Background: Apela, a newly identified peptide hormone, is important during zebrafish embryogenesis.
Results: Apela binds directly to APJ and acts through the Gi pathway. Apela is expressed exclusively in adult kidney and regulates fluid homeostasis.
Conclusion: Apela regulates fluid homeostasis through Gi signaling pathway.
Significance: Apela is a kidney ligand more potent than apelin in regulating fluid homeostasis.
Keywords: G protein, G protein-coupled receptor (GPCR), homeostasis, kidney, peptide hormone, APJ receptor, Gi pathway, apela, apelin, fluid homeostasis
Abstract
Apela (APJ early endogenous ligand, also known as elabela or toddler) is a recently discovered peptide hormone. Based on genetic studies in zebrafish, apela was found to be important for endoderm differentiation and heart development during embryogenesis. Although common phenotypes of apela and APJ-null zebrafish during embryonic development suggested that apela interacts with the APJ receptor, kinetics of apela binding to APJ and intracellular signaling pathways for apela remain unknown. The role of apela in adults is also uncertain. Using a chimeric apela ligand, we showed direct binding of apela to APJ with high affinity (Kd = 0.51 nm) and the ability of apelin, the known peptide ligand for APJ, to compete for apela binding. Apela, similar to apelin, acts through the inhibitory G protein pathway by inhibiting forskolin-stimulated cAMP production and by inducing ERK1/2 phosphorylation. In adult rats, apela is expressed exclusively in the kidney, unlike the wide tissue distribution of apelin. In vivo studies demonstrated the ability of apela to regulate fluid homeostasis by increasing diuresis and water intake. Dose-response studies further indicated that apela induces 2- and 5-fold higher maximal responses than apelin in ERK1/2 phosphorylation and diuresis/water intake, respectively. After designing an apela antagonist, we further demonstrated the role of endogenous ligand(s) in regulating APJ-mediated fluid homeostasis. Our results identified apela as a potent peptide hormone capable of regulating fluid homeostasis in adult kidney through coupling to the APJ-mediated Gi signaling pathway.
Introduction
Apelin, originally isolated from the bovine stomach (1, 2), encodes a mature peptide hormone of 13 amino acids conserved in vertebrates. Apelin was found to be important in the regulation of water and food intake (3–6) by binding and activating APJ (apelin receptor), a 7-transmembrane G-protein-coupled receptor. APJ and its ligand apelin play diverse roles in the central and peripheral regulation of the cardiovascular system, the release of hormones/neuropeptides, the modulation of immune functions, as well as the maintenance of cardiac contractility during pressure overload and aging (7–11).
Apela (APJ early endogenous ligand, also known as elabela or toddler) is a recently identified gene, encoding conserved C-terminal mature peptide hormones of 32 or 21 amino acids (12). Two groups independently showed that apela is an embryonic hormone capable of regulating heart development and promoting the movement of mesendodermal cells during gastrulation, respectively (13, 14). Because the APJ-null zebrafish shares the same phenotype as apela mutants during embryogenesis, apela was identified as the cognate peptide ligand for the APJ receptor (13, 14). In addition, apela binds to APJ and induces the internalization of APJ in receptor-transfected cell lines (13, 14).
Although genetic studies demonstrated the important role of apela during embryogenesis in zebrafish, the expression pattern and function of apela in adult mammals still remain unknown. In addition, whether apela functions through coupling to APJ to modulate the G protein signaling pathways has not been investigated. Here, we demonstrated the exclusive expression of apela in kidney of adult rats, and the ability of apela to activate the Gi signaling pathway. In addition, apela regulated diuresis and water intake, and a newly designed apela antagonist modulated fluid homeostasis in vivo.
Experimental Procedures
Peptides, Plasmids, and Animals
Apela-32 (all apela peptides in this report used is apela-32 except as otherwise indicated), apela-21, angiotensin II (AngII),2 apelin, apelin-FA, and other related peptides were purchased from Phoenix Pharmaceuticals, Inc. and Chinese Peptide Company (China). Apela analogs (apela-FA, PA, AA) were synthesized by the PAN facility at Stanford University and Chinese Peptide Company (China). APJ, GPR15, and GPR25 full-length cDNAs were subcloned into the pcDNA3.1 (+) plasmid and verified by DNA sequencing. Apela C-terminal coding sequences were subcoloned into the AP-tag vector (GenHunter's AP-TAG technology) and verified by DNA sequencing. Sprague-Dawley rats were used for in vivo studies. All procedures involving animals were carried out in accordance with institutional guidelines for the care and use of laboratory animals.
Real-time q-RT-PCR
Sprague-Dawley rats were sacrificed for tissue preparations. Total RNAs were extracted from stomach, lung, heart, muscle, intestine, testis, bladder, kidney, brain, spleen, liver, ovary, and uterus. 500 ng of total RNA were reverse-transcribed to cDNAs using the following master mix: 6 μl of RNase-free water, 2 μl of 5× buffer (Takara, China), 0.5 μl random 6 mers (100 μm) (Takara, China), 0.5 μl of oligo dT primer (50 μm) (Takara, China), 0.5 μl of primer Script RT Enzyme Mix I, and total RNA (500 ng). A master mix of the following reaction components was prepared: 6.8 μl of water, 0.4 μl of forward primer (10 μm), 0.4 μl of reverse primer (10 μm), 10 μl of SYBR Premix Ex Taq (Tli RnaseH Plus) (Takara, China), and 0.4 μl of ROX Reference Dye I before adding 2 μl of PCR templates. The following real-time PCR protocol was used: denaturation for 30 s. at 95 °C, 40 cycles of a three segmented amplification and quantification program (denaturation for 30 s at 95 °C, annealing for 5 s at the primer specific temperature, elongation for 30 s at 60 °C), and a melting step by slow heating from 60 to 99 °C.
