Background: Expression of x2 glycosphingolipid (PX2) is elevated on erythrocytes from individuals with the rare P/P1/Pk-negative p phenotype.
Results: Globoside-deficient individuals with mutated P synthase (β1,3GalNAc-T1) lack PX2 and have anti-PX2 in plasma. Transfection of B3GALNT1 induces P and PX2 expression.
Conclusion: PX2 synthesized by β1,3GalNAc-T1 fulfills blood group criteria.
Significance: β1,3GalNAc-T1 uses different acceptors to form immunologically distinct glycosphingolipids.
Keywords: blood, erythrocyte, glycolipid, glycolipid structure, glycosyltransferase, blood group, glycosphingolipid, transfusion medicine
Abstract
The x2 glycosphingolipid is expressed on erythrocytes from individuals of all common blood group phenotypes and elevated on cells of the rare P/P1/Pk-negative p blood group phenotype. Globoside or P antigen is synthesized by UDP-N-acetylgalactosamine:globotriaosyl-ceramide 3-β-N-acetylgalactosaminyltransferase encoded by B3GALNT1. It is the most abundant non-acid glycosphingolipid on erythrocytes and displays the same terminal disaccharide, GalNAcβ3Gal, as x2. We encountered a patient with mutations in B3GALNT1 causing the rare P-deficient P1k phenotype and whose pretransfusion plasma was unexpectedly incompatible with p erythrocytes. The same phenomenon was also noted in seven other unrelated P-deficient individuals. Thin-layer chromatography, mass spectrometry, and flow cytometry were used to show that the naturally occurring antibodies made by p individuals recognize x2 and sialylated forms of x2, whereas x2 is lacking on P-deficient erythrocytes. Overexpression of B3GALNT1 resulted in synthesis of both P and x2. Knockdown experiments with siRNA against B3GALNT1 diminished x2 levels. We conclude that x2 fulfills blood group criteria and is synthesized by UDP-N-acetylgalactosamine: globotriaosylceramide 3-β-N-acetylgalactosaminyltransferase. Based on this linkage, we proposed that x2 joins P in the GLOB blood group system (ISBT 028) and is renamed PX2 (GLOB2). Thus, in the absence of a functional P synthase, neither P nor PX2 are formed. As a consequence, naturally occurring anti-P and anti-PX2 can be made. Until the clinical significance of anti-PX2 is known, we also recommend that rare P1k or P2k erythrocyte units are preferentially selected for transfusion to Pk patients because p erythrocytes may pose a risk for hemolytic transfusion reactions due to their elevated PX2 levels.
Introduction
Glycosyltransferases are enzymes that add sugar moieties to acceptors on protein, lipid, carbohydrate, DNA, or other small acceptor molecules, e.g. steroids (1). The human genome encodes more than 200 different glycosyltransferases, and the field of glycodiversification is constantly expanding with both in vitro synthesis and modifications of natural glycoconjugates used in pharmaceuticals for example (2). However, glycosyltransferases appear to be more promiscuous than previously believed as they are able to use different donor and acceptor molecules (3).
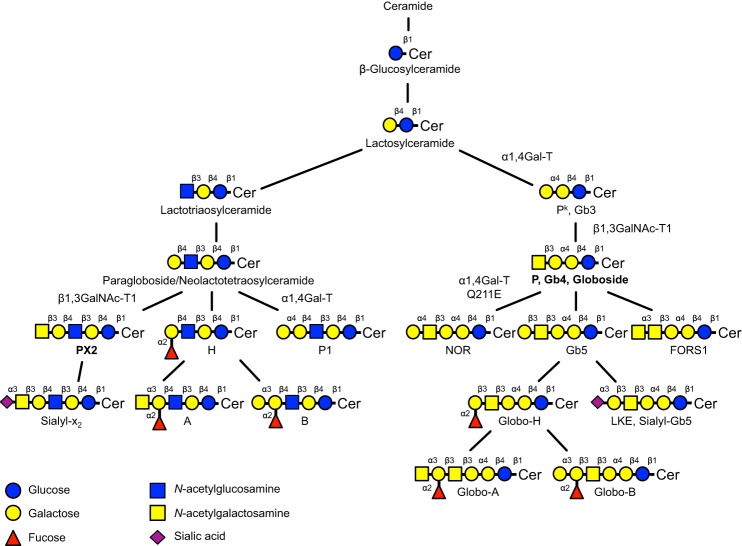
Glycosphingolipids are amphipathic compounds consisting of a hydrophilic oligosaccharide linked to a hydrophobic ceramide (4). The structures of both components (oligosaccharide and ceramide) vary, resulting in great molecular heterogeneity. To date, over 300 glycosphingolipids with different carbohydrate chains have been characterized. Glycosphingolipids are found in all mammalian cell membranes, and they are also present in intracellular compartments, such as the Golgi apparatus and mitochondria. The glycosphingolipids are divided into acid and non-acid glycosphingolipids where the acid glycosphingolipids are further subdivided into sialic acid-containing glycosphingolipids (gangliosides) and sulfate ester-conjugated glycosphingolipids (sulfatides). In addition, the glycosphingolipids are classified on the basis of their carbohydrate core chains. In humans, the globo (Galα4Gal), lacto (Galβ3GlcNAc), and neolacto (Galβ4GlcNAc) core chains are the most common among non-acid glycosphingolipids, whereas the gangliosides have mainly ganglio (Galβ3GalNAc) or neolacto core chains.
Glycosphingolipids on erythrocytes express several clinically important blood group antigens, and the absence of one of these structures results in naturally occurring antibodies against this antigen. These antibodies can cause hemolytic transfusion reactions and may result in hemolytic disease of the fetus or newborn and even recurrent spontaneous abortions (5).
Blood group antigens of carbohydrate nature are the products of glycosyltransferases. These enzymes are mainly present as type II transmembrane proteins in the Golgi apparatus (6, 7). The antigens are often present on other tissues in addition to erythrocytes and can be referred to as histo-blood group antigens (8). The most common non-acid glycosphingolipid on erythrocytes is globoside (globotetraosylceramide (Gb4)4), also known as the P antigen (9). It is currently the only antigen in the GLOB blood group system (ISBT 028) (10). The P antigen is the product of UDP-N-acetylgalactosamine: globotriaosylceramide 3-β-N-acetylgalactosaminyltransferase (P synthase; β1,3GalNAc-T1; EC 2.4.1.79) encoded by the gene B3GALNT1 on chromosome 3q26.1 (11–13). The P antigen is part of the globo series of glycosphingolipids and is a β1,3GalNAc elongation of the Pk antigen (globotriaosylceramide (Gb3)). The Pk antigen is synthesized by an α1,4-galactosyltransferase (lactosylceramide 4-α-galactosyltransferase; EC 2.4.1.228) encoded by A4GALT on chromosome 22q13.2 (14–16), which also synthesizes the P1 antigen (17). In addition, a mutated form of α1,4-galactosyltransferase (Q211E) shows a modified acceptor specificity and can therefore also add an α1,4Gal to the P antigen to form NOR antigen, which makes erythrocytes polyagglutinable (18) (Fig. 1). The three antigens synthesized by α1,4-galactosyltransferase are members of the P1PK blood group system (ISBT 003) (19). The GLOB blood group system is closely related to the P1PK system, and their null phenotypes are denoted Pk and p, respectively. The Pk phenotype is characterized by the absence of P antigen due to mutations in B3GALNT1, whereas the p phenotype is due to mutations in A4GALT, which lead to the absence of P/P1/Pk antigens. The Pk phenotype is further divided into P1k and P2k depending on the presence or absence of the P1 antigen. These phenotypes are very rare with a prevalence of ∼1 per million (5, 19), although they are much more common in selected population groups. Interestingly, the ceramide of lactosylceramide from p erythrocytes has mostly C22:0, C24:0, and C24:1 fatty acids, whereas the ceramide of lactosylceramide from normal individuals has predominantly C16:0, C18:0, and C18:1. Because Gb3 and Gb4 have primarily C22:0, C24:0, and C24:1 fatty acids (20), this suggests that glycosyltransferases that synthesize the more complex glycosphingolipids preferably use precursors with these fatty acids (21, 22).
FIGURE 1.
Schematic representation showing the synthesis pathways of selected glycosphingolipids. Structures relevant for this study are marked with bold text. Symbols are adopted from Varki (48). Cer represents ceramide. Structures carrying blood group antigens have been designated as such. In the case of the Pk, P, and LKE blood group antigens, an alternative name (Gb3, Gb4, and sialyl-Gb5, respectively) is given for increased recognition. The names of the involved key glycosyltransferases are given.
This project was initiated following an unexpected serological observation in a group A1B patient with the P1k phenotype and a strong anti-P in plasma, originally genetically defined by Hellberg et al. (11) and who had been transfused previously with blood of the p phenotype. The plasma from this patient reacted unexpectedly with p erythrocytes, which can be used as universal donor cells for individuals of the rare p and P1k/P2k phenotypes because they all lack globoside (19, 23). We hypothesized the presence of another glycosphingolipid present on p erythrocytes but absent on erythrocytes of P1k/P2k phenotype, to which the antibodies in this rare individual's plasma were directed. Already in 1977, Naiki et al. (24) suggested the presence of a structure on p erythrocytes that was strongly agglutinated by an unusual IgM paraprotein with specificity for glycosphingolipids possessing a terminal non-reducing N-acetylgalactosaminyl residue apparently absent on Pk erythrocytes. In 1982, Kannagi et al. (25) described a new neolacto series glycosphingolipid, which they named x2, following observations of additional reactivity between rabbit anti-P and erythrocyte membranes. The structure was determined as GalNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer, and the authors proposed the presence of a second P antigen. Ten years later, the structure was characterized further by Thorn et al. (26). These investigators also noted an increased amount of x2 on erythrocytes of the p phenotype. Following our presentation of the original A1B P1k case mentioned above, the x2 glycosphingolipid received status as an orphan blood group antigen in 2010 under the name PX2 (ISBT collection 209004) (27). However, the specificity of the antibodies in this patient or whether it was an isolated incident has remained unclear as has the enzymatic and genetic basis of x2 synthesis.
Experimental Procedures
Hemagglutination of Human Erythrocytes
Standard hemagglutination methods were used to generate the data in Table 1. Anonymized test erythrocytes from individuals with p and Pk phenotypes as well as plasmas from Pk individuals were available from the in-house collection of rare cells and fluids at the blood group reference laboratories in Paris (France) and Lund (Sweden). A semiquantitative scale from 0 to 1+, 2+, 3+, and 4+ was used to score hemagglutination reactions when performing the indirect antiglobulin test. If destruction of cells was visible with the naked eye, then H for hemolysis was scored. The two reference laboratories used different conditions (temperature and papain treatment) to enhance antibody reactivity against glycosphingolipid antigens. Therefore, all samples were not tested under all conditions.
