Background: Post-transcriptional modification of N6-threonylcarbamoyl-adenosine (t6A) is required for decoding function of tRNAs that pair A-starting codons.
Results: t6A-modified tRNAs are required for growth, to modulate TOR activity and translation efficiency.
Conclusion: Levels of t6A-modified tRNAs establish growth potential in eukaryotes.
Significance: Recognition in eukaryotes of a conserved mechanism of growth control that relies on tRNA post-transcriptional modification.
Keywords: cell growth, Drosophila, transfer RNA (tRNA), translation, yeast
Abstract
N6-Threonylcarbamoyl-adenosine (t6A) is a universal modification occurring at position 37 in nearly all tRNAs that decode A-starting codons, including the eukaryotic initiator tRNA (tRNAiMet). Yeast lacking central components of the t6A synthesis machinery, such as Tcs3p (Kae1p) or Tcs5p (Bud32p), show slow-growth phenotypes. In the present work, we show that loss of the Drosophila tcs3 homolog also leads to a severe reduction in size and demonstrate, for the first time in a non-microbe, that Tcs3 is required for t6A synthesis. In Drosophila and in mammals, tRNAiMet is a limiting factor for cell and animal growth. We report that the t6A-modified form of tRNAiMet is the actual limiting factor. We show that changing the proportion of t6A-modified tRNAiMet, by expression of an un-modifiable tRNAiMet or changing the levels of Tcs3, regulate target of rapamycin (TOR) kinase activity and influences cell and animal growth in vivo. These findings reveal an unprecedented relationship between the translation machinery and TOR, where translation efficiency, limited by the availability of t6A-modified tRNA, determines growth potential in eukaryotic cells.
Introduction
Transfer RNAs (tRNAs) are an essential part of protein synthesis machinery, decoding the linear information contained in mRNA into the three-dimensional information of proteins. Upon their discovery in the 1950s, tRNAs were considered to be simple adaptors that did not contain regulatory potential (1). Since then, tRNAs have been linked to diverse regulatory processes (2): uncharged tRNAs regulate gene expression in response to nutrient availability (3); tRNAs directly bind cytochrome c inhibiting apoptosis (4); reduced levels of the initiator tRNA (tRNAiMet) represses cell growth in yeast (5) and it is a limiting factor for growth in mammalian cells (6) and whole organism growth in Drosophila (7). Additionally, cytoplasmic tRNA availability has been shown to influence TORC12 (target of rapamycin complex 1) activity (8). Altogether these results reveal that in eukaryotic cells tRNAs are not only passive decoders of genetic information but also act as key regulators. The concentrations and charged levels of tRNAs are only one aspect of their potential as regulators. tRNAs can harbor a subset of the more than 100 known modifications (9). These range from simple methylations inserted by a single methyltransferase to complex modifications like wybutosine (yW) and queuosine (Q) that are synthesized in multistep pathways (10). tRNA modifications occur both in the body of the tRNA affecting its stability and folding, and in the anticodon stem loop (ASL) influencing mRNA decoding (11). To date, 15 tRNA modifications have been associated with human diseases, including Type 2 diabetes and Familial dysautonomia (12).
One of the few universally conserved modifications found in tRNA is N6-threonylcarbamoyl-adenosine (t6A) occurring at position 37 in tRNAs that decode A-starting codons (ANN) (10), including tRNAiMet (Fig. 1A). It stabilizes codon-anticodon interaction (13), suggesting that t6A might have a role regulating protein synthesis initiation. The modification was discovered over 40 years ago (14), but the enzymes involved in t6A synthesis were only identified and characterized in all domains of life in the last few years (15–21) and recently renamed (22). In yeast, components of the threonyl-carbamoyl transferase complex (TCTC, previously named KEOPS/EKC (kinase, endopeptidase and other proteins of small size/endopeptidase-like and kinase associated to transcribed chromatin) are required for t6A synthesis. Tcs3p (Kae1p) and Tcs5p (Bud32p) are part of the TCTC complex. Mutation of either gene eliminates t6A in tRNA and cause strong slow-growth phenotypes (15). Recently, we showed that Prpk (p53-related protein kinase), the Drosophila homolog of Tcs5p, is required for TORC1 activity and cell growth (23), suggesting t6A could be required for growth in metazoans.
FIGURE 1.
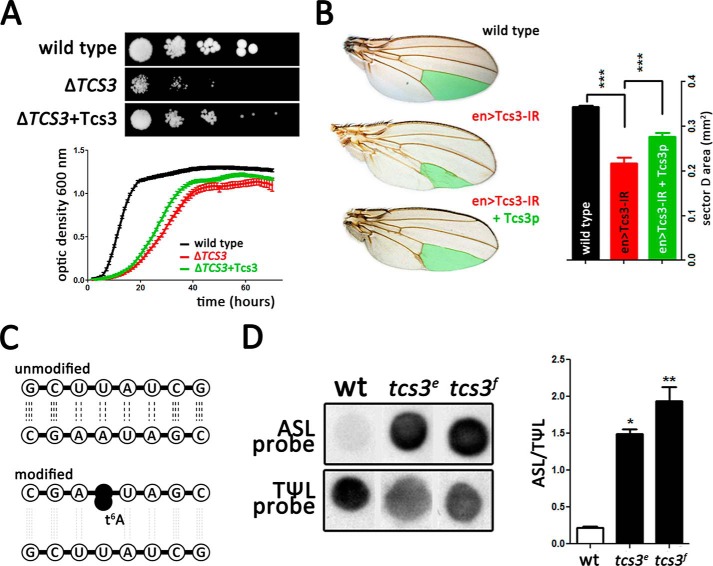
tcs3 mutants exhibit severe growth phenotypes. A, schematic representation of anti-codon stem loop of tRNAs that pair A-starting codons, indicating position 37 where t6A is present. Anti-codon (red) and codon (black) are also depicted. B, comparison between larvae from wild-type (wt), homozygous mutants for tcs3 (tcs3f and tcs3e) and a trans heterozygous genetic background (tcs3f/tcs3e). Ubiquitous Tcs3 expression using the armadillo-Gal4 driver (tcs3e+Tcs3) rescued the growth phenotype. C, Tcs3 mRNA was not detected by RT-PCR in tcs3f. Actin was amplified as loading control. D, mosaic fat body generated by FRT/FLP-mediated recombination shows that tcs3 is required for growth cell autonomously. Cell area quantification shows that mutant cells (GFP−, white arrowhead) are significantly smaller than wild-type cells (GFP+) (n = 50 cells).