Cell Culture, cAMP Assay, and Immunoblotting
Chinese hamster ovary (CHO) cells were grown in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). APJ was transfected into CHO cells using Lipofectamine 2000 (Invitrogen) before selection for their resistance to the antibiotic G418. G418-resistant clones were screened for the expression of APJ. For cAMP assay, CHO cells expressing APJ were pretreated for 30 min. with apela or apelin at the indicated concentration before treatment with 1 μm forskolin (FSK) in the presence of 0.2 mm IBMX. At 30 min. later, total cAMP levels were determined. To determine the effects of PTX pretreatment, cells were pretreated overnight with PTX (200 ng/ml) before cAMP assay. For determining the effects of apela-PA, cells were pretreated with apela-PA at the indicated concentration for 30 min. before cAMP assay. For immunoblotting, CHO cells expressing APJ at subconfluence were serum-deprived or pretreated with pertussis toxin at 200 ng/ml for 12 h before peptide exposure for different times. Cells were washed once in PBS and lysed for 15 min on ice in a RIPA Lysis and Extraction Buffer, and the mixture was gently agitated for 10 min. before centrifugation at 13,000 × g for 15 min. The same amount of proteins was fractionated on a 10% SDS-PAGE gel before immunoblotting. Anti-p44/42 ERK1/2 and 44/42 ERK1/2 antibodies were from Beyotime Company (China). Experiments were repeated independently at least three times. The density of the bands corresponding to 44 and 42 kDa was quantified with an imaging densitometer. Immunoblotting data are expressed as percentages of the maximal value and represent the mean ± S.E. of three independent experiments.
Binding Assay
The assay for the production of alkaline phosphatase (AP)-tagged proteins and binding affinity measurements have been described (15). Briefly, the AP-apela plasmid was transiently transfected into HEK293T cells and, after 24 h, cells were cultured in serum-free medium for 2 days. The supernatant containing AP-apela was quantified using the AP activity assay (GenHunter Corp.). For the cell-based binding assay, CHO cells expressing APJ were grown to near confluence and incubated with AP-apela for 90 min at room temperature. At the end of incubation, cells were washed with HBSS containing 0.5 mg/ml BSA and 20 mm HEPES (pH 7.0), and bound AP activities were determined by using AP assay reagents (GenHunter Corp.). Data were analyzed using Graphpad Prism 5.0 and Kd values were calculated. For peptide competition assays, the same APJ-expressing CHO cells were incubated with AP-apela (5 nm) and different peptide analogs at indicated concentration for 90 min at room temperature. Finally the same AP activity assay was performed. Data were analyzed using SigmaPlot.
Measurement of Urine Flow Rate, Water Intake, and Food Intake
All experiments were performed using adult male rats (Sprague-Dawley). For animals in urine flow rate tests, a stabilizing period of 30 min was allowed after anesthesia (using 2,2,2-tribromoethanol by intraperitoneal injection), and an intravenous (tail vein) injection of saline (500 ul) was performed to determine baseline values of urine flow rates (2 h basal period). Some rats received 500 μl of different doses of apelin, apela, or apela-PA by intravenous (tail vein) injection for 2 h (the experimental period). Urine was collected to tube by pipetting from plastic sheets at the bottom of cage 2 h post-injection. Urine flow rate is expressed as μl/min per 100 g body weight. For animals in water intake and food intake tests, either saline or different doses of apelin, apela, or apela-PA was administered into rats using intraperitoneal injections. Twenty-four hours post-injection was taken as the end point. Water and food intake were measured by weighing water content and food pellets inside the cage, respectively. Water intake was expressed as μl/h per 100 g body weight, and food intake was expressed as g/day per 100 g body weight. Every group included at least five test animals.
Statistics
Experiments for in vitro tests including cAMP assay, binding assay, and immunoblotting were repeated independently at least three times. Every animal group for in vivo experiments included at least five animals for testing. Differences between two groups were compared using two-tailed Student's t test. One way ANOVA followed by a Fisher's LSD post-hoc test was used to evaluate differences among multiple groups. Data are expressed as mean ± S.E. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as a p value < 0.05 (*) or p value < 0.01 (**).
