Sciences. 2012;109:E2083–E2090. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti D, Suguitan AL, Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingwood D, McTamney PM, Yassine HM, Whittle JR, Guo X, Boyington JC, Wei CJ, Nabel GJ. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature. 2012;489:566–570. doi: 10.1038/nature11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefranc MP, Lefranc G. The Immunoglobulin FactsBook. London: Academic Press; 2001. [Google Scholar]
- 15.Watson CT, Breden F. The immunoglobulin heavy chain locus: genetic variation, missing data, and implications for human disease. Genes and Immunity. 2012;13:363–373. doi: 10.1038/gene.2012.12. [DOI] [PubMed] [Google Scholar]
- 16.Watson C, Steinberg K, Huddleston J, Warren R, Malig M, Schein J, Willsey J, Joy J, Scott J, Graves T, Wilson R, Holt R, Eichler E, Breden F. Complete haplotype sequence of the human immunoglobulin heavy-chain variable, diversity, and joining genes and characterization of allelic and copy-number variation. American journal of human genetics. 2013;92:530–546. doi: 10.1016/j.ajhg.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, Abdool Karim Q, Grobler A, Barnabas N, Iriogbe I, Abdool Karim SS f. t. C. A. I. S. Team. Establishing a Cohort at High Risk of HIV Infection in South Africa: Challenges and Experiences of the CAPRISA 002 Acute Infection Study. PLoS ONE. 2008;3:e1954+. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D o. b. o. t. C. T. Group. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L a. t. C. S. Team. The Neutralization Breadth of HIV-1 Develops Incrementally over Four Years and Is Associated with CD4+ T Cell Decline and High Viral Load during Acute Infection. Journal of Virology. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Jackson K, Gäeta B, Pomat W, Siba P, Sewell W, Collins A. Genomic screening by 454 pyrosequencing identifies a new human IGHV gene and sixteen other new IGHV allelic variants. Immunogenetics. 2011;63:259–265. doi: 10.1007/s00251-010-0510-8. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha R, Lubinsky B, Bansode V, Moinz M, McCormack G, Travers S. QTrim: a novel tool for the quality trimming of sequence reads generated using the Roche/454 sequencing platform. BMC Bioinformatics. 2014;15:33. doi: 10.1186/1471-2105-15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2013;30:btt593–btt620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright I, Travers S. RAMICS: trainable, high-speed and biologically relevant alignment of high-throughput sequencing reads to coding DNA. Nucleic Acids Research. 2014 doi: 10.1093/nar/gku473. gku473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd SD, Marshall EL, Merker JD, Maniar JM, Zhang LN, Sahaf B, Jones CD, Simen BB, Hanczaruk B, Nguyen KD, Nadeau KC, Egholm M, Miklos DB, Zehnder JL, Fire AZ. Measurement and Clinical Monitoring of Human Lymphocyte Clonality by Massively Parallel V-D-J Pyrosequencing. Science Translational Medicine. 2009;1:12ra23. doi: 10.1126/scitranslmed.3000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson K, Liu Y, Roskin K, Glanville J, Hoh R, Seo K, Marshall E, Gurley T, Moody A, Haynes B, Walter E, Liao H-X, Albrecht R, García-Sastre A, Chaparro-Riggers J, Rajpal A, Pons J, Simen B, Hanczaruk B, Dekker C, Laserson J, Koller D, Davis M, Fire A, Boyd S. Human Responses to Influenza Vaccination Show Seroconversion Signatures and Convergent Antibody Rearrangements. Cell Host & Microbe. 2014;16:105–114. doi: 10.1016/j.chom.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parameswaran P, Liu Y, Roskin K, Jackson K, Dixit V, Lee J-Y, Artiles K, Zompi S, Vargas M, Simen B, Hanczaruk B, McGowan K, Tariq M, Pourmand N, Koller D, Balmaseda A, Boyd S, Harris E, Fire A. Convergent antibody signatures in human dengue. Cell Host & Microbe. 2014;13:691–700. doi: 10.1016/j.chom.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Liu Y, Xu L, Jackson K, Roskin K, Pham T, Laserson J, Marshall E, Seo K, Lee J-Y, Furman D, Koller D, Dekker C, Davis M, Fire A, Boyd S. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. Journal of Immunology. 2014;192:603–611. doi: 10.4049/jimmunol.1301384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Jackson K, Davies J, Chen Z, Gaeta B, Rimmer J, Sewell W, Collins A. IgE-associated IGHV genes from venom and peanut allergic individuals lack mutational evidence of antigen selection. PLoS One. 2014;9:e89730. doi: 10.1371/journal.pone.0089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. [Accessed: June 2014];The International Immunogenetics Information System Database. Available at: http://www.imgt.org/.
- 30. [Accessed: June 2014];Immunoglobulin Polymorphism Database. Available at: http://cgi.cse.unsw.edu.au/~ihmmune/IgPdb/.
- 31. [Accessed: June 2014];National Center for Biotechnology Information dbSNP - Short Genetic Variation Database. Available at: http://www.ncbi.nlm.nih.gov/SNP/.
- 32. [Accessed: June 2014];National Center for Biotechnology Information GenBank. Available at: http://www.ncbi.nlm.nih.gov/genbank/
- 33. [Accessed: June 2014];National Center for Biotechnology Information Immunoglobulin BLAST Tool. Available at: http://www.ncbi.nlm.nih.gov/igblast/.
- 34.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, P. G. Principal Investigators. Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao H-X, Lynch R, Zhou T, Gao F, Alam M, Boyd S, Fire A, Roskin K, Schramm C, Zhang Z, Zhu J, Shapiro L, Mullikin J, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia S-M, Montefiori D, Parks R, Lloyd K, Scearce R, Soderberg K, Cohen M, Kamanga G, Louder M, Tran L, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce G, Srivatsan S, Zhang B, Zheng A, Shaw G, Hahn B, Kepler T, Korber B, Kwong P, Mascola J, Haynes B. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doria-Rose N, Schramm C, Gorman J, Moore P, Bhiman J, DeKosky B, Ernandes M, Georgiev I, Kim H, Pancera M, Staupe R, Altae-Tran H, Bailer R, Crooks E, Cupo A, Druz A, Garrett N, Hoi K, Kong R, Louder M, Longo N, McKee K, Nonyane M, O’Dell S, Roark R, Rudicell R, Schmidt S, Sheward D, Soto C, Wibmer C, Yang Y, Zhang Z, Mullikin J, Binley J, Sanders R, Wilson I, Moore J, Ward A, Georgiou G, Williamson C, Abdool Karim S, Morris L, Kwong P, Shapiro L, Mascola J. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014 doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuire A, Glenn J, Lippy A, Stamatatos L. Diverse recombinant HIV-1 envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D. Journal of Virology. 2014;88:2645–2657. doi: 10.1128/JVI.03228-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.