Insulin and its downstream signaling (PI3K/TOR) play a paramount role in organ and cell growth in invertebrates and vertebrates. This signaling pathway allows transduction of hormonal and nutritional cues into the protein synthesis machinery (24–26). After activation, insulin receptor recruits Chico/IRS allowing PI3K activation, which increases the levels of phosphatidylinositol (3,4,5)-triphosphate causing Pdk1 to translocate and activate Akt1/Pdk1 at the plasma membrane. Akt1 influences protein synthesis in two major ways. First, it restricts 4E-BP transcription, an inhibitory factor of the elongation factor 4E, through inhibition of FOXO transcription factor. Second, Akt1 inhibits tuberous sclerosis complex (Tsc1–Tsc2), allowing the accumulation of GTP-Rheb and the subsequent activation of TOR (27). TOR activity is also regulated by the nutrient and energy status of the cell, as amino acid levels regulate the Rag GTPases and ATP/AMP ratio the AMP-activated protein kinase (28, 29). TOR ultimately enhances protein synthesis through an activating phosphorylation on S6K (which phosphorylates S6 ribosomal protein) and inhibiting 4E-BP, also by phosphorylation. In addition, TOR ensures a capable translational machinery controlling ribosome biogenesis and tRNA transcription (30).
Absence of t6A leads to an increase in +1 and −1 frameshifts and increases non-AUG start sites (16), which could explain its requirement for growth in eukaryotes. In addition, considering the particular limiting nature of the initiator tRNA for cell and animal growth (5, 7) and as this tRNA is modified by t6A, we were prompted to investigate the impact of t6A in whole animal context using Drosophila. All previous studies on t6A function and synthesis have been performed in unicellular organisms or cells in culture (15–21). In this study, we address for the first time the role of t6A in a metazoan.
Experimental Procedures
Husbandry, Fly Stocks, and Morphological Analysis
Animals were raised at low density at 25 °C on standard meal containing wheat flour (50 g/liter), fresh yeast (100 g/liter), agar-agar (11 g/liter), dextrose monohydrate (80 g/liter), propionic acid (6 ml/liter), and Nipagin (1.56 g/liter). Stocks were obtained from Bloomington Stock Center, Exelixis Collection, and Vienna Drosophila Resource Center. tcs3 coding sequence was amplified using primers Tcs3f-F, 5′-ggatccatggtttgcgctttgggtattg, and Tcs3f-R, 5′-ggatccttagtcatcccgccagctgacc, cloned into TOPO-TA vector (Life Technologies), sequenced (Macrogen), and subcloned in the pUAST vector using a BamHI restriction site to later develop transgenic animals following the standard germ line transformation protocol (31). Drosophila wings were mounted in a 1:1 mixture of lactic acid:ethanol as described in Ref. 23 and photographed under a Olympus BX51 microscope using a Moticam 2500 digital camera (Motic).
Staining, Western Blot, and RT-PCR Analysis
Nuclei were stained with TO-PRO-3 (1:200, Invitrogen) and F-actin with TRITC-labeled phalloidin (1 μg/ml, Sigma). Larvae were dissected and fixed as described by de Celis and co-workers (32). Confocal images were captured using a Zeiss LSM 510 Meta confocal microscope. For Western blot, rabbit polyclonal phospho-S6K, S6K, phospho-Akt, Akt, and phospho-eIF2α (all 1:1000 from Cell Signaling) and mouse anti-actin (1:1000 from Santa Cruz Biotechnology) were used. Blotting was performed as described in Ref. 33. For RT-PCR, total RNA was extracted from third instar larvae using TRIzol reagent (Invitrogen). cDNAs were synthesized with the Improm-II kit (Promega). Primers used were Tsc3-F, 5′-ATGGTTTGCGCTTTGGGTATTG, and Tsc3-R, 5′-TTAGTCATCCCGCCAGCTGACC. As a loading control actin cDNA was amplified using actin-F, 5′-GCGTCGGTCAATTCAATCTT, and actin-R, 5′-AAGCTGCAACCTCTTCGTCA. The PCR protocol was: denaturation 30 s at 95 °C, and 25 cycles of denaturation 95 °C s, annealing 55 °C, and elongation for 45 s at 72 °C. The final elongation was 5 min at 72 °C. Non-conventional splicing of Xbp1 mRNA was analyzed as described in Ref. 34.
tRNA Extraction and Detection by HPLC
For yeast and Drosophila tRNA extraction the method by El Yacoubi et al. (16) was used. HPLC analysis was performed as described in Ref. 35. The retention time of t6A was determined using a t6A standard synthesized by Darrell Davis at the University of Utah.
Positive Hybridization in Absence of t6A-(PHAt6A) Assays
tRNAs were mixed with 3 volumes of incubation solution (65% formamide, 0.08% formaldehyde, 1.3× MOPS) and incubated for 5 min at 65 °C for denaturation, mixed with a volume of ice-cold ×20 SSC, and kept on ice until used. This mixture was spotted on Biodyne-A (Thermo Scientific) nylon membrane and cross-linked by exposing it for 3 min to UV radiation. Afterward, pre-hybridization was done using DIG Easy Hyb (Roche) for 1 h at 42 °C with constant shaking. Biotinylated probes were designed to complement the Drosophila tRNAiMet (tRNA:M-i:61D, CR32482) and synthetized by Integrated DNA Technologies (IA). Both ACL (/5Bios(G/T)C TGG GTT ATG GGC CCA GC) and TΨL (/5Bios(G/G)A GCA AGG TTT CGA TCC TCG) were prepared 1:10,000 in DIG Easy Hyb and incubated overnight at 42 °C with constant shaking. The membranes were rinsed three times for 10 min with 2× SSC, 0.2% SDS, followed by two extra washes, the first one a room temperature and the second at 55 °C. Detection was carried our using BrightStar kit from Ambion (TX) following the manufacturer's instructions.