Results
Apela Binds to APJ with High Affinity
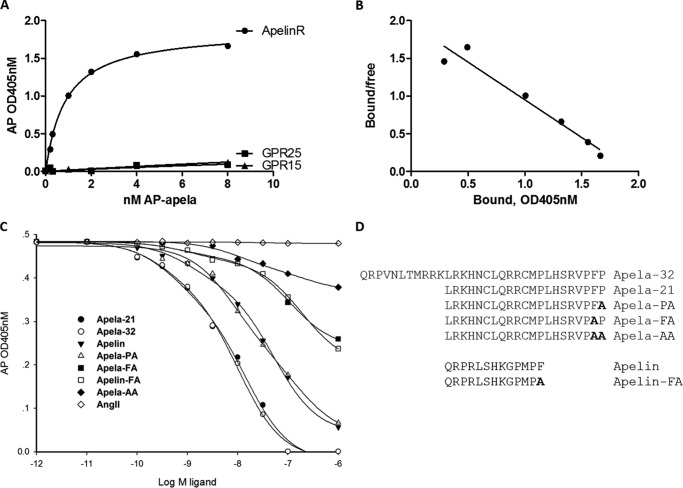
Apela induces the internalization of APJ in transfected cell lines overexpressing APJ (13, 14). However, binding kinetics between apela and APJ has not been demonstrated. Peptide ligands with alkaline phosphatase (AP) appended at its N-terminal end allows easy detection of chimeric probes for demonstrating direct ligand-receptor binding (16). To directly analyze the ligand-receptor relationship of apela and APJ, we introduced the alkaline phosphatase tag (AP-tag) to apela-32 and generated a chimeric apela ligand, AP-apela. In addition, we isolated CHO cells stably expressing APJ for binding tests. As shown in Fig. 1A, incubation of increasing levels of AP-apela led to a dose-dependent binding of the ligand to CHO cells expressing APJ. However, AP-apela did not bind to cells expressing phylogenetically related receptors, GPR15 and GPR25 (17) (Fig. 1A). Scatchard plot analyses (Fig. 1B) indicated an equilibrium-binding constant (Kd) of 0.51 nm for AP-apela, a high affinity comparable to the interaction between apelin-13 and APJ (Kd = 0.4 nm) (18).
FIGURE 1.
Direct binding of apela to the APJ receptor with high affinity. A, saturation curve for AP-apela binding to APJ and lack of binding to related receptors. CHO cells stably expressing APJ were incubated with increasing concentration of AP-apela for 90 min at room temperature before determination of alkaline phosphatase activities. Specific binding was calculated by subtracting the values from cells transfected with empty vectors. Similar tests were performed using cells stably expressing GPR15 and GPR25. Experiments were repeated independently at least three times. Data were analyzed using Graphpad Prism 5.0, and Kd values were calculated. The receptor density is 1.87 ± 0.025 (Bmax) and Kd value of apela is 0.51 ± 0.038 nm. B, Scatchard analysis of AP-apela binding to CHO cells expressing APJ. C, binding of AP-apela to APJ was competed by different apela- and apelin-related peptides, but not by AngII. For peptide competition assays, the same APJ-expressing CHO cells were incubated with AP-apela (5 nm) and different peptide analogs with indicated concentrations for 90 min at room temperature. Finally the AP activity assay was used, and data were analyzed using SigmaPlot. Experiments were repeated independently at least three times. D, sequences of apela, apelin, and their analogs. Altered amino acids are shown in bold.
To analyze the specificity of apela and apelin binding to APJ, we also checked for binding competition by related peptides. As shown in Fig. 1C, apela-32 and apela-21 competed for AP-apela binding to APJ with equal potency, whereas apelin only partially competed for AP-apela binding. In contrast, AngII, a peptide ligand capable of binding to the angiotensin receptor, with sequence similarity to APJ (19), was ineffective (Fig. 1C). Although both apela and apelin have mature peptides in their C-terminal region, they do not have homologous sequences (supplemental Fig. S1A). After alignment of their gene synteny in vertebrates, we also could not find their common origination in vertebrates (supplemental Fig. 1B). Thus, our data suggested that APJ binds to two phylogenetically unrelated peptide ligands, apela, and apelin.
Based on an early report showing the importance of the C-terminal region of apelin for receptor signaling (20), we designed apela analogs by modifying key residues in its C-terminal region (Fig. 1D). As shown in Fig. 1C, both apela and apelin analogs competed for AP-apela binding to the APJ receptor (apelin = apela-PA>apela-FA = apelin-FA>apela-AA).
Apela Activates Gi Signaling by Interacting with the APJ Receptor
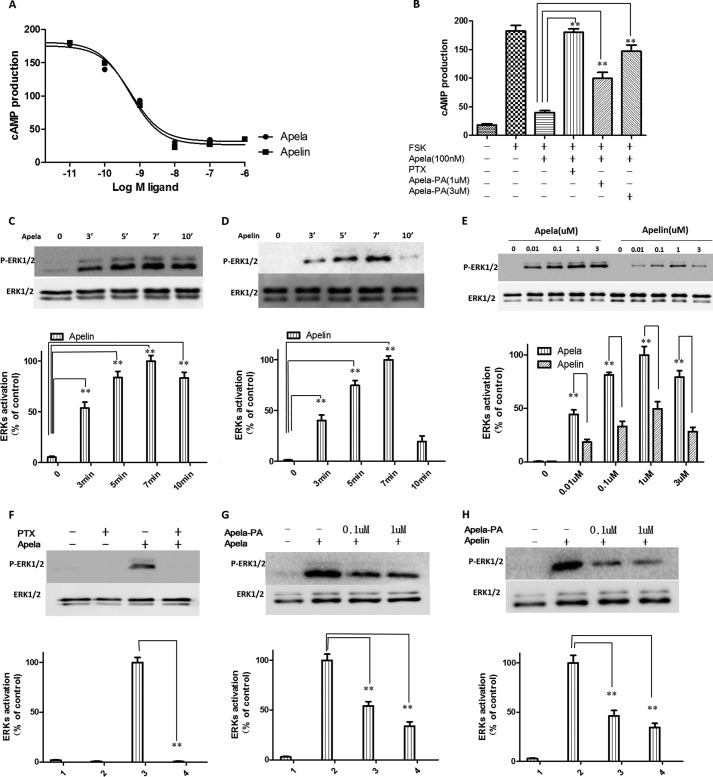
Apelin binds to APJ and activates downstream Gi signaling (1, 21, 22). We also checked whether apela activates Gi signaling via APJ. As shown in Fig. 2A, apela treatment inhibited forskolin-stimulated cAMP production in APJ-expressing CHO cells in a dose-dependent manner with IC50 value of 0.27 nm, showing a similar potency as apelin (IC50 = 0.29 nm) (1). Furthermore, pretreatment of APJ-expressing cells with pertussis toxin (PTX), which selectively ADP-ribosylates Gi/Go proteins and uncouples them from their associated receptors, abrogated the inhibitory effects of apela on cAMP production (Fig. 2B). Also, the newly designed apela analog (apela-PA) dose-dependently reduced inhibitory effects of apela on cAMP production (Fig. 2B), indicating apela-PA is an antagonist of Apela-APJ functions.