TABLE 1.
Hemagglutination reactivity (0 to 4+) of sera from eight unrelated individuals with the Pk phenotype when tested against ABO-compatible p erythrocytes from up to 20 individuals
| Pk phenotype of plasma | ABO phenotype | Reactivity with p erythrocytes (from n individuals) |
|||
|---|---|---|---|---|---|
| Untreated |
Papain-treated |
||||
| 20 °C | 4 °C | 20 °C | 37 °C | ||
| P1k | O | 0 (1) | 4+ (1) | ||
| P1k | A | 4+ (2) | 3+ (2) | ||
| P1k | AB | 2–3+ (3) | 2–4+ (3) | ||
| P2k | B | 0/1+ (2) | 0/3+ (2) | ||
| P1k | O | 1+ (4) | 0 (6) | ||
| P1k | A | 1+ (5) | 1+ (4) | ||
| P1k | A | 2+ (6) | Ha (6) | ||
| P1k | A | 3+ (20) | 1+ (4) | ||
a Hemolysis.
Isolation of Glycosphingolipids from Human Erythrocytes
Acid and non-acid glycosphingolipids were isolated from human blood group A erythrocytes of p (App) and P1k (AP1k) phenotype, respectively, as described previously (28). Briefly, the erythrocytes were lyophilized and then extracted in two steps in a Soxhlet apparatus with chloroform and methanol (2:1 and 1:9 by volume, respectively). The material obtained was subjected to mild alkaline hydrolysis and dialysis followed by separation on a silicic acid column. Acid and non-acid glycosphingolipid fractions were obtained by chromatography on a DEAE-cellulose column. To separate the non-acid glycosphingolipids from alkali-stable phospholipids, the non-acid fraction was acetylated and separated on a second silicic acid column followed by deacetylation and dialysis. Final purifications were done by chromatographies on DEAE-cellulose and silicic acid columns. Thereby, 46 mg of total acid and 10.4 mg of total non-acid glycosphingolipids were obtained from 60 g dry weight of App erythrocytes, whereas 28 mg of total acid and 34.7 mg of total non-acid glycosphingolipids were obtained from 60 g dry weight of AP1k erythrocytes.
The major part of the mono- and diglycosylceramides was removed from the non-acid glycosphingolipid fractions by chromatography on silicic acid columns eluted with increasing amounts of methanol in chloroform, giving fractions containing glycosphingolipids migrating as diglycosylceramides and below on thin-layer plates. This fraction from App erythrocytes (2.2 mg) was designated fraction App-4, whereas the fraction from AP1k erythrocytes (13.1 mg) was designated fraction AP1k-4 and used for binding studies and structural characterization.
The total acid glycosphingolipid fraction from App erythrocytes was separated on three subsequent silicic acid columns eluted with increasing amounts of methanol in chloroform. The final column gave two fractions containing compounds migrating as sialylneolactotetraosylceramide and below. These fractions were designated fraction A-7 (0.4 mg) and fraction A-8 (0.1 mg), respectively.
Endoglycoceramidase Digestion of Glycosphingolipids
Endoglycoceramidase II from Rhodococcus spp. (Takara Bio Europe S.A., Gennevilliers, France) was used for hydrolysis of the non-acid glycosphingolipid fraction. Briefly, 50 μg of the non-acid glycosphingolipid fractions App-4 and AP1k-4 and the lower phase fractions from B3GALNT1-transfected and mock-transfected MEG-01 cells were resuspended in 100 μl of 0.05 m sodium acetate buffer, pH 5.0 containing 120 μg of sodium cholate and sonicated briefly. Thereafter, 1 milliunit of endoglycoceramidase II from Rhodococcus spp. was added, and the mixture was incubated at 37 °C for 48 h. The reaction was stopped by addition of chloroform/methanol/water to the final proportions 8:4:3 (by volume). The oligosaccharide-containing upper phase thus obtained was separated from detergent on a Sep-Pak QMA cartridge (Waters, Milford, MA). The eluate containing the oligosaccharides was dried under nitrogen and under vacuum.
LC-ESI/MS of Oligosaccharides
The glycosphingolipid-derived oligosaccharides were resuspended in 50 μl of water and analyzed by LC-ESI/MS as described (29). The oligosaccharides were separated on a column (200 × 0.180 mm) packed in house with 5-μm porous graphite particles (Hypercarb, Thermo-Hypersil, Runcorn, UK). An autosampler, HTC-PAL (CTC Analytics AG, Zwingen, Switzerland), equipped with a Cheminert valve (0.25-mm bore) and a 2-μl loop was used for sample injection. An Agilent 1100 binary pump (Agilent Technologies, Palo Alto, CA) delivered a flow of 250 μl/min that was split down in a -inch microvolume-T (0.15-mm bore) (Vici AG International, Schenkon, Switzerland) by a 50-cm × 50-μm-inner diameter fused silica capillary before the injector of the autosampler, allowing a flow rate of ∼2–3 μl/min through the column. The oligosaccharides (3 μl) were injected onto the column and eluted with an acetonitrile gradient (A, 10 mm ammonium bicarbonate; B, 10 mm ammonium bicarbonate in 80% acetonitrile). The gradient (0–45% B) was eluted for 46 min followed by a wash step with 100% B and equilibration of the column for 24 min. A 30-cm × 50-μm-inner diameter fused silica capillary was used as transfer line to the ion source. The saccharides were analyzed in negative ion mode on an LTQ linear quadrupole ion trap mass spectrometer (Thermo Electron, San José, CA). The IonMax standard ESI source on the LTQ mass spectrometer was equipped with a stainless steel needle kept at −3.5 kV. Compressed air was used as nebulizer gas. The heated capillary was kept at 270 °C, and the capillary voltage was −50 kV. A full scan (m/z 380–2000; two microscans; maximum, 100 ms; target value of 30,000) was performed followed by data-dependent MS2 scans of the three most abundant ions in each scan (two microscans; maximum, 100 ms; target value of 10,000). The threshold for MS2 was set to 500 counts. Normalized collision energy was 35%, and an isolation window of 3 units, an activation q of 0.25, and an activation time of 30 ms were used. Data acquisition and processing were conducted with Xcalibur software (Version 2.0.7). Manual assignment of glycan sequences was done on the basis of knowledge of mammalian biosynthetic pathways with the assistance of the GlycoWorkbench tool (Version 2.1) and by comparison of retention times and MS2 spectra of oligosaccharides from reference glycosphingolipids (29).
LC-ESI/MS of Native Glycosphingolipids
Glycosphingolipids were dissolved in methanol/acetonitrile (75:25 by volume) and separated on a 200 × 0.250-mm column packed in house with 5-μm polyamine II particles (YMC Europe GmbH, Dinslaken, Germany). An autosampler, HTC-PAL, equipped with a Cheminert valve (0.25-mm bore) and a 2-μl loop was used for sample injection. An Agilent 1100 binary pump delivered a flow of 250 μl/min, which was split down in a -inch microvolume-T (0.15-mm bore) by a 50-cm × 50-μm-inner diameter fused silica capillary before the injector of the autosampler, allowing for a flow rate of ∼2–3 μl/min through the column. Samples were eluted with an aqueous gradient (A, 100% acetonitrile, to B, 10 mm ammonium bicarbonate). The gradient (0–50% B) was eluted for 40 min followed by a wash step with 100% B and equilibration of the column for 20 min. The samples were analyzed in negative ion mode on an LTQ linear quadrupole ion trap mass spectrometer with an IonMax standard ESI source equipped with a stainless steel needle kept at −3.5 kV. Compressed air was used as nebulizer gas. The heated capillary was kept at 270 °C, and the capillary voltage was −50 kV. A full scan (m/z 500–2000; two microscans; maximum, 100 ms; target value of 30,000) was performed followed by data-dependent MS2 scans (two microscans; maximum, 100 ms; target value of 10,000) with normalized collision energy of 35%, isolation window of 2.5 units, activation q of 0.25, and activation time of 30 ms. The threshold for MS2 was set to 500 counts. Data acquisition and processing were conducted with Xcalibur software (Version 2.0.7). Manual assignment of glycosphingolipid sequences was done with the assistance of the GlycoWorkbench tool (Version 2.1) and by comparison of retention times and MS2 spectra of reference glycosphingolipids.
Reference Glycosphingolipids
Total acid and non-acid glycosphingolipid fractions were isolated as described (28). Individual glycosphingolipids were isolated by repeated chromatography on silicic acid columns and by HPLC and identified by mass spectrometry (30) and 1H NMR spectroscopy (31).
Thin-layer Chromatography
Thin-layer chromatography was done on aluminum- or glass-backed silica gel 60 high performance thin-layer chromatography plates (Merck). Glycosphingolipid mixtures (40 μg) or pure glycosphingolipids (4 μg) were applied to the plates and eluted with chloroform/methanol/water (60:35:8 by volume) as the solvent system. Chemical detection was done with anisaldehyde (32).
Chromatogram Binding Assays
Binding of human sera or eluates to glycosphingolipids on thin-layer chromatograms was performed as described previously (33, 34). Dried chromatograms were dipped in diethyl ether/n-hexane (1:5, v/v) containing 0.5% (w/v) polyisobutylmethacrylate for 1 min. To diminish background binding, the chromatograms were blocked for 2 h at room temperature with phosphate-buffered saline (PBS, pH 7.3) containing 2% (w/v) bovine serum albumin, 0.1% (w/v) NaN3, and 0.1% (w/v) Tween 20 (BSA/PBS/TWEEN). Then the plates were incubated with human sera or eluates (diluted 10 times in BSA/PBS/TWEEN) for another 2 h at room temperature. After washing with PBS, a second 2-h incubation followed with 125I-labeled (labeled according to the IODO-GEN protocol of the manufacturer (Pierce)) goat anti-human antibodies (Pierce) diluted to 2 × 106 cpm/ml in BSA/PBS/TWEEN. Finally, the plates were washed six times with PBS. Dried chromatograms were autoradiographed for 12–24 h using XAR-5 x-ray films (Eastman Kodak Co.). Binding of the mouse monoclonal antibody anti-PX2 clone TH2 (26) (kindly provided by Ulla Mandel at the Copenhagen Center for Glycomics) to glycosphingolipids on thin-layer chromatograms was done as described (34).
