Background: Thrombin stimulates protease-activated receptors (PAR) on endothelial cells, and this pathway becomes dysregulated in disease.
Results: Ca2+ influx through the Na+/Ca2+ exchanger (NCX) regulated angiogenesis and endothelial permeability in response to thrombin via reactive oxygen species generation and ERK1/2 activation.
Conclusion: NCX activity is a novel determinant of thrombin signaling in the endothelium.
Significance: Inhibiting NCX could improve conditions involving unregulated thrombin signaling.
Keywords: angiogenesis, calcium, endothelium, extracellular-signal-regulated kinase (ERK), NADPH oxidase, permeability, sodium-calcium exchange, thrombin
Abstract
Thrombin acts on the endothelium by activating protease-activated receptors (PARs). The endothelial thrombin-PAR system becomes deregulated during pathological conditions resulting in loss of barrier function and a pro-inflammatory and pro-angiogenic endothelial phenotype. We reported recently that the ion transporter Na+/Ca2+ exchanger (NCX) operating in the Ca2+-influx (reverse) mode promoted ERK1/2 activation and angiogenesis in vascular endothelial growth factor-stimulated primary human vascular endothelial cells. Here, we investigated whether Ca2+ influx through NCX was involved in ERK1/2 activation, angiogenesis, and endothelial barrier dysfunction in response to thrombin. Reverse-mode NCX inhibitors and RNAi-mediated NCX1 knockdown attenuated ERK1/2 phosphorylation in response to thrombin or an agonist of PAR-1, the main endothelial thrombin receptor. Conversely, promoting reverse-mode NCX by suppressing Na+-K+-ATPase activity enhanced ERK1/2 activation. Reverse-mode NCX inhibitors and NCX1 siRNA suppressed thrombin-induced primary human vascular endothelial cell angiogenesis, quantified as proliferation and tubular differentiation. Reverse-mode NCX inhibitors or NCX1 knockdown preserved barrier integrity upon thrombin stimulation in vitro. Moreover, the reverse-mode NCX inhibitor SEA0400 suppressed Evans' blue albumin extravasation to the lung and kidneys and attenuated edema formation and ERK1/2 activation in the lungs of mice challenged with a peptide activator of PAR-1. Mechanistically, thrombin-induced ERK1/2 activation required NADPH oxidase 2-mediated reactive oxygen species (ROS) production, and reverse-mode NCX inhibitors and NCX1 siRNA suppressed thrombin-induced ROS production. We propose that reverse-mode NCX is a novel mechanism contributing to thrombin-induced angiogenesis and hyperpermeability by mediating ERK1/2 activation in a ROS-dependent manner. Targeting reverse-mode NCX could be beneficial in pathological conditions involving unregulated thrombin signaling.
Introduction
The vascular endothelium dynamically controls the passage of solutes, macromolecules, and immune cells from plasma to surrounding tissues (1, 2). Endothelial barrier function is crucial for maintaining plasma volume and an anti-inflammatory and anti-atherogenic tissue environment (2, 3). Impaired endothelial barrier function is also the hallmark of a number of diseases, including tumor progression (4), chronic inflammation (3), and acute lung injury (5).
Thrombin, the major protease of the coagulation cascade, can stimulate protease-activated receptors (PARs3; a subfamily of G-protein coupled receptors) on the endothelial cell (EC) surface (6). PAR activation regulates important processes, including hemostasis, responses to inflammation, vasorelaxation, angiogenesis, and the development of the embryonic vasculature (6, 7). Of the four known PAR isoforms, PAR-1 and PAR-4 are functional in the endothelium (8); however, PAR-1 is believed to be the main thrombin endothelial receptor (1, 2). The endothelial thrombin-PAR system becomes deregulated during atherosclerosis (9), acute lung injury (10), and tumor angiogenesis (11). Better understanding of this signaling axis could lead to improved interventions for these conditions.
PAR stimulation by thrombin activates multiple downstream effectors, including phospholipase Cβ3 (PLCβ3), c-Src, protein kinase Cs (PKC), and extracellular signal-regulated kinase 1/2 (ERK1/2), and mobilizes Ca2+ and small GTPases (2). This alters EC cytoskeletal dynamics and adhesion molecule expression and disrupts adherens and tight junctions, resulting in a motile, pro-inflammatory, and pro-angiogenic endothelial phenotype (2).
The Na+/Ca2+ exchanger (NCX) is a plasmalemma protein that extrudes cytosolic Ca2+ in exchange for extracellular Na+ (forward mode) or, in the reverse mode, leads to Ca2+ influx, depending on transmembrane Na+ and Ca2+ concentrations and membrane potential (12). The mammalian NCX family comprises three genes (NCX1–3) that are expressed in a tissue-specific manner (12). Under physiological conditions, NCX operates primarily in the Ca2+-extrusion mode maintaining a low cytosolic Ca2+ concentration ([Ca2+]i) (12). However, in conditions that promote Na+ overload and/or membrane depolarization (such as ischemia-reperfusion or heart disease), NCX can reverse its action leading to Ca2+ influx exacerbating the severity of the disease (13). Consequently, reverse-mode NCX is considered a therapeutic target for cardiovascular diseases, and pharmacological inhibitors have been developed (14).
The role of NCX is mainly studied in excitable cells (12), although it is also expressed in nonexcitable cells such as kidney tubular cells, β cells, and glial cells (12). ECs also exhibit NCX activity (15, 16) and primarily express the splice variants of the cardiac isoform NCX1.3 and NCX1.7 (17). The role of endothelial NCX in physiology and pathophysiology remains largely unknown.
We recently reported that ion channel activity and in particular Ca2+ influx through reverse-mode NCX were required for ERK1/2 activation and angiogenesis in human ECs stimulated with vascular endothelial growth factor (VEGF) (18, 19). Thrombin also activates ERK1/2 in a Ca2+-dependent manner (20). Therefore, we investigated whether Ca2+ influx through reverse-mode NCX could modulate ERK1/2 activation downstream of thrombin and its main endothelial receptor PAR-1 and whether NCX activity is required for thrombin-induced angiogenesis and endothelial barrier disruption.
Experimental Procedures
Materials
Primary HUVECs were obtained from TCS Cellworks. Thrombin from human plasma was from Sigma. The PAR-1 peptide agonist TFLLR-NH2 was from Tocris. SEA0400 was synthesized initially by Taisho Pharmaceuticals (Saitama, Japan) and later (for in vivo studies) at the Institute for Cancer Research by O. B. SN-6, BAPTA-AM, Gö6983, DPI, U73122, and SKF96365 were from Tocris. N-Acetyl-l-cysteine, EGTA-AM, and PD98059 were from Calbiochem. The Nox2 inhibitory peptide gp91ds-tat and its scrambled control were from Anaspec. KB-R7943, ouabain, l-NAME, ionomycin, and human VEGF-A were from Sigma. All other chemicals used were from Sigma unless otherwise indicated.
Cell Culture and Protein Extraction
HUVECs (TCS Cellworks) were cultured in complete large vessel EC growth medium with the addition of endothelial growth supplements and antibiotics (TCS Cellworks) as we described previously (18, 19). For the thrombin stimulation assays, cells, washed twice with PBS, were subsequently serum-starved in a physiological buffer containing 144 mm NaCl, 5.4 mm KCl, 2.5 mm CaCl2, 1 mm MgCl2, 5.6 mm d-glucose, and 5 mm Tris-HCl, pH 7.4, at 37 °C for 1 h. Inhibitors or vehicle was added 30 min prior to stimulation with thrombin, unless otherwise indicated. HUVECs were activated by the addition of 0.5 units/ml thrombin and maintained for the indicated times at 37 °C. Cells were subsequently washed once with ice-cold PBS, placed on ice, and lysed by applying an ice-cold buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% v/v Triton X-100, 0.5 mm DTT, 1 mm PMSF, 1% v/v protease inhibitor mixture (Sigma), 1 mm NaF, 5 mm bpVphen (Calbiochem), 5 μm fenvalerate (Calbiochem), 1 mm Na3VO4, and 1% v/v phosphatase inhibitor mixtures I and III (Sigma). The cell homogenate was placed for 10 min on ice and then centrifuged at 5,000 × g for 10 min at 4 °C. The supernatant was aliquoted and stored at −80 °C until further use. The bicinchoninic acid assay (BCA) (Pierce), with BSA (Sigma) as the protein standard, was used for determining protein concentration. In all the experiments described, HUVECs were used between passages 3 and 9.
Western Blot
Western blotting was performed as we have described previously (18, 19), using the NuPAGE electrophoresis system and buffers (Invitrogen). Protein bands were visualized using the ECLTM prime detection kit (GE Healthcare). In this study, we used the following antibodies: rabbit anti-phospho-p44/p42 ERK1/2 (Thr-202/Tyr-204); rabbit anti-p44/p42 (total ERK); rabbit anti-phospho-PLCβ3 (Ser-537); and peroxidase-conjugated secondary antibodies from Cell Signaling. The mouse anti-NCX1 was from Swant; mouse anti-β-actin was from Sigma. Mouse anti-GAPDH, rabbit anti-gp91Phox (Nox2), and rabbit anti-Nox5 were from Abcam Labs. Films were scanned, and the optical densities of bands of interest were determined using ImageJ 1.46r (National Institutes of Health). Phosphoprotein optical density was normalized against the corresponding protein loading controls. The ratio of phospho/total protein of the unstimulated controls in each experiment was arbitrarily set as 1. The values of the experimental conditions are represented as fold × normalized unstimulated controls from at least three independent experiments.
Immunoprecipitation
Immunoprecipitation was performed as we described previously (18, 19). Briefly, protein A-agarose beads (Roche Applied Science), washed twice with ice-cold PBS, were incubated with 10 μl of anti-NCX1 antibody for 1 h at 4 °C. Subsequently, the antibody-conjugated beads were washed twice in ice-cold PBS and added to the corresponding cell lysates. Following overnight incubation at 4 °C, the beads were washed twice with lysis buffer and once with PBS, and proteins were extracted by boiling in sample buffer at 95 °C for 5 min.
siRNA Transfection
siRNA duplexes (siGENOME SMARTpool) from Dharmacon were used to selectively suppress NCX1, Nox2 (gp91Phox), and Nox5 expression in HUVECs with OligofectamineTM as the transfectant, as we described previously (18, 19). Cells for the NCX1 immunoprecipitation experiments were plated in T-75 flasks. The NCX1 siRNA duplexes targeted the following sequences: GAAAUGUUAUCGUUCCAUA, GCAGAAGCAUCCAGAUAAA, GCAGACGCCUCCAUAGGUA, and GUGAGGAUCUGGAAUGAAA. Nox2 siRNA duplexes targeted the following sequences: GAAGACAACUGGACAGGAA, GGAACUGGGCUGUGAAUGA, GUGAAUGCCCGAGUCAAUA, and GAAACUACCUAAGAUAGCG. Nox5 siRNA duplexes targeted the following sequences: GGAGCAAGGUGUUCCAGAA, CUAUAGACCUGGUGACUAC, GCUUCUUUGCAGAGCGAUU, and CCUUCUUUGCAGAGCGAUU. Nontargeting siRNA pool, also from Dharmacon, was used as the control.
Ca2+ Assays
Ca2+ assays were performed essentially as described previously (18, 19). Briefly, HUVECs (1 × 104 cells/well), were seeded into a flat clear-bottomed black-walled 96-well plate (Corning Glass). The following day, cells were washed twice with PBS and loaded with the Ca2+ dye indicator Fluo-4NW (Life Sciences) for 45 min at 37 °C in the dark in Hanks' balanced salt solution (HBSS) in the presence of probenecid (2.5 mm) to enhance dye loading. Subsequently, equal volumes of HBSS containing inhibitors or vehicle were added, and cells were incubated for a further 15 min. The plate was then transferred to the assay chamber of a FLIPR plate reader (Molecular Devices), and HUVECs were challenged with 0.5 units/ml thrombin in HBSS for 200 s at 37 °C. Fluorescence intensity (excitation 485 nm, emission 525 nm, cutoff 515 nm), as a measure of [Ca2+]i, was monitored immediately after the addition of thrombin every 2 s. Uniform sample loading was determined by an end point fluorescence reading (excitation 485 nm and emission 525 nm) immediately before thrombin stimulation. Background fluorescence was measured for 20 s prior to the addition of thrombin, and Ca2+ transients were presented as the ratio of sample fluorescence at any given time point (F) divided by background fluorescence (F0). All of the experimental conditions were in triplicate and were assayed simultaneously on the same plate.
