Background: Pseudomonas aeruginosa flagellin binds to the membrane-tethered mucin, MUC1.
Results: Flagellin drives NEU1 to desialylate MUC1, thereby increasing its adhesiveness for Pseudomonas aeruginosa and its shedding.
Conclusion: P. aeruginosa hijacks host NEU1 through its flagellin.
Significance: P. aeruginosa mobilizes NEU1 to enhance its pathogenicity, but the host retaliates by releasing MUC1 as a hyperadhesive decoy receptor.
Keywords: adhesin; mucin; mucin 1, cell surface-associated (MUC1); neuraminidase; sialic acid
Abstract
Airway epithelia express sialylated receptors that recognize exogenous danger signals. Regulation of receptor responsiveness to these signals remains incompletely defined. Here, we explore the mechanisms through which the human sialidase, neuraminidase-1 (NEU1), promotes the interaction between the sialoprotein, mucin 1 (MUC1), and the opportunistic pathogen, Pseudomonas aeruginosa. P. aeruginosa flagellin engaged the MUC1 ectodomain (ED), increasing NEU1 association with MUC1. The flagellin stimulus increased the association of MUC1-ED with both NEU1 and its chaperone/transport protein, protective protein/cathepsin A. Scatchard analysis demonstrated NEU1-dependent increased binding affinity of flagellin to MUC1-expressing epithelia. NEU1-driven MUC1-ED desialylation rapidly increased P. aeruginosa adhesion to and invasion of the airway epithelium. MUC1-ED desialylation also increased its shedding, and the shed MUC1-ED competitively blocked P. aeruginosa adhesion to cell-associated MUC1-ED. Levels of desialylated MUC1-ED were elevated in the bronchoalveolar lavage fluid of mechanically ventilated patients with P. aeruginosa airway colonization. Preincubation of P. aeruginosa with these same ex vivo fluids competitively inhibited bacterial adhesion to airway epithelia, and MUC1-ED immunodepletion completely abrogated their inhibitory activity. These data indicate that a prokaryote, P. aeruginosa, in a ligand-specific manner, mobilizes eukaryotic NEU1 to enhance bacterial pathogenicity, but the host retaliates by releasing MUC1-ED into the airway lumen as a hyperadhesive decoy receptor.
Introduction
Pseudomonas aeruginosa is a Gram-negative, flagellated, and opportunistic human pathogen that typically colonizes and/or infects debilitated and immunocompromised patients (1). In the respiratory tract, P. aeruginosa is one of the most common and lethal pathogens responsible for acute ventilator-associated pneumonia with directly attributable mortality rates of 40% (2). P. aeruginosa infections worsen the prognosis for bronchiectasis and chronic obstructive pulmonary disease patients (3). P. aeruginosa also adheres to and invades extrapulmonary epithelia (4–8). Despite its recognized clinical impact, the molecular mechanisms that underlie P. aeruginosa pathogenesis and the host response to P. aeruginosa infection remain incompletely understood.
Bacterial adhesion to epithelial cells (EC)2 is prerequisite to establishment of invasive infection and is mediated through interactions between microbial adhesins and their cognate host cell receptors (9). One P. aeruginosa adhesin, flagellin, is the structural protein that forms the major portion of the flagellar filament. Flagellin contributes to the virulence of pathogenic bacteria through increased motility, adhesion, and invasion (10). P. aeruginosa flagellin engages Toll-like receptor (TLR) 5 (11) and the transmembrane mucin 1 (MUC1) (12), and each receptor-ligand interaction is coupled to intracellular signaling. MUC1 consists of a >250-kDa ectodomain (ED), with a variable number of highly sialylated tandem repeats, which is proteolytically processed and shed from the EC surface (13). Three MUC1 sheddases have been identified, including matrix metalloproteinase (MMP) 14, a disintegrin and metalloproteinase (ADAM) 17, and γ-secretase (14–16).
Glycoprotein receptors for bacteria often contain glycan chains terminating with sialic acid (Sia). Here, Sia residues are strategically positioned to influence cell-cell and intermolecular interactions (17). Sia residues can mask binding sites for pathogens, their toxins, endogenous lectins, and protease recognition sites through protein conformational changes, electrostatic repulsion, and/or steric hindrance (18). The sialylation state of glycoconjugates is dynamically and coordinately regulated through the opposing catalytic activities of sialyltransferases and neuraminidases (NEU). NEUs constitute a large family of prokaryotic and eukaryotic glycolytic enzymes that hydrolyzes the linkages between Sia and its subterminal sugars (19).
Prokaryotic NEUs are established virulence factors for viral and bacterial pathogens (18). P. aeruginosa NEU, referred to as NanPs, contributes to bacterial pathogenesis and its expression has been linked to biofilm formation and airway colonization (20). Although much is known about prokaryotic NEUs as virulence factors, a role for mammalian host NEUs in bacterial pathogenesis, to our knowledge, has never been considered. Of the four known mammalian NEUs, NEU1 is the predominant sialidase expressed by human airway ECs, and the second most abundant, NEU3, is expressed at much lower levels (12). NEU1 is localized both to lysosomes and the cell surface (19) and is only active in association with its chaperone/transport protein, protective protein/cathepsin A (PPCA) (21). PPCA is a multipurpose protein that targets NEU1 to the lysosome and is absolutely required for proper folding, stability, oligomerization, and activation of NEU1 (21). We previously demonstrated intense NEU1 immunostaining at the superficial surface of the human airway epithelium, including the brush border of the trachea and bronchus (12). This NEU1 expression pattern closely correlated with that known for MUC1 in these same tissues (22, 23). Furthermore, we established that forced NEU1 overexpression increases MUC1-ED desialylation and MUC1-ED-dependent P. aeruginosa adhesion to airway ECs in vitro (12). To extend these findings to a physiologically relevant context, we asked whether the MUC1 ligand, P. aeruginosa flagellin, might promote NEU1-mediated MUC1-ED desialylation and/or P. aeruginosa adhesion to and invasion of airway ECs. We now present evidence, for the first time, that a bacterial pathogen, P. aeruginosa, exploits the human sialidase, NEU1, to amplify its own pathogenicity through desialylation of its receptor, MUC1. At the same time, NEU1-mediated desialylation of MUC1-ED increases its shedding into the airway lumen to generate a soluble, hyperadhesive decoy receptor for P. aeruginosa. That NEU1 can act on a range of sialylated glycans, not only mucin-type, O-linked glycans abundant in MUC1 but also sialylated N-linked glycan chains, may implicate other sites through which NEU1 might influence the P. aeruginosa-airway EC interaction.
Experimental Procedures
Reagents
NEU1-, NEU3-, and MUC1-targeting small interfering (si)RNAs, and their respective control siRNAs, were from Dharmacon (Lafayette, CO). Lipofectamine and protein G-agarose were from Invitrogen. Oligonucleotide primers for quantitative (q)RT-PCR were synthesized at the Biopolymer and Genomics Core Facility, University of Maryland. Reagents for qRT-PCR were from Qiagen (Valencia, CA), Promega (Madison, WI), and Invitrogen. Precast SDS-polyacrylamide gels were from Invitrogen. Polyvinylidene difluoride (PVDF) membrane was from Millipore (Bedford, MA). Enhanced chemiluminescence reagents and prestained protein molecular weight markers were from Amersham Biosciences. Rabbit anti-human NEU1 antibody was from Rockland Immunochemicals (Gilbertsville, PA). Anti-MUC1 antibodies (Table 1) were from Biomeda (Foster City, CA) and ThermoFisher Scientific (Waltham, MA). Anti-β-tubulin antibody was from Boehringer-Mannheim (Indianapolis, IN). Mouse anti-FLAG and rabbit anti-hemagglutinin (HA) antibodies were from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were from BD Biosciences. Maackia amurensis lectin II (MAL) and Sambucus nigra agglutinin (SNA), Arachis hypogaea (peanut agglutinin (PNA)), and PNA-agarose were from Vector Laboratories (Burlingame, CA).
TABLE 1.
MUC1 antibodies used in this study
Bacteria
Overnight cultures of P. aeruginosa strain K (26), its flagellin-deficient fliC− isogenic mutant (26), Streptococcus pneumoniae type 3 (American Type Culture Collection (ATCC), Manassas, VA), Haemophilus influenzae type b (ATCC), Legionella pneumophila (provided by Dr. H. Steinman, Albert Einstein College of Medicine, Bronx, NY), Staphylococcus aureus provided by Dr. M. Shirtliff, University of Maryland, Baltimore, MD), or Klebsiella pneumoniae (provided by Dr. B. Evrard, CHU, Clermont-Ferrand, France) were resuspended in PBS containing 2.0 mg/ml glucose and quantified spectrophotometrically at A600.
Airway ECs and Ad Constructs
Human A549 cells (ATCC) and primary small airway ECs (SAEC) isolated from the distal portion of the human respiratory tract (PromoCell, Heidelberg, Germany) were cultured as described (12). The ECs were infected with recombinant Ad encoding FLAG-tagged NEU1 (Ad-NEU1-FLAG), HA-tagged NEU3 (Ad-NEU3-HA), catalytically inactive NEU1 containing a Gly68-to-Val substitution (Ad-NEU1-G68V), or green fluorescent protein (GFP) as described (12).
Bacterial Adhesion Assays
Human airway ECs and ECs infected with Ad constructs, transfected with siRNAs, and/or stimulated with flagellin (2.0 × 105 ECs/well) were washed with PBS, fixed for 10 min with 2.5% glutaraldehyde, and washed, and bacterial adhesion was assayed as described (12). The ECs were incubated for 40 min at 37 °C with bacteria (multiplicity of infection (m.o.i.) = 100) and washed, and adherent bacteria were released with 0.05% trypsin, and colony forming units (CFUs) in the releasate were enumerated. Alternatively, the ECs were incubated with GFP-expressing P. aeruginosa (m.o.i. = 100) and washed, and bound bacteria were examined by immunofluorescence microscopy. In other experiments, bacteria were preincubated for 30 min with culture supernatants from ECs infected with Ad constructs, transfected with siRNAs, and/or stimulated with flagellin or with human bronchoalveolar lavage fluids (BALFs) harvested from noncolonized patients, P. aeruginosa-colonized patients, or patients colonized with microorganisms other than P. aeruginosa, prior to adhesion assays. In still other experiments, EC culture supernatants or BALFs containing 2.5 μg/ml MUC1-ED were preincubated overnight with 100 μg/ml anti-MUC1-ED antibody or a species- and isotype-matched nonimmune IgG, after which Igs were immobilized on protein G-agarose for 2 h and removed by centrifugation prior to adhesion assays.
Bacterial Invasion Assays
Human airway ECs and ECs infected with Ad constructs, transfected with siRNAs, or stimulated with P. aeruginosa or flagellin (2.0 × 105 ECs/well) were incubated for 40 min at 37 °C with P. aeruginosa (m.o.i. = 100), washed, and incubated for 1 h with 200 μg/ml gentamicin (Sigma) (27). The ECs were washed, lysed with 0.1% Triton X-100, and CFUs in the lysate quantified.