PX2 Antigen Expression on Erythrocytes
Erythrocytes were papain-treated for 15 min at 37 °C to remove most of the negative charge carried on the glycophorins and thereby make the glycosphingolipids more accessible. Washed papain-treated erythrocytes were diluted to ∼10,000 cells/μl in PBS in 96-well plates (Nunc, Apogent, Roskilde, Denmark) and fixed with glutaraldehyde at a final concentration of 0.07% for 10 min at room temperature. Cells were centrifuged for 2 min at 350 × g, resuspended in 50 μl of PBS, and incubated with mouse monoclonal anti-PX2 (clone TH2) for 10 min at room temperature. Erythrocytes were washed twice in PBS, resuspended in 50 μl of PBS, and incubated with phycoerythrin-conjugated rat anti-mouse κ (clone X36, BD Biosciences) for 10 min at room temperature. All room temperature incubations were performed in the dark on a shaker. Data from 10,000 events were collected using FACSCalibur (BD Biosciences) and analyzed using Cell Quest (Version 3.1, BD Biosciences).
Preparation of Anti-P and Anti-PX2 Eluates
Anti-P (+PX2) was affinity-purified by adsorbing plasma from either a P1k or a P2k individual onto aliquots of pooled normal group O erythrocytes for 15 min at room temperature. Following stringent washing, the erythrocytes were eluted using Gamma® Elu-Kit II® reagent according to the manufacturer's instructions (Immucor, Inc., Norcross, GA). Anti-PX2 was prepared similarly following adsorption-elution of the plasmas on aliquots of group O p erythrocytes.
Blocking of Anti-PX2 (Clone TH2) Binding to Group O p Erythrocytes
Group O erythrocytes of p phenotype were incubated for 30 min at room temperature with either P1k plasma or anti-P + PX2 eluate. Following this incubation, the erythrocytes were washed and labeled with the monoclonal anti-PX2 and phycoerythrin-labeled rat anti-mouse secondary antibodies described above. AB plasma was used as a control for unspecific inhibition.
Transfection of MEG-01 Cells
The megakaryoblastic leukemia cell line MEG-01 was chosen for transfection experiments based on its very weak endogenous P and PX2 antigen expression. Four different B3GALNT1 constructs were made, including a consensus allele (B3GALNT1 open reading frame (ORF)) and three known naturally occurring mutants, 202C→T (R68stop), 376G→A (D126N) and 449A→G (D150G), as described previously (35). The 202C→T and 449A→G mutants are null alleles because they were identified in samples of the Pk phenotype. The 376G→A mutant (rs2231257) is a functionally active variant also found in the 1000 Genomes project database (accessed on February 2, 2015) with a frequency of ∼5.2%. Constructs were evaluated by DNA sequencing and cloned into the high copy bicistronic plasmid pEF1α-IRES-ZsGreen1 (Clontech). MEG-01 cells were grown in RPMI 1640 medium + l-glutamine (Life Technologies) supplemented with 10% FBS (Life Technologies), 10 units/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) prior to transfection.
Expression of B3GALNT1 Constructs in MEG-01 Cells and PX2 Antigen Detection by Flow Cytometry
MEG-01 cells were electroporated at a density of 7.5 × 106 cells/ml in 400 μl of RPMI 1640 medium with 10% FBS using 10 μg of each of the B3GALNT1 constructs at 0.28 kV and 960 microfarads using a Gene Pulser (Bio-Rad). Mock transfection was performed using 10 μg of pEF1α-IRES-ZsGreen1. Following electroporation, the cells were grown in 4.9 ml of RPMI 1640 medium with 10% FBS for 48 h in 37 °C 5% CO2 prior to flow cytometric analysis. Washed transfected MEG-01 cells were diluted to ∼10,000 cells/μl in 50 μl of Dulbecco's PBS with 0.1% sodium azide (NaN3) and 0.2% BSA in 96-well plates (Nunc) and incubated with the primary antibody anti-PX2 (clone TH2) for 10 min at room temperature followed by 50 min at 4 °C. The cells were washed twice in PBS, resuspended in 50 μl of PBS, and incubated with the secondary antibody phycoerythrin-conjugated rat anti-mouse κ for 10 min at room temperature. All room temperature incubations were performed in the dark on a shaker. In addition, cells were stained with 5 μl of 7-aminoactinomycin D (BD Biosciences) to exclude non-viable cells. A total of 50,000 events were collected using FACSCalibur and analyzed with Cell Quest software (Version 3.1). Statistical analysis was performed using the Student's t test (IBM SPSS Statistics 20, IBM Corp., Armonk, NY). All experiments were performed in triplicate on three separate occasions.
Overexpression and siRNA Silencing of B3GALNT1 in MEG-01 Cells
The MEG-01 cells were electroporated at a density of 7.5 × 106 cells/ml in Gene Pulser Electroporation Buffer (catalog number165-2677, Bio-Rad) using 10 μg of mock or B3GALNT1 ORF together with 125 nm siRNA. The siRNAs used were Silencer® Select B3GALNT1 siRNA (s16584, Life Technologies), Silencer Select Negative Control Number 1 (catalog number 4390843, Life Technologies), and positive control Silencer Select GAPDH siRNA (s5573, Life Technologies). Cells were electroporated at 0.30 kV and 500 microfarads with square wave for 20 ms using the Gene Pulser MXcellTM Electroporation System (Bio-Rad). Following electroporation, cells were grown and analyzed on the flow cytometer as described above. In addition, RNA was extracted from a 1.5-ml aliquot of the transfected cells. Samples were analyzed in triplicate on three separate occasions (n = 9) with the exception of the control cells transfected with the vectors alone, mock, and B3GALNT1 ORF (n = 3).
Real Time PCR
RNA was extracted using the RNeasy Plus Mini kit (Qiagen, GmbH, Hilden, Germany) followed by DNase treatment with TURBO DNA-freeTM kit (Life Technologies), and cDNA was synthesized using the High-Capacity RNA-to-cDNATM kit (Life Technologies) according to the manufacturer's instructions. Quantification of B3GALNT1 and GAPDH transcripts was performed on 3 μl of cDNA with the B3GALNT1 and GAPDH TaqMan Gene Expression Assays (Hs00364202_s1 and Hs02758991_g1, respectively; Life Technologies). Ct values were normalized to β-actin transcripts (ACTB 4333762F, Life Technologies). Samples were run in duplicate using real time PCR (7500 System, Life Technologies).
Overexpression of B3GALNT1 Constructs in MEG-01 Cells for Isolation of Glycosphingolipids
The MEG-01 cells were electroporated at a density of 23.75 × 106 cells/ml in 400 μl of RPMI 1640 medium (without FBS) using 33 μg of either B3GALNT1 ORF or mock vector. Cells were electroporated as described above for siRNA transfection. Following electroporation, the cells were grown for 48 h in 9 ml of RPMI 1640 medium with 10% FBS in 6-well plates in 37 °C at 5% CO2. The cells were pooled, and 1 ml was taken out for flow cytometric analysis as described above. The rest of the cells were washed once in PBS, and the pellet was frozen at −80 °C before glycosphingolipid extraction.
Isolation of Glycosphingolipids from B3GALNT1-transfected and Mock-transfected MEG-01 Cells
Approximately 1 × 108 cells of each variant were lyophilized, giving a dry weight of 213 mg for the B3GALNT1-transfected MEG-01 cells and 254 mg for the mock-transfected MEG-01 cells. The lyophilized materials were extracted in a Soxhlet apparatus as described above. After drying, the extracts were subjected to mild alkaline hydrolysis followed by dialysis. Thereafter, the extracts were acetylated, and the acetylated lipids were separated on a silicic acid column eluted first with dichloromethane to remove non-polar material. Non-acid and acid glycosphingolipids were eluted with 5, 10, and 15% methanol in chloroform (by volume). The 5, 10, and 15% fractions were combined and deacetylated using 0.2 m KOH in methanol. This was followed by a Folch partition (36), and the resulting lower phase was thereafter dried and redissolved in 150 μl of chloroform/methanol (2:1 by volume). Approximately 30 μl were used for chromatogram binding assays, and the remaining materials were thereafter hydrolyzed by endoglycoceraminidase and analyzed by LC-ESI/MS.
Results
Serological Investigations
A positive reaction was observed when a cross-match was performed between plasma from eight unrelated Pk individuals and erythrocytes from up to 20 individuals of the p phenotype, indicating the presence of an antibody directed toward a structure other than the P antigen (Table 1). The intensity of reactions ranged from 0 to 4+ depending on the temperature and technical procedure (untreated or papain-treated erythrocytes). These results confirmed the observations in the index case and showed that the reactivity with p erythrocytes was not a single event but rather a general phenomenon among all Pk individuals tested.
Isolation of Glycosphingolipids from Human App and AP1k Erythrocytes
The non-acid glycosphingolipid fractions purified by chromatography gave fractions (designated fractions App-4 and AP1k-4, respectively) with a number of glycosphingolipids migrating in the diglycosylceramide region and below (exemplified in Fig. 2, lane 5). Separation of the acid fraction from App erythrocytes on silicic acid columns gave two acid fractions (designated fractions A-7 and A-8) containing compounds migrating as sialylneolactotetraosylceramide and below (Fig. 2, lanes 6 and 7).
FIGURE 2.
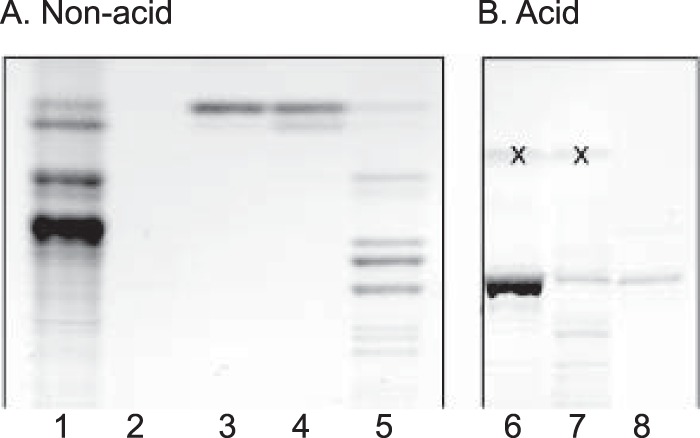
Thin-layer chromatogram of glycosphingolipids isolated from human App erythrocytes. The chromatograms were developed with chloroform/methanol/water (60:35:8 by volume), and detection of glycosphingolipids was done with anisaldehyde reagent. Non-acid fractions are shown in A: lane 1, non-acid glycosphingolipids of human blood group AB control erythrocytes, 80 μg; lane 2, non-acid fraction 1 from App erythrocytes (App-1), 10 μg; lane 3, non-acid fraction App-2, 10 μg; lane 4, non-acid fraction App-3, 10 μg; lane 5, non-acid fraction App-4, 40 μg. Acid fractions are shown in B: lane 6, acid fraction A-7, 40 μg; lane 7, acid fraction A-8, 40 μg; lane 8, reference sialylneolactotetraosylceramide (NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) from human control erythrocytes, 4 μg. The bands marked with “X” in B are non-glycosphingolipid contaminants.