FIGURE 2.
Apela is equipotent as apelin in suppressing cAMP production but more potent than apelin in the stimulation of ERK1/2 phosphorylation mediated by the APJ receptor. A, inhibition of forskolin-stimulated cAMP production by apela or apelin in CHO cells stably expressing APJ. CHO cells expressing APJ were pretreated for 30 min with apela or apelin at indicated concentrations before treatment with 1 μm forskolin (FSK) in the presence of 0.2 mm IBMX. At 30 min later, total cAMP levels were determined. Experiments were repeated independently at least three times. Data were analyzed using Graphpad Prism 5.0 and IC50 values were calculated. IC50 of apela is 0.27 nm ±0.011, and IC50 of apelin is 0.29 nm ±0.013, respectively; Imax value of aplea is 178.8 ± 5.3 and Imax value of aplea is 183.3 ± 4.6, respectively. B, pretreatment with PTX and apela-PA abrogated the inhibition of forskolin-stimulated cAMP production by apela. To determine the effects of PTX pretreatment, cells were pretreated overnight with PTX (200 ng/ml) before testing. For determining the effects of apela-PA, cells were pretreated with apela-PA (1 μm or 3 μm) for 30 min. before testing. Experiments were repeated independently at least three times. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value <0.05 (*) or p value <0.01 (**). C and D, apela and apelin treatment promoted a time-dependent phosphorylation of ERKs in CHO cells expressing APJ. E, apela induced higher maximal phosphorylation of ERK1/2 than apelin. CHO cells stably expressing APJ were changed into serum-free conditions for 12 h, followed by stimulation with different concentrations of apela or apelin for 7 min or indicated time course before immunoblotting analyses (upper panel, representative blots). The density of the bands corresponding to 44 kDa and 42 kDa were quantified with an imaging densitometer. Data shown in the lower panel of C, D, and E are expressed as percentages of the maximal value and represent the mean ± S.E. of three independent experiments. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value <0.05 (*) and p value <0.01 (**). F, PTX pretreatment abrogated the phosphorylation of ERKs induced by apela. CHO cells stably expressing APJ were changed into serum-free conditions with or without pretreatment with PTX (200 ng/ml) for 12h before stimulation with 1 μm apela for 7 min. Cells were lysed and immunoblotting analyses were performed using specific antibodies. G and H, apela-PA dose-dependently reduced effects of both apela and apelin on ERKs phosphorylation. CHO cells stably expressing APJ were changed into serum-free conditions for 12 h and were pretreated with apela-PA (0.1 μm or 1 μm) for 2 min before stimulation with 10 nm apela or apelin for 7 min. Cells were lysed, and immunoblotting analyses were performed using specific antibodies. The density of the bands corresponding to 44 and 42 kDa was quantified with an imaging densitometer. Data shown in the lower panel of F, G, and H are expressed as percentages of the maximal value and represent the mean ± S.E. of three independent experiments. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value <0.05 (*) and p value <0.01 (**).
In addition, we monitored apela induction of the phosphorylation of p42/44 ERKs, known to be downstream of Gi/Go signaling (23). As shown in Fig. 2, C and D, both apela and apelin treatment of APJ-expressing cells promoted a time-dependent phosphorylation of p42/44 ERKs with the highest stimulation at 7 min, which is consistent with previous study (24). Dose-dependence tests further indicated that treatment of APJ-expressing CHO cells with either apela or apelin for 7 min. promoted a dose-dependent phosphorylation of p42/44 ERKs (Fig. 2E), with apela showing a 2-fold higher maximal increase than apelin (Fig. 2E). Furthermore, PTX pretreatment abrogated this activation (Fig. 2F). Also, the newly designed apela analog (apela-PA) dose-dependently reduced effects of both apela and apelin on ERKs phosphorylation (Fig. 2, G and H), further indicating apela-PA is an antagonist of apela/apelin actions and also APJ receptor functions. In addition, other G protein pathways (Gs, Gq, and G12) were tested but apela show no stimulation (data not shown). Taken together, our results suggest that apela activates APJ by coupling to the Gi signaling pathway.