FLP-out Clonal Analysis and Mitotic Clones
All the fly stocks employed were generated by standard crosses. To generate FLP-out clones in fat body cells, offspring was subjected to heat shock (37 °C) at 36 ± 12 h after egg laying for 2 min. To generate mitotic clones in fat body cells, the offspring was heat shocked (37 °C) from 0 to 6 h after egg laying for 1 h. Third instar larvae with clones were processed and tissues were visualized by confocal microscopy.
Yeast Strains and Growth Conditions
Yeast strains were grown on YPD (DIFCO Laboratories) at 30 °C. Synthetic minimal, with or without agar, S.D. base or S.D. base Gal/raf with or without dropout (-uracil, -ura; -leucine, -leu; -histidine, -his) were purchased from Clontech and prepared as recommended by the manufacturer. Transformations were carried out as described in the pYES-DEST52 manual (Invitrogen). Growth curves were obtained using a Bioscreen C MBR (Oy Growth Curves AB Ltd., Finland) at 30 °C and at maximum shaking. 300 μl of culture was used in each well, and 10 replicates were used for each condition. Yeast cultures were grown on S.D. Gal/Raf −ura to saturation and diluted 500 times in S.D. Gal/Raf −ura before loading on the Bioscreen. The growth curves presented are the average of 10 independent replications.
Morphometric and Statistical Analysis
Wing area and hairs were quantified with Adobe Photoshop CS5 Extended using an analysis tool from at least 30 samples from female flies. Cell area was measured from fat body cells using ImageJ software (36). All data presented are mean ± S.D. and were subjected to Student's two-tailed t test. p values lower than 0.01 were considered to be significant, unless otherwise indicated. Flow cytometry was analyzed using FlowJo program.
Polysome Profiles
Gradients and ultracentrifugation were carried out as in Ref. 7. Polysome profiles were constructed by measuring 260 nm absorbance in samples from wild-type and tcs3 mutants. The polysome fraction (indicated with a black line) was identified in divalent cation-free extraction buffer supplemented with chelating agents.
Bioinformatics
The Blast tools and resources at NCBI were used (37). Multiple protein alignments were performed with the ClustalW tool (38). Structure-based alignment was performed using the ESPript platform online (39). For domain recognition we used InterPro Scan (40).
Results
Drosophila Tcs3 Is Required for Organismal and Cell Autonomous Growth
BLASTP analysis using the Saccharomyces cerevisiae Tcs3p (Kae1p) sequence (NP_012964.2 YKR038C) as query, on the Drosophila proteome identified the CG4933-PA (95% coverage, E = 5 × 10−154) as best match. The CG4933 locus corresponds to an unnamed protein-coding sequence located in the left arm of the third chromosome at cytological position 72E2. The locus is predicted to produce a single transcript with 3 exons encoding a 347-amino acid protein. InterPro identified CG4933-PA as a member of Kae1/YgjD family, which is involved in the biosynthesis of t6A (15, 41). Sequence alignment revealed conservation in several domains, particularly for amino acids that constitute the active center (data not shown). In FlyBase, CG4933-PA has been predicted to be part of TCTC (formerly KEOPS/EKC) and have threonylcarbamoyl transferase activity (42). Two insertional mutant alleles are available for CG4933; one has a PiggyBac transposon inserted in the 5′-UTR (f01978, BDSC) and the other in the coding sequence (e01173, Exelixis). Homozygous mutant larvae for these alleles exhibit a smaller size compared with wild-type animals (Fig. 1B). Mutants do not pupariate and die at larvae stages. These results are coherent with our previous reports on deficiency in another TCTC subunit, Tcs5 (Prpk) in the fly (23). We propose to name CG4933 as tcs3 (to be consistent with the new nomenclature and to reflect the ubiquitous role of this protein family). The two insertion alleles do not complement each other (Fig. 1B), confirming that both are mutant alleles of the same gene. Tcs3 mRNA was not detected by RT-PCR in tcs3e mutant larvae (Fig. 1C). In addition, we could rescue the mutant phenotype by ubiquitously expressing the Tcs3 cDNA using the Gal4/UAS system (Fig. 1B, right side). To elucidate if the reduction in size phenotype is caused by a systemic failure or if it is a cell autonomous phenomenon, we generated mosaic animals by mitotic recombination and measured the cell area in fat body clones. Homozygous mutant cells presented a significantly smaller size than controls (Fig. 1D) indicating the cellular requirement of this gene.
Functional Complementation between Yeast and Drosophila Genes: Tcs3 Is Required to Modify the Initiator tRNA (tRNAiMet)
To establish the functional conservation between these two proteins, we carried out inter-species complementation experiments. Yeast tcs3 mutants present a slow-growth phenotype (15) that can be partially, but significantly rescued, by ectopic expression of Drosophila tsc3 (Fig. 2A). Consistently, the phenotype caused by Tcs3 knockdown in Drosophila, using an inverted repeated construct, was rescued in a similar extent by co-expressing yeast Tcs3p in the posterior compartment of the wing primordia using the engrailed-Gal4 driver (Fig. 2B). Altogether these results support the notion that tcs3 is the Drosophila TCS3 homolog and implicate its encoded protein in t6A synthesis, which is required cell autonomously to sustain growth. Our in silico analysis and the interspecies complementation tests suggest that Drosophila Tcs3 is implicated in t6A synthesis. This modification was detected using an adaptation of a hybridization method used to detect the presence of N6-isopentenyl-adenosine (i6A), another modification found at position 37 of specific tRNAs (43). Briefly, when the threonyl-carbamoyl moiety is present in position Ala-37, it impairs Watson-Crick pairing between the tRNA and the specific DNA probe designed to complement the ASL. In the absence of t6A, no interference occurs and base pairing can be complete. Consequently, a dimmer signal reveals a higher level of t6A modification (Fig. 2C). We have named this technique PHAt6A (positive hybridization in the absence of t6A), and it correlates with the presence of t6A in tRNAs.3 Signal intensity obtained from tRNAs extracted from tcs3 mutants was significantly stronger than control, suggesting a reduction in t6A modification on tRNAiMet (Fig. 2D). Our results indicate that Tcs3 is required for the specific modification of the tRNAiMet, however, this likely extends to the other tRNAs that recognize A-starting codons.