Mass Spectrometry of the Non-acid Glycosphingolipid Fractions App-4 and AP1k-4
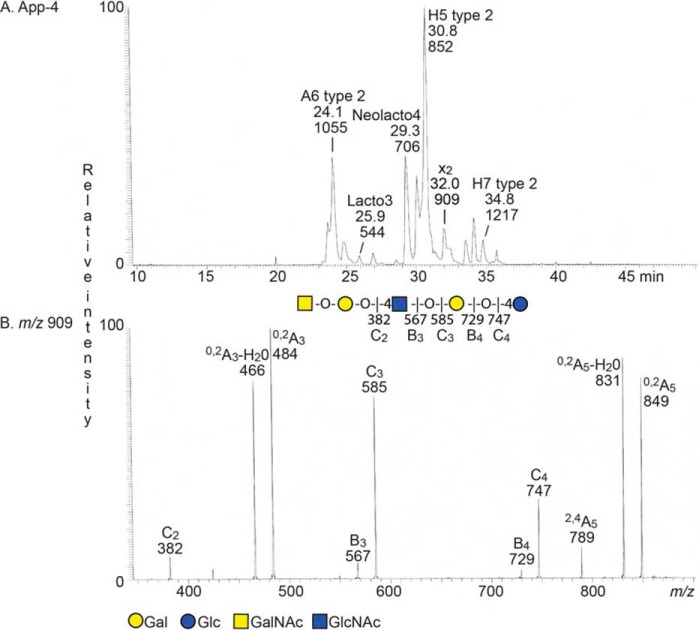
Aliquots of fractions App-4 and AP1k-4 were hydrolyzed with Rhodococcus endoglycoceramidase II, and the oligosaccharides thereby obtained were analyzed by LC-ESI/MS. The base peak chromatogram of the oligosaccharides obtained by hydrolysis of fraction App-4 is shown in Fig. 3A. Molecular ions corresponding to oligosaccharides ranging from trisaccharides (detected as [M − H+]− ions at m/z 544) to heptasaccharides (detected as [M − H+]− ions at m/z 1217) were found.
FIGURE 3.
LC-ESI/MS of the oligosaccharides obtained by digestion of the non-acid glycosphingolipid fraction App-4 with Rhodococcus endoglycoceramidase II. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction App-4. The identification of individual glycosphingolipid-derived oligosaccharides was based on their determined molecular masses and subsequent MS2 sequencing. The structures identified in the chromatogram are: A6 type 2, GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glc; Lacto3, GlcNAcβ3Galβ4Glc; Neolacto4, Galβ4GlcNAcβ3Galβ4Glc; H5 type 2, Fucα2Galβ4GlcNAcβ3Galβ4Glc; x2, GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc; and H7 type 2, Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc. B, MS2 spectrum of the ion at m/z 909 (retention time, 31.9 min). The interpretation formula shows the deduced carbohydrate sequence.
The MS2 spectrum of the [M − H+]− ion at m/z 909 at retention time 33.7 min (Fig. 3B) had a C-type fragment ion series (C2 at m/z 382, C3 at m/z 585, and C4 at m/z 747), demonstrating a pentasaccharide with HexNAc-Hex-HexNAc-Hex-Hex sequence. Cross-ring 0,2A-type fragments are diagnostic for carbohydrates substituted at C-4 (29, 37, 38), and here the 0,2A3 fragment ion at m/z 484 and the 0,2A3 − H2O ion at m/z 466, demonstrated a 4-substituted internal HexNAc, whereas the 0,2A5 ion at m/z 849 and the 0,2A5 − H2O ion at m/z 831 were derived from cross-ring cleavage of the 4-substituted Glc of the lactose unit at the reducing end. The features of this MS2 spectrum thus identified an x2 pentasaccharide (GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc). In a similar manner, MS2 of the [M − H+]− ions at m/z 544, m/z 706, m/z 852, m/z 1055, m/z 1217, and m/z 1420 allowed a tentative identification of a lactotrisaccharide, a neolactotetrasaccharide, an H type 2 pentasaccharide, an A type 2 hexasaccharide, and an H type 2 heptasaccharide, respectively (data not shown).
Mass Spectrometry of the Acid Glycosphingolipid Fractions A-7 and A-8 from Human App Erythrocytes
The native acid glycosphingolipid fractions A-7 and A-8 were analyzed by LC-ESI/MS. The base peak chromatogram of fraction A-7 was dominated by an [M − 2H+]2− ion at m/z 813, and MS2 of this ion demonstrated a ganglioside with NeuAc-Hex-HexNAc-Hex-Hex sequence and d18:1–24:1 ceramide, most likely sialylneolactotetraosylceramide (data not shown).
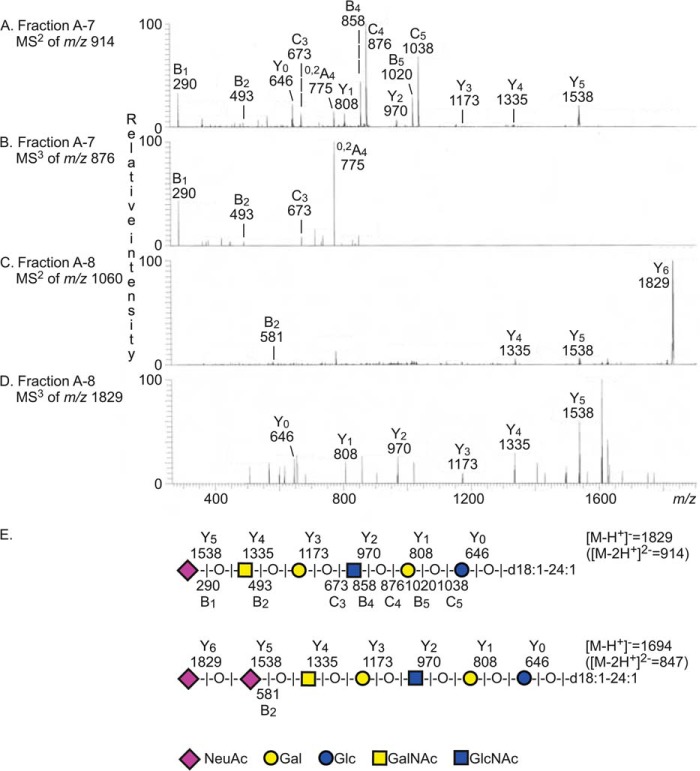
The base chromatogram also had an [M − 2H+]2− ion at m/z 914. MS2 of this ion gave a series of B and C ions (B1 at m/z 290, B2 at m/z 493, C3 at m/z 673, B4 at m/z 858, C4 at m/z 876, B5 at m/z 1020, and C5 at m/z 1038) and a series of Y ions (Y0 at m/z 646, Y1 at m/z 808, Y2 at m/z 970, Y3 at m/z 1173, Y4 at m/z 1335, and Y5 at m/z 1538) (Fig. 4A). There was also an 0,2A4 fragment ion at m/z 775, indicating a 4-substituted internal HexNAc (Fig. 4, A and B). Taken together, this clearly demonstrated a ganglioside with NeuAc-HexNAc-Hex-4HexNAc-Hex-Hex carbohydrate sequence and d18:1–24:1 ceramide as the sialyl-x2 ganglioside.
FIGURE 4.
LC-ESI/MS of the acid glycosphingolipid fractions A-7 and A-8 from human App erythrocytes. A, MS2 spectrum of the [M − 2H+]2− ion at m/z 914 of fraction A-7 (retention time, 23.2 min). B, MS3 of m/z 876. C, MS2 spectrum of the [M − 2H+]2− ion at m/z 1060 of fraction A-8 (retention time, 31.4 min). D, MS3 of m/z 1829. E, interpretation formulas showing the deduced glycosphingolipid sequences.
The major [M − 2H+]2− ion in the base peak chromatogram of fraction A-8 was found at m/z 959, and MS2 of this ion identified a ganglioside with NeuAc-NeuAc-Hex-HexNAc-Hex-Hex sequence and d18:1–24:1 ceramide as disialylneolactotetraosylceramide (data not shown) (39). The base chromatogram also had a minor [M − 2H+]2− ion at m/z 1060, and here MS2 gave a B2 ion at m/z 581, indicating a NeuAc-NeuAc sequence (Fig. 4C). There was also a series of Y ions (Y4 at m/z 1335, Y5 at m/z 1538, and Y6 at m/z 1829) identifying a terminal NeuAc-NeuAc-HexNAc sequence. MS3 of the Y6 ion at m/z 1829 (Fig. 4D) gave a series of Y ions (Y0 at m/z 646, Y1 at m/z 808, Y2 at m/z 970, Y3 at m/z 1173, Y4 at m/z 1335, and Y5 at m/z 1538). Taken together, these spectral features indicated a ganglioside with NeuAc-NeuAc-HexNAc-Hex-HexNAc-Hex-Hex carbohydrate sequence and d18:1–24:1 ceramide, i.e. a disialyl-x2 ganglioside. However, it should be noted that the B2 ion at m/z 581, indicating a terminal NeuAc-NeuAc sequence, was relatively weak, and thus the position of the sialic acids could not be clearly established. The glycosphingolipid structures identified by mass spectrometry in fraction App-4 (Fig. 3) or A-7 and A-8 (Fig. 4) of human App erythrocytes are indicated on a thin-layer chromatogram in Fig. 5.
FIGURE 5.