Apela Is Exclusively Expressed in Kidney of Adult Rats
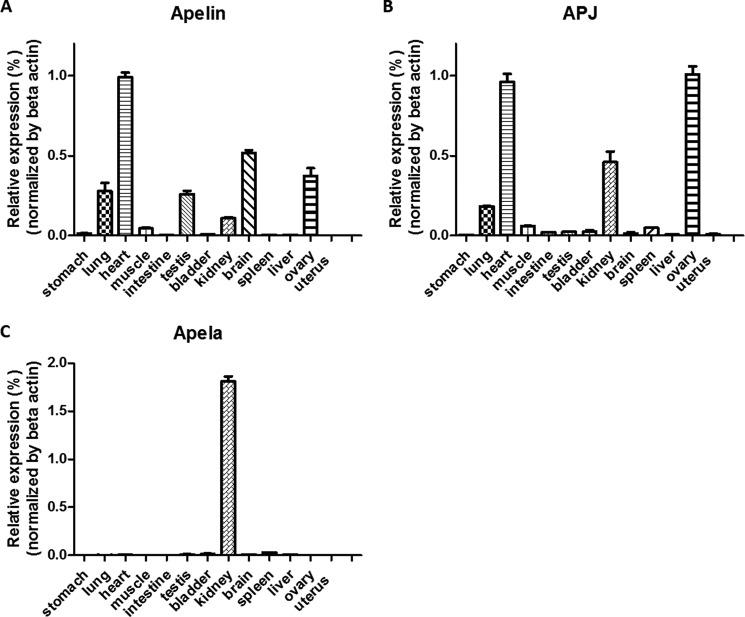
Both apelin and APJ are expressed in the central nervous system and diverse other tissues including stomach, gastrointestinal tract, heart, kidney, adipose, and lung (10, 25–27). As showed in Fig. 3, A and B, we confirmed the wide tissue expression pattern of apelin and APJ in adult rats. As another natural peptide ligand for APJ, apela was found to be expressed during zebrafish embryogenesis (13, 14). To investigate apela expression in adults, we performed quantitative-PCR screening of diverse tissues in adult rats, and found exclusive expression of apela in the kidney (Fig. 3C), suggesting apela may play important roles in fluid homeostasis, in addition to its role in heart development during embryogenesis.
FIGURE 3.
Apelin, APJ, and apela expression in diverse tissues of adult rats. Expression of apelin (A), APJ (B), and apela (C) mRNAs in different rat tissues (stomach, lung, heart, muscle, intestine, testis, bladder, kidney, brain, spleen, liver, ovary, and uterus). Quantitative RT-PCR was carried out with specific primer sets for apelin, APJ and apela. Expression of these three transcripts was normalized based on beta actin levels.
Effects of Apela on Diuresis and Water Intake
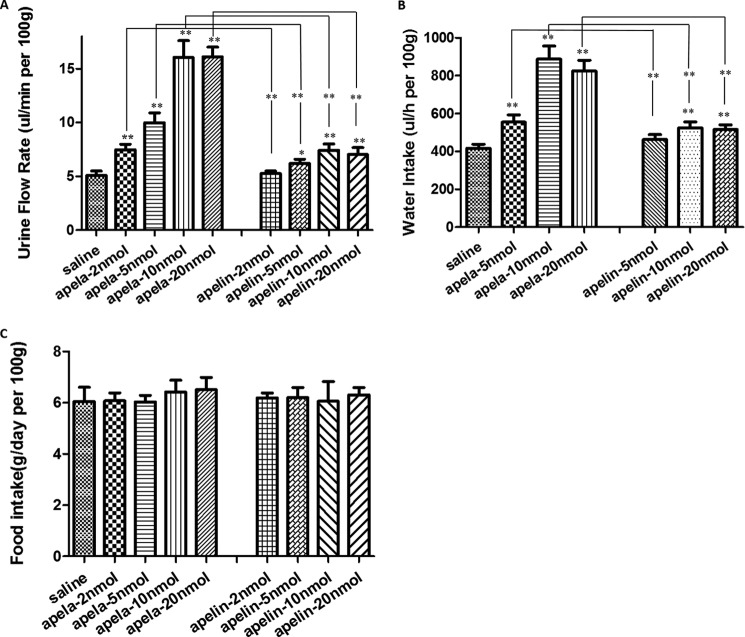
Earlier studies demonstrated that the apelin-APJ pair regulates kidney functions (3–5). Because apela is exclusively expressed in the kidney during adult life (Fig. 3C), we further checked its regulation of fluid homeostasis. As shown in Fig. 4A, urine flow rate was increased following intravenous injection of adult rats with apelin as compared with saline-injected animals, consistent with earlier results (3). Of interest, apela injection induced about 5-fold higher maximal increases in plateau-responses of urine flow rates than apelin (Fig. 4A).
FIGURE 4.
Apela is more potent than apelin in regulating fluid homeostasis. A, effect of intravenous injections of increasing doses of apela or apelin on urine flow rates. Control period (2 h) corresponded to intravenous injection of saline. Experimental period (2 h) corresponded to intravenous injection of increasing doses of apela or apelin (2, 5, 10, and 20 nmol). Urine flow rates were expressed as μl/min per 100 g body weight. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value < 0.05 (*) and p value < 0.01 (**). At least five animals for each group were used. * or ** on the top of columns denotes apela or apelin versus saline; * or ** between broken lines denotes apela versus apelin. B, effect of intraperitoneal injections of increasing doses of apela or apelin on water intake. Either saline or different doses of apelin or apela was administered into rats using intraperitoneal injection. Water intake was measured by weighing the water inside the cage at 24 h post-intraperitoneal injection. Water intake was expressed as μl/h per 100 g body weight. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value < 0.05 (*) and p value < 0.01 (**). At least five animals for each group were used, and P represents the number of animals. * or ** on the top of column was made concerning apela or apelin versus saline; * or ** between the broken lines denotes apela versus apelin. C, effect of intraperitoneal injections of increasing doses of apela or apelin on food intake. Either saline or different doses of apelin or apela was administered into rats using intraperitoneal injection. Food intake was measured by weighing food pellets inside the cage at 24 h post-intraperitoneal injection. Food intake was expressed as g/day per 100 g body weight. At least five animals for each group were used. Calculations were done with a standard statistical package (SPSS for Windows, version 21).