Knockdown of NEU1 and MUC1
Airway ECs (5.0 × 105) were centrifuged, and the cell pellet was resuspended in 100 μl of Amaxa Nucleofector solution (Lonza, Walkersville, MD) with 2.7 μg of NEU1-targeting or control siRNAs as described (12). The EC-siRNA mixture was transferred to an Amaxa-certified cuvette and subjected to programmed electroporation, and the transfected ECs were cultured for 24–72 h. In other experiments, airway ECs (5.0 × 105) were transfected with 1.5 μg of MUC1-targeting or control siRNAs using Lipofectamine2000 (Invitrogen) according to the manufacturer's instructions.
Immunoblotting Assays
Airway ECs infected with Ad constructs or transfected with siRNAs were lysed, and equal protein aliquots of lysates were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were probed with primary antibody followed by HRP-conjugated secondary antibody and enhanced chemiluminescence reagents as described (12).
Purification of P. aeruginosa Flagellin
An overnight culture of P. aeruginosa strain PA01 was centrifuged at 5,000 × g for 30 min, resuspended in Krebs-Ringer buffer, and incubated for 1 h at 37 °C as described (26). The bacteria were pelleted by centrifugation, and the supernatant was filtered through a 0.22-μm pore membrane and the filtrate boiled for 20 min. The filtrate was concentrated by centrifugal ultrafiltration, adjusted to pH 6.0, and flagellin was purified by sequential ion exchange chromatography using Macro-Prep High S and Macro-Prep High Q support (Bio-Rad). Aliquots of column fractions were resolved by SDS-PAGE and stained with Coomassie Blue to detect the 50-kDa flagellin protein band. Other column aliquots were processed for flagellin immunoblotting and for P. aeruginosa pilin immunoblotting to confirm the absence of pilin contamination. Flagellin-containing fractions were incubated with polymyxin B-agarose (Pierce) to remove lipopolysaccharide (LPS), after which less than 0.1 endotoxin unit/μg of protein was detected by the Limulus amebocyte lysate assay.
Flagellin Binding Assays
Airway ECs infected with Ad constructs (2.0 × 105 ECs/well) were exposed to Alexa Fluor 594-labeled flagellin (26). The ECs were washed; bound flagellin was determined by fluorometry (λex = 591 nm, λex = 615 nm), and Scatchard analysis of the binding data was performed.
qRT-PCR
Total cellular RNA was extracted from SAECs using TRIzol reagent (Invitrogen). RNA purity was established with the 260:280 nm absorption ratio (>1.90). Total RNA (1.0 μg) was treated with DNase I (Invitrogen) for 15 min and reverse-transcribed using avian myeloblastosis virus-reverse transcriptase and poly(T) primer (Promega). The resulting cDNA was amplified by PCR using pre-validated RT2 qPCR primers (Qiagen, Frederick, MD) for detection of transcripts encoding human MMP14, ADAM17, γ-secretase (PSEN1, PSEN2), and 18S rRNA. All qPCR reagents were from Qiagen. Thermal cycling was performed using the StepOnePlus qPCR system (ThermoFisher Scientific). The 2−ΔΔCtt method was used to assess the amplitude of changes in gene expression (12).
Co-immunoprecipitation Assays
Ad-NEU1-FLAG-infected airway ECs (1.2 × 106 ECs/well) were incubated with P. aeruginosa (m.o.i. = 100), 10 ng/ml flagellin, or medium alone. The ECs were lysed and the lysates incubated with anti-MUC1 antibody (12). Immune complexes were immobilized on protein G-agarose, washed, resolved by SDS-PAGE, and processed for FLAG (NEU1) immunoblotting.
Lectin Blotting Assays
Airway ECs infected with Ad constructs or transfected with siRNAs were lysed and the lysates immunoprecipitated with anti-MUC1 antibody. The MUC1 immunoprecipitates were analyzed by MAL, SNA, or PNA lectin blotting as described (12). In other experiments, airway ECs and ECs transfected with NEU1-targeting or control siRNAs were incubated with flagellin or medium alone, washed, and lysed. The lysates were incubated with PNA-agarose and bound proteins processed for MUC1 immunoblotting.
Enzyme-linked Immunosorbent Assays (ELISAs)
A549 cells were incubated for 6 h with 10 ng/ml flagellin or medium alone, and culture supernatants were processed for IL-8 ELISA as described (28). For MUC1-ED ELISA, airway EC supernatants and BALFs were added to ELISA plates, and wells were blocked with PBS, pH 7.0, containing 10 mg/ml BSA, and washed with PBS containing 0.05% Tween 20 (PBS-T). The wells were incubated with anti-MUC1 antibody, washed with PBS-T, and incubated with peroxidase-conjugated goat anti-mouse IgG antibody. Bound antibodies were detected with tetramethylbenzidine substrate at A450. MUC1 levels were calculated from a standard curve of serial dilutions of known concentrations of purified MUC1.
BAL
BAL was performed on mechanically ventilated patients who underwent standard of care diagnostic bronchoscopy using a modification of our previously described procedure (29). The study was approved by the University of Maryland Institutional Review Board (protocol number HP-00059183). After conscious sedation with fentanyl and midazolam and local anesthesia with 2% lidocaine, the bronchoscope was wedged in a 3rd or 4th order bronchus, after which 125 ml of sterile, pyrogen-free 0.9% NaCl was injected in 25-ml aliquots. The BALF was retrieved with gentle suction and pooled; the volume was recorded and transported to the laboratory on ice. The BALF was filtered through sterile gauze, centrifuged at 450 × g to remove cells, and the supernatants concentrated 25-fold by passage through membrane filters (pore size, 100 kDa) mounted in Centricon tubes (Millipore). Single use BALF aliquots were stored at −70 °C. To identify microbial cultures, the BALF was plated onto culture media in the following order as described (30): CHROMagar Acinetobacter, trypticase soy agar with 5% sheep's blood, chocolate agar, and MacConkey agar. BALF MUC1-ED levels were quantified by ELISA as described above.
Statistical Analysis
All values were expressed as means ± S.E. Differences between means were compared using the Student's t test and considered significant at p < 0.05.
Results
NEU1 Overexpression Selectively Enhances MUC1-dependent P. aeruginosa Adhesion to and Invasion of Airway ECs
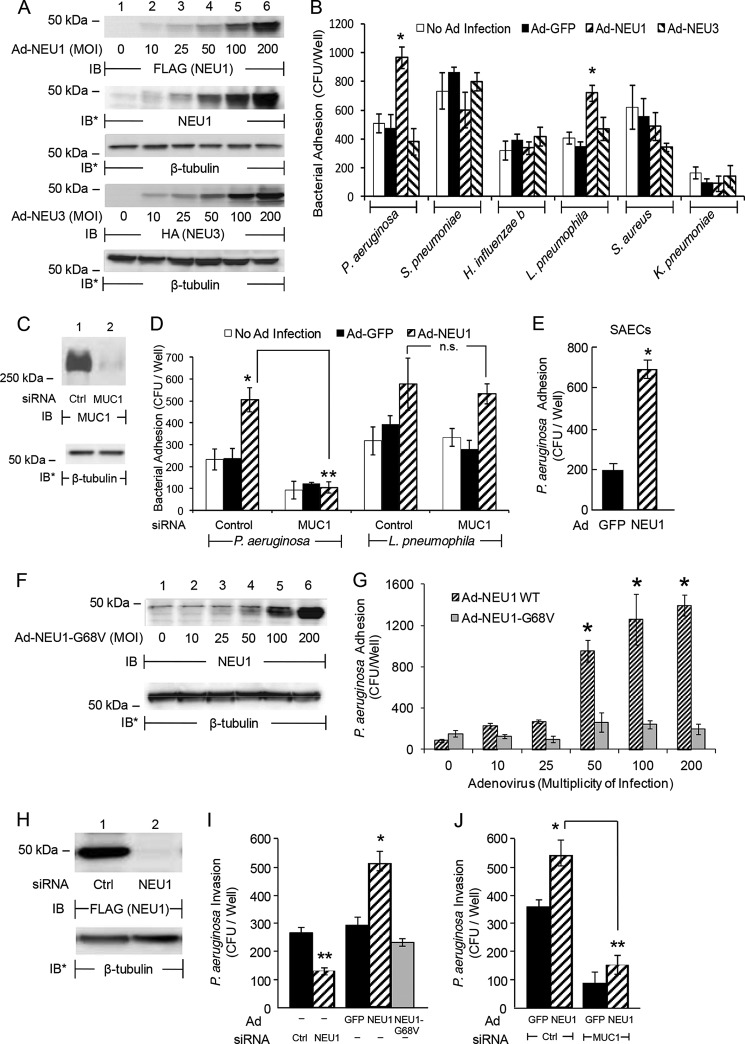
NEU1 regulates P. aeruginosa adhesion to primary human airway ECs (12). We asked whether NEU1 and/or NEU3 might regulate airway EC adhesiveness for other respiratory pathogens. NEU1 (Fig. 1A, upper 3 panels) and NEU3 (Fig. 1A, lower 2 panels) were each overexpressed in primary SAECs and A549 cells using recombinant Ad. Ad-NEU1 infection of A549 cells enhanced the adhesion of P. aeruginosa and L. pneumophila by 2.0- and 1.8-fold, respectively, compared with adhesion to Ad-GFP-infected ECs (Fig. 1B). These P. aeruginosa adhesion results are quantitatively comparable with prior control baseline P. aeruginosa adhesion in the absence of NEU1 manipulation (31, 32), as well as to increased P. aeruginosa adhesion following NEU1 overexpression (12). NEU1-mediated increases in adhesion was not observed for S. pneumoniae, H. influenzae, S. aureus, or K. pneumoniae. NEU3 overexpression did not influence adhesion of any bacteria tested (Fig. 1B). MUC1 silencing (Fig. 1C) completely protected against the NEU1-mediated increase in adhesion of P. aeruginosa but not of L. pneumophila (Fig. 1D). NEU1 overexpression also enhanced P. aeruginosa adhesion to primary SAECs 3.4-fold compared with Ad-GFP-infected SAECs (Fig. 1E). Infection with Ad-NEU1, but not with Ad-NEU1-G68V that encodes for a catalytically inert NEU1 mutant (Fig. 1F) (33), increased P. aeruginosa adhesion to airway ECs in a dose-dependent manner (Fig. 1G). P. aeruginosa adhesion to airway ECs is a prerequisite to their invasion (9). Prior NEU1 silencing (Fig. 1H) diminished P. aeruginosa invasion of airway ECs compared with control siRNA-transfected ECs (Fig. 1I). In contrast, Ad-NEU1 infection enhanced P. aeruginosa invasion compared with either Ad-GFP- or Ad-NEU1-G68V-infected ECs (Fig. 1I). MUC1 silencing (Fig. 1C) reduced NEU1-dependent P. aeruginosa invasion compared with control siRNA-transfected ECs (Fig. 1J). Together, these data indicate the following: 1) P. aeruginosa binds to MUC1, and the P. aeruginosa-MUC1 interaction is influenced by NEU1; 2) NEU1-augmented bacterial adhesion to MUC1 is specific for P. aeruginosa; 3) L. pneumophila adhesion is also NEU1-dependent but not MUC1-dependent; 4) NEU1 catalytic activity is required for its ability to influence MUC1-dependent P. aeruginosa adhesion; and finally 5) NEU1 increases MUC1-dependent P. aeruginosa invasion of airway ECs.
FIGURE 1.