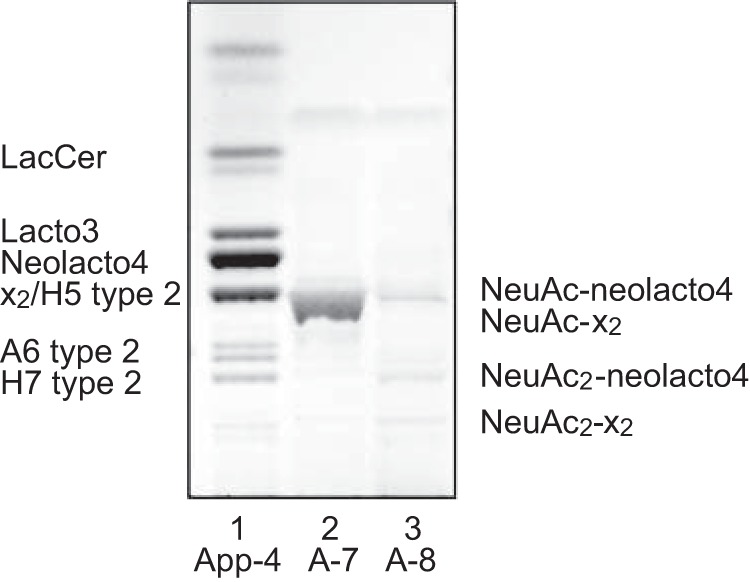
Summary of the glycosphingolipids identified in fractions App-4, A-7, and A-8 of human App erythrocytes by mass spectrometry as indicated on a thin-layer chromatogram. Lacto3, GlcNAcβ3Galβ4Glcβ1Cer; Neolacto4, Galβ4GlcNAcβ3Galβ4Glcβ1Cer; x2, GalNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; H5 type 2, Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer; A6 type 2, GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer; H7 type 2, Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; NeuAc-neolacto4, NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; NeuAc-x2, NeuAcα3GalNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; NeuAc2-neolacto4, NeuAcα8NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; NeuAc2-x2, NeuAcα8NeuAcα3GalNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer.
Binding of Antibodies from Pk Plasma and the Monoclonal Anti-PX2 to Glycosphingolipids Isolated from Human App Erythrocytes
Having established the presence of the x2 pentaglycosylceramide in the non-acid fraction App-4 from App erythrocytes, the sialyl-x2 ganglioside in the acid fraction A-7, and disialyl-x2 ganglioside in fraction A-8, we next examined the binding of eluates made from human P1k and P2k plasmas to these fractions in the chromatogram binding assay. Plasma from human blood group AB blood was used as a control and gave no binding to the glycosphingolipids on the chromatograms (data not shown).
Both the P1k and P2k eluates from pooled normal erythrocytes gave the expected distinct binding to reference P antigen (Fig. 6, B and C, lane 1) but also a binding in the pentaglycosylceramide region of the non-acid fraction App-4 (Fig. 6, B and C, lane 3). The binding-active pentaglycosylceramide co-migrated with the glycosphingolipid recognized by the x2-binding antibody TH2 (Fig. 6D, lane 3). Taken together with the identification of the x2 pentaglycosylceramide in fraction App-4 described above, these findings thus demonstrate an anti-x2 reactivity in the P1k and P2k eluates.
FIGURE 6.
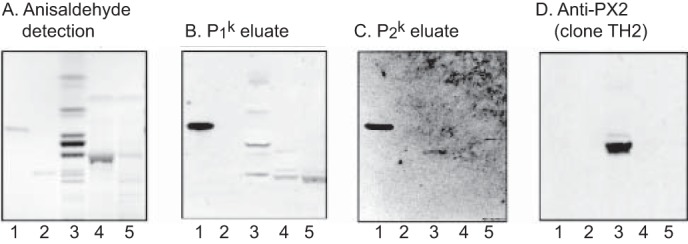
Binding of human sera and monoclonal antibodies to the glycosphingolipids isolated from human App erythrocytes. The thin-layer chromatogram after chemical detection by anisaldehyde (A) and autoradiograms obtained by binding of polyclonal anti-P (+PX2) eluate made from P1k plasma (B), eluate made from P2k plasma (C), and the monoclonal anti-PX2 clone TH2 (D) are shown. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system, and the binding assays were performed as described under “Experimental Procedures.” Autoradiography was for 12 h. Lane 1, reference globoside (GalNAcβ3Galα4Galβ4Glcβ1Cer), 4 μg; lane 2, reference A type 2 hexaosylceramide (GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 4 μg: lane 3, non-acid glycosphingolipids (fraction App-4) of human App erythrocytes, 40 μg; lane 4, acid glycosphingolipids (fraction A-7) of human App erythrocytes, 40 μg; lane 5, acid glycosphingolipids (fraction A-8) of human App erythrocytes, 40 μg.
The P1k eluate also gave a binding in the heptaglycosylceramide region in fraction App-4 (Fig. 6B, lane 3) and to a slow migrating ganglioside in the acid fractions A-7 and A-8 (Fig. 6B, lanes 4 and 5). Although the nature of the non-acid heptaglycosylceramide is elusive, the ganglioside recognized is most likely sialyl-x2. A further observation is that there was no binding of the TH2 antibody to the acid fractions containing sialyl-x2 and disialyl-x2 (Fig. 6D, lanes 4 and 5); i.e. substitution of the terminal GalNAc of the x2 sequence by a NeuAcα3 is not tolerated by the TH2 antibody.
PX2 Antigen Expression on Different Types of Human Erythrocytes
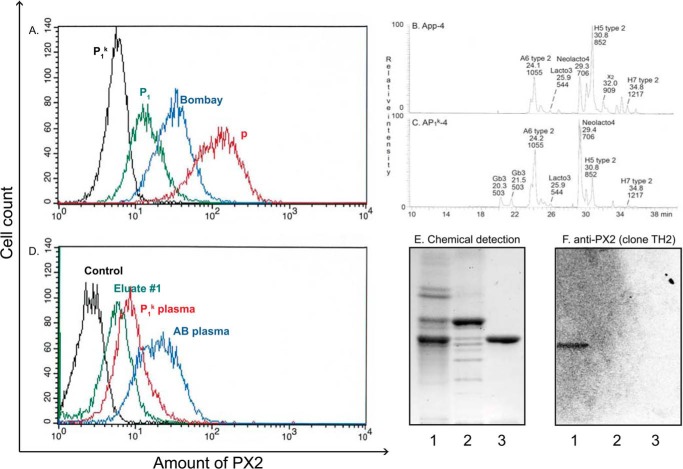
The expression of the PX2 antigen on human erythrocytes of variant phenotypes was investigated in several ways. First, flow cytometry using the anti-PX2 antibody demonstrated that erythrocytes of the p phenotype have the highest PX2 antigen expression followed by Oh (Bombay phenotype) erythrocytes in which paragloboside cannot be used for synthesis of H (and subsequently A and/or B) antigens and P1+ erythrocytes. In contrast, P1k erythrocytes were negative (Fig. 7A).
FIGURE 7.
Analysis of PX2 expression on erythrocytes of P1k and p phenotypes. The y axis in the flow cytometry histograms (A and D) shows the number of cells on a linear scale, and the x axis displays the fluorescence intensity on a logarithmic scale. A shows the reactivity of papain-treated P1k (black), P1 (green), Bombay (blue), and p erythrocytes (red) with the mouse monoclonal anti-PX2 (clone TH2). B and C show a comparison of non-acid glycosphingolipid fractions from App and AP1k erythrocytes. B, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction App-4 from human App erythrocytes. The identification of individual glycosphingolipid-derived oligosaccharides was based on their determined molecular masses and subsequent MS2 sequencing. A6 type 2, GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glc; Lacto3, GlcNAcβ3Galβ4Glc; Neolacto4, Galβ4GlcNAcβ3Galβ4Glc; H5 type 2, Fucα2Galβ4GlcNAcβ3Galβ4Glc; x2, GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc; H7 type 2, Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc. C, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction AP1k-4. Gb3, Galα4Galβ4Glc; the other designations are given under B. D illustrates a partial blocking of the monoclonal anti-PX2 binding to p erythrocytes following incubation with P1k whole plasma or an eluate thereof. Pooled AB plasma was used as a control for unspecific blocking and incubation with secondary antibody only (phycoerythrin-conjugated rat anti-mouse κ-labeled control). E and F show binding of the monoclonal anti-PX2 to glycosphingolipids isolated from human AP1k erythrocytes and controls. The thin-layer chromatogram after chemical detection by anisaldehyde (E) and autoradiogram obtained by binding of the mouse monoclonal antibody clone TH2 (F) are shown. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system, and the binding assay was done as described under “Experimental Procedures.” Autoradiography was for 12 h. Lane 1, non-acid glycosphingolipids of human blood group AB erythrocytes, 40 μg; lane 2, non-acid glycosphingolipid fraction AP1k-4 from human AP1k erythrocytes, 40 μg; lane 3, reference P antigen (GalNAcβ3Galα4Galβ4Glcβ1Cer), 4 μg.
Second, we compared the subfractions containing complex glycosphingolipids (fractions App-4 and AP1k-4) isolated from App and AP1k erythrocytes by hydrolysis with endoglycoceramidase and analysis of the oligosaccharides obtained by LC-ESI/MS. The base peak chromatogram obtained from fraction AP1k-4 is shown in Fig. 7C. This chromatogram had [M − H+]− ions at m/z 544, m/z 706, m/z 852, m/z 1055, and m/z 1217 like the base peak chromatogram of fraction App-4 (Figs. 3A and 7B). In addition, the base peak chromatogram of fraction AP1k-4 (Fig. 7C) had [M − H+]− ions at m/z 503 (corresponding to globotrisaccharides) but no [M − H+]− ion, indicating an x2 pentasaccharide at m/z 909. Furthermore, no binding of the TH2 antibody to the non-acid glycosphingolipid fraction AP1k-4 was obtained (Fig. 7F, lane 2).
Finally, group O erythrocytes of p phenotype were preincubated with P1k plasma or anti-P + PX2 eluate, washed, labeled with the monoclonal anti-PX2, and analyzed by flow cytometry (Fig. 7D). A partial inhibition of the binding of monoclonal anti-PX2 was clearly demonstrated both with the P1k plasma and an eluate from Pk plasma. This confirmed the presence of antibodies directed to the x2 glycosphingolipid in P1k individuals. Pooled AB plasma was used as a control for unspecific inhibition.