Tubulogenesis Assays
HUVEC tubular differentiation assays were carried out according to a previously published method (21) with minor modifications. HUVECs (2.5 × 103 per sample) in phenol red-free Opti-MEM® (Life Sciences) containing 2% FCS were plated in 24-well plates precoated with 250 μl of growth factor-reduced, phenol red-free MatrigelTM (BD Biosciences) for 1 h at 37 °C. Where indicated, 0.5 units/ml thrombin and the appropriate concentrations of reverse-mode NCX inhibitors or vehicle were added. The cells were incubated in a humidified incubator at 37 °C and 5% CO2. After 16 h, bright field images were captured with an Olympus LX70 microscope from five random fields of view per sample at ×10 magnification. HUVECs transfected with NCX1 siRNA for 48 h were plated on MatrigelTM as above, but tubule length was quantified after 6 h of thrombin addition. Tubule length and number of branching points for each condition were quantified using Image Pro-Plus 5.0 software (Media Cybernetics).
Proliferation Assays
96-Well plates (Nunc) were precoated with 50 μg/ml rat tail collagen-I (BD Biosciences) for 2 h at 37 °C. Subsequently, 3 × 103 HUVECs were seeded per well in complete EC medium (TCS Cellworks). The next day, the medium was discarded, and 100 μl of phenol red-free Opti-MEM® (Life Sciences) medium containing 0.1% (w/v) BSA and 1 μg/ml hydrocortisone were added. Where appropriate, the medium contained 0.5 units/ml thrombin and the indicated concentrations of reverse-mode NCX inhibitors or vehicle. Each condition was assayed in triplicate. After 48 h in a humidified incubator at 37 °C and 5% CO2 in air, the number of cells in each well was determined by the alkaline phosphatase assay as we described previously (18, 19).
In Vitro Endothelial Barrier Function Assays
Endothelial barrier function in vitro was assessed by determining the passage of FITC-labeled dextran through a confluent endothelial monolayer according to a published method (22) with minor modifications. HUVECs (1 × 104) were seeded into modified Boyden transwell inserts (Costar, 0.4 μm pore size) precoated with 50 μg/ml rat tail collagen-I (BD Biosciences) and cultured in complete medium for 2 days until confluent. Subsequently, HUVECs monolayers were serum-starved in physiological buffer (described under “Cell Culture and Protein Extraction”) for 1 h. Appropriate concentrations of drugs or vehicle were added into the top chamber 30 min prior to thrombin stimulation. Thrombin (0.5 units/ml) was applied to the top chamber simultaneously with 1 mg/ml FITC-dextran (Mr 40,000) (Sigma). Fluorescence in the bottom chamber of each sample was measured 30 min after thrombin stimulation in an Infinite M200PRO (Tecan) fluorescent plate reader (excitation 485 nm and emission 525 nm). For the siRNA experiments, HUVECs transfected with NCX1 targeting or control siRNA, as described under “siRNA Transfection,” were seeded into collagen-I-coated transwell inserts at a density of 2 × 104 cells/well, 24 h post-transfection. The following day, endothelial barrier function in response to thrombin was determined as above.
Immunofluorescence
HUVECs plated on collagen-I (BD Biosciences)-coated glass coverslips and cultured until 80–90% confluent were serum-starved in physiological buffer, preincubated for 30 min with inhibitors or vehicle, and stimulated with thrombin (0.5 units/ml) for 30 min. Cells were washed with prewarmed PBS and fixed for 20 min with 4% w/v paraformaldehyde at room temperature. After an additional wash step with PBS, fixed HUVECs were permeabilized with 0.5% v/v Triton X-100 in PBS for 5 min at room temperature. Subsequently, cells were washed in PBS, blocked in 0.5% w/v BSA in PBS, and incubated for 15 min at room temperature with FITC-conjugated phalloidin (1:40 v/v) in PBS containing 0.1% v/v BSA. Cells were then washed twice with PBS and once with PBS containing 0.025% v/v Tween 20. Finally, HUVECs were washed for 10 min in PBS containing 1:10,000 v/v DAPI (Invitrogen) to visualize nuclei, and after two wash steps in PBS, slides were air-dried in the dark and mounted on glass slides with ProLong® Gold anti-fade (Invitrogen). Images were captured with a Hamamatsu digital camera (Improvision) on a Zeiss Axioplan microscope at ×40 magnification.
RhoA Activity Assay
RhoA activity was determined with the ELISA-based kit G-LISA® RhoA (Cytoskeleton) according to the manufacturer's instructions. Serum-starved HUVECs (1 × 105/sample) were preincubated with inhibitors or vehicle for 30 min and then stimulated with thrombin (0.5 units/ml) for the indicated times. Cells were then lysed with the lysis buffer provided in the kit. Cell lysates were precleared by centrifugation (5000 × g for 1 min at 4 °C). A small sample was used for protein concentration determination with the BCA assay, and the remaining supernatant was snap-frozen in liquid N2 and stored at −80 °C until used for the assay. Absorbance was corrected for protein concentration.
ROS Assays
For DCF, HUVECs were plated at a density of 104 cells per well in clear bottom black 96-well plates (Corning). The following day, the medium was aspirated, and cells were serum-starved in physiological buffer (described under “Cell Culture and Protein Extraction”) in a humidified incubator at 37 °C, 5% CO2. After 30 min, the appropriate inhibitors or vehicle was added, and HUVECs were serum-starved for a further 30 min at 37 °C. Next, the medium was removed, and fresh medium, containing 10 μm DCF, was added for 10 min at 37 °C in the dark. Finally, the DCF solution was removed; cells were washed twice with prewarmed buffer and stimulated with 0.5 units/ml thrombin in the presence of inhibitors or vehicle for 5 min at 37 °C. Fluorescence was measured immediately in an Infinite M200PRO (Tecan) fluorescent plate reader (excitation 485 nm and emission 525 nm). All the buffers contained 0.2 mm l-NAME to prevent peroxynitrite formation, which is reported to interfere with DCF fluorescence (23).
For DHE fluorescence, HUVECs, plated at a density of 104 cells per well in clear bottom black 96-well plates (Corning Glass) were serum-starved the following day in physiological buffer for 1 h. Appropriate inhibitors or vehicle was added 30 min prior to stimulation with a solution containing thrombin and DHE. The final concentrations of thrombin and DHE were 0.5 units/ml and 5 μm, respectively. After 10 min, fluorescence was measured in an Infinite M200PRO (Tecan) fluorescent plate reader (excitation 520 nm and emission 590 nm).
MCLA chemiluminescence measurements were performed as described previously (24). Briefly, HUVECs (104 cells/well) were plated in clear bottom black 96-well plates (Corning Glass). The following day, cells were serum-starved in physiological buffer for 1 h. The appropriate inhibitors or vehicle were added for the last 30 min of the starvation step. HUVECs were then stimulated with a solution of 0.5 units/ml thrombin containing 1 μm MCLA, and the plate was immediately transferred to the measuring chamber of an Infinite M200PRO plate reader (Tecan). The photons emitted from each sample, including a blank sample containing 1 μm MCLA, were measured simultaneously, for 2 s/min for a total of 10 min. The value of the blank sample was subtracted from each sample, and emitted photons were expressed as percentage of the unstimulated control (arbitrarily set to 100%) corrected for protein concentration. In the case of siRNA-transfected HUVECs, cells were plated 24-h post-transfections and assayed the following day as above.
In Vivo Assays
All animal experiments were conducted in accordance with the United Kingdom Home Office Animals (1986 Scientific Procedures) with local ethical committee approval. Vascular permeability in response to a specific peptide agonist of PAR-1 (TFLLR-NH2) was evaluated according to previously published methods (25, 26), with minor modifications. Male C57BL/6J mice (8–10 weeks old) received 10 mg/kg SEA0400 dissolved in 4% Arabic gum (Sigma) or vehicle by oral gavage, as described previously (27). After 2 h, the conscious animals were placed in a restrainer, and 20 mg/kg Evans blue albumin (EBA) with or without 1 mg/kg PAR-1 peptide agonist, as indicated, in a 100-μl final volume, was injected through the tail vein. After 30 min, animals were terminally anesthetized and sacrificed by cardiac puncture after thoracotomy. Blood was collected in heparinized syringes, and EBA in the plasma was evaluated against an EBA standard curve to ensure comparable EBA loading. Subsequently, mice were perfused with 12 ml of ice-cold sterile PBS containing 5 mm EDTA (4 ml/min) to remove red blood cells. The lungs and the kidneys were removed, washed in cold PBS, dried with sterile blotting paper, weighed, snap-frozen in liquid nitrogen, and stored at −80 °C until analyzed as described below.
EBA Tissue Leakage
Right superior lung lobes or kidneys of mice treated as described above were homogenized in 1 ml of PBS per 0.1 mg of tissue using a Polytron homogenizer. Two volumes of formamide were subsequently added to the lysates, and EB dye was extracted from the tissues by incubation at 60 °C for 18 h (25, 26). The lysates were centrifuged for 30 min at 5,000 × g in a benchtop centrifuge. The EBA content of the tissues was determined by measuring the absorbance of the supernatant at 620 nm corrected at 740 nm for hemoglobin content in a plate reader (Infinite M200PRO, Tecan) against standards of known EBA content.
Lung Edema Formation
Left lungs from the animals used for EBA determination were dried by incubation in an oven for 16 h at 60 °C. The ratio of wet tissue weight (obtained immediately after the harvest of tissues) divided by the dry tissue weight was used as an indication of the water content (and hence edema formation) of the lungs (25, 26).
Lung Western Blot
The middle and inferior right lobes of experimental animals were homogenized in mammalian protein extraction buffer (GE Healthcare) containing protease and phosphatase inhibitors. Protein homogenization of tissues and Western blotting were performed as we described previously (28).
Statistical Analysis
The data are expressed as the means ± S.E. Statistical significance was determined by one- or two-way analysis of variance and Tukey's post hoc test using GraphPad Prism 5 software, as appropriate. Values of p < 0.05 were deemed statistically significant.
Results
Ca2+ Influx through Reverse-mode NCX Is Required for ERK1/2 Activation by Thrombin
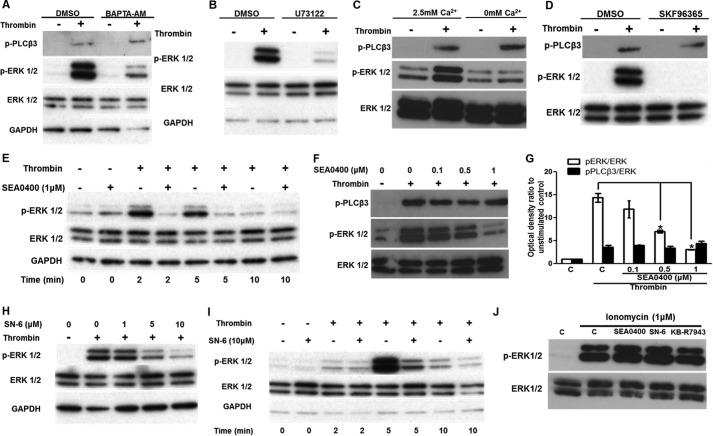
Loading HUVECs with the Ca2+ chelator BAPTA-AM suppressed ERK1/2 activation (Fig. 1A). Suppression of [Ca2+]i signaling with BAPTA-AM did not affect PLCβ3 phosphorylation at a site (Ser-537) downstream of PAR-1-mediated Gαi2 activation (29), indicating that [Ca2+]i does not affect PAR-1 activity directly. Because phosphorylation of PLCβ3 at Ser-537 is required for activity (29) and a PLC inhibitor suppressed thrombin-induced ERK1/2 phosphorylation (Fig. 1B), Ca2+ signaling probably affects ERK1/2 activation downstream of PLCβ3.
FIGURE 1.