NEU1 overexpression selectively enhances MUC1-dependent P. aeruginosa adhesion to and invasion of airway ECs. A, A549 cells were infected with Ad-NEU1-FLAG or Ad-NEU3-HA at the indicated m.o.i., cultured for 48 h, and lysed. The lysates were processed for FLAG (NEU1) or HA (NEU3) immunoblotting. To demonstrate ectopically overexpressed NEU1 levels relative to endogenous NEU1 expression, the FLAG (NEU1) blot (top panel) was stripped and reprobed with anti-NEU1 antibody (2nd panel). B, A549 cells and A549 cells infected with Ad-GFP, Ad-NEU1-FLAG, or Ad-NEU3-HA (m.o.i. = 100) were cultured for 48 h, fixed, washed, and incubated for 30 min with each of the indicated bacteria (m.o.i. = 100). Nonadherent bacteria were removed by washing, and CFUs bound to the ECs were quantified. C, A549 cells were transfected with MUC1-targeting or control siRNAs, cultured for 48 h, and lysed. The lysates were processed for MUC1 immunoblotting. D, A549 cells and A549 cells infected with Ad-GFP or Ad-NEU1 (m.o.i. = 100) were cultured for 24 h. The ECs were transfected with MUC1-targeting or control siRNAs and cultured for an additional 48 h. Adhesion of P. aeruginosa and L. pneumophila to the ECs was assayed. E, P. aeruginosa adhesion to SAECs infected for 48 h with Ad-GFP or Ad-NEU1 (m.o.i. = 100) was determined. F, A549 cells infected with increasing m.o.i. of Ad-NEU1-G68V were cultured for 48 h, lysed, and the lysates processed for NEU1 immunoblotting. G, P. aeruginosa adhesion to A549 cells infected for 48 h with increasing m.o.i. of Ad-NEU1 wild type (WT) or Ad-NEU1-G68V was assayed. H, A549 cells were infected with Ad-NEU1-FLAG (m.o.i. = 100) and cultured for 24 h. The ECs were transfected with NEU1-targeting or control siRNAs, cultured for an additional 48 h, and lysed, and the lysates were processed for FLAG (NEU1) immunoblotting. I, A549 cells were transfected with NEU1-targeting or control siRNAs or infected with Ad-NEU1, Ad-NEU1-G68V, or Ad-GFP (m.o.i. = 100) and cultured for 48 h. The ECs were incubated for 1 h with P. aeruginosa (m.o.i. = 100), washed, incubated for 1 h with 200 μg/ml gentamicin, and lysed, and CFUs were quantified. J, A549 cells infected with Ad-GFP or Ad-NEU1 (m.o.i. = 100) were cultured for 24 h and transfected with MUC1-targeting or control siRNAs. After 48 h, P. aeruginosa invasion was quantified. A, C, F, and H, to control for loading and transfer, blots were stripped and reprobed for β-tubulin. IB, immunoblot. IB*, immunoblot after stripping. Molecular mass in kDa is indicated on the left. Each blot is representative of three independent experiments. B, D, E, G, I, and J, bars represent mean ± S.E. CFUs/well (n = 4). *, increased bacterial adhesion to or invasion of Ad-NEU1-infected ECs compared with Ad-GFP, Ad-NEU3, or Ad-NEU1-G68V at p < 0.05. **, decreased P. aeruginosa adhesion to or invasion of MUC1 or NEU1 siRNA-transfected ECs compared with adhesion to or invasion of control siRNA-transfected ECs at p < 0.05.
Flagellin Is Required for NEU1-responsive P. aeruginosa Adhesion and Invasion
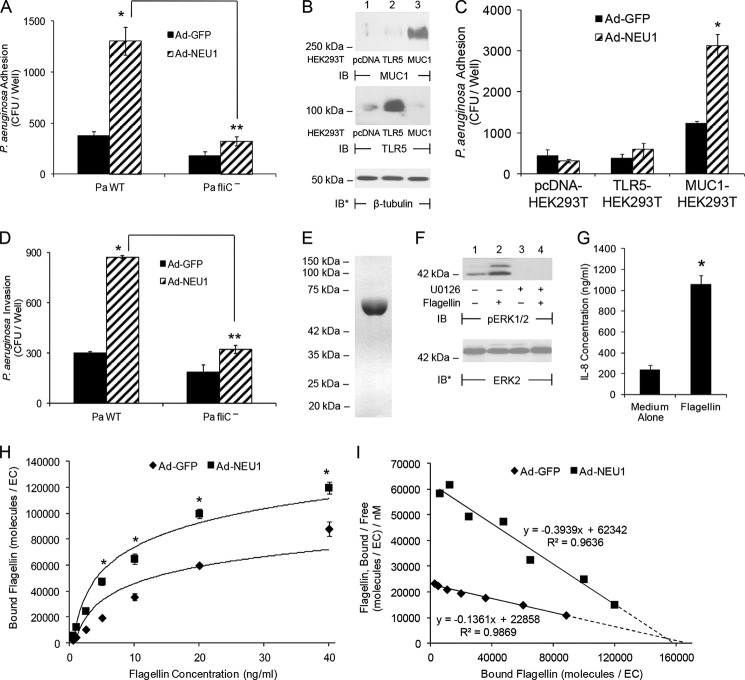
Flagellin is necessary for P. aeruginosa adhesion to airway ECs (26), and NEU1 promotes this adhesion (Fig. 1, B, D, E, and G) (12). Because P. aeruginosa expresses multiple EC adhesins (9), we asked whether flagellin is required for NEU1-responsive P. aeruginosa adhesion, and if so, might NEU1 increase the binding of flagellin itself to airway ECs. NEU1 overexpression of airway ECs increased the adhesion of flagellin-expressing P. aeruginosa but not that of the flagellin-deficient P. aeruginosa fliC− isogenic mutant (Fig. 2A). Because P. aeruginosa flagellin is an established ligand for TLR5 (11), we examined whether TLR5 contributes to the NEU1 effect on P. aeruginosa adhesion. Given that primary SAECs and A549 cells both co-express MUC1 and TLR5 (13), HEK293T cells that express extremely low or undetectable endogenous MUC1 or TLR5 were individually transfected for MUC1 expression (Fig. 2B, upper panel) or for TLR5 expression (Fig. 2B, middle panel). Infection of MUC1-expressing HEK293T cells with Ad-NEU1 increased P. aeruginosa adhesion compared with Ad-GFP-infected cells, whereas Ad-NEU1 infection of TLR5-expressing HEK293T cells did not (Fig. 2C). Because flagellin is required for NEU1-regulated P. aeruginosa adhesion to airway ECs (Fig. 2A), we tested whether flagellin is also required for NEU1-responsive P. aeruginosa invasion. NEU1 overexpression increased invasion of flagellin-expressing P. aeruginosa but not P. aeruginosa fliC− (Fig. 2D). Finally, we investigated whether NEU1 promotes the binding of purified flagellin to airway ECs. Our flagellin preparation migrated as a single band on Coomassie Blue-stained SDS-polyacrylamide gels (Fig. 2E) and elicited two previously established P. aeruginosa flagellin-stimulated activities, extracellular signal-regulated kinase 1/2 (ERK1/2) activation (Fig. 2F, lane 2 versus 1) (34) and IL-8 synthesis (Fig. 2G) (28). Infection of airway ECs with Ad-NEU1 increased the binding of Alexa Fluor 594-labeled flagellin compared with Ad-GFP-infected controls (Fig. 2H). Scatchard analysis of the binding data revealed a 2.9-fold reduction in the dissociation constant (Kd) for flagellin binding to Ad-NEU1-infected ECs (Kd = 2.54 nm) compared with Ad-GFP-infected ECs (Kd = 7.35 nm) (Fig. 2I). The sialylation state of a cell surface receptor can regulate its surface residency (35). However, the number of flagellin-binding sites on ECs infected with the two Ad constructs were comparable as indicated by extrapolation of the best fit binding curves to the abscissa (Ad-NEU1, 158,000 sites/EC; Ad-GFP, 168,000 sites/EC) (Fig. 2I, dashed lines). These data indicate that flagellin is the P. aeruginosa adhesin that participates in NEU1-responsive P. aeruginosa adhesion to and invasion of MUC1-expressing ECs and that NEU1 promotes flagellin binding to the ECs through increased binding affinity of its receptor, MUC1, not through increased receptor number. Although the binding affinity of monomeric flagellin for airway ECs was clearly increased by NEU1 expression, the magnitude of this effect with P. aeruginosa flagella is likely greater than that measured here given the clustering/density-related polyvalent binding interaction between the multimeric P. aeruginosa flagellum and the multiple tandem repeats of MUC1.
FIGURE 2.
NEU1 increases flagellin-dependent P. aeruginosa adhesion to and invasion of MUC1-expressing ECs. A, A549 cells were infected with Ad-GFP or Ad-NEU1 (m.o.i. = 100), cultured for 48 h, and adhesion of P. aeruginosa WT or P. aeruginosa fliC− quantified. B, HEK293T cells transfected with plasmids encoding for MUC1, TLR5, or the pcDNA empty vector control were cultured for 48 h and lysed. Lysates were processed for MUC1 (upper panel) or TLR5 (middle panel) immunoblotting (IB). C, HEK293T cells transfected with plasmids encoding for MUC1 or TLR5, or the empty vector control, were cultured for 24 h and infected with Ad-GFP or Ad-NEU1 (m.o.i. = 100). After 48 h, P. aeruginosa WT adhesion was quantified. D, A549 cells were infected with Ad-GFP or Ad-NEU1 (m.o.i. = 100), cultured for 48 h, and invasion of P. aeruginosa WT or P. aeruginosa fliC− quantified. A, C, and D, bars represent mean ± S.E. CFUs/well (n = 4). *, increased P. aeruginosa WT adhesion to or invasion of Ad-NEU1-infected ECs compared with Ad-GFP-infected controls at p < 0.05. **, decreased adhesion or invasion of P. aeruginosa fliC− compared with adhesion or invasion of P. aeruginosa WT at p < 0.05. E, Coomassie Blue-stained SDS-polyacrylamide gel of purified P. aeruginosa flagellin. F, A549 cells were preincubated for 30 min with 5.0 μm U0126 or medium alone, washed, incubated for 30 min with 10 ng/ml P. aeruginosa flagellin or medium alone, lysed, and the lysates processed for phospho-ERK1/2 immunoblotting. To control for loading and transfer, the blots were stripped and reprobed for total ERK2. G, A549 cells were incubated for 6 h with 10 ng/ml flagellin or medium alone and culture supernatants processed for IL-8 ELISA. Bars represent mean ± S.E. IL-8 ng/ml (n = 3). *, increased IL-8 concentrations compared with simultaneous medium control at p < 0.05. H, A549 cells were infected with Ad-GFP or Ad-NEU1 (m.o.i. = 100), cultured for 48 h, and binding of increasing concentrations of Alexa Fluor 594-flagellin for 40 min at 4 °C was determined. Data points represent mean ± S.E. of bound flagellin (n = 3). I, Scatchard analysis of the binding data in H. The equation and R2 value are indicated adjacent to each line. Extrapolations to the x axis intercepts as an estimation of the number of binding sites per cell are indicated by broken lines. The results are representative of three independent experiments.