Previous study showed the role of apelin in water and food intake (6). However, contrary data also exists (28). To further elucidate apela function in vivo, we investigated the effect of apela and apelin on water and food intake. As shown in Fig. 4B, intraperitoneal injection of adult rats with either apela or apelin increased water intake with apela showing about 5-fold higher maximal plateau responses than apelin (Fig. 4B). In contrast, no effect of either apela or apelin on food intake was found (Fig. 4C).
To further confirm the roles of endogenous APJ ligands on urine flow rate and water intake, we treated adult rats with the antagonistic analog apela-PA. Interestingly, urine flow rates measured after apela-PA treatment were significantly lower than saline-treated animals (Fig. 5A). Likewise, treatment with apela-PA dose-dependently decreased water intake of rats following intraperitoneal injections (Fig. 5B). Our results further confirmed importance roles of the APJ receptor in fluid regulation by the kidney. Combined with the high expression pattern of apela in adult kidney, our results suggested that apela and APJ may play important roles in fluid regulation by the kidney.
FIGURE 5.
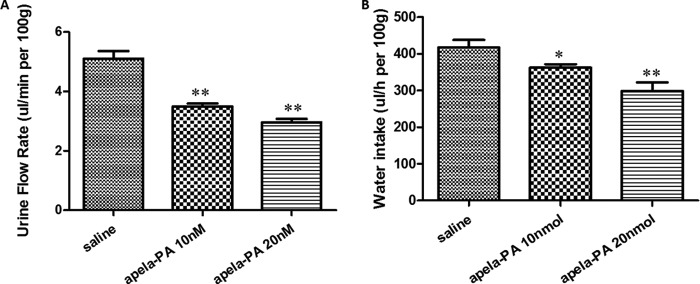
Effect of treatment with an apela antagonist (apela-PA) on fluid homeostasis. A, effect of intravenous injections of increasing doses of apela-PA on urine flow rates. Basal control period (2 h) corresponds to intravenous injection of saline. Experimental period corresponds to intravenous injection of increasing doses of apela-PA (10 and 20 nmol). Urine flow rates were expressed as μl/min per 100 g body weight. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value <0.05 (*) and p value <0.01 (**). At least five animals for each group were used. B, effect of intraperitoneal injections of increasing doses of apela-PA on water intake. Either saline or different doses of apela-PA was administered into rats using intraperitoneal injection. At 24 h post-intraperitoneal injection, water intake was measured by weighing water inside the cage. Water intake was expressed as μl/h per 100g body weight. Calculations were done with a standard statistical package (SPSS for Windows, version 21). Statistical significance was defined as p value <0.05 (*) and p value <0.01 (**). At least five animals for each group were used.
Discussion
Our results demonstrated binding kinetics of apela to its cognate receptor-APJ and apela inhibition of forskolin-stimulated cAMP production and induction of ERK1/2 phosphorylation though coupling of APJ to the PTX-sensitive Gi pathway. In addition, we identified the exclusive expression of apela in kidney of adult rodents and the role of apela in regulating diuresis and water intake.
Apela and apelin, two phylogenetically unrelated, natural peptide ligands for APJ, are both conserved in vertebrates and represent a rare case of GPCR ligand-receptor pairs showing one receptor interacting with two distinct ligands (29). Because apelin can compete for the binding of AP-apela to the APJ receptor (Fig. 1C), it is likely that these two peptides bind to overlapping sites in APJ, and it is interesting to investigate the exact binding sites of APJ for apela and apelin based on structural analysis.
Angiotensin, bradykinin, and apelin bind to angiotensin II receptor types 1/2, bradykinin receptor B1/2, and APJ, respectively (19). Receptors for these three ligands constitute a subfamily of peptide GPCRs due to their close sequence homology (30). Early studies demonstrated the role of this GPCR subfamily in fluid homeostasis. Treatment with angiotensin II in mice inhibits drinking of water or saline, whereas administration of angiotensin II receptor antagonists stimulates diverse responses including water drinking, vasopressin secretion, and natriuresis (31, 32). In addition, experiments on dogs showed that bradykinin regulates proximal tubular sodium reabsorption (33).
Although apelin is the cognate ligand for APJ and abnormal fluid homeostasis was found in APJ null mice (34, 35), the exact role of apelin in the regulation of fluid homeostasis has been controversial. Several reports showed the ability of apelin to regulate fluid homeostasis in rodents (3–6) whereas other studies showed that apelin does not have reliable or robust effects on fluid intake or blood pressure in rats (28). Our studies demonstrated that apela is expressed exclusively in adult kidney and apela is more potent than apelin in regulating diuresis and water intake. Because apelin is expressed in low levels in the adult kidney, our findings suggest that apela is more important than apelin in regulating fluid homeostasis in adults.