Ca2+ influx through reverse-mode NCX is required for thrombin-induced ERK1/2 activation. A, HUVECs, serum-starved for 40 min, were treated with BAPTA-AM (10 μm) or vehicle for a further 20 min prior to challenge with thrombin (0.5 units/ml) for 5 min. ERK1/2 and PLCβ3 activation were demonstrated by Western blot. Membranes were subsequently stripped and re-probed for total ERK1/2 and GAPDH proteins. B, serum-starved HUVECs were preincubated with the PLC inhibitor U73122 (1 μm) for 30 min prior to challenge with thrombin (0.5 units/ml) for 5 min. C, HUVECs were serum-starved for 45 min and then incubated for a further 15 min in Ca2+-free medium, prior to challenge with 0.5 units/ml thrombin (5 min). ERK1/2 and PLCβ3 activation was determined as in A. D, HUVECs were incubated with the broad-spectrum inhibitor of nonselective cation channels SKF96365 (30 μm) and treated as in B. E, serum-starved HUVECs were preincubated with the reverse-mode NCX inhibitor SEA0400 (1 μm) or vehicle for 30 min prior to challenge with thrombin (0.5 units/ml) for the times indicated. F, SEA0400 was also applied over a range of concentrations, and ERK1/2 and PLCβ3 activation was assayed after 5 min. G, optical densities of the phospho-ERK1/2 and phospho-PLCβ3 bands in F were normalized against the ERK1/2 total protein of the corresponding sample. The normalized density of the unstimulated control (bar C) was set to 1. The mean value of the ratio for each condition is expressed as fold × unstimulated control value. Bars represent the means ± S.E. from n = 3 experiments. *, p < 0.05 versus the thrombin-stimulated control. A second reverse-mode NCX inhibitor, SN-6, also suppressed thrombin-induced ERK1/2 activation in a dose-dependent (H) and time-dependent manner (I). HUVECs were stimulated with ionomycin (1 μm for 5 min) in the presence of SEA0400 (1 μm), SN-6 (10 μm), KB-R7943 (10 μm), or vehicle (J), and ERK1/2 activation was assessed by Western blot. n = 3.
HUVEC stimulation by thrombin in a nominal Ca2+-free physiological medium (Ca2+ was omitted) resulted in a substantial reduction in phospho-ERK1/2 levels compared with controls (Fig. 1C). Thrombin-induced ERK1/2 activation was also inhibited by the nonselective inhibitor of Ca2+ influx SKF96365 (Fig. 1D). Neither of these treatments visibly altered pPLCβ3 levels, suggesting that Ca2+ influx does not affect PAR activity. Thus, extracellular Ca2+ was required for ERK1/2 phosphorylation in response to thrombin, in agreement with a previous study (20).
Next, we investigated whether a specific inhibitor of reverse-mode NCX, SEA0400 (14), attenuates ERK1/2 activation by thrombin, as we reported previously for VEGF (18). Thrombin stimulation (0.5 units/ml) increased phospho-ERK1/2 levels, peaking between 2 and 5 min and declining toward control levels after 10 min (Fig. 1E). In HUVECs preincubated with SEA0400 as described previously (18), ERK1/2 activation was suppressed in a time-dependent (Fig. 1E) and dose-dependent (Fig. 1F) manner. Thrombin stimulation for 5 min resulted in a 14.11 ± 0.95-fold normalized phospho-ERK1/2 ratio increase versus the unstimulated control. Pretreatment with 0.5 or 1 μm SEA0400 significantly attenuated phospho-ERK1/2 levels compared with vehicle-treated control (7.43 ± 0.30- and 2.95 ± 0.04-fold, respectively) (Fig. 1G). SEA0400 did not significantly affect phospho-PLCβ3 levels (Fig. 1, F and G) suggesting that SEA0400 does not affect PAR activity. Another chemically distinct reverse-mode NCX inhibitor, SN-6 (14, 18), also suppressed thrombin-induced ERK1/2 activation both dose- and time-dependently (Fig. 1, H and I). To exclude the possibility that these inhibitors affect nonspecifically the intracellular enzymes that couple Ca2+ influx with ERK1/2 activation in HUVECs, we stimulated them with the Ca2+ ionophore ionomycin (1 μm for 5min) in the presence of SEA0400 (1 μm), SN-6 (10 μm), or KB-R7943 (10 μm) (Fig. 1J). Ionomycin treatment resulted in ERK1/2 activation, as we have reported previously (19), whereas SEA0400 or SN-6 pretreatment had no apparent effect on ERK1/2 phosphorylation. KB-R7943 had a small nonsignificant effect.
Effect of Reverse-mode NCX Inhibitors on Thrombin-induced Ca2+ Transients
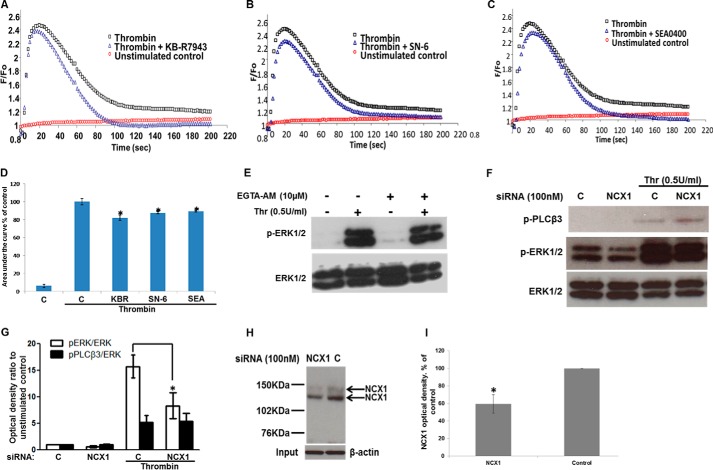
Next, we investigated the effect of reverse-mode NCX inhibitors on thrombin-induced Ca2+ transients. HUVECs were serum-starved for 1 h in physiological buffer in the presence of probenecid (2.5 mm). With the exception of probenecid (included for improved dye loading) conditions were similar to the ERK1/2 stimulation experiments to allow for direct comparisons (see under “Experimental Procedures”).
Thrombin stimulation of HUVECs loaded with the Ca2+ dye indicator Fluo-4NW resulted in a rapid increase in emitted fluorescence. The Ca2+ response peaked at ∼20–30 s and then declined toward the baseline within 120 s. Steady-state bulk [Ca2+]i remained slightly higher than baseline for the duration of the experiment (200 s). Preincubation of HUVECs with the reverse-mode NCX inhibitors KB-R7943 (10 μm), SN-6 (10 μm), or SEA0400 (1 μm) for 15 min prior to stimulation resulted in a modest decrease to the tonic phase of the Ca2+ response (Fig. 2, A–C). In contrast, there was a sharper decline and a lower steady state of the Ca2+ transient in comparison with controls. The Ca2+ response (quantified as area under the curve) was modestly, but significantly, diminished by pretreatment with KB-R7943 (10 μm), SN-6 (10 μm), and SEA0400 (1 μm) by 17.8 ± 2.96, 12.4 ± 1.21, and 10.9 ± 0.6%, respectively, compared with thrombin-stimulated control (arbitrarily set to 100%) (Fig. 2D).
FIGURE 2.
Effect of reverse-mode NCX inhibitors on Ca2+ transients and NCX1 siRNA on ERK1/2 phosphorylation. Serum-starved HUVECs were challenged with 0.5 units/ml thrombin at time 0. Representative time courses of Fluo-4NW Ca2+-sensitive fluorescence of unstimulated control (red circles), thrombin-stimulated control (black squares), or 10 μm KB-R7943 (A), 10 μm SN-6 (B), or 1 μm SEA0400 (C) preincubation prior to thrombin stimulation (blue triangles). D, area under the curve of the Ca2+ signal in A–C was calculated. Bars represent means ± S.E. (n = 3 in triplicate). *, p < 0.05 versus thrombin-stimulated control. E, HUVECs serum-starved for 40 min in physiological buffer and treated with EGTA-AM (10 μm) or vehicle (0.5% v/v Me2SO) for a further 20 min were subsequently challenged with thrombin (0.5 units/ml) for 5 min. ERK1/2 activation was demonstrated as in Fig. 1A. n = 3. F, HUVECs transfected with nontargeting or NCX1-targeting siRNA were serum-starved and then stimulated with thrombin. ERK1/2 and PLCβ3 activation were demonstrated as in Fig. 1 (n = 3). G, optical densities of the phospho-ERK1/2 and phospho-PLCβ3 bands in F were quantified as in Fig. 1G. *, p < 0.05 versus the thrombin-stimulated control (n = 3). H, NCX1 protein was immunoprecipitated from control- or NCX1-siRNA-treated cells in experiments run in parallel to those shown in F. NCX1 protein levels were determined by Western blot. Equal protein in the input was ensured by probing for β-actin. I, optical densities of the NCX1 bands in H (n = 3). Control optical density was set to 100%. The optical density of the NCX1 protein bands from NCX1-siRNA treated cells is expressed as % change relative to the corresponding control value. Bars represent the means ± S.E. from n = 3 experiments. *, p < 0.05 versus the control.
The potential involvement of Ca2+ microdomains was investigated using EGTA-AM, which does not affect local Ca2+ transients within 100 nm of the source of Ca2+ entry (30). Loading HUVECs with EGTA-AM had no apparent effect on ERK1/2 phosphorylation in response to thrombin (Fig. 2E). Conversely, when HUVECs were loaded, under similar conditions, with the “fast” Ca2+ chelator BAPTA-AM, which suppresses localized Ca2+ signals (22), ERK1/2 activation was inhibited (Fig. 1A).
Knockdown of NCX1 by siRNA Suppresses Thrombin-induced ERK1/2 Activation
Next, we genetically targeted expression of NCX1, the predominant NCX isoform in HUVECs (17, 18). Transfection of HUVECs with NCX1-targeting siRNA significantly attenuated ERK1/2 activation in response to thrombin compared with control siRNA (15.71 ± 2.14-fold down to 8.31 ± 2.46-fold of unstimulated control; Fig. 2, F and G). Phospho-PLCβ3 levels were unaffected, indicating that knockdown of NCX1 does not affect PAR activity. Knockdown of NCX1 by ∼50% at the protein level, as reported previously (18), in parallel transfection experiments (Fig. 2, H and I) was confirmed by Western blot.
Ouabain Augments ERK1/2 Activation by Thrombin
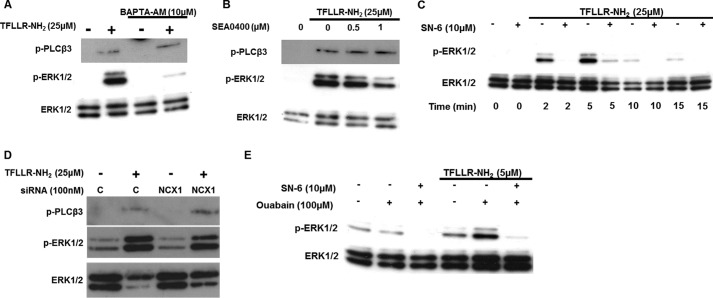
HUVECs were then treated with the Na+-K+-ATPase inhibitor ouabain, reported to rapidly increase [Na+]i in HUVECs and to promote reverse-mode NCX (31). Ouabain alone did not affect ERK1/2 phosphorylation for the duration of the experiment (3 min), as we have reported previously (18). However, preincubation of HUVECs with ouabain (100 μm for 1min) prior to short term stimulation with thrombin (0.5 units/ml for 2 min) augmented ERK1/2 phosphorylation (13.3 ± 2.15- to 20.10 ± 2.54-fold of unstimulated control). Preincubation of the thrombin-stimulated, ouabain-treated cells with SN-6 suppressed ERK1/2 phosphorylation (2.4 ± 0.71-fold versus the control) indicating that the effect of ouabain was probably due to the induction of reverse-mode NCX (Fig. 3, A and B).
FIGURE 3.

Promoting reverse-mode NCX enhances thrombin-induced ERK1/2 phosphorylation. A, HUVECs were preincubated with 100 μm ouabain for 1 min prior to thrombin stimulation (0.5 units/ml) for 2 min. ERK1/2 activation was determined as in Fig. 1. Where indicated, samples were preincubated for 30 min with SN-6 (10 μm). B, optical densities of the phospho-ERK1/2 bands in A were normalized against the ERK1/2 total protein of the corresponding sample. The normalized density of the unstimulated control (1st bar) was set to 1. The mean value of the ratio for each condition is expressed as fold × unstimulated control value. Bars represent the means ± S.E. from n = 3 experiments. *, p < 0.05 versus the thrombin-stimulated control.