Expression of PX2 Antigen on Transiently Transfected MEG-01 Cells
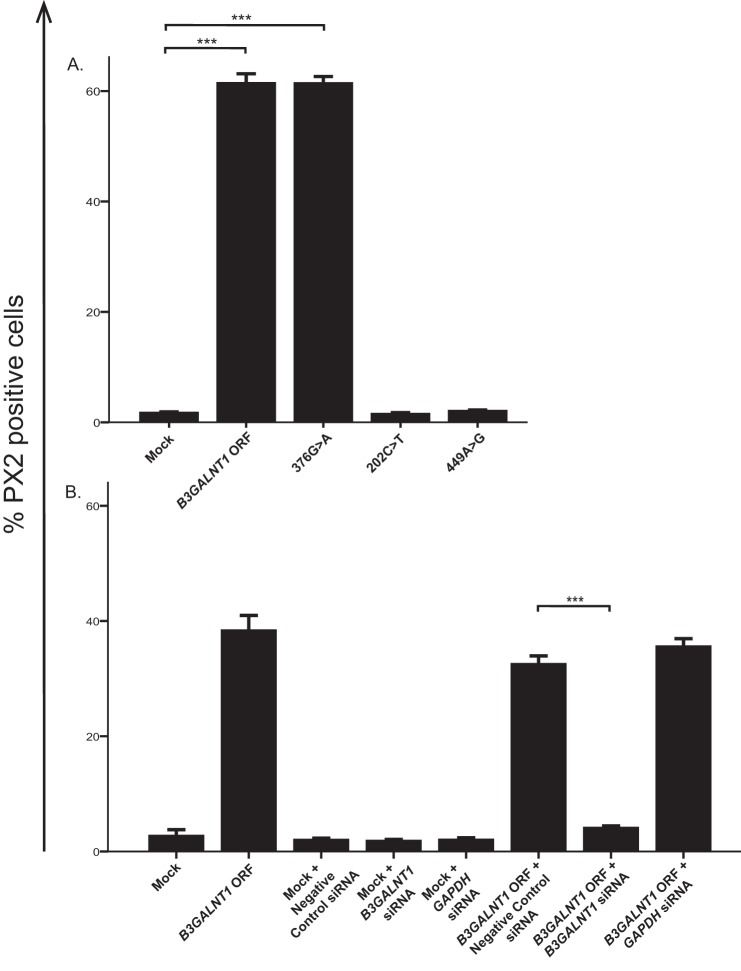
We hypothesized that the β1,3GalNAc-T1 was responsible for synthesizing the PX2 antigen based on the facts that (a) the PX2 antigen is absent on erythrocytes of Pk phenotype lacking the P antigen and (b) the two glycosphingolipids both terminate with a GalNAc in a β1,3-linkage. Therefore, we decided to overexpress the β1,3GalNAc-T1 in a cell line with very low endogenous P and PX2 expression. Flow cytometry using the TH2 antibody showed that MEG-01 cells transfected with constructs containing the B3GALNT1 ORF or an enzymatically active mutant (376G→A) gave rise to high PX2 antigen expression (approximately 60% positive cells), whereas cells transfected with a mock vector or the null mutant ORF constructs 202C→T and 449A→G were negative for TH2 staining (Fig. 8A). Co-transfection of the B3GALNT1 ORF and siRNA targeting B3GALNT1 resulted in significantly lower PX2 antigen expression on MEG-01 cells compared with co-transfection with B3GALNT1 ORF and a negative control siRNA (Fig. 8B). Transcript levels of B3GALNT1 were also significantly decreased in the cells co-transfected with B3GALNT1 ORF and B3GALNT1 siRNA as compared with the B3GALNT1 ORF- and negative control siRNA- or GAPDH siRNA-transfected cells, respectively (data not shown). This shows that the enzyme encoded by B3GALNT1 is capable of PX2 antigen synthesis in MEG-01 cells in addition to synthesizing the P antigen as shown previously (35). This glycosyltransferase can thereby use two different acceptor substrates, neolactotetraosylceramide (paragloboside) or Gb3 (Pk) to form either a neolacto or a globo series glycosphingolipid.
FIGURE 8.
PX2 antigen expression following overexpression of B3GALNT1 and siRNA knockdown in MEG-01 cells. A, expression of the PX2 antigen after transfection of MEG-01 cells with the various B3GALNT1 constructs. Transfection with mock (empty vector) was used as a negative control. B, expression of PX2 antigen following transfection with mock or B3GALNT1 ORF combined with siRNA targeting B3GALNT1. Negative Control Number 1 siRNA and GAPDH siRNA were used as negative and positive controls, respectively. PX2 antigen expression was measured in cells gated for 7-aminoactinomycin D negativity and ZsGreen positivity, indicating the viable transfected cells. Error bars represent the S.E. Asterisks (***) indicate p < 0.001 (unpaired Student's t test).
Glycosphingolipids from B3GALNT1-transfected MEG-01 Cells
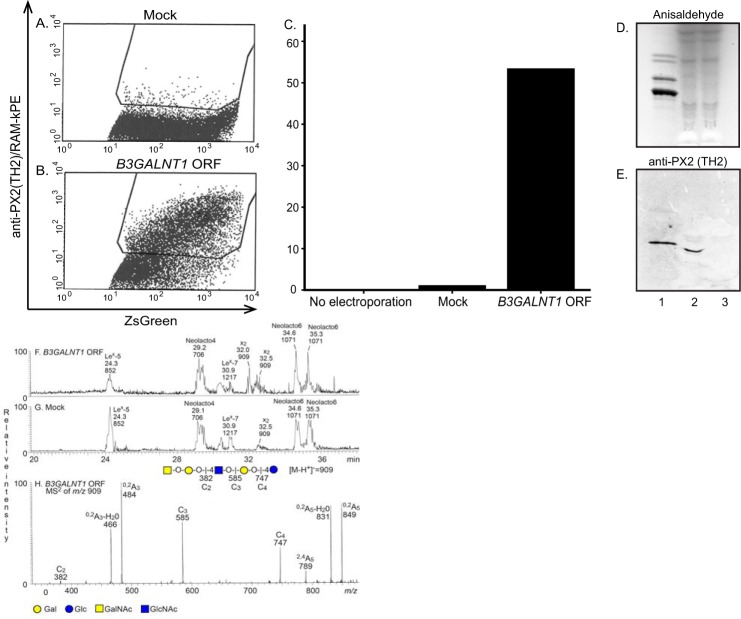
A clear difference in PX2 antigen expression on the cell surface of MEG-01 cells was seen comparing the mock-transfected and B3GALNT1-transfected cells using the TH2 antibody in flow cytometry (Fig. 9, A–C). In addition, a distinct binding of the TH2 antibody to the crude fraction containing acid and non-acid glycosphingolipids from B3GALNT1-transfected MEG-01 cells was obtained (Fig. 9E, lane 2), whereas there was no binding to the glycosphingolipids from mock-transfected MEG-01 cells (Fig. 9E, lane 3). Furthermore, LC-ESI/MS analyses of the glycosphingolipid-derived oligosaccharides from the B3GALNT1- and mock-transfected MEG-01 cells (Fig. 9, F and G) showed that both cell types gave mainly glycosphingolipid-derived oligosaccharides with type 2 core chain like Lex penta- (m/z 852) and heptaosylsaccharides (m/z 1217), neolactotetra- (m/z 706) and hexaosylsaccharides (m/z 1071), and the x2 pentaosylsaccharide (m/z 909). The identity of the x2 pentaosylsaccharide was confirmed by the MS2 spectrum of the ion at m/z 909 (Fig. 9H). The increased relative intensity of the ion at m/z 909 in the base peak chromatogram from the B3GALNT1-transfected cells (Fig. 9F) when compared with the mock-transfected cells (Fig. 9G) demonstrated an increased production of the x2 glycosphingolipid upon B3GALNT1 transfection.
FIGURE 9.
Detection of glycosphingolipids in MEG-01 cells following mock and B3GALNT1 ORF transfection. The amounts of PX2-positive MEG-01 cells following mock or B3GALNT1 transfection are shown in A and B, respectively. C illustrates the percentage of PX2-positive cells comparing MEG-01 cells not electroporated, mock-transfected cells, and B3GALNT1 ORF-transfected cells. The thin-layer chromatogram after chemical detection by anisaldehyde (D) and autoradiogram obtained by binding of the monoclonal antibody TH2 (E) are shown. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system, and the binding assays were performed as described under “Experimental Procedures.” Autoradiography was for 12 h. Lane 1, non-acid glycosphingolipids of human blood group O erythrocytes, 40 μg; lane 2, acid and non-acid glycosphingolipids of B3GALNT1-transfected MEG-01 cells, 10 μl/150 μl; lane 3, acid and non-acid glycosphingolipids of mock-transfected MEG-01 cells, 10 μl/150 μl. F, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained by digestion of the B3GALNT1-transfected MEG-01 cells with Rhodococcus endoglycoceramidase II. The identification of individual glycosphingolipid-derived oligosaccharides was based on their determined molecular masses and subsequent MS2 sequencing. Lex-5, Galβ4(Fucα3)GlcNAcβ3Galβ4Glc; Neolacto4, Galβ4GlcNAcβ3Galβ4Glc; Lex-7, Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc; x2, GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc; Neolacto6, Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc. G, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained by digestion of the mock-transfected MEG-01 cells with Rhodococcus endoglycoceramidase II. The identification of individual glycosphingolipid-derived oligosaccharides was performed as described for F (not shown). H, MS2 spectrum of the ion at m/z 909 (retention time, 32.5 min) from LC-ESI/MS of the oligosaccharides derived from B3GALNT1-transfected MEG-01 cells. The interpretation formula shows the deduced carbohydrate sequence. RAM-kPE, phycoerythrin-conjugated rat anti-mouse κ.
Discussion
The results presented here suggest that the B3GALNT1-encoded β1,3GalNAc-T1, which is known to synthesize the P antigen (globoside, Gb4) with Pk (Gb3) as its acceptor, is also capable of using a different acceptor, paragloboside, to produce x2 glycosphingolipid, now also known as the PX2 antigen. When mutations in this gene abolish P synthesis, PX2 is also lost as indicated by different methods here. When B3GALNT1 is overexpressed in a cell line, PX2 and P antigens are made. When B3GALNT1 transcripts are being suppressed by siRNA technology, PX2 levels go down. At the same time, we define the target for p-erythrocyte-reactive antibodies found in the plasma of all tested Pk individuals. In addition to the naturally occurring anti-P mentioned in textbooks for many decades, these rare human B3GALNT1 knock-out individuals also make antibodies directed at the PX2 and possibly also its mono- and/or disialylated derivatives. This body of data was presented to the Working Party for Red Cell Immunogenetics and Blood Group Terminology, and based on the available evidence, the committee voted for adopting PX2 as the second blood group antigen in the GLOB system (GLOB2) where P antigen (GLOB1) was previously the only member (49).