FIGURE 2.
Tcs3 is required for t6A synthesis in Drosophila. A, yeasts were plated in solid media in serial dilutions of 1:10 factor from left to right. Growth differences of wild-type, TCS3 mutant cells (Δtcs3), and mutant yeasts expressing the Drosophila Tcs3 homolog (Δtcs3+Tcs3). Growth parameter of each strain was also analyzed in liquid media. Each strain has a color code (n = 10, p < 0.005). B, in Drosophila, Tcs3 was knocked-down using a specific inverted repeat construct expressed in the posterior wing compartment with the Gal4/UAS system (Tcs3-IR) and the growth phenotype evoked was rescued by yeast Tcs3p co-expression (en> Tcs3-IR+Tcs3). The area of sector D (colored in green) was measured and plotted following the same color code used in wing image labels (n = 50, mean ± S.D., t test p < 0.005). C, PHAt6A. In brief, probes designed against different regions of the initiator tRNA evidenced the presence of t6A modification. The strength of ASL probe (anti-codon stem loop) hybridization depends on the presence of t6A, whereas the TΨL probe hybridizes an unmodified base stretch and serves as an internal loading control. When t6A is absent, the tRNA-probe interaction is maximal, whereas its existence weakens the interaction. D, tRNAs from wild-type (wt) and mutant tcs3 animals (tcs3e and tcs3f) were probed with ASL and TΨL probes. Dot blots were merged to make a better composition. Also a plot depicting the change in the ASL/TΨL signal ratio is shown. ASL signals obtained from tcs3 mutants are significantly stronger than control counterparts (n = 6 samples).
t6A-modified tRNAs Are a Limiting Factor for Growth
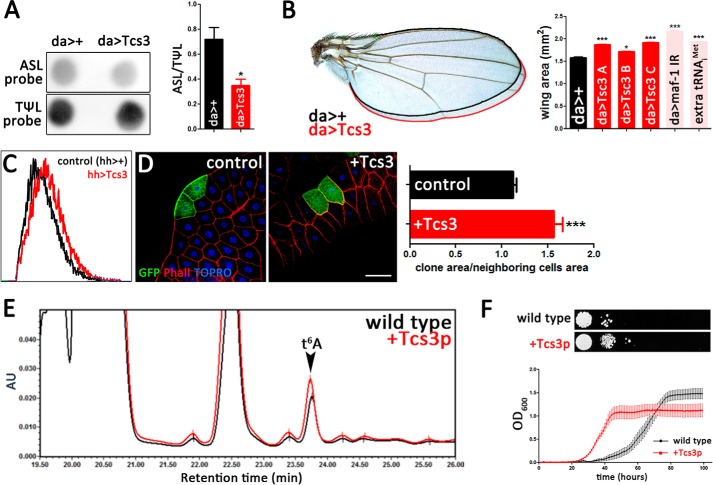
tRNAiMet is a limiting factor for growth in Drosophila (7) and is, in this organism, modified by t6A (9). Hence, the t6A-modified tRNAiMet could be the actual limiting factor. To address this, a synthetic mutant tRNAiMet that cannot be conjugated with t6A was inserted in the Drosophila genome. In this construct, the Ala at position 37 was mutated to Gly (tRNAiMet(A37G)) thus interfering with the consensus sequence required for t6A modification (44). Transcriptional regulatory sequences of this synthetic initiator tRNA, as well as 5′ and 3′ regions were taken from the endogenous sequences present in one of the four Drosophila initiator tRNA locus (tRNA:M-i:61D, CR32482) (45). Animals homozygous for the tRNAiMet(A37G) insertion had smaller wings than control (Fig. 3A). Likewise, counting the wing hairs in an area showed that wings of transgenic animals have more cells by unit of area, indicating that transgenic wings are composed of smaller cells than wild-type animals (Fig. 3B). These results not only support the notion that initiator tRNA is limiting for growth (7), but suggests that the proportion of t6A-modified initiator tRNA is the actual limiting factor. We then sought to increase the levels of t6A-modified tRNA by overexpressing tcs3 and asked whether this was sufficient to promote growth. Ubiquitous overexpression of tcs3 correlated with a significant increase in t6A-modified tRNAiMet (Fig. 4A) and with larger wings (Fig. 4B). To confirm that growth enhancement was specifically caused by tcs3 overexpression and not an indirect consequence of the insertion site of the UAS construct, we tested three different UAS insertions in combination with daughterless>Gal4 driver. Overexpression of tcs3 with all transgenes promoted similar increased growth, indicating that overgrowth was caused specifically by tcs3 overexpression (Fig. 4B). Also, overgrowth caused by tcs3 was comparable with the effect caused by maf1 (Pol III repressor) knockdown or the addition of an extra initiator tRNA locus (Fig. 4B), two experimental conditions that were previously shown to promote growth in Drosophila (7). tcs3-overexpressing animals were larger and composed of larger cells than control siblings, as flow cytometry analysis showed when control wing progenitor cells were compared with tcs3-overexpressing cells (Fig. 4C). Importantly, tcs3-overexpressing clones grew larger than controls, showing that the overgrowth response is a cell autonomous phenomenon (Fig. 4D). Altogether, these results show that t6A-modified tRNAs are a cell autonomous limiting factor for growth. An equivalent result was obtained overexpressing TCS3 in S. cerevisiae, as these cells grew faster than the control and contained more t6A (Fig. 4, E and F). Overall our results indicate t6A-modified tRNAiMet is a limiting factor for growth and not only supports the hypothesis that the proportion of t6A-modified tRNAiMet determine the potential for cell growth, but also reveals the conserved nature of this feature among eukaryotic cells.