Ca2+ Influx through Reverse-mode NCX Is Required for ERK1/2 Activation Downstream of PAR-1
Thrombin can also activate EC signaling independently of PAR ligation. For example, thrombin-binding thrombomodulin EC receptors can elicit Ca2+ signals and ERK1/2 activation (32). Consequently, HUVECs were stimulated with the specific PAR-1 peptide agonist TFLLR-NH2 (8). The Ca2+ chelator BAPTA-AM suppressed ERK1/2 activation in response to TFLLR-NH2 (Fig. 4A), and the reverse-mode NCX inhibitors SEA0400 and SN-6 inhibited ERK1/2 phosphorylation in a dose-dependent (Fig. 4B) and time-dependent manner (Fig. 4C). Moreover, siRNA-mediated knockdown of NCX1 expression attenuated TFLLR-NH2-induced ERK1/2 phosphorylation (Fig. 4D). NCX1 protein levels were assessed by Western blot in parallel transfections (Fig. 2H). Ouabain pretreatment also augmented ERK1/2 activation, and this was inhibited by SN-6 (Fig. 4E). Pretreatment with BAPTA-AM, SEA0400, and NCX1 siRNA did not affect phospho-PLCβ3 levels in response to TFLLR-NH2, suggesting that, as in the case of thrombin, these agents act downstream of PAR-1.
FIGURE 4.
Reverse-mode NCX activity is required for ERK1/2 activation by a peptide agonist of PAR-1. A, HUVECs loaded with BAPTA-AM were challenged with the PAR-1 peptide agonist TFLLR-NH2 (25 μm for 5 min). ERK1/2 and PLCβ3 activation was determined as in Fig. 1A (n = 3). B, HUVECs, preincubated with SEA0400, were stimulated with TFLLR-NH2 (25 μm for 5 min). ERK1/2 and PLCβ3 phosphorylation was determined as before (n = 3). C, HUVECs were preincubated with SN-6 and stimulated with TFLLR-NH2 for the indicated times. D, HUVECs transfected with NCX1 or control siRNA as in Fig. 2F were stimulated with TFLLR-NH2 (25 μm for 5 min); ERK1/2 and PLCβ3 activation was evaluated as in Fig. 1A. NCX1 protein knockdown for this experiment is shown in Fig. 2H, in cells transfected in parallel. E, HUVECs were treated with ouabain (100 μm for 1 min) prior to stimulation with TFLLR-NH2 (5 μm for 2 min). Where indicated, samples were preincubated with SN-6 (10 μm) for 30min. For A–F, n = 3.
Inhibitors of Reverse-mode NCX Suppress Thrombin-induced Angiogenesis
Thrombin and/or PAR-1 activation induces angiogenesis (11, 21, 33), and some of the pro-angiogenic effects of PAR activation are ERK1/2-mediated (34). Hence, we investigated the effect of reverse-mode NCX inhibitors on thrombin-induced angiogenesis. KB-R7943, SN-6, or SEA0400 dose-dependently suppressed thrombin-induced EC tubular differentiation (Fig. 5, A and B). Thrombin (0.5 units/ml for 18 h) potentiated EC cord formation on growth factor-reduced MatrigelTM by 1.41 ± 0.02-fold compared with controls (n = 3 in duplicate), in agreement with a previous study (21). Conversely, a panel of reverse-mode NCX inhibitors, KB-R7984 (10 μm), SN-6 (10 μm), and SEA0400 (1 μm), attenuated EC cord length to 0.33 ± 0.05-, 0.56 ± 0.02-, and 0.66 ± 0.02-fold of control, respectively (n = 3 in duplicate). Reverse-mode NCX inhibitors also significantly reduced EC tubular differentiation quantified as the number of endothelial branches per field of view (Fig. 5C). SEA0400 and SN-6, at the maximal concentrations used here, did not significantly affect HUVEC tubulogenesis under baseline conditions (Fig. 5, D and E). On the contrary, KB-R7984 significantly reduced HUVEC tubule length to 47.9 ± 13.9% of control (Fig. 5, D and E). This is not surprising because KB-R7984 is known to affect nonspecifically other ionic mechanisms at concentrations that also inhibit NCX (12, 14, 18).
FIGURE 5.
Reverse-mode NCX is required for thrombin-induced angiogenesis. HUVECs in Opti-MEM-I® medium containing 2% v/v FCS, 0.1% w/v BSA with or without thrombin (0.5 units/ml) were seeded in 24-well plates precoated with growth factor-reduced MatrigelTM in the presence of KB-R7943, SN-6, SEA0400, or vehicle. Images were obtained after 18 h. A, representative images at ×4 magnification are shown for key conditions. Tubule length (B) or branching points per field of view (C) from five fields of view at ×10 magnification were quantified. Mean tubule length of unstimulated controls (C) was set at 1. Bars represent means ± S.E. (n = 3 in duplicate). *, p < 0.05 versus the thrombin-stimulated control. D, representative images of HUVECs treated with the indicated concentrations of reverse-mode NCX inhibitors as in A without thrombin stimulation. E, quantification of tubule length for the cells in D was done as in B. F, images of HUVECs transfected with control (C) or NCX1-targeting (NCX1) siRNA with or without thrombin (Thr; 0.5 units/ml) and seeded on MatrigelTM as above for 6 h. G, quantification of tubule length from n = 4 in duplicate experiments was carried out as in B. H, HUVECs in Opti-MEM-I® containing 0.1% w/v BSA, 1 μg/ml hydrocortisone, and the indicated concentrations of KB-R7943, SN-6, SEA0400, or vehicle were seeded in 96-well plates precoated with collagen-I, with or without thrombin (0.5 units/ml). Cell numbers were determined by alkaline phosphatase assay after 48 h. The absorbance of the no-thrombin control was set to 1. Bars represent means ± S.E. (n = 3 in triplicate). *, p < 0.05 versus the thrombin-stimulated control. I, effect of KB-R7943 (10 μm), SN-6 (10 μm), or SEA0400 (1 μm) on HUVEC proliferation/viability in the absence of thrombin was assessed as in H. *, p < 0.05 versus the control.
Similarly, in HUVECs transfected with control siRNA (100 nm for 48 h) thrombin application (0.5 units/ml) for 6 h resulted in a significant increase in the length of EC tubules to 1.34 ± 0.04-fold of the control value, set at 1 (n = 4 in duplicate) (Fig. 5, F and G). Conversely, when HUVECs transfected with NCX1 targeting siRNA were stimulated with thrombin, we did not observe any significant increase in tubule length in comparison with the unstimulated control (0.98 ± 0.09; n = 4 in duplicate), whereas NCX1 knockdown did not have any discernible effects on tubule length at baseline condition (1.01 ± 0.07-fold of unstimulated control; n = 4 in duplicate).
Thrombin is also mitogenic for HUVECs (21). Thrombin (0.5 units/ml for 48 h) increased the number of viable HUVECs to 1.94 ± 0.37-fold compared with controls, similar to a previous report (21). KB-R7943, SN-6, or SEA0400 dose-dependently suppressed thrombin-induced HUVEC proliferation (Fig. 5H). Specifically, KB-R7943 (10 μm), SN-6 (10 μm), and SEA0400 (1 μm) reduced the number of cells to 0.63 ± 0.11-, 0.93 ± 0.082-, and 1.13 ± 0.08-fold, respectively, compared with controls. Incubation of SEA0400 (1 μm) and SN-6 (10 μm) for 48 h did not significantly affect the viability of HUVECs in the absence of thrombin stimulation (Fig. 5I). Conversely, KB-R7943 (10 μm) treatment for 48 h reduced the number of viable HUVECs to 0.62 ± 0.04-fold of control levels (Fig. 5I), as in the tubulogenesis assays (Fig. 5E).
Effect of Reverse-mode NCX on Thrombin-induced Endothelial Permeability
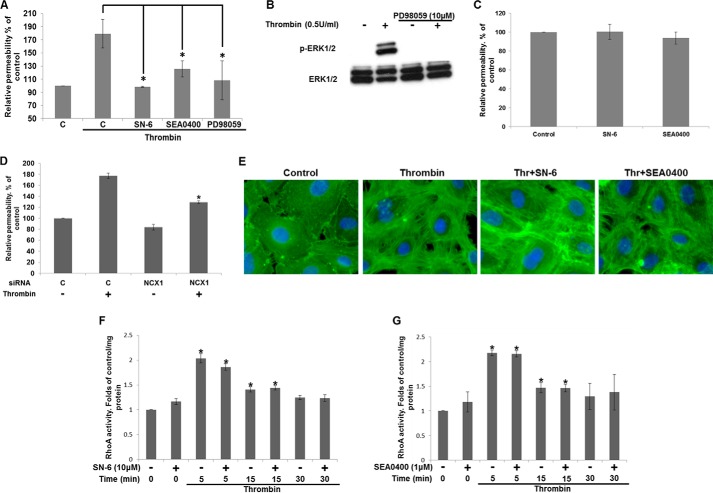
Because ERK1/2 activation has been implicated in EC barrier disruption in response to thrombin (35), we investigated the effect of reverse-mode NCX inhibitors on barrier function. Stimulation of confluent HUVEC monolayers on transwell inserts with thrombin resulted in an ∼2-fold increase in permeability after 30 min (179.26 ± 21.74% in comparison with control values arbitrarily set to 100%) (Fig. 6A), quantified as the fluorescence intensity of FITC-labeled dextran (Mr 40,000) in the lower chamber. Preincubation of the HUVEC monolayer with SN-6 (10 μm) or SEA0400 (1 μm) resulted in a significant decrease in fluorescence compared with controls (98.49 ± 1.22 and 125.46 ± 12.24% fluorescence of unstimulated control, respectively) (Fig. 6A). The ERK1/2 pathway inhibitor PD98059 at a concentration (10 μm) that abolished thrombin-induced ERK1/2 activation (Fig. 6B) also significantly suppressed EC permeability to 108.19 ± 29.7 of control (Fig. 6A), in agreement with a previous report (35). SEA0400 (1 μm) and SN-6 (10 μm) did not significantly affect unstimulated HUVEC monolayer barrier function (Fig. 6C). Thus, our results suggest that reverse-mode NCX activity is required for thrombin-induced endothelial barrier disruption, potentially upstream of ERK1/2 activation. This was confirmed using NCX1 siRNA.
FIGURE 6.
Effect of reverse-mode NCX activity on EC permeability. A, HUVECs seeded into the top chamber of transwell inserts (0.4 μm pore size), coated with collagen-I, were cultured for 2 days. Cells were preincubated with SN-6 (10 μm), SEA0400 (1 μm), or PD98059 (10 μm) prior to thrombin stimulation. FITC-dextran was simultaneously added to the top chamber, and fluorescence in the bottom chamber was measured after 30 min. The absorbance of the control was set to 100%. Bars represent the means ± S.E. (n = 3 in duplicate). *, p < 0.05 versus thrombin-stimulated control. B, serum-starved HUVECs were preincubated with the MEK inhibitor PD98059 (10 μm) or vehicle (0.5% v/v Me2SO) for 30 min prior to thrombin stimulation (0.5 units/ml for 5 min). ERK1/2 phosphorylation was determined by Western blot, n = 3. C, effect of SN-6 (10 μm) or SEA0400 (1 μm) on barrier function at baseline conditions was determined as in A. Bars represent the means ± S.E. (n = 3 in duplicate). D, HUVECs transfected with control or NCX1 targeting siRNA were seeded on transwells, and after 24 h changes in permeability in response to thrombin were assessed as in A. E, subconfluent HUVEC monolayers on collagen-I coated coverslips were serum-starved, preincubated for 30 min with SEA0400 (1 μm), SN-6 (10 μm), or vehicle and stimulated with thrombin (0.5 units/ml) for 30 min. Cells were fixed with 4% paraformaldehyde, permeabilized, and incubated with FITC-conjugated phalloidin. Nuclei were stained with DAPI. Images were captured at ×40 magnification. HUVECs preincubated with SN-6 (10 μm) (F) or SEA0400 (1 μm) (G) were stimulated with thrombin (0.5 units/ml) as indicated. RhoA activity was determined with an ELISA-based kit. Absorbance was corrected for protein concentration and expressed as -fold control levels (set to 1). Bars are the means ±S.E. *, p < 0.05 versus control (n = 3).
Thrombin stimulation of HUVECs transfected with nontargeting siRNA duplexes resulted in a 177.37 ± 4.60% fluorescence increase compared with unstimulated controls (set to 100%). The permeability of the thrombin-stimulated NCX knockdown cells was reduced to 129.28 ± 2.72% (Fig. 6D). Knocking down NCX1 in unstimulated HUVECs reduced permeability to 83.88 ± 5.12% of control, but this was not statistically significant.