Thirty years ago, a β1,3-N-acetylgalactosaminyltransferase involved in globoside synthesis was isolated from canine spleen and shown to contain two subunits with molecular masses of 57 and 64 kDa. The enzyme could add a β1,3GalNAc onto Galα4-Gal-O-R where the R moiety seemed to have little effect on the enzyme activity, also indicating the possibility of different acceptors (40). However, we show here that β1,3GalNAc-T1 can use not only α4Gal- but also β4Gal-terminating acceptors. Our findings are analogous with the A4GALT-encoded α1,4-galactosyltransferase, which is capable of using both lactosylceramide and paragloboside as acceptors to synthesize the Pk and P1 antigens, respectively (17). Such examples indicate that these enzymes are capable of using different acceptors with various sizes and hydrophobicity while sharing the same terminal sugar. It has also been suggested that perhaps ceramide steers the enzyme specificity toward different acceptors and that each transferase has specific lipophilic properties affecting the association with different lipid bilayer regions and thereby different glycolipids (41).
Naturally occurring globoside-deficient mutant individuals with the P1k or P2k phenotype lack both P and PX2 antigens on the surface of erythrocytes, and the two individuals whose eluates were tested here had made both anti-P and anti-PX2 (Fig. 6, B and C, lanes 1 and 3). The presence of x2 glycosphingolipid on erythrocytes of p phenotype as the target for anti-PX2 is clinically disturbing because p phenotype red cell units are often used for transfusion to P1k and P2k patients due to the extreme rarity of P1k and P2k donors. Thus, even if the current study does not address the potential clinical significance of anti-PX2 when it comes to hemolytic potential in the transfusion or pregnancy setting, our findings may influence blood selection for these rare patients. Until more evidence is available, we therefore propose that blood units of P1k or P2k phenotype should be selected for Pk patients if available. A unit of p erythrocytes could pose a risk due to their high expression levels of PX2. In this way, cross-match-negative units can be selected for safe transfusion. Because the anti-P response is typically much stronger than anti-PX2, p red cell units are the obvious second choice despite the PX2 incompatibility before random P-positive units are given. Because both the p and Pk phenotypes are so rare, finding matching blood has always been problematic, and this usually requires international collaboration. Pk blood is even rarer than p units, so the findings presented here will not make blood selection any easier.
Plasma from the index P1k patient also contains specificities toward structures in the acid glycosphingolipid preparations (Fig. 6B, lanes 4 and 5) containing sialyl and disialyl versions of PX2 (Fig. 4), which may also implicate these structures as novel blood group antigens. α2,3-Sialylation of the terminal GalNAc of x2 by the α2,3-sialyltransferase ST3Gal II has been reported (42). However, so far, no sialyltransferase has been found to constitute the basis of a blood group system in humans. Based on the findings in this study, the polyclonal blood group antibody response present in Pk patients' sera should theoretically be a mixture of antibodies directed toward P, PX2, sialyl-PX2, and disialyl-PX2 (plus anti-P1 in the case of P2k individuals). However, in the P2k patient tested here, an antibody targeting only PX2 could be detected, and no reactivity toward acid glycosphingolipids was shown (Fig. 6C, lanes 4 and 5). One possible explanation is that the P1k patient had been transfused with several units of p erythrocytes and thereby possibly boosted an antibody response toward the missing structures.
Tonegawa et al. (43) first reported that “anti-globoside” produced in rabbits could react with glycoproteins prepared from human erythrocytes. However, Yang et al. (44) reported that the Galα4Gal epitope, terminating both P1 and Pk antigens, is only present on glycolipids, thereby suggesting the globo series epitopes to be absent from glycoproteins.
The cell line MEG-01 used in the transfection experiments has a weak endogenous P and PX2 antigen expression. P antigen is believed to be the main product of the glycosyltransferase encoded by B3GALNT1, although this enzyme is now also shown to be capable of PX2 antigen synthesis. However, to be able to induce a high P antigen expression in MEG-01 cells, double transfection with A4GALT was needed as reported previously (35) to enhance the expression of its precursor, Pk. One may therefore conclude from these observations that A4GALT appears to be a limiting step component of lactosylceramide elongation, and if the enzyme expression is low, it directs its enzymatic attention elsewhere (45), e.g. to the neolacto series of glycosphingolipids and in this case PX2 antigen synthesis. Furthermore, the effect of macromolecular crowding cannot be ignored because the effect on the glycosyltransferase is not known when overexpressing it in a cellular system (46). The Drosophila melanogaster β1,4-N-acetylgalactosaminyltransferase B (β4GalNAcTB) has been shown to require a cofactor, the GalNAcTB pilot, and specific hydrophobic amino acids in the stem region to be functionally important. The GalNAcTB pilot also seems to be essential for Golgi localization of the β4GalNAcTB (47). Whether this is crucial for the human GalNAc-transferases is not known, but because attempts to synthesize the soluble part of β1,3GalNAc-T1 has not been successful, other cofactors may be needed for its proper function (12).
In conclusion, the glycosyltransferase encoded by B3GALNT1 is capable of both P and PX2 antigen synthesis, and mutations in this gene render an individual's erythrocytes to become PX2-negative as well as globoside-deficient. The results of this study are of interest to the fields of glycobiology and enzymology in general but also specifically to the field of transfusion medicine because we propose a new blood selection principle for Pk individuals based on these data.
Author Contributions
J. S. W. performed and interpreted the B3GALNT1 overexpression experiments, siRNA knockdown, RNA extraction, real time PCR, and flow cytometric analyses of transfected cells. J. B. performed and interpreted the glycosphingolipid isolations, thin-layer chromatography, chromatogram binding assays, and LC-ESI/MS analyses. J. R. S. performed and interpreted the serological investigation in Lund, Sweden and the TH2 blocking experiments. T. P. performed and interpreted the serological investigation in Paris, France. A. K. H. performed and interpreted the flow cytometric analysis on erythrocytes. Å. H., J. R. S., S. T., and M. L. O. designed and coordinated the study. J. S. W., J. B., J. R. S., S. T., and M. L. O. wrote the paper. All authors read, edited, and approved the final version of the manuscript.
Acknowledgments
We express our gratitude to Sylvie Poupel in Paris, France for technical assistance with serological investigation and to Ulla Mandel in Copenhagen, Denmark for the generous donation of the monoclonal antibody TH2. We also acknowledge Rut Norda and Jan Säfwenberg in Uppsala, Sweden; Birgitta Nilsson Sojka in Umeå, Sweden; Marja-Kaisa Auvinen in Kuala Lumpur, Malaysia; and many others involved in the original clinical case that spurred our first interest. The use of the LTQ linear quadrupole ion trap mass spectrometer (obtained by Swedish Research Council Grant 342-2004-4434 to Gunnar Hansson), is gratefully acknowledged.
This work was supported by Swedish Medical Research Council Grants K2014-14251 (to M. L. O.) and K2013-12628 (to S. T.), the HOPE program at the Medical Faculty of Lund University (to M. L. O.), the Swedish Cancer Foundation (to S. T.), governmental ALF research grants to Lund and Sahlgrenska University Hospitals (to M. L. O. and S. T.), and the Skåne County Council's Research and Development foundation, Sweden (to M. L. O.). The authors declare that they have no conflicts of interest with the contents of this article.
- Gb4
- globotetraosylceramide
- PX2
- x2 glycosphingolipid
- β1,3GalNAc-T1
- UDP-N-acetylgalactosamine: globotriaosylceramide 3-β-N-acetylgalactosaminyltransferase (P synthase)
- Gb3
- globotriaosylceramide
- App
- A erythrocytes of p phenotype
- AP1k
- A erythrocytes of P1k phenotype
- ESI
- electrospray ionization
- LTQ
- linear trap quadrupole
- Hex
- hexose
- HexNAc
- N-acetylhexosamine
- GalNAcTB
- N-acetylgalactosaminyltransferase B.