FIGURE 3.

Expression of an Ala-37 mutant initiator tRNA reduces growth. A, comparison between wings from wild-type and from tRNAiMet(A37G) homozygous transgenic animals. A significant reduction in wing area, together with a higher cell density (B) is detected in transgenic animals compared with control wings (n = 50 wings).
FIGURE 4.
An increment of t6A-modified tRNAs promotes growth cell-autonomously. A, representative image of PHAt6A assay in samples from control and Tcs3-overexpressing animals. Signal intensity was quantified and plotted as ASL/TΨL ratio (n = 4 samples). B, a comparison between control (black line) and Tcs3-overexpressing wings (red line). Wing area was plotted showing that Tcs3 significantly promotes growth (n = 50 wings). As controls we used two other UAS insertions located in the second and third chromosome (Tcs3(B) and Tcs3(C)) were able to promote animal growth. Likewise other interventions with known effects on animal growth are: Maf1 knockdown and the addition of an extra locus of tRNAiMet. Measurements of wing area from control (da>+) and experimental animals show that these manipulations favor animal growth in a comparable range (n = 50, p < 0.005 to control). C, flow cytometry was used to compare cell size in Tcs3-overexpressing and control cells. hedgehog-Gal4 driver (hh>Gal4) was used to express Tcs3 and GFP in the wing posterior compartment, whereas control cells were obtained from the anterior compartment of the same imaginal discs (no GFP). Histogram are representative of 3 independent experiments. D, FLP-out Tcs3-overexpressing mosaic analysis (bar = 100 μm). Control clones express GFP only, whereas Tcs3-overexpressing clones express Tcs3 and GFP. Clone area was measured and normalized by the area of its neighbor cells; these ratios are presented in the chart (n = 50 cells). Wild-type yeast strain (BY4741) was transformed with empty pDEST52 vector (control) or with a pDEST52 construct to overexpress the D. melanogaster Tcs3p coding sequence (+Tcs3p). E, plot representing an archetypal HPLC elution profile of nucleosides from control (black line) or Tsc3p-overexpressing yeast (red line). AU, arbitrary units. F, serial dilution assays (1:10) were made from a suspension of cells with A600 = 0.6. Growth assay in liquid media were made measuring simultaneously the A600 of 10 independent wells and the plot is representative of 3 independent experiments.
Levels of t6A-modified tRNAs Alter TOR Kinase Activity
Because tcs3 overexpression promotes growth (Fig. 4) and the phenotypes observed in tcs3 mutants (Fig. 1, B and D) resemble deficiencies in positive regulators of the insulin/TOR pathway (46, 47), we examined if changes in t6A-modified tRNA levels could modulate TOR activity. TOR is present in two structural and functionally different complexes (TORC1–2). TORC1 is related to growth control and one of its phosphorylation targets is S6K at Thr-398 (48). S6K phosphorylation was strongly diminished in tcs3 mutants (Fig. 5A), suggesting the lack of TORC1 activity as a potential underlying cause of the growth deficiency. To directly activate TORC1, independently of t6A levels, we overexpressed Rheb in homozygous tsc3 mutants (49). Expression of Rheb in a tcs3 mutant background was unable to rescue animal growth (Fig. 5C), even though TORC1 activity was enhanced in these animals (Fig. 5D). Also TORC2 activity was strongly reduced in tcs3 mutants (Fig. 5A). These results indicate that tRNAs lacking t6A caused a failure in the translation apparatus, probably due to poor or inaccurate codon recognition that disable it to sustain growth. To get insights about the protein synthesis status we performed polysome-profiling experiments and observed that tcs3 mutants have a strongly reduced polysome fraction (Fig. 5B), further supporting the notion of insufficient protein synthesis in tcs3 mutants. This reduction of protein synthesis was not dependent on eIF2α phosphorylation, because it did not change in tcs3 mutant larvae (Fig. 5A). On the other hand, increasing the proportion of t6A-modified tRNAs by overexpressing tcs3 stimulates TORC1 activity, as shown by the augmented phosphorylation of S6K (Fig. 5E). These findings expose a regulatory relationship between TOR activity and the availability of t6A-modified tRNAs.
FIGURE 5.

Variations of TOR activity in animals with different levels of t6A-modified tRNAs. A, Western blot of S6K and Akt phosphorylation performed by TORC1 and TORC2, respectively, which in tcs3 mutants (tcs3e and tcsf) was strongly reduced in comparison to wild-type animals. Total S6K, Akt, and actin were used as loading controls. Also we detected eIF2α phosphorylation at Ser-51 in these genetic backgrounds; actin was used as loading control. B, polysome profiles were constructed measuring 260 nm absorbance in samples obtained after ultracentrifugation in sucrose gradient. The polysome fraction (indicated with a black line) was identified in divalent cation-free extraction buffer and in the presence of chelating agents, wild-type (green) and tcs3e mutant (red) larvae. C, comparison between wild-type and tcs3e mutants expressing Rheb (tcs3e+Rheb) using the armadillo>Gal4 driver. D, Western blot detection of S6K phosphorylation in mutant (tcs3e) and mutant overexpressing Rheb (tcs3e+Rheb). E, Western blot detecting S6K phosphorylation in control (da>+) and Tcs3-overexpressing animals (da>Tcs3). Western blot images are representative of 3 independent experiments in each case.
Discussion
tRNA molecules have a paramount role in protein synthesis and prior studies have centered on structural and biochemical features, overlooking their regulatory properties and functional interplay with cellular physiology. However, recent technology advances and shifts in research paradigms have opened new venues to investigate the non-canonical, ancestral, and divergent functions of tRNAs. The results presented here suggest a previously unexpected causal relationship by which changes in tRNA isoacceptors or their degree of modifications could not only be the result of cell differentiation processes or homeostatic responses to cellular stress, but also could channel an ontology trajectory that modulates the mode cells respond to environmental cues. Specifically, our results show a clear relationship between the level of t6A modification, particularly of the initiator tRNA, and the growth potential of eukaryotic cells.