Effect of Reverse-mode NCX on Stress Fiber Formation and RhoA Activity in Response to Thrombin
In ECs, thrombin promotes stress fiber formation, resulting in contraction and the formation of inter-endothelial gaps (1, 2). Visualization of polymerized actin with FITC-phalloidin revealed a primarily peripheral distribution (Fig. 6E). Thrombin (0.5 units/ml, 30 min) induced marked morphological changes characterized by stress fiber formation, an apparent EC contraction, and the appearance of inter-endothelial gaps (Fig. 6E). Preincubation with SEA0400 or SN-6, did not apparently inhibit the formation of stress fibers; however, they appeared to remain at the cell periphery in association with more spread EC phenotypes and preserved cell-cell contacts (Fig. 6E).
The small Rho-GTPase RhoA is activated by thrombin and required for stress fiber formation (1, 2); moreover, Ca2+ influx regulates RhoA activity (36). Exposure to thrombin for 5 min resulted in a 2-fold increase in RhoA activity (2.03 ± 0.10) compared with controls (set to 1), gradually declining over time (Fig. 6F), similar to a previous study (37). SN-6 or SEA0400 had no significant effect on RhoA activity (Fig. 6, F and G, respectively). Thus, the differences in permeability following reverse-mode NCX inhibition were probably independent of RhoA activity.
Involvement of Reverse-mode NCX in ROS Generation
We have shown that Ca2+ influx through reverse-mode NCX regulates VEGF-induced ERK1/2 activation by the targeting and subsequent activation of PKCα to the plasma membrane (18), and thrombin-induced PKCα activation has also been reported to require Ca2+ influx and regulate barrier integrity (38). The broad spectrum PKC inhibitor Gö6983 had no discernible effect on thrombin-mediated ERK1/2 activation (Fig. 7A) but did inhibit VEGF-induced ERK1/2 activation in parallel experiments (data not shown), as we recently described (18). Thus, we infer that reverse-mode NCX possibly suppressed ERK1/2 activation independently of PKCs.
FIGURE 7.
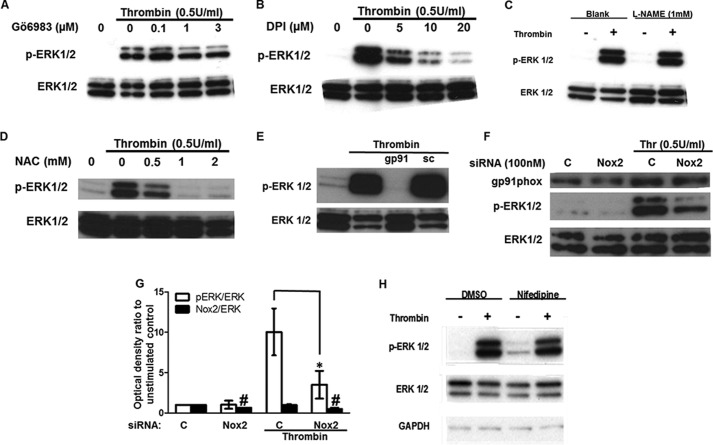
ROS generation by NADPH oxidase 2 is required for ERK1/2 activation downstream of thrombin. A, HUVECs preincubated with the PKC inhibitor Gö6983 were stimulated with thrombin (0.5 units/ml) for 5 min. Serum-starved HUVECs were preincubated the inhibitor of NADPH oxidases DPI (B), the inhibitor of NOS l-NAME (1 mm) (C), the ROS scavenger NAC (D), or the peptide inhibitor of Nox2 assembly gp91 ds-tat (50 μm), or its scrambled analogue (sc; E), prior to thrombin stimulation. F, HUVECs transfected with nontargeting or Nox2-targeting siRNA were serum-starved and then stimulated with thrombin. ERK1/2 activation and Nox2 protein levels were determined by Western blot (n = 3). G, optical densities of the phospho-ERK1/2 and gp91Phox (Nox2) bands in F were normalized against the ERK1/2 total protein of the corresponding sample. The normalized density of the unstimulated control (bar C) was set to 1. The mean value of the ratio for each condition is expressed as fold × unstimulated control value. Bars represent the means ± S.E. from n = 3 experiments. #, p < 0.05 when compared with control Nox2 levels; *, p < 0.05 versus the thrombin-stimulated control. H, HUVECs were incubated with 1 μm nifedipine for 30 min prior to stimulation with thrombin (0.5 units/ml) for 5 min (n = 3).
NADPH oxidase 2 (Nox2) activation and subsequent ROS production has been implicated in thrombin-induced angiogenesis (33). Thus, we investigated the effect of Nox inhibition on ERK1/2 phosphorylation by thrombin. The inhibitor of ROS formation by flavoenzyme DPI dose-dependently suppressed ERK1/2 activation by thrombin (Fig. 7B). Because DPI would also inhibit the endothelial nitric oxide synthase, l-NAME, a NOS inhibitor had no effect on phospho-ERK1/2 levels (Fig. 7C). Moreover, the ROS scavenger N-acetyl-l-cysteine (NAC), dose-dependently attenuated ERK1/2 phosphorylation (Fig. 7D), and gp91 ds-tat, a specific peptide inhibitor of Nox2 assembly, suppressed thrombin-induced ERK1/2 activation, although a scrambled peptide had no effect (Fig. 7E). To further corroborate the involvement of Nox2-generated ROS in thrombin-induced ERK1/2 activation in HUVECs, we knocked down the catalytic subunit of Nox2 gp91Phox (Nox2 thereafter) with siRNA. Targeting Nox2 expression resulted in an ∼50% decrease at the protein level (Fig. 7, F and G). Thrombin stimulation of HUVECs transfected with nontargeting control siRNA increased ERK1/2 phosphorylation by 10.05 ± 2.9 when compared with the unstimulated control (set to 1), and this was reduced to 3.50 ± 1.7 of control in cells transfected with Nox2 siRNA (Fig. 7, F and G).
The Ca2+-sensitive NADPH oxidase Nox5 has been implicated in ERK1/2 phosphorylation downstream of angiotensin-II (39). We attempted to investigate the role of Nox5 in thrombin-induced ERK1/2 activation by knocking down Nox5 with siRNA. However, although Nox5 siRNA had no effect on ERK1/2 phosphorylation (data not shown), we could not confirm Nox5 protein knockdown using a commercially available antibody; hence, the involvement of Nox5 in this pathway cannot be categorically excluded.
In the study of Montezano et al. (39), Ca2+ influx through L-type voltage-gated Ca2+ channels was required for ERK1/2 activation by angiotensin-II upstream of Nox5. Preincubation of HUVECs with the L-type voltage-gate Ca2+ channel inhibitor nifedipine had no apparent effect on thrombin-induced ERK1/2 phosphorylation (Fig. 7H).
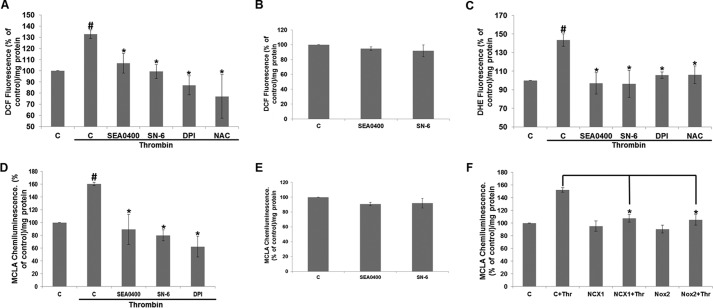
Next, we investigated the effect of SEA0400 (1 μm) and SN-6 (10 μm) on thrombin-induced ROS generation. Thrombin stimulation resulted in an increase in the emitted fluorescence of the ROS indicator DCF to 132 ± 3.9% of the unstimulated control (set to 100%) per mg of protein (Fig. 8A). SEA0400 and SN-6 significantly attenuated ROS production in response to thrombin to 106.7 ± 8.8 and 99.3 ± 6.3% of control, respectively. The Nox inhibitor DPI (10 μm) and the ROS scavenger NAC (1 mm) also suppressed DCF fluorescence to 87.0 ± 8.5 and 77.0 ± 19.5% of control value, respectively. Conversely, SEA0400 and SN-6 did not have a significant effect on DCF fluorescence under baseline conditions (Fig. 8B). Similar results were obtained with a second ROS indicator, DHE. Thrombin stimulation increased DHE fluorescence to 143.4 ± 6.8% of control (set to 100%) corrected for protein, and SEA0400, SN-6, DPI, or NAC preincubation suppressed DHE fluorescence to 96.9 ± 11.6, 96.4 ± 14.5, 105.8 ± 3.3, and 106.1 ± 9.4% of control, respectively (Fig. 8C). The involvement of reverse-mode NCX in thrombin-induced ROS production was further corroborated by the use of the lucigenin derivative MCLA that emits light when reacting with superoxide. Stimulation of HUVECs with thrombin resulted in an increase in the emitted chemiluminescence to 160.62 ± 2.65% of control (arbitrarily set to 100%) corrected for protein and measured over 10 min. Preincubation of HUVECs for 30 min prior to thrombin stimulation with SEA0400 (1 μm), SN-6 (10 μm), or DPI (10 μm) attenuated chemiluminescence to 89.41 ± 23.76, 79.95 ± 8.54, and 62.36 ± 16.27% of control, respectively (Fig. 8D). As in the case of DCF, SEA0400 or SN-6 did not significantly affect MCLA chemiluminescence of nonstimulated serum-starved HUVECs (Fig. 8E). The involvement of NCX1 and Nox2 activities in thrombin-induced superoxide generation was further supported by the use of RNAi. Thrombin stimulation of HUVECs transfected with control siRNA resulted in an increase in the emitted chemiluminescence of 152.0 ± 3.5% when compared with unstimulated controls (set to 100%). Stimulation of HUVECs transfected with NCX1- or Nox2-targeting siRNA significantly reduced chemiluminescence to 107.8 ± 6.2 and 105.2 ± 8.3% of controls, respectively (Fig. 8D). Knockdown of NCX1 or Nox2 protein did not appear to have a significant effect on HUVEC baseline superoxide production (Fig. 8F). Taken together, our data suggest that Ca2+ influx through reverse-mode NCX could be facilitating thrombin-induced ERK1/2 phosphorylation by modulating Nox2 activity and subsequent ROS production.
FIGURE 8.
Reverse-mode NCX regulates ROS production in response to thrombin. Reverse-mode NCX inhibitors SEA0400 (1 μm) or SN-6 (10 μm), the Nox inhibitor DPI (10 μm), and the ROS scavenger NAC (1 mm) attenuated thrombin-induced ROS generation measured by the fluorescent indicators DCF (A) or DHE (C), or by chemiluminescence (D) as detailed under “Experimental Procedures.” SEA0400 (1 μm) or SN-6 (10 μm) did not have a significant effect on basal ROS generation as measured by fluorescence (B) or chemiluminescence (E). F, HUVECs were transfected with siRNA targeting NCX1 (NCX1) or Nox2 (Nox2). 24 h post-transfection, the cells were plated in 96-well plates and the following day were stimulated with thrombin (Thr) or vehicle as indicated. ROS production was measured with chemiluminescence as described under “Experimental Procedures.” A–D, fluorescence or chemiluminescence was corrected for protein concentration and expressed as % of control levels (set to 100%). Bars are the means ±S.E. #, *, p < 0.05 versus the unstimulated or stimulated controls, respectively (n = 3 in triplicate).