References
- 1. Breton C., Fournel-Gigleux S., Palcic M. M. (2012) Recent structures, evolution and mechanisms of glycosyltransferases. Curr. Opin. Struct. Biol. 22, 540–549 [DOI] [PubMed] [Google Scholar]
- 2. Palcic M. M. (2011) Glycosyltransferases as biocatalysts. Curr. Opin. Chem. Biol. 15, 226–233 [DOI] [PubMed] [Google Scholar]
- 3. Keusch J. J., Manzella S. M., Nyame K. A., Cummings R. D., Baenziger J. U. (2000) Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. J. Biol. Chem. 275, 25308–25314 [DOI] [PubMed] [Google Scholar]
- 4. Merrill A. H., Jr. (2011) Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111, 6387–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daniels G. (2013) Human Blood Groups, pp. 162–181, Blackwell Scientific, Oxford, UK [Google Scholar]
- 6. Paulson J. C., Colley K. J. (1989) Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J. Biol. Chem. 264, 17615–17618 [PubMed] [Google Scholar]
- 7. Lairson L. L., Henrissat B., Davies G. J., Withers S. G. (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 77, 521–555 [DOI] [PubMed] [Google Scholar]
- 8. Clausen H., Hakomori S. (1989) ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 56, 1–20 [DOI] [PubMed] [Google Scholar]
- 9. Fletcher K. S., Bremer E. G., Schwarting G. A. (1979) P blood group regulation of glycosphingolipid levels in human erythrocytes. J. Biol. Chem. 254, 11196–11198 [PubMed] [Google Scholar]
- 10. Daniels G. L., Fletcher A., Garratty G., Henry S., Jørgensen J., Judd W. J., Levene C., Lomas-Francis C., Moulds J. J., Moulds J. M., Moulds M., Overbeeke M., Reid M. E., Rouger P., Scott M., Sistonen P., Smart E., Tani Y., Wendel S., Zelinski T., and International Society of Blood Transfusion (2004) Blood group terminology 2004: from the International Society of Blood Transfusion committee on terminology for red cell surface antigens. Vox Sang. 87, 304–316 [DOI] [PubMed] [Google Scholar]
- 11. Hellberg A., Poole J., Olsson M. L. (2002) Molecular basis of the globoside-deficient Pk blood group phenotype. Identification of four inactivating mutations in the UDP-N-acetylgalactosamine:globotriaosylceramide 3-β-N-acetylgalactosaminyltransferase gene. J. Biol. Chem. 277, 29455–29459 [DOI] [PubMed] [Google Scholar]
- 12. Amado M., Almeida R., Carneiro F., Levery S. B., Holmes E. H., Nomoto M., Hollingsworth M. A., Hassan H., Schwientek T., Nielsen P. A., Bennett E. P., Clausen H. (1998) A family of human β3-galactosyltransferases. Characterization of four members of a UDP-galactose:β-N-acetyl-glucosamine/β-N-acetyl-galactosamine β-1,3-galactosyltransferase family. J. Biol. Chem. 273, 12770–12778 [DOI] [PubMed] [Google Scholar]
- 13. Okajima T., Nakamura Y., Uchikawa M., Haslam D. B., Numata S. I., Furukawa K., Urano T., Furukawa K. (2000) Expression cloning of human globoside synthase cDNAs. Identification of β3Gal-T3 as UDP-N-acetylgalactosamine:globotriaosylceramide β1,3-N-acetylgalactosaminyltransferase. J. Biol. Chem. 275, 40498–40503 [DOI] [PubMed] [Google Scholar]
- 14. Steffensen R., Carlier K., Wiels J., Levery S. B., Stroud M., Cedergren B., Nilsson Sojka B., Bennett E. P., Jersild C., Clausen H. (2000) Cloning and expression of the histo-blood group Pk UDP-galactose: Gal-β1–4Glc-β1-Cer α1,4-galactosyltransferase. Molecular genetic basis of the p phenotype. J. Biol. Chem. 275, 16723–16729 [DOI] [PubMed] [Google Scholar]
- 15. Keusch J. J., Manzella S. M., Nyame K. A., Cummings R. D., Baenziger J. U. (2000) Cloning of Gb3 synthase, the key enzyme in globo-series glycosphingolipid synthesis, predicts a family of α1,4-glycosyltransferases conserved in plants, insects, and mammals. J. Biol. Chem. 275, 25315–25321 [DOI] [PubMed] [Google Scholar]
- 16. Kojima Y., Fukumoto S., Furukawa K., Okajima T., Wiels J., Yokoyama K., Suzuki Y., Urano T., Ohta M., Furukawa K. (2000) Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo series glycosphingolipids. J. Biol. Chem. 275, 15152–15156 [DOI] [PubMed] [Google Scholar]
- 17. Thuresson B., Westman J. S., Olsson M. L. (2011) Identification of a novel A4GALT exon reveals the genetic basis of the P1/P2 histo-blood groups. Blood 117, 678–687 [DOI] [PubMed] [Google Scholar]
- 18. Suchanowska A., Kaczmarek R., Duk M., Lukasiewicz J., Smolarek D., Majorczyk E., Jaskiewicz E., Laskowska A., Wasniowska K., Grodecka M., Lisowska E., Czerwinski M. (2012) A single point mutation in the gene encoding Gb3/CD77 synthase causes a rare inherited polyagglutination syndrome. J. Biol. Chem. 287, 38220–38230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reid M. E., Lomas-Francis C., Olsson M. L. (2012) The Blood Group Antigen FactsBook, pp. 135–146, 609–613, Elsevier, New York [Google Scholar]
- 20. Ando S., Yamakawa T. (1973) Separation of polar glycolipids from human red blood cells with special reference to blood group-A activity. J. Biochem. 73, 387–396 [PubMed] [Google Scholar]
- 21. Marcus D. M., Naiki M., Kundu S. K. (1976) Abnormalities in the glycosphingolipid content of human Pk and p erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 73, 3263–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kościelak J., Krauze R., Maśliński W., Zdebska E., Zieleński J., Brudzyński T., Miller-Podraza H. (1978) Neutral glycosphingolipids of serum and plasma [proceedings]. Arch. Immunol. Ther. Exp. 26, 119. [PubMed] [Google Scholar]
- 23. Olsson M. L., Peyrard T., Hult A. K., Hellberg Å., Nilsson Sojka B., Norda R., Auvinen M.-K., Storry J. R. (2011) PX2: a new blood group antigen with implications for transfusion recommendations in P1k and P2k individuals. Vox Sang. 101 (Suppl. 1), 53 (4A-S16–04) [Google Scholar]
- 24. Naiki M., Marcus D. M. (1977) Binding of N-acetylgalactosamine-containing compounds by a human IgM paraprotein. J. Immunol. 119, 537–539 [PubMed] [Google Scholar]
- 25. Kannagi R., Fukuda M. N., Hakomori S. (1982) A new glycolipid antigen isolated from human erythrocyte membranes reacting with antibodies directed to globo-N-tetraosylceramide (globoside). J. Biol. Chem. 257, 4438–4442 [PubMed] [Google Scholar]
- 26. Thorn J. J., Levery S. B., Salyan M. E., Stroud M. R., Cedergren B., Nilsson B., Hakomori S., Clausen H. (1992) Structural characterization of x2 glycosphingolipid, its extended form, and its sialosyl derivatives: accumulation associated with the rare blood group p phenotype. Biochemistry 31, 6509–6517 [DOI] [PubMed] [Google Scholar]
- 27. Storry J. R., Castilho L., Daniels G., Flegel W. A., Garratty G., Francis C. L., Moulds J. M., Moulds J. J., Olsson M. L., Poole J., Reid M. E., Rouger P., van der Schoot E., Scott M., Smart E., Tani Y., Yu L. C., Wendel S., Westhoff C., Yahalom V., Zelinski T. (2011) International Society of Blood Transfusion Working Party on red cell immunogenetics and blood group terminology: Berlin report. Vox Sang. 101, 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karlsson K. A. (1987) Preparation of total nonacid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 138, 212–220 [DOI] [PubMed] [Google Scholar]
- 29. Karlsson H., Halim A., Teneberg S. (2010) Differentiation of glycosphingolipid-derived glycan structural isomers by liquid chromatography/mass spectrometry. Glycobiology 20, 1103–1116 [DOI] [PubMed] [Google Scholar]
- 30. Samuelsson B. E., Pimlott W., Karlsson K. A. (1990) Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 193, 623–646 [DOI] [PubMed] [Google Scholar]
- 31. Koerner T. A., Jr., Prestegard J. H., Demou P. C., Yu R. K. (1983) High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry 22, 2676–2687 [DOI] [PubMed] [Google Scholar]
- 32. Waldi D. (1962) in Dünnschicht-Chromatographie (Stahl E., ed), pp. 496–515, Springer-Verlag, Berlin [Google Scholar]
- 33. Zhou D., Mattner J., Cantu C., 3rd, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y. P., Yamashita T., Teneberg S., Wang D., Proia R. L., Levery S. B., Savage P. B., Teyton L., Bendelac A. (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 [DOI] [PubMed] [Google Scholar]
- 34. Hansson G. C., Karlsson K. A., Larson G., McKibbin J. M., Blaszczyk M., Herlyn M., Steplewski Z., Koprowski H. (1983) Mouse monoclonal antibodies against human cancer cell lines with specificities for blood group and related antigens. Characterization by antibody binding to glycosphingolipids in a chromatogram binding assay. J. Biol. Chem. 258, 4091–4097 [PubMed] [Google Scholar]
- 35. Westman J. S., Hellberg A., Peyrard T., Hustinx H., Thuresson B., Olsson M. L. (2013) P1/P2 genotyping of known and novel null alleles in the P1PK and GLOB histo-blood group systems. Transfusion 53, 2928–2939 [DOI] [PubMed] [Google Scholar]
- 36. Folch J., Lees M., Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 37. Chai W., Piskarev V., Lawson A. M. (2001) Negative-ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal. Chem. 73, 651–657 [DOI] [PubMed] [Google Scholar]
- 38. Robbe C., Capon C., Coddeville B., Michalski J. C. (2004) Diagnostic ions for the rapid analysis by nano-electrospray ionization quadrupole time-of-flight mass spectrometry of O-glycans from human mucins. Rapid Commun. Mass Spectrom. 18, 412–420 [DOI] [PubMed] [Google Scholar]
- 39. Kundu S. K., Samuelsson B. E., Pascher I., Marcus D. M. (1983) New gangliosides from human erythrocytes. J. Biol. Chem. 258, 13857–13866 [PubMed] [Google Scholar]
- 40. Taniguchi N., Makita A. (1984) Purification and characterization of UDP-N-acetylgalactosamine: globotriaosylceramide β-3-N-acetylgalactosaminyltransferase, a synthase of human blood group P antigen, from canine spleen. J. Biol. Chem. 259, 5637–5642 [PubMed] [Google Scholar]
- 41. Kannagi R., Nudelman E., Hakomori S. (1982) Possible role of ceramide in defining structure and function of membrane glycolipids. Proc. Natl. Acad. Sci. U.S.A. 79, 3470–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toivonen S., Aitio O., Renkonen O. (2001) α2,3-Sialylation of terminal GalNAc β1–3Gal determinants by ST3Gal II reveals the multifunctionality of the enzyme. The resulting Neu5Acα2–3GalNAc linkage is resistant to sialidases from Newcastle disease virus and Streptococcus pneumoniae. J. Biol. Chem. 276, 37141–37148 [DOI] [PubMed] [Google Scholar]
- 43. Tonegawa Y., Hakomori S. I. (1977) “Ganglioprotein and globoprotein”: the glycoproteins reacting with anti-ganglioside and anti-globoside antibodies and the ganglioprotein change associated with transformation. Biochem. Biophys. Res. Commun. 76, 9–17 [DOI] [PubMed] [Google Scholar]
- 44. Yang Z., Bergström J., Karlsson K. A. (1994) Glycoproteins with Galα4Gal are absent from human erythrocyte membranes, indicating that glycolipids are the sole carriers of blood group P activities. J. Biol. Chem. 269, 14620–14624 [PubMed] [Google Scholar]
- 45. Takematsu H., Yamamoto H., Naito-Matsui Y., Fujinawa R., Tanaka K., Okuno Y., Tanaka Y., Kyogashima M., Kannagi R., Kozutsumi Y. (2011) Quantitative transcriptomic profiling of branching in a glycosphingolipid biosynthetic pathway. J. Biol. Chem. 286, 27214–27224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ellis R. J. (2001) Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 26, 597–604 [DOI] [PubMed] [Google Scholar]
- 47. Kraft B., Johswich A., Kauczor G., Scharenberg M., Gerardy-Schahn R., Bakker H. (2011) “Add-on” domains of Drosophila β1,4-N-acetylgalactosaminyltransferase B in the stem region and its pilot protein. Cell. Mol. Life Sci. 68, 4091–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varki A. (2009) Essentials of Glycobiology, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 49. Working Party for Red Cell Immunogenetics and Blood Group Terminology (2014) in Congress of the International Society of Blood Transfusion in Seoul, South Korea, May 31, 2014, meeting report, International Society of Blood Transfusion, Amsterdam [Google Scholar]