The machinery that synthetizes t6A is conserved in all domains of life. In accordance, we identified tcs3, the Drosophila homolog of yeast TCS3, and established that tcs3 is involved in t6A synthesis. Furthermore, and in agreement with the severe slow-growth phenotype reported in yeast (15), we have determined that this enzyme is necessary for cell autonomous growth in Drosophila, as we previously showed for Tcs5 (Prpk) (23). Metazoans are composed of different cell types, therefore making the phenotype observed in tcs3 mutants even more complex than the ones observed in yeast and other unicellular organisms (15, 16, 18, 21, 50, 51). However, in every case analyzed, the deficiency of these enzymes strongly impairs cellular growth and proliferation. Interestingly, the possibility that fast growing or proliferating cells could have special requirements for t6A-modified tRNAs is further hinted by the fact that human PRPK was originally identified in a transcriptional screen performed in IL-2-activated lymphocytes (52). In Drosophila, tcs3 is differentially expressed in different anatomical structures and developmental times (53). Also OSGEP, the human homolog of TCS3, is deferentially expressed in human tissues (The Human Protein Atlas) (54). From a wider perspective, this indicates that t6A-modified tRNAs have a relevant role modulating protein expression in different cellular contexts and in consequence, the study of the relationship of t6A modification with protein synthesis regulation and growth control in physiological and pathological conditions, as in cancer, may emerge as a fertile area of research. In accordance with this, t6A had been proposed in the past as a prognosis marker for breast cancer (55).
Changes in tRNA levels are well documented in physiological (56) and pathological (57) processes, as well as a cellular homeostatic response to face stress conditions (58). These variations allow cells to adapt to determined conditions regulating protein synthesis, a process termed adaptive translation (59). Also, changes in isoacceptors can promote or decrease translation of specific mRNAs. This type of behavior has been recently documented; proliferating cells present a different tRNA transcriptome compared with differentiating ones, and this is correlated with epigenetic landmarks in specific tRNAs loci (60). Thus, isoacceptor profiles would permit the cell to fine-tune gene expression to establish a particular proteome to face differentiation programs. In this regard, changes in the levels of tRNA post-transcriptional modification have not been considered enough and our findings invite to think that tRNA modifications, or at least t6A-modified tRNAs, may have an important role in this regard. Changes in tRNA modification profiles have been reported to help cells cope with stressful conditions (58). Modification levels may have a direct role over cellular processes by conditioning, among others, the growth potential of a determined cell population.
Experiments published by the Grewal group (7) showed that the addition of an extra initiator tRNA locus, but no other tRNA, is sufficient to promote organismal growth in Drosophila. Our results showed that apparently the actual limiting factor for cell growth is the proportion of t6A-modified initiator tRNA, as the addition of an extra locus that codes for a mutant initiator tRNA, which cannot be modified with t6A, in fact inhibits growth. Thus, our results suggest that variations in the proportion of t6A-modified initiator tRNA is able to condition the ability of cells to grow. Likewise tcs3 overexpression consistently enhanced the levels of t6A-modified initiator tRNA and promoted autonomous cell growth in accordance with our proposal.
It has been described, and it is widely accepted, that TORC1 is the central regulator of cell growth in eukaryotes. Because the phenotypes observed in tcs3 mutants are remarkably similar to tor mutants (61) or some of its positive regulators (49, 62), an inter-relationship between the function of these elements would be expected. It has been shown that reductions in cytoplasmic tRNAs reduce TORC1 activity (8), suggesting the existence of an uncharacterized molecular feedback mechanism between the upstream protein synthesis controller, TORC1, and the canonical structural decoding blocks, the tRNAs. Furthermore, we found that tcs3 mutants have a severe decrease in S6K phosphorylation, a direct target of TORC1, whereas tcs3 overexpression enhanced it. Hence, levels of t6A-modified tRNAs strongly influence TORC1 activity. This implies that the translation machinery, possibly through initiator complex assembly, is tightly linked with TORC1 and the activation of its downstream targets. Also tcs3 mutants present a strong reduction of polysome fraction supporting a general failure in protein synthesis initiation caused by deficient ribosome assembly. In this respect, it has been described that the interaction of TORC2 with ribosome is required for its activation (63) and consequently, TORC2 activity was also reduced in tcs3 mutants. However, neither the specific phosphorylation of Akt1 catalyzed by TORC2, nor the phenotypes of mutants in TORC2 components support a functional relationship that could explain our observations. Mistranslation and unfolded protein response activation have been reported in yeast TCS3 mutants (18) and in tcs3 and tcs5 knockdown conditions in Drosophila (64), respectively. The results obtained with tcs3 mutants indicate that the growth phenotype evidenced is complex and most likely based on the poor translation initiation, not induced by eIF2α activation, but due to a fundamental structural defect produced by the absence of t6A-modified tRNAs.
Recently, Scheidt et al. (65) showed in yeast that absence of another tRNA modification altered TOR activity as well. Methoxycarbonylmethyl-2-thiouridine (mcm5s2U) is a modification present at position 34 and is required for the efficient decoding by tRNALysUUU (10). The absence of mcm5s2U mislocalizes Gln3p due to reductions in TOR activity (65). Together with our results, these observations suggest that TOR activity is probably affected by the aberrant translation caused by deficiencies in either modification. The molecular nature of the components that connect translation and TOR are currently unknown. However, our findings indicate a tight association between the availability of t6A-modified tRNAs and TOR to establish the general capability of the cell to grow.
Author Contributions
D. R. B. conceived and performed the experiments and wrote the paper. P. C. T. helped draft the article and revised it for critically important intellectual content. V. d. C. L. helped draft the article and revised it for critically important intellectual content. A. G. conceived experiments, wrote the paper, and helped draft the article and revised it critically for important intellectual content.