Reverse-mode NCX Inhibitor Preserves Endothelial Barrier Function in Vivo
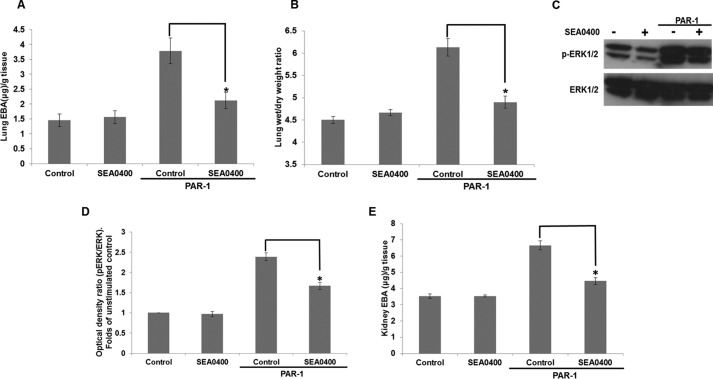
Finally, we investigated the in vivo relevance of our cell-based data. C57BL/6 mice were treated with vehicle or 10 mg/kg SEA0400 by oral gavage for 2 h (27) prior to administration of the PAR-1-activating peptide (1 mg/kg) together with EBA (20 mg/kg) via the tail vein to measure vascular permeability according to published methods (38) with some modifications (see “Experimental Procedures”). PAR-1 activation significantly increased EBA extravasation from 1.46 ± 0.20 and 3.54 ± 0.14 μg of EBA/g of wet tissue in the lungs and kidneys of control animals to 3.78 ± 0.43 and 6.67 ± 0.27 μg as determined by formamide extraction. Conversely, SEA0400 decreased EBA to 2.12 ± 0.27 and 4.47 ± 0.21 μg of EBA/g of wet tissue in the lungs and kidneys (Fig. 9, A and E). Additionally, PAR-1 stimulation increased the lung wet/dry weight ratio, an indicator of edema formation (38), from 4.50 ± 0.08 to 6.13 ± 0.20, and SEA0400 attenuated this to 4.90 ± 0.14 (Fig. 9B). Moreover, PAR-1 increased ERK1/2 phosphorylation in the lungs of these animals to 2.39 ± 0.10-fold of vehicle-treated controls (arbitrarily set to 1), and SEA0400 decreased ERK1/2 phosphorylation to 1.67 ± 0.09 (Fig. 9, C and D).
FIGURE 9.
SEA0400 attenuates endothelial barrier dysfunction and edema formation in response to PAR-1 activation in vivo. C57BL/6 mice received 10 mg/kg SEA0400 by oral gavage. Two hours later, animals were injected i.p. with EBA (20 mg/kg) solution and, where appropriate, 1 mg/kg PAR-1 peptide agonist. After 30 min, animals were culled, and blood was collected for EBA concentration levels. Mice were then perfused with PBS containing 5 mm EDTA, and tissues were collected. A, EBA content of the lung tissue was determined with formamide extraction (data not shown). Bars represent means ± S.E. μg of EBA corrected for tissue weight (n = 4–7). *, p < 0.05 versus the PAR-stimulated control. B, pulmonary edema determination was assessed by the ratio of wet/dry lung weight (C). ERK1/2 activation was assessed by Western blot of lung tissue as described under “Experimental Procedures.” D, densitometric analysis of ERK1/2 phosphorylation of Western blots in C was performed as in Fig. 1E, n = 4. E, EBA extravasation in the kidneys of experimental animals was determined as in A, n = 4–7.
Our findings suggest, in agreement with our in vitro studies, that reverse-mode NCX activity is required for enhanced vascular permeability in response to PAR-1 activation, possibly upstream of ERK1/2 activation.
Discussion
In this study, we report that Ca2+ influx through reverse-mode NCX can modulate EC responses to thrombin downstream of PAR-1 by influencing ERK1/2 activation, as we described previously for VEGF (18).
Role of Reverse-mode NCX in ERK1/2 Activation by Thrombin
Reverse-mode NCX activity was required for thrombin-induced ERK1/2 activation (Fig. 1). ERK1/2 phosphorylation was suppressed by siRNA-mediated knockdown of NCX1 (Fig. 2), whereas favoring reverse-mode NCX (by inhibiting the Na+-K+-ATPase, thus loading ECs with Na+), promoted ERK1/2 phosphorylation (Fig. 3). Similarly, reverse-mode NCX inhibitors and siRNA-suppressed ERK1/2 activation and ouabain treatment augmented ERK1/2 phosphorylation in response to a PAR-1 peptide agonist, indicating that NCX could act downstream of the G-protein-coupled receptor PAR-1 (Fig. 4).
The inhibitors used here are proposed to inhibit reverse-mode NCX by accelerating and stabilizing the Na+-dependent inactivated state of the exchanger (14, 40, 41), and at the concentrations used, they are not expected to affect the forward mode of NCX. Nonetheless, although the more recent inhibitors SN-6 and SEA0400 are more selective for the reverse mode NCX (14), there is evidence that they could have off-target effects, because in mouse embryos where the NCX1 gene was deleted, SEA0400 suppressed to some extent Ca2+ transients in cardiomyocytes (42).
To control for any off-target effects, we employed at least two chemically distinct inhibitors in each assay and used siRNA to knock down NCX1 in HUVECs. Nonetheless, knocking down NCX1 protein also abolishes the forward mode of the exchanger, and this could ultimately result in Ca2+ overload and/or compensatory changes in the gene expression of other Ca2+-handling proteins. Consequently, we opted for the siRNA transfection conditions (100 nm siRNA for 48 h) that produced the clearest result in the functional assays (ERK1/2 activation, permeability, and tubulogenesis) with the minimum knockdown of NCX1, as we have optimized previously (18).
As a further control we showed that by using ouabain to inhibit the Na+-K+-ATPase and thus load HUVECs with Na+, thrombin-induced ERK1/2 activation was enhanced, probably by promoting reverse-mode NCX (Fig. 3). Ouabain treatment in the absence of thrombin did not result in ERK1/2 activation (Fig. 3A), as we reported previously for VEGF-stimulated HUVECs (18). This is not surprising because human NCX1 requires an initial increase in [Ca2+]i for activation (12). Consequently, simply loading the cells with Na+ in isolation is not expected to activate reverse-mode NCX. Ouabain cannot be used for longer term functional assays (permeability and angiogenesis) because Na+ overload would also affect other ionic mechanisms.
Taken together, our findings suggest that reverse-mode NCX is implicated in thrombin-induced ERK1/2 activation, downstream of PAR-1, as we reported for VEGF-induced ERK1/2 activation and angiogenesis downstream of the receptor-tyrosine kinase VEGFR2 (18). In addition to our work in ECs, others have independently reported that reverse-mode NCX activity is required for ERK1/2 phosphorylation in cardiac fibroblasts (43) and neuroblastoma cells (44). Thus, reverse-mode NCX could modulate ERK1/2 activation downstream of multiple stimuli activating receptor-tyrosine kinases and G-protein-coupled receptors in a variety of cell types. Further work is required to support this exciting hypothesis and to elucidate the signaling pathways influenced by reverse-mode NCX in these diverse systems.
Role of Ca2+ Microdomains in ERK1/2 Activation
We demonstrated that Ca2+ influx through reverse-mode NCX was required for ERK1/2 phosphorylation in response to thrombin. However, although reverse-mode NCX inhibitors suppressed maximal ERK1/2 phosphorylation by almost 80% (Fig. 1, F and G), their impact on bulk [Ca2+]i was less pronounced (between 10 and 20%; Fig. 2D). This discrepancy could be explained by the contribution of Ca2+ microdomains in thrombin signaling in ECs, as our differential Ca2+ chelation experiments indicate (Figs. 1A and 2E). EGTA-AM is a “slow” Ca2+ chelator allowing Ca2+ to diffuse up to 100 nm from the source, although BAPTA-AM is a fast chelator binding Ca2+ (with similar affinity to EGTA-AM) within 2 nm from the source (30). The existence of Ca2+ microdomains regulating ERK1/2 activation in HUVECs is also supported by our previous work. Thapsigargin, an inhibitor of the sarcoendoplasmic reticulum Ca2+-ATPase that substantially increased [Ca2+]i due to Ca2+ leakage from the internal stores and subsequent store-operated Ca2+ entry, failed to induce ERK1/2 phosphorylation (18). Conversely, the Ca2+ ionophore ionomycin, which is expected to raise [Ca2+]i uniformly throughout the plasma membrane, induced ERK1/2 phosphorylation (19). Thus, in agreement with our work with VEGF (18, 19), local, as opposed to global, Ca2+ signals appear to affect ERK1/2 phosphorylation in response to thrombin. Consequently, because NCX is a high capacity Ca2+ transporter (12), inhibition of reverse-mode NCX could significantly modify Ca2+ signals in microdomains close to the plasma membrane, while modestly affecting global Ca2+ signals. Thus, reverse-mode NCX activity could conceivably have a major impact on signaling events requiring Ca2+ at the locality of the plasma membrane, providing spatial resolution to such a signal.
Interestingly, in the [Ca2+]i experiments shown in Fig. 2, thrombin stimulation appeared to result in a higher steady-state [Ca2+]i, after the initial peak of the Ca2+ signal, when compared with unstimulated controls. Moreover, reverse-mode NCX inhibitors suppressed this increase in steady-state [Ca2+]i, suggesting that reverse-mode NCX activity could be involved. Nonetheless, further experimental work with thrombin-stimulated ECs in nominal Ca2+-free buffers (to estimate the relative contribution of Ca2+ influx on bulk [Ca2+]i) and with the use of inhibitors or siRNA of other Ca2+-influx mechanisms known to be activated by thrombin in ECs, would be required to conclude that this steady-state increase in [Ca2+]i occurs primarily via NCX activation, as the pharmacology indicates.
Reverse-mode NCX in Thrombin-induced Angiogenesis
Thrombin promotes in vitro and in vivo angiogenesis (11, 21, 34). The complex angiogenic response can be broken down to elementary processes, including EC adhesion to substrate, proliferation, motility, tubular differentiation, and permeability (45). Reverse-mode NCX inhibitors and siRNA attenuated thrombin-induced tubular differentiation and the same inhibitors suppressed EC proliferation in response to thrombin (Fig. 5). ERK1/2 phosphorylation has been reported to be crucial for EC tubulogenesis (46) and mitogenesis (47) in response to thrombin. Moreover, in cancers, endothelial ERK1/2 activation by PAR signaling has been implicated in resistance to angiogenic therapy (34), and activation of PAR-1 expressed on ECs by tumor-secreted matrix metalloproteinases promotes tumor progression (48). Consequently, it is probable that reverse-mode NCX activity regulates the angiogenic response to thrombin by promoting ERK1/2 activation.
Interestingly, the more selective reverse-mode NCX inhibitors SEA0400 and SN-6 significantly reduced tubulogenesis below unstimulated control levels only in thrombin-stimulated HUVECs (Fig. 5B), although their effect on tubulogenesis in unstimulated controls was minimal (Fig. 5E). A crucial difference of the tubulogenesis experiments shown in Fig. 5 compared with all the other assays (where the effect of SEA0400 or SN-6 under basal conditions is minimal) is that inhibitors and stimulant were applied when cells were in suspension. It is feasible that thrombin has an additional effect on EC adhesion and/or spreading on extracellular matrix components that is also inhibited by SEA0400 and SN-6, thus resulting in more pronounced suppression of tubule formation in the thrombin-stimulated samples. More experimental work is needed to investigate this intriguing possibility.
Anti-angiogenic monotherapy often fails due to adaptive resistance (e.g. ECs switching to a different pro-angiogenic pathway) (4). Taken together, our findings with thrombin described here and our previous work with VEGF (18, 19) suggest that reverse-mode NCX activity could be required for ERK1/2 activation and the angiogenic response downstream of diverse pathways. Thus, inhibitors of reverse-mode NCX could potentially be advantageous for the treatment of cancers resistant to conventional anti-angiogenic agents. The use of reverse-mode NCX inhibitors in in vivo tumor models merit further investigation.
Thrombin-induced ERK1/2 Activation and Vascular Permeability
Reverse-mode NCX inhibitors and siRNA targeting NCX1 preserved EC barrier function in response to thrombin stimulation in vitro (Fig. 6) and in vivo (Fig. 9), probably due to the inhibition of ERK1/2 activation. Unlike the role of ERK1/2 in thrombin-induced angiogenesis (34, 46, 47), its function as a regulator of endothelial permeability is less clear. Suppression of ERK1/2 phosphorylation prevented endothelial barrier disruption and maintained cortical actin levels in response to thrombin in HUVECs (35), in agreement with our data (Fig. 6A). This was further supported by the finding that ERK1/2 pathway inhibitors alleviated endothelial hyperpermeability in response to endotoxin insult in vivo (49), a process proposed to involve PAR-1 transactivation by matrix metalloproteinases (50). Nonetheless, the issue is still controversial, and the role of ERK1/2 activity in thrombin-induced HUVEC barrier disruption has been challenged (37). Moreover, there is no conclusive mechanistic evidence linking ERK1/2 activity and permeability in ECs. Recently, ERK1/2 was shown to directly regulate barrier function by modulating the association of the tight junction protein zona occludens-1 (ZO-1) with occludin in ECs stimulated with the arachidonic acid metabolite 15(S)-hydroxyeicosatetraenoic acid (51). Whether a similar mechanism operates in thrombin-stimulated ECs remains to be determined.