Flagellin Stimulates NEU1 Association with MUC1
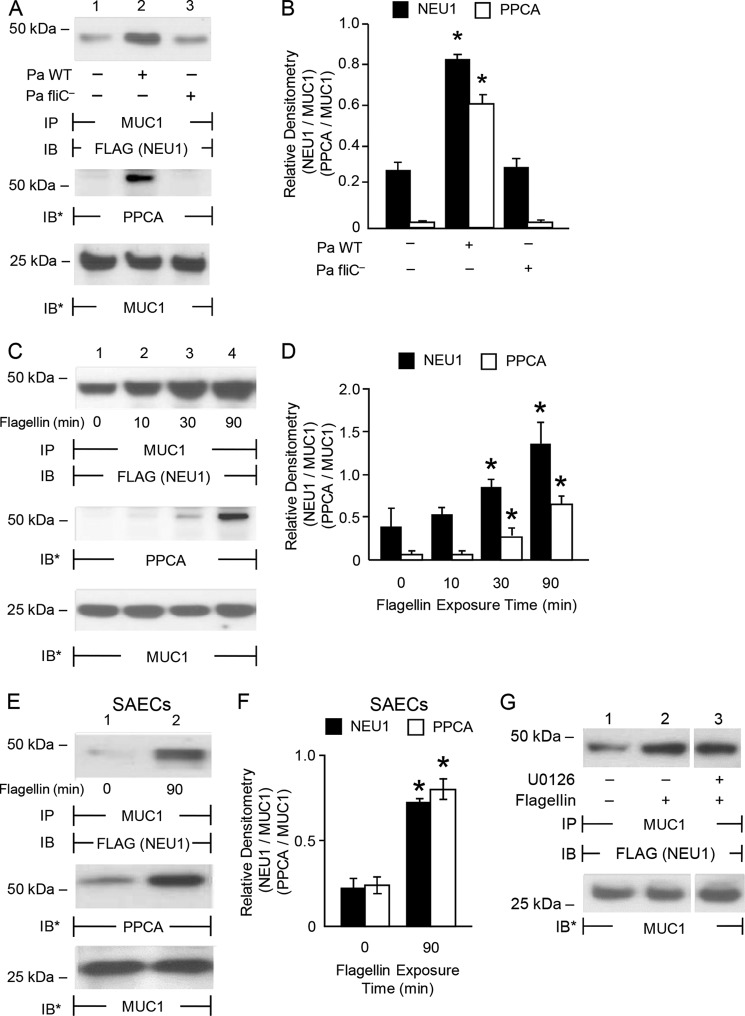
Forced NEU1 overexpression increases adhesion of flagellin-expressing P. aeruginosa to airway ECs (Figs. 1, B, D, E, and G, and 2A). We asked whether engagement of MUC1 by P. aeruginosa flagellin up-regulates NEU1 expression and/or catalytic activity. Flagellin stimulation of airway ECs failed to alter NEU1 mRNA or protein levels or the catalytic activity for the fluorogenic substrate, 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid, compared with the simultaneous medium controls (data not shown). We then hypothesized that flagellin induces the association of a preformed pool of NEU1 to MUC1. Incubation of airway ECs for 30 min with flagellin-expressing P. aeruginosa, but not with the P. aeruginosa fliC− mutant, increased MUC1-NEU1 co-immunoprecipitation compared with the simultaneous medium controls (Fig. 3, A, upper panel, and B). Flagellin-expressing P. aeruginosa also increased MUC1-PPCA association compared with P. aeruginosa fliC− (Fig. 3, A, middle panel, and B). In A549 cells (Fig. 3, C and D) and primary SAECs (Fig. 3, E and F), flagellin increased MUC1-NEU1 (Fig. 3, C, E, upper panels, D, and F) and MUC1-PPCA (Fig. 3, C, E, middle panels, D, and F) association within 30 min compared with the simultaneous medium controls. Because flagellin-induced ERK1/2 activation (Fig. 2F, lane 2 versus 1) temporally coincided with MUC1-NEU1 association (Fig. 3, C–F), we tested whether MUC1-NEU1 association requires ERK1/2 activation. Preincubation of airway ECs with an inhibitory concentration of the MEK1/2 inhibitor, U0126 (Fig. 2F, lane 4 versus 2), failed to alter MUC1-NEU1 co-immunoprecipitation (Fig. 3G). These data indicate that flagellin rapidly stimulates the association of a preformed pool of NEU1, accompanied by PPCA, to MUC1 in an ERK1/2-independent manner.
FIGURE 3.
Flagellin increases NEU1 association with MUC1. A, A549 cells infected with Ad-NEU1-FLAG (m.o.i. = 100) were cultured for 48 h, incubated for 30 min with P. aeruginosa WT or P. aeruginosa fliC− bacteria (m.o.i. = 100), or medium alone, and lysed. The lysates were immunoprecipitated with anti-MUC1 antibody and the immunoprecipitates processed for FLAG (NEU1) immunoblotting (IB) (upper panel). The blots were stripped and reprobed for PPCA (middle panel) or MUC1 (lower panel). B, densitometric analyses of the blots in A. C, A549 cells and E, SAECs infected with Ad-NEU1-FLAG (m.o.i. = 100) were cultured for 48 h, incubated for the indicated times with 10 ng/ml P. aeruginosa flagellin or medium alone, and lysed. The lysates were immunoprecipitated with anti-MUC1 antibody and the immunoprecipitates processed for FLAG (NEU1) immunoblotting (upper panel). The blots were stripped and reprobed for PPCA (middle panel) followed by MUC1 (lower panel). D and F, densitometric analyses of the blots in C and E, respectively. *, increased normalized FLAG (NEU1)/PPCA signal of P. aeruginosa WT-stimulated ECs compared with P. aeruginosa fliC−, or flagellin-stimulated ECs compared with medium controls, at p < 0.05. G, A549 cells infected with Ad-NEU1-FLAG (m.o.i. = 100) were preincubated for 30 min with 5.0 μm U0126 or medium alone, washed, incubated for 30 min with 10 ng/ml P. aeruginosa flagellin or medium alone, and lysed. The lysates were immunoprecipitated with anti-MUC1 antibody and the immunoprecipitates processed for FLAG (NEU1) immunoblotting. A, C, E, and G, to control for loading and transfer, blots were stripped and reprobed for MUC1. IP, immunoprecipitation. Each blot is representative of three independent experiments. B, D, and F, bars represent mean ± S.E. FLAG (NEU1) or PPCA signal normalized to MUC1 signal in the same lane on the same stripped and reprobed blot (n = 3).
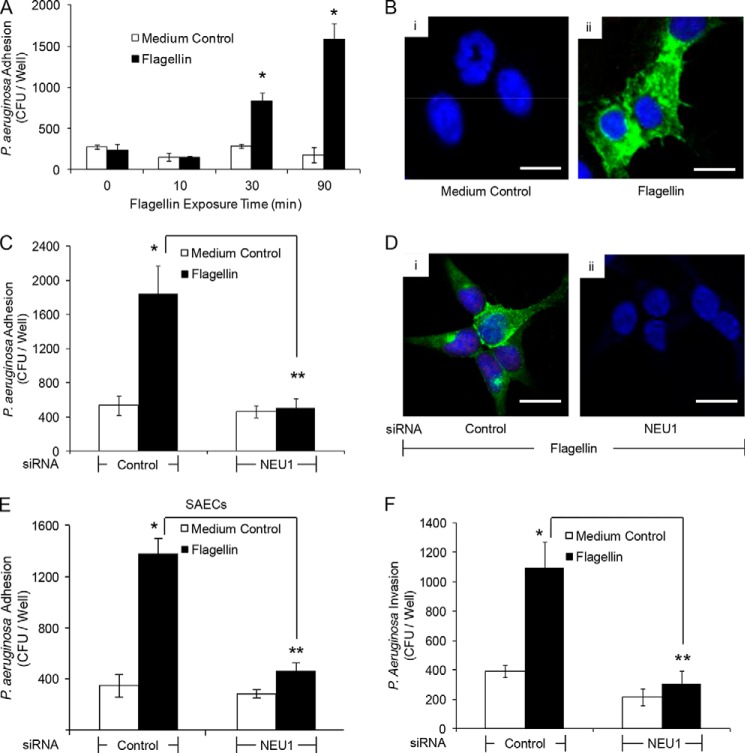
Flagellin Increases NEU1-dependent MUC1-ED Desialylation, P. aeruginosa Adhesion, and Invasion
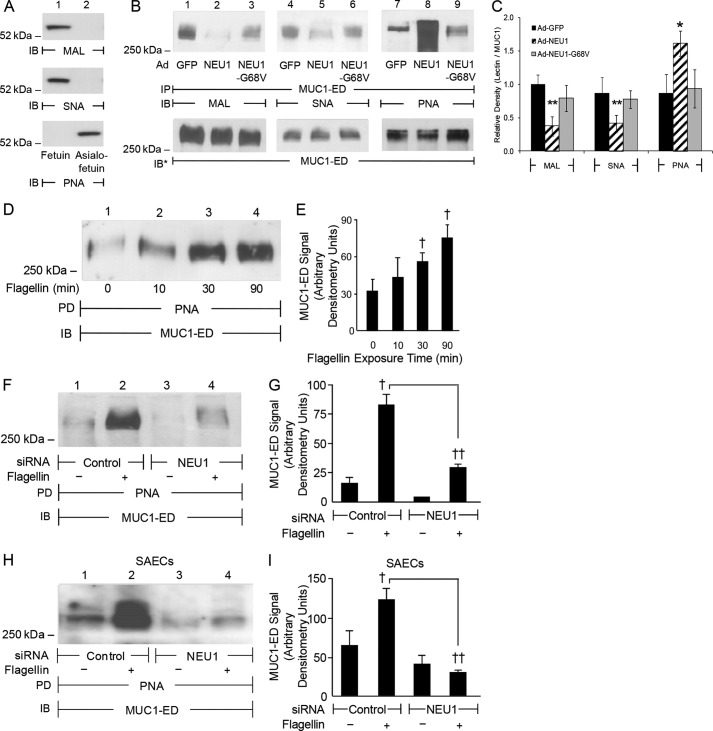
Because NEU1 overexpression increases MUC1 adhesiveness for P. aeruginosa (Figs. 1, B, D, E, and G, and 2, A and C) and its flagellin (Fig. 2, H and I), and because MUC1 is sialylated on its ED (13), we next tested whether MUC1-ED is a NEU1 substrate. Infection of airway ECs with Ad-NEU1 diminished the binding of MUC1-ED with MAL and SNA, which recognize α2,3- and α2,6-linked Sia residues, respectively, compared with Ad-GFP-infected ECs (Fig. 4, B, lanes 2 versus 1, and C, lanes 5 versus 4) and Ad-NEU1-G68V-infected ECs (Fig. 4B, lanes 2 versus 3, and C, lanes 5 versus 6). Conversely, NEU1 overexpression increased the binding of MUC1-ED with PNA, which recognizes subterminal galactose after removal of terminal Sia, compared with Ad-GFP-infected ECs (Fig. 4, B, lanes 8 versus 7, and C) and Ad-NEU1-G68V-infected ECs (Fig. 4, B, lanes 8 versus 9, and C). Because flagellin promotes MUC1-NEU1 association (Fig. 3, A–G) and NEU1 overexpression desialylates MUC1-ED (Fig. 4, B and C), we asked whether flagellin increases NEU1-mediated MUC1-ED desialylation. Incubation of airway ECs for 30 or 90 min with flagellin enhanced PNA binding to MUC1-ED compared with medium alone (Fig. 4, D and E). NEU1 knockdown (Fig. 1H) protected against flagellin-stimulated MUC1-ED desialylation in A549 cells (Fig. 4, F, lanes 4 versus 2, and G) and SAECs (Fig. 4, H, lanes 4 versus 2, and I) compared with control siRNA-transfected ECs. Because flagellin increases NEU1-mediated MUC1-ED desialylation (Fig. 4, D–I), we postulated that flagellin increases P. aeruginosa adhesion to and invasion of airway ECs. Flagellin stimulation of airway ECs rapidly (≤30 min) increased P. aeruginosa adhesion compared with medium alone (Fig. 5, A and B). NEU1 silencing protected against the flagellin-induced increase in P. aeruginosa adhesion compared with control siRNA-transfected A549 cells (Fig. 5, C and D) and SAECs (Fig. 5E). Stimulation of airway ECs with flagellin increased P. aeruginosa invasion in a NEU1-dependent manner compared with controls (Fig. 5F). These results indicate the following: 1) in its endogenous state, MUC1-ED contains α2,3- and α2,6-linked Sia as well as subterminal galactose; 2) overexpression of catalytically active NEU1 and flagellin treatment each desialylates MUC1-ED; and 3) NEU1-dependent MUC1-ED desialylation increases P. aeruginosa adhesion to and invasion of airway ECs.