Acknowledgments
We thank The Company of Biologists and International Union of Biochemistry and Molecular Biology for travel funding (to D. R. B.). Stocks obtained from the Bloomington Drosophila Stock Center (National Institutes of Health Grant P40OD018537) were used in this study.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM70641 (to V. d. C-L.), FONDECYT (Fondo Nacional de Desarrollo Científico y Tecnológico) 1140522 and FONDAP (Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias) 15090007 grants (to A. G.), and CONICYT (Consejo Nacional de Investigación Científica y Tecnológica) Grant AT24121519 (to D. R. B.). The authors declare that they have no conflicts of interest with the contents of this article.
P. Thiaville, J. Bacusmo, D. Rojas-Benítez, A. Glavic, and V. de Crecy-Lagard, manuscript in preparation.
- TORC1
- target of rapamycin complex 1
- ASL
- anticodon stem loop
- t6A
- N6-threonylcarbamoyl-adenosine
- TCTC
- threonyl-carbamoyl transferase complex
- KEOPS/EKC
- kinase, endopeptidase and other proteins of small size/endopeptidase-like and kinase associated to transcribed chromatin
- Prpk
- p53-related protein kinase
- Tsc
- tuberous sclerosis complex
- TRITC
- tetramethylrhodamine isothiocyanate
- PHAt6A
- positive hybridization in the absence of t6A.
References
- 1. Crick F. H. (1968) The origin of the genetic code. J. Mol. Biol. 38, 367–379 [DOI] [PubMed] [Google Scholar]
- 2. Raina M., Ibba M. (2014) tRNAs as regulators of biological processes. Front. Genet. 5, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wek S. A., Zhu S., Wek R. C. (1995) The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 15, 4497–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mei Y., Yong J., Liu H., Shi Y., Meinkoth J., Dreyfuss G., Yang X. (2010) tRNA binds to cytochrome c and inhibits caspase activation. Mol. Cell 37, 668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Unger M. W., Hartwell L. H. (1976) Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc. Natl. Acad. Sci. U.S.A. 73, 1664–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pavon-Eternod M., Gomes S., Rosner M. R., Pan T. (2013) Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA 19, 461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rideout E. J., Marshall L., Grewal S. S. (2012) Drosophila RNA polymerase III repressor Maf1 controls body size and developmental timing by modulating tRNAiMet synthesis and systemic insulin signaling. Proc. Natl. Acad. Sci. U.S.A. 109, 1139–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huynh L. N., Thangavel M., Chen T., Cottrell R., Mitchell J. M., Praetorius-Ibba M. (2010) Linking tRNA localization with activation of nutritional stress responses. Cell Cycle 9, 3112–3118 [DOI] [PubMed] [Google Scholar]
- 9. Machnicka M. A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K. M., Helm M., Bujnicki J. M., Grosjean H. (2013) MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res. 41, D262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Yacoubi B., Bailly M., de Crécy-Lagard V. (2012) Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev. Genet. 46, 69–95 [DOI] [PubMed] [Google Scholar]
- 11. Phizicky E. M., Alfonzo J. D. (2010) Do all modifications benefit all tRNAs? FEBS Lett. 584, 265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres A. G., Batlle E., Ribas de Pouplana L. (2014) Role of tRNA modifications in human diseases. Trends Mol. Med. 20, 306–314 [DOI] [PubMed] [Google Scholar]
- 13. Weissenbach J., Grosjean H. (1981) Effect of threonylcarbamoyl modification (t6A) in yeast tRNA Arg III on codon-anticodon and anticodon-anticodon interactions. A thermodynamic and kinetic evaluation. Eur. J. Biochem. 116, 207–213 [DOI] [PubMed] [Google Scholar]
- 14. Schweizer M. P., Chheda G. B., Baczynskyj L., Hall R. H. (1969) Aminoacyl nucleosides: VII. N-(Purin-6-ylcarbamoyl)threonine. A new component of transfer ribonucleic acid. Biochemistry 8, 3283–3289 [DOI] [PubMed] [Google Scholar]
- 15. El Yacoubi B., Hatin I., Deutsch C., Kahveci T., Rousset J. P., Iwata-Reuyl D., Murzin A. G., de Crécy-Lagard V. (2011) A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 30, 882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Yacoubi B., Lyons B., Cruz Y., Reddy R., Nordin B., Agnelli F., Williamson J. R., Schimmel P., Swairjo M. A., de Crécy-Lagard V. (2009) The universal YrdC/Sua5 family is required for the formation of threonylcarbamoyladenosine in tRNA. Nucleic Acids Res. 37, 2894–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perrochia L., Guetta D., Hecker A., Forterre P., Basta T. (2013) Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A tRNA modification. Nucleic Acids Res. 41, 9484–9499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daugeron M. C., Lenstra T. L., Frizzarin M., El Yacoubi B., Liu X., Baudin-Baillieu A., Lijnzaad P., Decourty L., Saveanu C., Jacquier A., Holstege F. C., de Crécy-Lagard V., van Tilbeurgh H., Libri D. (2011) Gcn4 misregulation reveals a direct role for the evolutionary conserved EKC/KEOPS in the t6A modification of tRNAs. Nucleic Acids Res. 39, 6148–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perrochia L., Crozat E., Hecker A., Zhang W., Bareille J., Collinet B., van Tilbeurgh H., Forterre P., Basta T. (2013) In vitro biosynthesis of a universal t6A tRNA modification in Archaea and Eukarya. Nucleic Acids Res. 41, 1953–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauhon C. T. (2012) Mechanism of N6-threonylcarbamoyladenonsine (t6A) biosynthesis: isolation and characterization of the intermediate threonylcarbamoyl-AMP. Biochemistry 51, 8950–8963 [DOI] [PubMed] [Google Scholar]
- 21. Thiaville P. C., El Yacoubi B., Perrochia L., Hecker A., Prigent M., Thiaville J. J., Forterre P., Namy O., Basta T., de Crécy-Lagard V. (2014) Cross kingdom functional conservation of the core universally conserved threonylcarbamoyladenosine tRNA synthesis enzymes. Eukaryot. Cell 13, 1222–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiaville P. C., Iwata-Reuyl D., de Crécy-Lagard V. (2014) Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t6A), a universal modification of tRNA. RNA Biol. 11, 1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibar C., Cataldo V. F., Vásquez-Doorman C., Olguín P., Glavic A. (2013) Drosophila p53-related protein kinase is required for PI3K/TOR pathway-dependent growth. Development 140, 1282–1291 [DOI] [PubMed] [Google Scholar]