Both apelin and APJ are widely expressed in the nervous system in adults, including the frontal cortex, striatum, midbrain, hippocampus, medulla pons, cerebellum, pituitary, olfactory tubercle, and septum. They are also expressed in peripheral tissues including adrenal, vas deferens, testis, intestine, and kidney (26). For the adult kidney, apelin showed low expression levels, as compared with the high and exclusive expression of apela (Fig. 3C). Furthermore, apela stimulated a 5-fold higher maximal response than apelin in regulating diuresis and water intake, suggesting apela of kidney origin is more important than apelin in controlling APJ functions in the kidney (3–5). Also, apela showed 2-fold higher plateau responses in stimulating phosphorylation of ERK1/2 than apelin (Fig. 2E), although both of them inhibited FSK-stimulated cAMP production with the same potency (Fig. 2A). Further study can investigate additional apela effects on kidney functions and the exact kidney cell types expressing apela and APJ.
Our results showed the newly designed apela antagonist, apela-PA, antagonized ERKs activation of both apela and apelin (Fig. 2, G and H), presumably by binding to the APJ receptor. Also, in vivo, apela-PA inhibits the urine flow rate and water intake after injection in adult rats. Studies on the antagonistic functions of apela-PA and related peptides could allow the formulation of new therapy for kidney diseases.
Author Contributions
CD and AH designed the study and wrote the paper. CD, HC, and NY designed, performed and analyzed the experiments shown in Fig. 1, Fig. 2, and Fig. 3. CD and YF designed, performed, and analyzed the experiments shown in Figs. 4 and 5. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Note Added in Proof
Cheng Deng and Aaron J. W. Hsueh were listed as co-corresponding authors on the version of this article that was published on May 20, 2015 as a Paper in Press. Aaron J. W. Hsueh is the sole corresponding author of the final version.
This work was supported by the National Natural Science Foundation of China (31401207), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the One Hundred Person Project of Nanjing Normal University. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Fig. S1.
- Ang II
- angiotensin II
- AP
- alkaline phosphatase
- PTX
- pertussis toxin.
References
- 1. Habata Y., Fujii R., Hosoya M., Fukusumi S., Kawamata Y., Hinuma S., Kitada C., Nishizawa N., Murosaki S., Kurokawa T., Onda H., Tatemoto K., Fujino M. (1999) Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta 1452, 25–35 [DOI] [PubMed] [Google Scholar]
- 2. Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M. X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., Kurokawa T., Onda H., Fujino M. (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 251, 471–476 [DOI] [PubMed] [Google Scholar]
- 3. Hus-Citharel A., Bouby N., Frugière A., Bodineau L., Gasc J. M., Llorens-Cortes C. (2008) Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 74, 486–494 [DOI] [PubMed] [Google Scholar]
- 4. Soltani Hekmat A., Najafipour H., Nekooian A. A., Esmaeli-Mahani S., Javanmardi K. (2011) Cardiovascular responses to apelin in two-kidney-one-clip hypertensive rats and its receptor expression in ischemic and non-ischemic kidneys. Regulatory Peptides 172, 62–68 [DOI] [PubMed] [Google Scholar]
- 5. Najafipour H., Soltani Hekmat A., Nekooian A. A., Esmaeili-Mahani S. (2012) Apelin receptor expression in ischemic and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney-one-clip hypertension in rats. Regulatory Peptides 178, 43–50 [DOI] [PubMed] [Google Scholar]
- 6. Taheri S., Murphy K., Cohen M., Sujkovic E., Kennedy A., Dhillo W., Dakin C., Sajedi A., Ghatei M., Bloom S. (2002) The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem. Biophys. Res. Commun. 291, 1208–1212 [DOI] [PubMed] [Google Scholar]
- 7. Horiuchi Y., Fujii T., Kamimura Y., Kawashima K. (2003) The endogenous, immunologically active peptide apelin inhibits lymphocytic cholinergic activity during immunological responses. J. Neuroimmunol. 144, 46–52 [DOI] [PubMed] [Google Scholar]
- 8. Szokodi I., Tavi P., Foldes G., Voutilainen-Myllyla S., Ilves M., Tokola H., Pikkarainen S., Piuhola J., Rysä J., Tóth M., Ruskoaho H. (2002) Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circulation Research 91, 434–440 [DOI] [PubMed] [Google Scholar]
- 9. Sunter D., Hewson A. K., Dickson S. L. (2003) Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neuroscience Letters 353, 1–4 [DOI] [PubMed] [Google Scholar]
- 10. Reaux A., De Mota N., Skultetyova I., Lenkei Z., El Messari S., Gallatz K., Corvol P., Palkovits M., Llorens-Cortès C. (2001) Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem. 77, 1085–1096 [DOI] [PubMed] [Google Scholar]
- 11. Kuba K., Zhang L., Imai Y., Arab S., Chen M., Maekawa Y., Leschnik M., Leibbrandt A., Markovic M., Schwaighofer J., Beetz N., Musialek R., Neely G. G., Komnenovic V., Kolm U., Metzler B., Ricci R., Hara H., Meixner A., Nghiem M., Chen X., Dawood F., Wong K. M., Sarao R., Cukerman E., Kimura A., Hein L., Thalhammer J., Liu P. P., Penninger J. M. (2007) Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circulation Research 101, e32–42 [DOI] [PubMed] [Google Scholar]