FIGURE 4.
Flagellin increases NEU1-dependent MUC1-ED desialylation. A, fetuin and asialofetuin (1.0 μg) were processed for MAL, SNA, and PNA lectin blotting as controls to validate lectin specificity. B, A549 cells infected with Ad-GFP, Ad-NEU1, or Ad-NEU1-G68V (m.o.i. = 100) were cultured for 48 h and lysed. The lysates were immunoprecipitated (IP) with anti-MUC1 antibody and the immunoprecipitates processed for MAL (lanes 1–3), SNA (lanes 4–6), or PNA (lanes 7–9) lectin blotting. To control for loading and transfer, blots were stripped and reprobed for MUC1. C, densitometric analyses of the blots in B. Bars represent mean ± S.E. lectin signal normalized to MUC1 signal in the same lane in the same stripped and reprobed blot (n = 3). *, increased normalized PNA signal of Ad-NEU1-infected ECs compared with Ad-GFP or Ad-NEU1-G68V at p < 0.05. **, decreased normalized MAL/SNA signal of Ad-NEU1-infected ECs compared with Ad-GFP or Ad-NEU1-G68V-infected at p < 0.05. D, A549 cells were incubated for the indicated times with 10 ng/ml P. aeruginosa flagellin or medium alone and lysed. The lysates were incubated with PNA-agarose and the PNA-binding proteins processed for MUC1 immunoblotting (IB). E, densitometric analyses of the blots in D. F and H, A549 cells (F) and SAECs (H) transfected with NEU1-targeting or control siRNAs were cultured for 48 h, incubated for 30 min with 10 ng/ml P. aeruginosa flagellin or medium alone, and lysed. The lysates were incubated with PNA-agarose, and the PNA-binding proteins processed for MUC1 immunoblotting. G and I, densitometric analysis of the blots in F and H, respectively. PD, PNA pulldown. E, G, and I, bars represent mean ± S.E. MUC1 signal (n = 3). †, increased MUC1 signal of flagellin-stimulated ECs compared with the medium control at p < 0.05. ††, decreased MUC1 signal of NEU1 siRNA-transfected ECs compared with control siRNA-transfected ECs at p < 0.05. The results are representative of three independent experiments.
FIGURE 5.
NEU1 is required for flagellin-induced increases in P. aeruginosa adhesion and invasion. A, A549 cells were incubated for increasing times with 10 ng/ml P. aeruginosa flagellin or medium alone, washed, and P. aeruginosa adhesion quantified. B, A549 cells were incubated for 30 min with medium alone (panel i) or 10 ng/ml P. aeruginosa flagellin (panel ii). The ECs were washed, incubated for 40 min with GFP-P. aeruginosa (m.o.i. = 100), washed, counterstained with DAPI, and processed for fluorescence microscopy. C, A549 cells transfected with NEU1-targeting or control siRNAs were cultured for 48 h, incubated for 30 min with 10 ng/ml P. aeruginosa flagellin or medium alone, and P. aeruginosa adhesion was quantified. D, A549 cells transfected with control (panel i) or NEU1-targeting (panel ii) siRNAs were cultured for 48 h and incubated for 30 min with 10 ng/ml P. aeruginosa flagellin. The ECs were incubated with GFP-P. aeruginosa, washed, counterstained with DAPI, and processed for fluorescence microscopy. E, SAECs transfected with NEU1-targeting or control siRNAs were cultured for 48 h, incubated for 30 min with 10 ng/ml P. aeruginosa flagellin or medium alone, and P. aeruginosa adhesion was quantified. F, A549 cells transfected with NEU1-targeting or control siRNAs were cultured for 48 h, incubated for 30 min with 10 ng/ml P. aeruginosa flagellin or medium alone, and P. aeruginosa invasion was quantified. A, C, E, and F, bars represent mean ± S.E. CFUs/well (n = 4). *, increased P. aeruginosa adhesion to or invasion of flagellin-stimulated ECs compared with the medium controls at p < 0.05. **, decreased P. aeruginosa adhesion to or invasion of NEU1-targeting siRNA-transfected ECs compared with control siRNA-transfected ECs at p < 0.05. B and D, scale bars, 25 μm. The results are representative of three independent experiments.
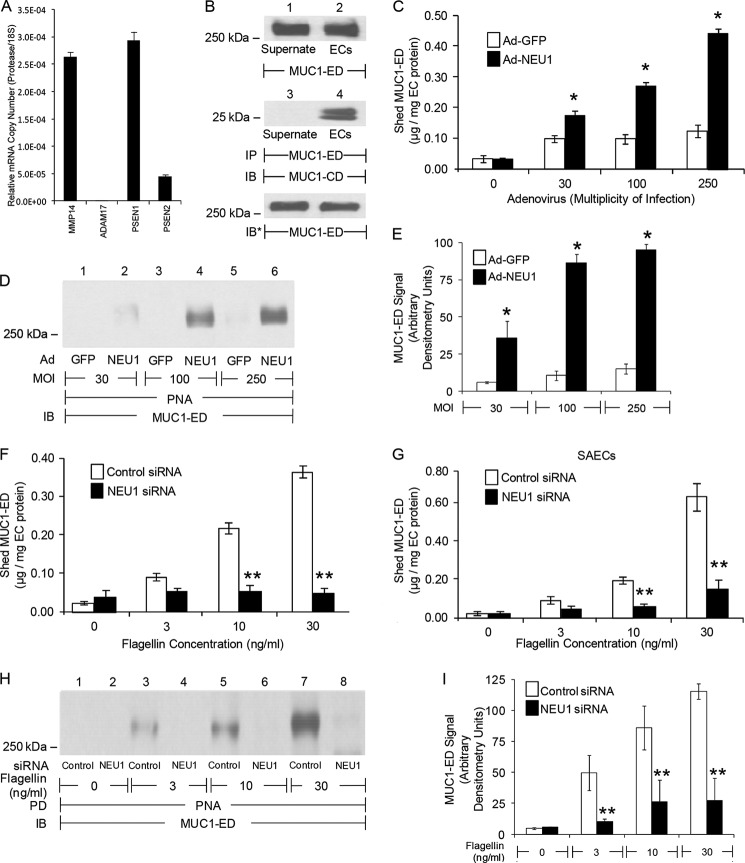
Flagellin Increases NEU1-dependent MUC1-ED Shedding
Three MUC1-ED sheddases have been identified in extrapulmonary tissue, MMP14, ADAM17, and γ-secretase (14–16). We asked whether one or more of these proteases might be expressed in human airway ECs. Transcripts for MMP14 and γ-secretase, but not ADAM17, were detected (Fig. 6A). Furthermore, MUC1-ED was shed into culture supernatants of airway ECs (Fig. 6B, top panel). Co-immunoprecipitation assays using antibodies against the noncovalently associated MUC1-ED and MUC1-cytoplasmic domain (CD) demonstrated that shed MUC1-ED is not associated with MUC1-CD, thereby ruling out nonspecific release of full-length MUC1 from dying ECs (Fig. 6B, middle panel). NEU1 overexpression (Fig. 4, B and C) and flagellin stimulation (Fig. 4, D–I) each desialylate the MUC1-ED. Because Sia residues can mask protease recognition sites (36), we inquired whether the same experimental conditions that promote MUC1-ED desialylation might also increase its shedding. Both NEU1 overexpression (Fig. 6, C–E) and flagellin stimulation of A549 cells (Fig. 6, F, H, and I) and SAECs (Fig. 6G) increased shedding of desialylated MUC1-ED into airway EC culture supernatants over 24 h in a dose-dependent manner compared with Ad-GFP-infected ECs and medium controls, respectively. NEU1 silencing protected against flagellin-induced increases in shed MUC1-ED desialylation (Fig. 6, H and I) and MUC1-ED shedding in A549 cells (Fig. 6F) and SAECs (Fig. 6G) compared with control siRNA-transfected ECs. Therefore, the identical experimental conditions that promote NEU1-mediated MUC1-ED desialylation also provoke its shedding.
FIGURE 6.
Flagellin stimulates NEU1-dependent MUC1-ED shedding. A, qRT-PCR for MMP14, ADAM17, and γ-secretase (PSEN1 and PSEN2) transcripts. The mRNA levels for each protease were normalized to the 18S rRNA internal control. Bars represent mean ± S.E. normalized mRNA levels (n = 2). B, A549 culture supernatants and EC lysates were processed for MUC1-ED immunoblotting (IB) (top panel) or were immunoprecipitated (IP) with anti-MUC1-ED antibody, and the immunoprecipitates were processed for MUC1-CD immunoblotting (middle panel). To control for loading and transfer, the blot was stripped and reprobed with anti-MUC1-ED antibody (bottom panel). C, A549 cells were infected with increasing m.o.i. of Ad-GFP or Ad-NEU1 and incubated for 24 h. MUC1-ED levels in EC culture supernatants were quantified by ELISA and normalized to total EC protein. D, culture supernatants (100 μl) were incubated with PNA-agarose and the PNA-binding proteins processed for MUC1-ED immunoblotting. E, densitometric analyses of the blots in D. A549 cells (F, H, and I) and SAECs (G) were transfected with NEU1-targeting or control siRNAs and cultured for 24 h. The transfected ECs were incubated for 30 min with increasing concentrations of P. aeruginosa flagellin, or medium alone, washed, and incubated for 24 h. F and G, culture supernatants were processed for MUC1-ED ELISA. H, supernatants (100 μl) from flagellin-stimulated A549 cells were incubated with PNA-agarose and the PNA-binding proteins processed for MUC1-ED immunoblotting. I, densitometric analyses of the blots in H. C, F, and G, bars represent mean ± S.E. MUC1-ED levels normalized to total EC protein (n = 9). E and I, bars represent mean ± S.E. MUC1-ED signal (n = 3). *, increased MUC1-ED level or signal in supernatants from Ad-NEU1-infected ECs compared with Ad-GFP, or flagellin-stimulated ECs compared with medium control, at p < 0.05; **, decreased MUC1-ED level or signal in supernatants from NEU1 siRNA-transfected compared with control siRNA-transfected ECs at p < 0.05. The results are representative of three independent experiments.