Ca2+ Influx through Reverse-mode NCX as a Mediator of Vascular Permeability
A role for Ca2+ in endothelial barrier disruption by thrombin has been demonstrated in numerous in vitro and in vivo models. The most well documented is the role of Ca2+ influx through the nonselective cation channel canonical transient receptor potential (TRPC)-1 and -6. In particular, TRPC6 was required for RhoA and PKCα activation and subsequent EC barrier dysfunction, and TRPC1 was required for NF-κB activation downstream of thrombin (1, 2, 36, 38). In endotoxin-induced acute lung injury, a model characterized by extensive endothelial hyperpermeability attributed, at least partially, to PAR-1 activation (50), inhibition of Ca2+ signaling through TRPC6 (52) or stromal interaction molecule-1 (STIM1) (53) enhanced EC barrier function and improved survival.
In a previous study investigating the role of ERK1/2 activation in EC thrombin signaling (35), ERK1/2 inhibition did not affect myosin light chain kinase activity, which is downstream of RhoA activation and regulates stress fiber formation (1, 2). Moreover, the investigators reported that inhibiting the ERK1/2 pathway activity resulted in the appearance of stress fibers at the EC periphery (35), similar to our findings with reverse-mode NCX inhibitors (Fig. 6E). Given that Ca2+ influx through TRPC6 activates RhoA by thrombin (36), and reverse-mode NCX inhibitors did not affect RhoA activation (Fig. 6, F and G), reverse-mode NCX and TRPC6 possibly regulate distinct Ca2+ influx pathways. Alternatively, given the increasing evidence suggesting that TRPC3- and/or TRPC6-mediated Na+ entry promotes reverse-mode NCX in smooth muscle cells (54), cardiomyocytes (55), and recently platelets (56), a proportion of TRPCs could be functionally coupled with NCX linking PLC activation to reverse-mode NCX in ECs. The role of TRPC3 and/or TRPC6 in ERK1/2 activation in ECs is unclear and merits investigation. Additionally, reverse-mode NCX could also be functionally associated with other ionic mechanisms, such as voltage-gated Na+ channels, via the membrane potential, which could also influence the direction of the exchanger (12, 19), or the Na+-K+-ATPase, by controlling [Na+] (Fig. 3A).
Our research suggests that reverse-mode NCX is a novel, complementary Ca2+ influx mechanism contributing to thrombin-induced angiogenesis and permeability, at least partially by mediating ERK1/2 activation. Thus, reverse-mode NCX, in cooperation with other associated ionic mechanism(s) would further fine-tune the complex EC response to PAR-1 activation, providing spatiotemporal resolution to the Ca2+ signal and subsequent ERK1/2 activation and also linking the signal intensity to transmembrane [Na+] and [Ca2+], the membrane potential, and the energy status of the cell.
Mechanistic Studies
ROS generation and Nox activity (presumably Nox2) were required for thrombin-induced ERK1/2 phosphorylation (Fig. 7); also reverse-mode NCX inhibitors and siRNA suppressed thrombin-induced ROS production (Fig. 8). ROS are well established to cause barrier dysfunction (57), and thrombin is reported to increase ROS production in ECs primarily via Nox2 (33). Moreover, Ca2+ signaling and Nox2 activity have been implicated in endothelial hyperpermeability in models of liposaccharide-induced vascular inflammation (53) and lung ischemia-reperfusion injury (58) in mice. An inhibitory peptide of Nox2 assembly and knockdown of its catalytic subunit attenuated thrombin-induced ERK1/2 activation (Fig. 7, E and F), whereas siRNA targeting Nox5 had no effect, although Nox5 protein knockdown could not be confirmed by Western blot (data not shown). Consequently, involvement of Nox5 on thrombin-induced ERK1/2 activation cannot be categorically excluded. Moreover, nifedipine, an inhibitor of L-type voltage-gated Ca2+ channels showed no effect on ERK1/2 activation (Fig. 7H), suggesting that these channels do not play a role in ERK1/2 activation by thrombin.
Collectively, our data suggest that reverse-mode NCX could regulate Nox (presumably Nox2)-mediated ROS production, subsequent ERK1/2 activation, and thrombin-induced angiogenesis and hyperpermeability, possibly in a spatially restricted manner (Fig. 10). However, Nox2 is not Ca2+-sensitive per se, and further work is required to delineate the putative Ca2+-sensitive pathway linking reverse-mode NCX to Nox2 activation.
FIGURE 10.
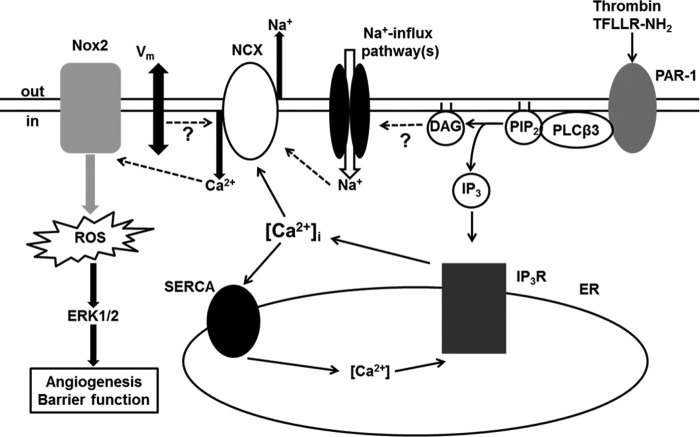
Proposed mechanistic model. Bold lines show direct interactions. Dashed lines show indirect interactions. Our work is consistent with the following simplified mechanistic model. Activation of PLCβ3 downstream of protease-activated receptor 1 (PAR-1) leads to the generation of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 binds to and activates the endoplasmic reticulum (ER) IP3 receptors (IP3Rs), leading to Ca2+ release from the internal stores, a rise in cytosolic Ca2+ concentration ([Ca2+]i), and the activation of the plasma membrane NCX. Concurrently, associated Na+-influx pathways that could be diacylglycerol-sensitive and/or plasma membrane depolarization (Vm) could lead to induction of reverse-mode NCX and Ca2+ influx. This Ca2+ influx promotes indirectly, via a yet unidentified Ca2+-sensitive mechanism, the assembly and subsequent activation of the NADPH oxidase 2 complex, resulting in ROS generation, ERK1/2 activation, and ultimately diminished endothelial barrier function and a pro-angiogenic phenotype. Ca2+ uptake via the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) could switch off the Ca2+ response. Other associated ionic mechanisms not depicted here for the sake of simplicity, such as mitochondrial Ca2+-uptake and/or store operated Ca2+ entry could further fine-tune indirectly the degree of NCX activation/reversal impacting on the functional output.
In Vivo Studies
The physiological relevance of our cell-based studies was strengthened by our finding that SEA0400 application in vivo attenuated EBA extravasation, edema formation, and ERK1/2 activation in the lungs of mice stimulated with a PAR-1-specific agonist (Fig. 9). SEA0400 also enhanced endothelial barrier function in the kidneys of these animals suggesting that reverse-mode NCX could be playing a functional role in diverse tissue vascular beds. PAR-1 activation was preferred to global thrombin application because thrombin would also activate the coagulation cascade, and thrombin-induced microvascular permeability in mouse lung has been attributed primarily to PAR-1 activation (59).
Limitations of the Study and Concluding Remarks
To summarize, the major findings of our study are as follows: 1) reverse-mode NCX is required for thrombin-induced ERK1/2 activation; 2) reverse-mode NCX activity regulates angiogenesis in vitro and endothelial permeability in vitro and in vivo in response to thrombin or PAR-1 stimulation; and 3) reverse-mode NCX activity is required for the generation of ROS downstream of thrombin via NADPH oxidases (presumably Nox2), as summarized in Fig. 10.
Despite the new information reported here, two main questions remain unanswered. First, we have not directly shown how ERK1/2 activation influences thrombin-induced EC permeability. Our work suggests that the NCX-Nox2-ROS-ERK1/2 axis acts independently of RhoA activity and adherens junction stability. An alternative mechanism is the modulation of EC tight junction disassembly. ERK1/2 has been shown to regulate the association of the tight junction proteins ZO-1 and occludin in response to 15(S)-hydroxyeicosatetraenoic acid (51) or hydrogen peroxide (60), suggesting that ROS can modulate ERK1/2 activation in ECs and impact on tight junction stability and endothelial permeability, as we propose here. Second, although the cross-talk between Nox2 activity, Ca2+ signaling, and vascular permeability has been reported in models of endothelial barrier dysfunction (53, 58), the exact molecular mechanism, especially in the case of thrombin, is not clear. Thus, further experimental work is required to firmly establish the role of ERK1/2 and determine the Ca2+-sensitive mechanism(s) regulating Nox2 activity/assembly in thrombin signaling in ECs.
Nonetheless, our work consistently suggests that Ca2+ influx through reverse-mode NCX is a novel determinant of thrombin-induced EC angiogenesis and barrier dysfunction. Collectively, given our previous work on the role of reverse-mode NCX in VEGF signaling (18, 19), inhibition of this pathway could be beneficial in conditions characterized by deregulated PAR-1 and VEGF activity, such as tumor angiogenesis and sepsis.
This work was supported by Barts and London Charity Grant 578/1128 (to M. M. Y.) and National Health Service funding to the National Institute of Health Research Biomedical Research Centre. This work forms part of the research themes contributing to the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, which is supported and funded by the National Institute of Health Research. The authors declare that they have no conflicts of interest with the contents of this article.
- PAR
- protease-activated receptor
- HUVEC
- primary human vascular endothelial cell
- NCX
- Na+/Ca2+ exchanger
- ROS
- reactive oxygen species
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester
- EC
- endothelial cell
- DPI
- diphenyleneiodonium
- MCLA
- 2-methyl-6-(p-methoxyphenyl)3,7-dihydroimidazo[1,2-a]pyrazin-3-one
- DHE
- dihydroethidium
- DCF
- 2′,7′-dichlorofluorescin diacetate
- EBA
- Evans blue albumin
- NAC
- N-acetyl-l-cysteine
- HBSS
- Hanks' balanced salt solution.