- 12. Reichman-Fried M., Raz E. (2014) Small proteins, big roles: the signaling protein Apela extends the complexity of developmental pathways in the early zebrafish embryo. BioEssays 36, 741–745 [DOI] [PubMed] [Google Scholar]
- 13. Chng S. C., Ho L., Tian J., Reversade B. (2013) ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell 27, 672–680 [DOI] [PubMed] [Google Scholar]
- 14. Pauli A., Norris M. L., Valen E., Chew G. L., Gagnon J. A., Zimmerman S., Mitchell A., Ma J., Dubrulle J., Reyon D., Tsai S. Q., Joung J. K., Saghatelian A., Schier A. F. (2014) Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 343, 1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Q., Wang Y., Dabdoub A., Smallwood P. M., Williams J., Woods C., Kelley M. W., Jiang L., Tasman W., Zhang K., Nathans J. (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 [DOI] [PubMed] [Google Scholar]
- 16. Flanagan J. G., Leder P. (1990) The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell 63, 185–194 [DOI] [PubMed] [Google Scholar]
- 17. Jung B. P., Nguyen T., Kolakowski L. F., Jr., Lynch K. R., Heng H. H., George S. R., O'Dowd B. F. (1997) Discovery of a novel human G protein-coupled receptor gene (GPR25) located on chromosome 1. Biochem. Biophys. Res. Commun. 230, 69–72 [DOI] [PubMed] [Google Scholar]
- 18. Katugampola S. D., Maguire J. J., Matthewson S. R., Davenport A. P. (2001) [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br. J. Pharmacol. 132, 1255–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Metpally R. P., Sowdhamini R. (2005) Cross genome phylogenetic analysis of human and Drosophila G protein-coupled receptors: application to functional annotation of orphan receptors. BMC Genomics 6, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee D. K., Saldivia V. R., Nguyen T., Cheng R., George S. R., O'Dowd B. F. (2005) Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 146, 231–236 [DOI] [PubMed] [Google Scholar]
- 21. Masri B., Lahlou H., Mazarguil H., Knibiehler B., Audigier Y. (2002) Apelin (65–77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem. Biophys. Res. Commun. 290, 539–545 [DOI] [PubMed] [Google Scholar]
- 22. Bai B., Tang J., Liu H., Chen J., Li Y., Song W. (2008) Apelin-13 induces ERK1/2 but not p38 MAPK activation through coupling of the human apelin receptor to the Gi2 pathway. Acta Biochimica et Biophysica Sinica 40, 311–318 [DOI] [PubMed] [Google Scholar]
- 23. Cheng Z., Garvin D., Paguio A., Stecha P., Wood K., Fan F. (2010) Luciferase Reporter Assay System for Deciphering GPCR Pathways. Current Chemical Genomics 4, 84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masri B., Morin N., Pedebernade L., Knibiehler B., Audigier Y. (2006) The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 281, 18317–18326 [DOI] [PubMed] [Google Scholar]
- 25. Wang G., Anini Y., Wei W., Qi X., O'Carroll A. M., Mochizuki T., Wang H. Q., Hellmich M. R., Englander E. W., Greeley G. H., Jr. (2004) Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology 145, 1342–1348 [DOI] [PubMed] [Google Scholar]
- 26. Lee D. K., Cheng R., Nguyen T., Fan T., Kariyawasam A. P., Liu Y., Osmond D. H., George S. R., O'Dowd B. F. (2000) Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 74, 34–41 [DOI] [PubMed] [Google Scholar]
- 27. Boucher J., Masri B., Daviaud D., Gesta S., Guigné C., Mazzucotelli A., Castan-Laurell I., Tack I., Knibiehler B., Carpéné C., Audigier Y., Saulnier-Blache J. S., Valet P. (2005) Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771 [DOI] [PubMed] [Google Scholar]
- 28. Mitra A., Katovich M. J., Mecca A., Rowland N. E. (2006) Effects of central and peripheral injections of apelin on fluid intake and cardiovascular parameters in rats. Physiology Behavior 89, 221–225 [DOI] [PubMed] [Google Scholar]
- 29. Ben-Shlomo I., Hsueh A. J. (2005) Three's company: two or more unrelated receptors pair with the same ligand. Mol. Endocrinol. 19, 1097–1109 [DOI] [PubMed] [Google Scholar]
- 30. Ben-Shlomo I., Yu Hsu S., Rauch R., Kowalski H. W., Hsueh A. J. (2003) Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transduction. Science's STKE 2003, RE9. [DOI] [PubMed] [Google Scholar]
- 31. Rowland N. E., Goldstein B. E., Robertson K. L. (2003) Role of angiotensin in body fluid homeostasis of mice: fluid intake, plasma hormones, and brain Fos. American Journal of Physiology. Regulatory, Integrative, and Comparative Physiology 284, R1586–1594 [DOI] [PubMed] [Google Scholar]
- 32. McKinley M. J., Allen A. M., Mathai M. L., May C., McAllen R. M., Oldfield B. J., Weisinger R. S. (2001) Brain angiotensin and body fluid homeostasis. The Japanese Journal of Physiology 51, 281–289 [DOI] [PubMed] [Google Scholar]
- 33. Stein J. H., Congbalay R. C., Karsh D. L., Osgood R. W., Ferris T. F. (1972) The effect of bradykinin on proximal tubular sodium reabsorption in the dog: evidence for functional nephron heterogeneity. J. Clin. Investig. 51, 1709–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberts E. M., Newson M. J., Pope G. R., Landgraf R., Lolait S. J., O'Carroll A. M. (2009) Abnormal fluid homeostasis in apelin receptor knockout mice. J. Endocrinol. 202, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts E. M., Pope G. R., Newson M. J., Landgraf R., Lolait S. J., O'Carroll A. M. (2010) Stimulus-specific neuroendocrine responses to osmotic challenges in apelin receptor knockout mice. J. Neuroendocrinol. 22, 301–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.