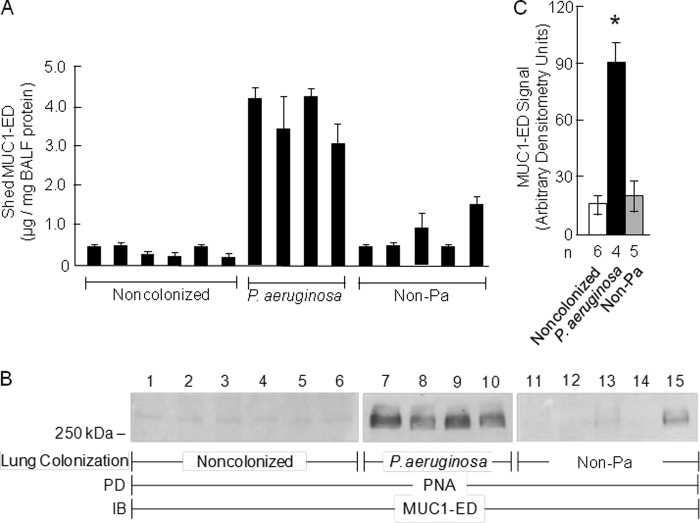
Levels of Desialylated MUC1-ED Are Increased in BALF from P. aeruginosa-colonized Patients
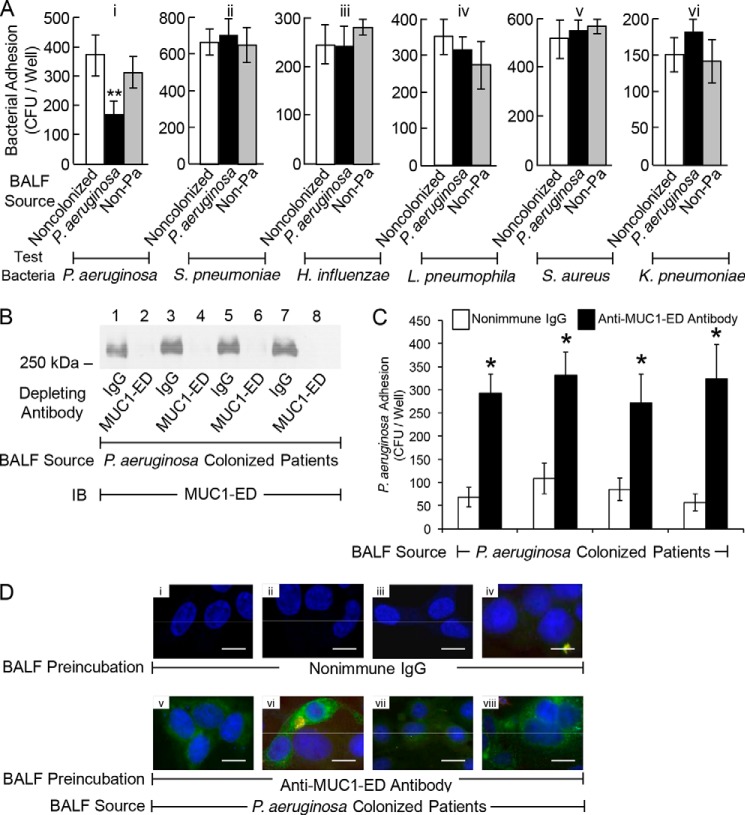
Because P. aeruginosa flagellin increases both MUC1-ED desialylation (Fig. 4, D–I) and its shedding (Fig. 6, F and G), we investigated whether MUC1-ED levels might be increased in BALF from patients with P. aeruginosa airway colonization. MUC1-ED levels were quantified by ELISA and normalized to total BALF protein, which ranged from 69 to 101 μg/ml. Mean MUC1-ED levels were 9.9-fold higher (range, 5.7–17.2-fold) in BALF from P. aeruginosa-colonized patients compared with levels in BALF from noncolonized subjects, and 5.5-fold greater (range, 2.5–9.6-fold) compared with levels in BALF from patients colonized with non-P. aeruginosa microorganisms (S. aureus, K. pneumoniae, Klebsiella oxytoca, Proteus mirabilis, or Candida albicans) (Fig. 7A). MUC1-ED in BALF from P. aeruginosa-colonized patients was desialylated compared with MUC1 ED in BALF from these same two control groups (Fig. 7, B and C). These data indicate that our in vitro results can be extended to in vivo human pathophysiology and that the increased MUC1-ED shedding is specific for P. aeruginosa airway colonization. Although LPS and other bacterial constituents reportedly alter NEU1 activity (37), our studies with the LPS-expressing P. aeruginosa fliC− mutant (Figs. 2, A and D, and 3, A and B), purified polymyxin B preadsorbed flagellin (Figs. 3, C–F, 4, D–I, 5, 6, H and I, and 8B), and BALFs harvested from patients colonized with bacteria other than P. aeruginosa (Figs. 7, A–C, and 9A) indicate that contribution of bacterial components other than P. aeruginosa-derived flagellin is unlikely. Given the dilutional effect inherent to the BAL procedure, these results may underestimate the true levels of desialylated MUC1-ED in the BALF of P. aeruginosa-colonized patients.
FIGURE 7.
Levels of desialylated MUC1-ED are increased in BALF from P. aeruginosa-colonized patients. A, MUC1-ED levels in BALF from noncolonized patients, P. aeruginosa-colonized patients, or patients colonized with non-P. aeruginosa microorganisms were quantified by ELISA and normalized to total BALF protein. B, equal protein aliquots (100 μg) of BALF from noncolonized patients, P. aeruginosa-colonized patients, or patients colonized with non-P. aeruginosa microorganisms were incubated with PNA-agarose and the PNA-binding proteins processed for MUC1-ED immunoblotting. C, densitometric analyses of the blots in B. Bars represent mean ± S.E. MUC1-ED signal (n = 3). The n for each condition is indicated. *, increased mean MUC1-ED levels or signals in BALF from P. aeruginosa-colonized patients compared with levels in BALF from noncolonized patients or patients colonized with non-P. aeruginosa (Non-Pa) microbes at p < 0.05. The results are representative of ≥2 independent experiments.
FIGURE 8.
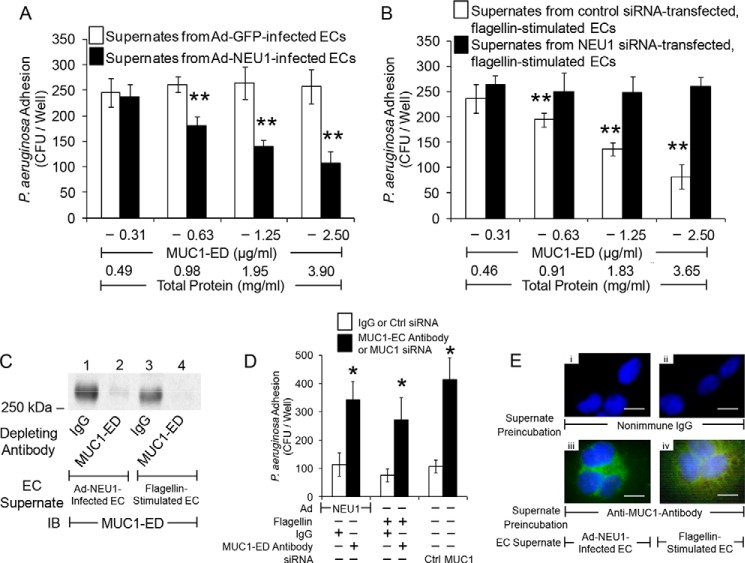
Desialylated MUC1-ED shed in response to flagellin competitively inhibits P. aeruginosa adhesion to EC-associated MUC1-ED. A, A549 cells were infected with Ad-GFP or Ad-NEU1 (m.o.i. = 250) and cultured for 24 h, and EC culture supernatants were collected and protein concentrations measured. P. aeruginosa was preincubated for 30 min with supernatant from Ad-NEU1-infected ECs containing the indicated concentrations of MUC1-ED or supernatant from Ad-GFP-infected ECs containing an equivalent amount of total protein and assayed for adhesion to fresh A549 cell monolayers. B, A549 cells were transfected with NEU1-targeting or control siRNAs and incubated for 24 h. The ECs were incubated for 30 min with 30 ng/ml P. aeruginosa flagellin, cultured for 24 h, and EC culture supernatants collected, and protein concentrations were measured. P. aeruginosa was preincubated for 30 min with supernatant from control siRNA-transfected ECs containing the indicated concentrations of MUC1-ED, or supernatant from NEU1 siRNA-transfected ECs containing an equivalent amount of total protein, and assayed for adhesion to fresh A549 cell monolayers. **, decreased P. aeruginosa adhesion following preincubation with supernatants from Ad-NEU1-infected compared with Ad-GFP-infected ECs, or control siRNA-transfected compared with NEU1-targeting siRNA-transfected ECs at p < 0.05. C, supernatants of A549 cells infected with Ad-NEU1 or stimulated by flagellin, each containing 2.5 μg/ml MUC1-ED, were incubated with 100 μg/ml anti-MUC1-ED antibody or a nonimmune IgG control. Immunoglobulins were immobilized on protein G-agarose and removed by centrifugation, and the supernatants were processed for MUC1-ED immunoblotting (IB). D, P. aeruginosa was preincubated for 30 min with the supernatants in C, or supernatants of A549 cells transfected with MUC1-targeting or control siRNAs, and assayed for adhesion to A549 cells. *, increased P. aeruginosa adhesion following MUC1-ED depletion compared with preincubation with IgG control or MUC1-targeting siRNA-transfected compared with control siRNA-transfected ECs at p < 0.05. A, B, and D, each bar represents mean ± S.E. CFUs/well (n = 6). E, supernatants of A549 cells infected with Ad-NEU1 or stimulated by flagellin were incubated with the IgG control (panels i and ii) or depleted with anti-MUC1-ED antibody (panels iii and iv). Immunoglobulins were immobilized on protein G-agarose and removed by centrifugation. GFP-P. aeruginosa were preincubated for 30 min with the supernatants, washed, and incubated with A549 cells. The ECs were washed, counterstained with DAPI, and processed for fluorescence microscopy. Scale bar, 25 μm.
FIGURE 9.