References
- 1. Mehta D., Malik A. B. (2006) Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86, 279–367 [DOI] [PubMed] [Google Scholar]
- 2. Komarova Y., Malik A. B. (2010) Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 72, 463–493 [DOI] [PubMed] [Google Scholar]
- 3. Pober J. S., Sessa W. C. (2007) Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 [DOI] [PubMed] [Google Scholar]
- 4. Goel S., Duda D. G., Xu L., Munn L. L., Boucher Y., Fukumura D., Jain R. K. (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldenberg N. M., Steinberg B. E., Slutsky A. S., Lee W. L. (2011) Broken barriers: a new take on sepsis pathogenesis. Sci. Transl. Med. 3, 88ps25. [DOI] [PubMed] [Google Scholar]
- 6. Coughlin S. R. (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814 [DOI] [PubMed] [Google Scholar]
- 7. Hirano K. (2007) The roles of proteinase-activated receptors in the vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 27, 27–36 [DOI] [PubMed] [Google Scholar]
- 8. Kataoka H., Hamilton J. R., McKemy D. D., Camerer E., Zheng Y. W., Cheng A., Griffin C., Coughlin S. R. (2003) Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 102, 3224–3231 [DOI] [PubMed] [Google Scholar]
- 9. Borissoff J. I., Spronk H. M., ten Cate H. (2011) The hemostatic system as a modulator of atherosclerosis. N. Engl. J. Med. 364, 1746–1760 [DOI] [PubMed] [Google Scholar]
- 10. Finigan J. H. (2009) The coagulation system and pulmonary endothelial function in acute lung injury. Microvasc. Res. 77, 35–38 [DOI] [PubMed] [Google Scholar]
- 11. Nierodzik M. L., Karpatkin S. (2006) Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 10, 355–362 [DOI] [PubMed] [Google Scholar]
- 12. Khananshvili D. (2013) The SLC8 gene family of sodium-calcium exchangers (NCX)- structure, function, and regulation in health and disease. Mol. Aspects Med. 34, 220–235 [DOI] [PubMed] [Google Scholar]
- 13. Imahashi K., Pott C., Goldhaber J. I., Steenbergen C., Philipson K. D., Murphy E. (2005) Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ. Res. 97, 916–921 [DOI] [PubMed] [Google Scholar]
- 14. Iwamoto T., Watanabe Y., Kita S., Blaustein M. P. (2007) Na+/Ca2+ exchange inhibitors: a new class of calcium regulators. Cardiovasc. Hematol. Disord. Drug. Targets 7, 188–198 [DOI] [PubMed] [Google Scholar]
- 15. Li L., van Breemen C. (1995) Na+-Ca2+ exchange in intact endothelium of rabbit cardiac valve. Circ. Res. 76, 396–404 [DOI] [PubMed] [Google Scholar]
- 16. Sedova M., Blatter L. A. (1999) Dynamic regulation of [Ca2+]i by plasma membrane Ca2+-ATPase and Na+/Ca2+ exchange during capacitative Ca2+ entry in bovine vascular endothelial cells. Cell Calcium 25, 333–343 [DOI] [PubMed] [Google Scholar]
- 17. Szewczyk M. M., Davis K. A., Samson S. E., Simpson F., Rangachari P. K., Grover A. K. (2007) Ca2+-pumps and Na2+-Ca2+-exchangers in coronary artery endothelium versus smooth muscle. J. Cell. Mol. Med. 11, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrikopoulos P., Baba A., Matsuda T., Djamgoz M. B., Yaqoob M. M., Eccles S. A. (2011) Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J. Biol. Chem. 286, 37919–37931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrikopoulos P., Fraser S. P., Patterson L., Ahmad Z., Burcu H., Ottaviani D., Diss J. K., Box C., Eccles S. A., Djamgoz M. B. (2011) Angiogenic functions of voltage-gated Na+ channels in human endothelial cells: modulation of vascular endothelial growth factor (VEGF) signaling. J. Biol. Chem. 286, 16846–16860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keogh R. J., Houliston R. A., Wheeler-Jones C. P. (2002) Thrombin-stimulated Pyk2 phosphorylation in human endothelium is dependent on intracellular calcium and independent of protein kinase C and Src kinases. Biochem. Biophys. Res. Commun. 294, 1001–1008 [DOI] [PubMed] [Google Scholar]
- 21. Caunt M., Hu L., Tang T., Brooks P. C., Ibrahim S., Karpatkin S. (2006) Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res. 66, 4125–4132 [DOI] [PubMed] [Google Scholar]
- 22. Itagaki K., Yun J. K., Hengst J. A., Yatani A., Hauser C. J., Spolarics Z., Deitch E. A. (2007) Sphingosine 1-phosphate has dual functions in the regulation of endothelial cell permeability and Ca2+ metabolism. J. Pharmacol. Exp. Ther. 323, 186–191 [DOI] [PubMed] [Google Scholar]
- 23. Wang H., Joseph J. A. (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 27, 612–616 [DOI] [PubMed] [Google Scholar]
- 24. Kimura C., Oike M., Ito Y. (2000) Hypoxia-induced alterations in Ca2+ mobilization in brain microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 279, H2310–H2318 [DOI] [PubMed] [Google Scholar]
- 25. Peng X., Hassoun P. M., Sammani S., McVerry B. J., Burne M. J., Rabb H., Pearse D., Tuder R. M., Garcia J. G. (2004) Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 169, 1245–1251 [DOI] [PubMed] [Google Scholar]
- 26. Knezevic N., Tauseef M., Thennes T., Mehta D. (2009) The G protein βγ subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J. Exp. Med. 206, 2761–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iwamoto T., Kita S., Zhang J., Blaustein M. P., Arai Y., Yoshida S., Wakimoto K., Komuro I., Katsuragi T. (2004) Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 10, 1193–1199 [DOI] [PubMed] [Google Scholar]
- 28. Byrne C. J., McCafferty K., Kieswich J., Harwood S., Andrikopoulos P., Raftery M., Thiemermann C., Yaqoob M. M. (2012) Ischemic conditioning protects the uremic heart in a rodent model of myocardial infarction. Circulation 125, 1256–1265 [DOI] [PubMed] [Google Scholar]
- 29. Hatziapostolou M., Koukos G., Polytarchou C., Kottakis F., Serebrennikova O., Kuliopulos A., Tsichlis P. N. (2011) Tumor progression locus 2 mediates signal-induced increases in cytoplasmic calcium and cell migration. Sci. Signal. 4, ra55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parekh A. B. (2008) Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J. Physiol. 586, 3043–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang G. H., Kim M. Y., Park S., Kim J. A., Choi S., Suh S. H. (2008) Intracellular Na+ modulates large conductance Ca2+-activated K+ currents in human umbilical vein endothelial cells. Pflugers Arch. 457, 67–75 [DOI] [PubMed] [Google Scholar]
- 32. David-Dufilho M., Millanvoye-Van Brussel E., Topal G., Walch L., Brunet A., Rendu F. (2005) Endothelial thrombomodulin induces Ca2+ signals and nitric oxide synthesis through epidermal growth factor receptor kinase and calmodulin kinase II. J. Biol. Chem. 280, 35999–36006 [DOI] [PubMed] [Google Scholar]
- 33. Diebold I., Djordjevic T., Petry A., Hatzelmann A., Tenor H., Hess J., Görlach A. (2009) Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ. Res. 104, 1169–1177 [DOI] [PubMed] [Google Scholar]
- 34. Svensson K. J., Kucharzewska P., Christianson H. C., Sköld S., Löfstedt T., Johansson M. C., Mörgelin M., Bengzon J., Ruf W., Belting M. (2011) Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 108, 13147–13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borbiev T., Verin A. D., Birukova A., Liu F., Crow M. T., Garcia J. G. (2003) Role of CaM kinase II and ERK activation in thrombin-induced endothelial cell barrier dysfunction. Am. J. Physiol. Lung. Cell. Mol. Physiol. 285, L43–L54 [DOI] [PubMed] [Google Scholar]
- 36. Singh I., Knezevic N., Ahmmed G. U., Kini V., Malik A. B., Mehta D. (2007) Gαq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J. Biol. Chem. 282, 7833–7843 [DOI] [PubMed] [Google Scholar]
- 37. Wang Z., Ginnan R., Abdullaev I. F., Trebak M., Vincent P. A., Singer H. A. (2010) Calcium/calmodulin-dependent protein kinase IIδ6 (CaMKIIδ6) and RhoA involvement in thrombin-induced endothelial barrier dysfunction. J. Biol. Chem. 285, 21303–21312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vandenbroucke St Amant E., Tauseef M., Vogel S. M., Gao X. P., Mehta D., Komarova Y. A., Malik A. B. (2012) PKCα activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ. Res. 111, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montezano A. C., Burger D., Paravicini T. M., Chignalia A. Z., Yusuf H., Almasri M., He Y., Callera G. E., He G., Krause K. H., Lambeth D., Quinn M. T., Touyz R. M. (2010) Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ. Res. 106, 1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iwamoto T., Inoue Y., Ito K., Sakaue T., Kita S., Katsuragi T. (2004) The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4-(4-nitrobenzyloxy) benzyl]thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol. Pharmacol. 66, 45–55 [DOI] [PubMed] [Google Scholar]
- 41. Matsuda T., Arakawa N., Takuma K., Kishida Y., Kawasaki Y., Sakaue M., Takahashi K., Takahashi T., Suzuki T., Ota T., Hamano-Takahashi A., Onishi M., Tanaka Y., Kameo K., Baba A. (2001) SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J. Pharmacol. Exp. Ther. 298, 249–256 [PubMed] [Google Scholar]
- 42. Reuter H., Henderson S. A., Han T., Matsuda T., Baba A., Ross R. S., Goldhaber J. I., Philipson K. D. (2002) Knockout mice for pharmacological screening: testing the specificity of Na+-Ca2+ exchange inhibitors. Circ. Res. 91, 90–92 [DOI] [PubMed] [Google Scholar]
- 43. Kamimura D., Ohtani T., Sakata Y., Mano T., Takeda Y., Tamaki S., Omori Y., Tsukamoto Y., Furutani K., Komiyama Y., Yoshika M., Takahashi H., Matsuda T., Baba A., Umemura S., et al. K (2012) Ca2+ entry mode of Na+/Ca2+ exchanger as a new therapeutic target for heart failure with preserved ejection fraction. Eur. Heart J. 33, 1408–1416 [DOI] [PubMed] [Google Scholar]
- 44. Nashida T., Takuma K., Fukuda S., Kawasaki T., Takahashi T., Baba A., Ago Y., Matsuda T. (2011) The specific Na+/Ca2+ exchange inhibitor SEA0400 prevents nitric oxide-induced cytotoxicity in SH-SY5Y cells. Neurochem. Int. 59, 51–58 [DOI] [PubMed] [Google Scholar]
- 45. Eccles S. A., Court W., Patterson L., Sanderson S. (2009) In vitro assays for endothelial cell functions related to angiogenesis: proliferation, motility, tubular differentiation, and proteolysis. Methods Mol. Biol. 467, 159–181 [DOI] [PubMed] [Google Scholar]
- 46. Caunt M., Huang Y. Q., Brooks P. C., Karpatkin S. (2003) Thrombin induces neoangiogenesis in the chick chorioallantoic membrane. J. Thromb. Haemost. 1, 2097–2102 [DOI] [PubMed] [Google Scholar]
- 47. Zania P., Papaconstantinou M., Flordellis C. S., Maragoudakis M. E., Tsopanoglou N. E. (2008) Thrombin mediates mitogenesis and survival of human endothelial cells through distinct mechanisms. Am. J. Physiol. Cell Physiol. 294, C1215–C1226 [DOI] [PubMed] [Google Scholar]
- 48. Foley C. J., Fanjul-Fernández M., Bohm A., Nguyen N., Agarwal A., Austin K., Koukos G., Covic L., López-Otín C., Kuliopulos A. (2014) Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Oncogene 33, 2264–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schuh K., Pahl A. (2009) Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochem. Pharmacol. 77, 1827–1834 [DOI] [PubMed] [Google Scholar]
- 50. Tressel S. L., Kaneider N. C., Kasuda S., Foley C., Koukos G., Austin K., Agarwal A., Covic L., Opal S. M., Kuliopulos A. (2011) A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol. Med. 3, 370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chattopadhyay R., Dyukova E., Singh N. K., Ohba M., Mobley J. A., Rao G. N. (2014) Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase Cϵ-mediated zona occludens-1 phosphorylation at threonine 770/772. J. Biol. Chem. 289, 3148–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tauseef M., Knezevic N., Chava K. R., Smith M., Sukriti S., Gianaris N., Obukhov A. G., Vogel S. M., Schraufnagel D. E., Dietrich A., Birnbaumer L., Malik A. B., Mehta D. (2012) TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 209, 1953–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gandhirajan R. K., Meng S., Chandramoorthy H. C., Mallilankaraman K., Mancarella S., Gao H., Razmpour R., Yang X. F., Houser S. R., Chen J., Koch W. J., Wang H., Soboloff J., Gill D. L., Madesh M. (2013) Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Invest. 123, 887–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poburko D., Liao C. H., Lemos V. S., Lin E., Maruyama Y., Cole W. C., van Breemen C. (2007) Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ. Res. 101, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 55. Eder P., Probst D., Rosker C., Poteser M., Wolinski H., Kohlwein S. D., Romanin C., Groschner K. (2007) Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc. Res. 73, 111–119 [DOI] [PubMed] [Google Scholar]
- 56. Harper M. T., Londoño J. E., Quick K., Londoño J. C., Flockerzi V., Philipp S. E., Birnbaumer L., Freichel M., Poole A. W. (2013) Transient receptor potential channels function as a coincidence signal detector mediating phosphatidylserine exposure. Sci. Signal. 6, ra50. [DOI] [PubMed] [Google Scholar]
- 57. Jin B. Y., Lin A. J., Golan D. E., Michel T. (2012) MARCKS protein mediates hydrogen peroxide regulation of endothelial permeability. Proc. Natl. Acad. Sci. U.S.A. 109, 14864–14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weissmann N., Sydykov A., Kalwa H., Storch U., Fuchs B., Mederos y Schnitzler M., Brandes R. P., Grimminger F., Meissner M., Freichel M., Offermanns S., Veit F., Pak O., Krause K. H., Schermuly R. T., et al. (2012) Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 3, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vogel S. M., Gao X., Mehta D., Ye R. D., John T. A., Andrade-Gordon P., Tiruppathi C., Malik A. B. (2000) Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol. Genomics 4, 137–145 [DOI] [PubMed] [Google Scholar]
- 60. Kevil C. G., Oshima T., Alexander B., Coe L. L., Alexander J. S. (2000) H(2)O(2)-mediated permeability: role of MAPK and occluding. Am. J. Physiol. Cell Physiol. 279, C21–C30 [DOI] [PubMed] [Google Scholar]