Desialylated MUC1-ED in BALF from P. aeruginosa-colonized patients competitively inhibits P. aeruginosa adhesion to EC-associated MUC1-ED. A, indicated test bacteria were preincubated for 30 min with BALF from P. aeruginosa-colonized patients containing 2.5 μg/ml MUC1-ED or BALF from noncolonized patients or patients colonized with non-P. aeruginosa microorganisms containing an equivalent amount of BALF total protein. The bacteria were washed and assayed for adhesion to A549 cells. **, decreased bacterial adhesion following preincubation with BALF from P. aeruginosa-colonized patients compared with BALF from either noncolonized patients or patients colonized with non-P. aeruginosa microbes at p < 0.05. B, BALF from P. aeruginosa-colonized patients was incubated with anti-MUC1-ED antibody or a nonimmune IgG control. Immunoglobulins were immobilized on protein G-agarose and removed by centrifugation, and the BALF supernatants were processed for MUC1-ED immunoblotting. C, P. aeruginosa were preincubated for 30 min with the BALFs in B and assayed for adhesion to A549 cells. *, increased P. aeruginosa adhesion following MUC1-ED depletion compared with preincubation with IgG control. A and C, each bar represents mean ± S.E. CFUs/well (n = 6). The results are representative of ≥2 independent experiments. D, BALF from P. aeruginosa-colonized patients were incubated with the IgG control (panels i–iv) or depleted with anti-MUC1-ED antibody (panels v–viii). Immunoglobulins were immobilized on protein G-agarose and removed by centrifugation. GFP-P. aeruginosa were preincubated for 30 min with the BALFs, washed, and incubated with A549 cells. The ECs were washed, counterstained with DAPI, and processed for fluorescence microscopy. Scale bar, 25 μm.
Desialylated MUC1-ED Shed in Response to Flagellin Competitively Inhibits P. aeruginosa Adhesion to EC-associated MUC1-ED
NEU1 desialylates the MUC1-ED, rendering it hyperadhesive for P. aeruginosa at 30 min (Figs. 1, B, D, E, and G, 2, A and C, and 5, A–E), yet after 24 h it increases MUC1-ED shedding (Fig. 6, C, F, and G). To establish whether the shed, hyperadhesive MUC1-ED competitively inhibits P. aeruginosa adhesion to EC-associated MUC1-ED, P. aeruginosa was preincubated with supernatants of airway ECs infected with Ad-NEU1 prior to assaying for adhesion. A MUC1-ED dose-dependent reduction of bacterial adhesion to fresh EC monolayers was observed (Fig. 8A). Preincubation of P. aeruginosa with supernatants of ECs infected with Ad-GFP did not diminish adhesion. Preincubation with supernatants of flagellin-stimulated ECs blocked P. aeruginosa adhesion in a dose-dependent manner (Fig. 8B). Prior NEU1 silencing abolished the ability of supernatants of flagellin-stimulated ECs to reduce P. aeruginosa adhesion compared with supernatants of control siRNA-transfected ECs (Fig. 8B). Preincubation of supernatants of either Ad-NEU1-infected ECs or ECs stimulated by flagellin, with anti-MUC1-ED antibody, depleted the samples of detectable MUC1-ED (Fig. 8C) and reduced their ability to inhibit P. aeruginosa adhesion compared with supernatants preincubated with the same concentration of a species- and isotype-matched nonimmune IgG (Fig. 8, D and E). Transfection of airway ECs with MUC1 siRNA also profoundly diminished the ability of their supernatants to inhibit P. aeruginosa adhesion compared with supernatants from control siRNA-transfected ECs (Fig. 8D). These data indicate that MUC1-ED shed in vitro in response to flagellin competitively inhibits P. aeruginosa adhesion to MUC1-ED-expressing airway ECs.
Desialylated MUC1-ED in BALF from P. aeruginosa-colonized Patients Competitively Inhibits P. aeruginosa Adhesion to EC-associated MUC1-ED
Because MUC1-ED shed in vitro in response to P. aeruginosa flagellin inhibits P. aeruginosa adhesion to EC-associated MUC1-ED (Fig. 8, B, D, and E), we examined whether BALF from P. aeruginosa-colonized patients containing increased levels of desialylated MUC1-ED might inhibit bacterial adhesion. P. aeruginosa adhesion was tested following preincubation of the bacteria with BALF from the same three groups of patients analyzed for desialylated MUC1-ED BALF levels (Fig. 7, A–C). Preincubation of P. aeruginosa with BALF from P. aeruginosa-colonized patients inhibited bacterial adhesion to airway ECs by 52.9%, compared with preincubation with BALF from noncolonized subjects, and by 42.1%, compared with preincubation with BALF from patients colonized with non-P. aeruginosa microorganisms (Fig. 9A, panel i). We next assessed the ability of these same BALFs to inhibit adhesion of the same non-P. aeruginosa bacteria tested for NEU1-responsive adhesion (Fig. 1B) to airway ECs. None of the BALFs tested inhibited the adhesion of these non-P. aeruginosa bacteria (Fig. 9A, panels ii–vi). Finally, preincubation of BALF from P. aeruginosa-colonized patients with anti-MUC1-ED antibody, which depleted the samples of detectable MUC1-ED (Fig. 9B), greatly reduced their ability to inhibit P. aeruginosa adhesion compared with preincubation of the same BALFs with the IgG control (Fig 9, C and D). These combined data indicate that desialylated MUC1-ED in BALF from P. aeruginosa-colonized patients acts as a soluble decoy receptor to competitively inhibit P. aeruginosa adhesion to MUC1-ED-expressing airway ECs and that MUC1-ED inhibitory activity for bacterial adhesion is specific for P. aeruginosa. Because the levels of total protein in the recovered BALFs reached 0.1 mg/ml, and the in vivo concentrations of shed MUC1-ED in BALF from P. aeruginosa-colonized subjects, normalized to total protein, reached 4.3 μg/mg (Fig. 7A), the levels of MUC1-ED in these same BALFs, up to 0.43 μg/ml, approached those MUC1-ED concentrations capable of inhibiting P. aeruginosa adhesion in vitro (≤0.63 μg/ml) (Fig. 8, A and B). Again, given the dilutional effect inherent to the BAL procedure, combined with the known sequestration of a fraction of MUC1-ED within the fluid lining the bronchoalveolar compartment (38), these data likely underestimate the true levels of shed MUC1-ED in the BALF from P. aeruginosa-colonized patients.
Discussion
Invasive P. aeruginosa lung infections remain an important worldwide cause of morbidity and mortality in debilitated and immunocompromised patients despite advances in antibiotic therapy and supportive care. P. aeruginosa adhesion to airway epithelia is mediated through a direct interaction between its flagellin and the cell surface sialoprotein, MUC1. We now report that treatment of airway ECs with P. aeruginosa flagellin rapidly (≤30 min) increases NEU1-dependent MUC1 desialylation, thereby promoting bacterial adhesion to and invasion of the ECs. We previously reported that under these identical experimental conditions, EC cytotoxicity in response to P. aeruginosa was not observed (31, 32). Because flagellin is an established MUC1 ligand, these results support an amplification process through which P. aeruginosa increases its pathogenicity. However, at a later time (24 h), MUC1-ED desialylation also promotes its release from the EC surface. Although MUC1-ED shedding might be expected to reduce EC-associated MUC1-ED and P. aeruginosa adhesion, prior pulse-chase studies have demonstrated that shed MUC1-ED molecules are rapidly replaced by newly synthesized molecules exported to the cell surface (39). Shedding of the MUC1-P. aeruginosa complex into the airway lumen prior to bacterial invasion, together with liberation of unligated MUC1 as a soluble decoy receptor, might well reduce invasive infection in vivo. To our knowledge, this is the first report demonstrating that a prokaryotic organism, P. aeruginosa, uses its flagellin to hijack human eukaryotic NEU1 to increase MUC1-dependent P. aeruginosa adhesion and invasion, although the host responds by generating a hyperadhesive decoy receptor limiting invasive infection. Although MUC1 silencing clearly abrogated NEU1-mediated changes in P. aeruginosa adhesion and invasion, and MUC1 immunodepletion of BALFs greatly reduced their ability to inhibit P. aeruginosa adhesion, it is still conceivable that NEU1 might also desialylate O- and/or N-linked glycans of other soluble or membrane-bound mucins, or non-mucin sialoproteins, that in turn contribute to these changes.
That NEU1 overexpression enhanced airway EC adhesiveness for P. aeruginosa and its flagellin, and bacterial invasion of these same ECs, suggests that removal of terminal Sia residues unmasks a cryptic binding site(s) within the MUC1-ED comprised of one or more sugars subterminal to Sia and/or its protein backbone. A549 airway ECs express the sialyl-Thomsen-nouveau antigen (40), and creation of the Thomsen-nouveau antigen through NEU1-mediated desialylation might expose a binding site for P. aeruginosa and its flagellin. MUC1-ED exhibits a β-turn helix resulting from its high proline content (41). This extended rod-like conformation allows MUC1 to protrude higher than most membrane-associated proteins above the EC surface where it is strategically positioned to interact with P. aeruginosa in the airway lumen. The degree of MUC1 extension into the lumen depends upon the number of tandem repeat units, a genetically inherited allelic polymorphism varying from 20 to 120 repeats (13). Whether upon desialylation, MUC1 molecules with more tandem repeats exhibit greater adhesiveness for P. aeruginosa and/or superior decoy receptor function, or whether individuals expressing different repeat numbers have altered susceptibility to invasive P. aeruginosa lung infection, is unknown.
Analysis of the mouse NEU1 gene promoter has identified potential positive and negative cis-acting elements that might regulate NEU1 expression (42). Although flagellin failed to increase NEU1 expression or catalytic activity, it did promote MUC1-NEU1 association. That flagellin promoted this association within 30 min of stimulation diminishes the likelihood of, but does not entirely exclude, de novo NEU1 mRNA or protein synthesis as a source for the increased NEU1 associated with MUC1. Although LPS reportedly promotes NEU1 association with TLR4 (37), the fact that our flagellin preparation was preadsorbed with polymyxin B (26) reduces the possibility that LPS contamination of flagellin is responsible for MUC1-NEU1 association. It is possible that undetectable amounts of LPS might synergize with other unknown mediators to provoke this effect. Although we previously reported that flagellin engagement of MUC1-ED activates ERK1/2 (34), pharmacologic blockade of ERK1/2 activation failed to prevent flagellin-stimulated NEU1 association with MUC1. Others have reported that c-Src and EGF receptor each regulate MUC1-CD-dependent intracellular signaling (43, 44). However, pharmacologic inhibition of either c-Src or EGF receptor activation did not block flagellin-induced increases in NEU1-MUC1 co-immunoprecipitation (data not shown). It is conceivable that flagellin activates one or more signaling pathways, possibly through TLR5, that promotes the association of preformed NEU1 with MUC1. Because NEU1 exists in a complex with PPCA and β-galactosidase (21), MUC1-NEU1 association might be indirectly mediated through either of these or other NEU1-binding partners. Alternatively, MUC1-binding partners (e.g. glycogen synthase kinase 3β, β-catenin) (13) may participate in the MUC1-NEU1 interaction.
P. aeruginosa NanPs might contribute to basal levels of P. aeruginosa adhesion seen in airway ECs in which NEU1 has been silenced (12). However, P. aeruginosa expression of NanPs has not been associated with increased bacterial adhesion to airway ECs (20). In contrast to other bacterial NEUs, which contain a catalytic pocket structurally similar to that of canonical NEUs, crystallographic analysis of NanPs has revealed a more open, atypical structure (45). We hypothesize that P. aeruginosa commandeers the human sialidase, NEU1, to compensate for its structurally and functionally distinct NanPs, to desialylate MUC1-ED, and to increase P. aeruginosa adhesion to and invasion of human airway ECs. Although manipulation of host cell pathways by bacteria to enhance their virulence has been recognized as a central tenet of bacterial pathogenesis for several decades (46), to the best of our knowledge these results are the first to implicate a eukaryotic NEU in bacterial pathogenesis. Interventions that target influenza-expressed NEU have entered clinical practice. Whether these NEU inhibitors interfere with the catalytic activity of human NEU1 or whether human NEU1-specific inhibitors can be designed are clinically important questions. Our results further suggest that the host responds to P. aeruginosa hijacking of human NEU1 by releasing desialylated MUC1-ED from the EC surface to competitively inhibit interaction between flagellin-expressing P. aeruginosa and EC-associated MUC1. It is tempting to speculate that the balance between these opposing processes, i.e. P. aeruginosa adhesiveness versus decoy receptor function, and how they are impacted by the activities of other NEUs, sialyltransferases, and/or proteases ultimately dictate the clinical outcome of an individual airway EC exposure to P. aeruginosa. Future studies are needed to establish whether similar pathogenic mechanism(s) might be extended to other flagellin-expressing airway pathogens not examined here, mucin gene products other than MUC1, additional adhesin-receptor interactions, and/or extrapulmonary mucosal surfaces.
Note Added in Proof
Avelino C. Verceles' contributions to this article fulfill the JBC authorship criteria, but his authorship was inadvertently omitted from the version of the article that was published on May 11, 2015 as a Paper in Press.
This work was supported, in whole or in part, by National Institutes of Health Grants HL084223 and HL070155 (to S. E. G.). The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as a Paper of the Week.
- EC
- epithelial cell
- Ad
- adenovirus
- BALF
- bronchoalveolar lavage fluid
- CD
- cytoplasmic domain
- ED
- ectodomain
- MAL
- M. amurensis lectin II
- m.o.i.
- multiplicity of infection
- MUC1
- mucin 1
- NEU
- neuraminidase
- PNA
- peanut agglutinin
- PPCA
- protective protein/cathepsin A
- qRT-PCR
- quantitative reverse transcriptase-polymerase chain reaction
- Sia
- sialic acid
- SAEC
- small airway EC
- siRNA
- small interfering RNA
- SNA
- S. nigra agglutinin
- m.o.i.
- multiplicity of infection.
References
- 1. Pier G. B., Ramphal R. (2010) in Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases (Mandell G. L., Bennett J. E., Dolin R., eds) 6th Ed., pp. 2835–2860, Elsevier/Churchill/Livingstone, Philadelphia [Google Scholar]
- 2. Crouch Brewer S., Wunderink R. G., Jones C. B., Leeper K. V., Jr. (1996) Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109, 1019–1029 [DOI] [PubMed] [Google Scholar]
- 3. Novosad S. A., Barker A. F. (2013) Chronic obstructive pulmonary disease and bronchiectasis. Curr. Opin. Pulm. Med. 19, 133–139 [DOI] [PubMed] [Google Scholar]
- 4. Woods D. E., Straus D. C., Johanson W. G., Jr., Berry V. K., Bass J. A. (1980) Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect. Immun. 29, 1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. (1990) Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect. Immun. 58, 2361–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rostand K. S., Esko J. D. (1993) Cholesterol and cholesterol esters: host receptors for Pseudomonas aeruginosa adherence. J. Biol. Chem. 268, 24053–24059 [PubMed] [Google Scholar]
- 7. Comolli J. C., Waite L. L., Mostov K. E., Engel J. N. (1999) Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect. Immun. 67, 3207–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang F. C. (2014) Differential regulation of interleukin-8 and human β-defensin 2 in Pseudomonas aeruginosa-infected intestinal epithelial cells. BMC Microbiol. 14, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prince A. (1992) Adhesins and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract. Microb. Pathog. 13, 251–260 [DOI] [PubMed] [Google Scholar]
- 10. Ramos H. C., Rumbo M., Sirard J. C. (2004) Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12, 509–517 [DOI] [PubMed] [Google Scholar]
- 11. Adamo R., Sokol S., Soong G., Gomez M. I., Prince A. (2004) Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 30, 627–634 [DOI] [PubMed] [Google Scholar]
- 12. Lillehoj E. P., Hyun S. W., Feng C., Zhang L., Liu A., Guang W., Nguyen C., Luzina I. G., Atamas S. P., Pasaniti A., Twaddell W. S., Puché A. C., Wang L. X., Cross A. S., Goldblum S. E. (2012) NEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signaling. J. Biol. Chem. 287, 8214–8231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lillehoj E. P., Kato K., Lu W., Kim K. C. (2013) Cellular and molecular biology of airway mucins. Int. Rev. Cell Mol. Biol. 303, 139–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thathiah A., Blobel C. P., Carson D. D. (2003) Tumor necrosis factor-α converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 278, 3386–3394 [DOI] [PubMed] [Google Scholar]
- 15. Thathiah A., Carson D. D. (2004) MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. Biochem. J. 382, 363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Julian J., Dharmaraj N., Carson D. D. (2009) MUC1 is a substrate for γ-secretase. J. Cell. Biochem. 108, 802–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schauer R. (2009) Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19, 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis A. L., Lewis W. G. (2012) Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell. Microbiol. 14, 1174–1182 [DOI] [PubMed] [Google Scholar]
- 19. Pshezhetsky A. V., Ashmarina L. I. (2013) Desialylation of surface receptors as a new dimension in cell signaling. Biochemistry 78, 736–745 [DOI] [PubMed] [Google Scholar]
- 20. Soong G., Muir A., Gomez M. I., Waks J., Reddy B., Planet P., Singh P. K., Kaneko Y., Kanetko Y., Wolfgang M. C., Hsiao Y. S., Tong L., Prince A. (2006) Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Invest. 116, 2297–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonten E. J., Annunziata I., d'Azzo A. (2014) Lysosomal multienzyme complex: pros and cons of working together. Cell. Mol. Life Sci. 71, 2017–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stonebraker J. R., Wagner D., Lefensty R. W., Burns K., Gendler S. J., Bergelson J. M., Boucher R. C., O'Neal W. K., Pickles R. J. (2004) Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J. Virol. 78, 13755–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Button B., Cai L. H., Ehre C., Kesimer M., Hill D. B., Sheehan J. K., Boucher R. C., Rubinstein M. (2012) A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norum L. F., Varaas T., Kierulf B., Nustad K. (1998) Carcinoma-associated MUC1 detected by immunoradiometric assays. Tumour Biol. 19, 134–146 [DOI] [PubMed] [Google Scholar]
- 25. Schroeder J. A., Thompson M. C., Gardner M. M., Gendler S. J. (2001) Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 276, 13057–13064 [DOI] [PubMed] [Google Scholar]
- 26. Lillehoj E. P., Kim B. T., Kim K. C. (2002) Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L751–L756 [DOI] [PubMed] [Google Scholar]
- 27. Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. (1989) Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J. Infect. Dis. 160, 452–459 [DOI] [PubMed] [Google Scholar]
- 28. Shanks K. K., Guang W., Kim K. C., Lillehoj E. P. (2010) Interleukin-8 production by human airway epithelial cells in response to Pseudomonas aeruginosa clinical isolates expressing type a or type b flagellins. Clin. Vaccine Immunol. 17, 1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luzina I. G., Todd N. W., Nacu N., Lockatell V., Choi J., Hummers L. K., Atamas S. P. (2009) Regulation of pulmonary inflammation and fibrosis through expression of integrins αVβ3 and αVβ5 on pulmonary T lymphocytes. Arthritis Rheum. 60, 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ranke T. D., Strassle P., Harris A. D., Zhu J., Johnson J. K. (2012) Recovery of Gram-negative bacilli in stored endotracheal aspirates. J. Clin. Microbiol. 50, 2791–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lillehoj E. P., Hyun S. W., Kim B. T., Zhang X. G., Lee D. I., Rowland S., Kim K. C. (2001) Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L181–L187 [DOI] [PubMed] [Google Scholar]
- 32. Kato K., Lillehoj E. P., Kai H., Kim K. C. (2010) MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front. Biosci. 2, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee C., Liu A., Miranda-Ribera A., Hyun S. W., Lillehoj E. P., Cross A. S., Passaniti A., Grimm P. R., Kim B. Y., Welling P. A., Madri J. A., DeLisser H. M., Goldblum S. E. (2014) NEU1 sialidase regulates the sialylation state of CD31 and disrupts CD31-driven capillary-like tube formation in human lung microvascular endothelia. J. Biol. Chem. 289, 9121–9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lillehoj E. P., Kim H., Chun E. Y., Kim K. C. (2004) Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L809–L815 [DOI] [PubMed] [Google Scholar]
- 35. Kitazume S., Imamaki R., Ogawa K., Komi Y., Futakawa S., Kojima S., Hashimoto Y., Marth J. D., Paulson J. C., Taniguchi N. (2010) α2,6-Sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J. Biol. Chem. 285, 6515–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bachert C., Linstedt A. D. (2013) A sensor of protein O-glycosylation based on sequential processing in the Golgi apparatus. Traffic 14, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amith S. R., Jayanth P., Franchuk S., Finlay T., Seyrantepe V., Beyaert R., Pshezhetsky A. V., Szewczuk M. R. (2010) Neu1 desialylation of sialyl α-2,3-linked β-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell. Signal. 22, 314–324 [DOI] [PubMed] [Google Scholar]
- 38. Ali M., Lillehoj E. P., Park Y., Kyo Y., Kim K. C. (2011) Analysis of the proteome of human airway epithelial secretions. Proteome Sci. 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Razawi H., Kinlough C. L., Staubach S., Poland P. A., Rbaibi Y., Weisz O. A., Hughey R. P., Hanisch F. G. (2013) Evidence for core 2 to core 1 O-glycan remodeling during the recycling of MUC1. Glycobiology 23, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Croce M. V., Colussi A. G., Price M. R., Segal-Eiras A. (1999) Identification and characterization of different subpopulations in a human lung adenocarcinoma cell line (A549). Pathol. Oncol. Res. 5, 197–204 [DOI] [PubMed] [Google Scholar]
- 41. Fontenot J. D., Mariappan S. V., Catasti P., Domenech N., Finn O. J., Gupta G. (1995) Structure of a tumor associated antigen containing a tandemly repeated immunodominant epitope. J. Biomol. Struct. Dyn. 13, 245–260 [DOI] [PubMed] [Google Scholar]
- 42. Champigny M. J., Johnson M., Igdoura S. A. (2003) Characterization of the mouse lysosomal sialidase promoter. Gene 319, 177–187 [DOI] [PubMed] [Google Scholar]
- 43. Li Y., Kuwahara H., Ren J., Wen G., Kufe D. (2001) The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β and β-catenin. J. Biol. Chem. 276, 6061–6064 [DOI] [PubMed] [Google Scholar]
- 44. Li Y., Ren J., Yu W., Li Q., Kuwahara H., Yin L., Carraway K. L., 3rd, Kufe D. (2001) The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and β-catenin. J. Biol. Chem. 276, 35239–35242 [DOI] [PubMed] [Google Scholar]
- 45. Hsiao Y. S., Parker D., Ratner A. J., Prince A., Tong L. (2009) Crystal structures of respiratory pathogen neuraminidases. Biochem. Biophys. Res. Commun. 380, 467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhavsar A. P., Guttman J. A., Finlay B. B. (2007) Manipulation of host-cell pathways by bacterial pathogens. Nature 449, 827–834 [DOI] [PubMed] [Google Scholar]