- 24. Saltiel A. R., Kahn C. R. (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 [DOI] [PubMed] [Google Scholar]
- 25. van Sluijters D. A., Dubbelhuis P. F., Blommaart E. F., Meijer A. J. (2000) Amino-acid-dependent signal transduction. Biochem. J. 351, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leevers S. J., Weinkove D., MacDougall L. K., Hafen E., Waterfield M. D. (1996) The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 15, 6584–6594 [PMC free article] [PubMed] [Google Scholar]
- 27. Oldham S., Hafen E. (2003) Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 13, 79–85 [DOI] [PubMed] [Google Scholar]
- 28. Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willis I. M., Lee J. (2012) Two new kinases in the TOR signaling network regulate ribosome and tRNA synthesis. Cell Cycle 11, 2769–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spradling A. C., Rubin G. M. (1982) Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218, 341–347 [DOI] [PubMed] [Google Scholar]
- 32. Cruz C., Glavic A., Casado M., de Celis J. F. (2009) A gain-of-function screen identifying genes required for growth and pattern formation of the Drosophila melanogaster wing. Genetics 183, 1005–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hennig K. M., Colombani J., Neufeld T. P. (2006) TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J. Cell Biol. 173, 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lisbona F., Rojas-Rivera D., Thielen P., Zamorano S., Todd D., Martinon F., Glavic A., Kress C., Lin J. H., Walter P., Reed J. C., Glimcher L. H., Hetz C. (2009) BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1α. Mol. Cell 33, 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pomerantz S. C., McCloskey J. A. (1990) Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 193, 796–824 [DOI] [PubMed] [Google Scholar]
- 36. Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gouet P., Courcelle E., Stuart D. I., Metoz F. (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 40. Mulder N., Apweiler R. (2007) InterPro and InterProScan: tools for protein sequence classification and comparison. Methods Mol. Biol. 396, 59–70 [DOI] [PubMed] [Google Scholar]
- 41. Srinivasan M., Mehta P., Yu Y., Prugar E., Koonin E. V., Karzai A. W., Sternglanz R. (2011) The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A. EMBO J. 30, 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. dos Santos G., Schroeder A. J., Goodman J. L., Strelets V. B., Crosby M. A., Thurmond J., Emmert D. B., Gelbart W. M., and FlyBase Consortium (2015) FlyBase: introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic Acids Res. 43, D690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lamichhane T. N., Blewett N. H., Maraia R. J. (2011) Plasticity and diversity of tRNA anticodon determinants of substrate recognition by eukaryotic A37 isopentenyltransferases. RNA 17, 1846–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morin A., Auxilien S., Senger B., Tewari R., Grosjean H. (1998) Structural requirements for enzymatic formation of threonylcarbamoyladenosine (t6A) in tRNA: an in vivo study with Xenopus laevis oocytes. RNA 4, 24–37 [PMC free article] [PubMed] [Google Scholar]
- 45. Sharp S., DeFranco D., Silberklang M., Hosbach H. A., Schmidt T., Kubli E., Gergen J. P., Wensink P. C., Söll D. (1981) The initiator tRNA genes of Drosophila melanogaster: evidence for a tRNA pseudogene. Nucleic Acids Res. 9, 5867–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oldham S., Montagne J., Radimerski T., Thomas G., Hafen E. (2000) Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 14, 2689–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Montagne J., Stewart M. J., Stocker H., Hafen E., Kozma S. C., Thomas G. (1999) Drosophila S6 kinase: a regulator of cell size. Science 285, 2126–2129 [DOI] [PubMed] [Google Scholar]
- 48. Pullen N., Thomas G. (1997) The modular phosphorylation and activation of p70s6k. FEBS Lett. 410, 78–82 [DOI] [PubMed] [Google Scholar]
- 49. Saucedo L. J., Gao X., Chiarelli D. A., Li L., Pan D., Edgar B. A. (2003) Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 5, 566–571 [DOI] [PubMed] [Google Scholar]
- 50. Naor A., Thiaville P. C., Altman-Price N., Cohen-Or I., Allers T., de Crécy-Lagard V., Gophna U. (2012) A genetic investigation of the KEOPS complex in halophilic Archaea. PLoS One 7, e43013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Na J. G., Pinto I., Hampsey M. (1992) Isolation and characterization of SUA5, a novel gene required for normal growth in Saccharomyces cerevisiae. Genetics 131, 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abe Y., Matsumoto S., Wei S., Nezu K., Miyoshi A., Kito K., Ueda N., Shigemoto K., Hitsumoto Y., Nikawa J., Enomoto Y. (2001) Cloning and characterization of a p53-related protein kinase expressed in interleukin-2-activated cytotoxic T-cells, epithelial tumor cell lines, and the testes. J. Biol. Chem. 276, 44003–44011 [DOI] [PubMed] [Google Scholar]
- 53. St Pierre S. E., Ponting L., Stefancsik R., McQuilton P., FlyBase C. (2014) FlyBase 102[en]advanced approaches to interrogating FlyBase. Nucleic Acids Res. 42, D780–D788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., Odeberg J., Djureinovic D., Takanen J. O., Hober S., Alm T., Edqvist P. H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J. M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. (2015) Proteomics: tissue-based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- 55. Vold B. S., Kraus L. E., Rimer V. G., Coombes R. C. (1986) Use of a monoclonal antibody to detect elevated levels of a modified nucleoside, N-[9-(β-d-ribofuranosyl)purin-6-yl-carbamoyl]-l-threonine, in the urine of breast cancer patients. Cancer Res. 46, 3164–3167 [PubMed] [Google Scholar]
- 56. Cooper H. L., Braverman R. (1980) Protein synthesis in resting and growth-stimulated human peripheral lymphocytes. Evidence for regulation by a non-messenger RNA. Exp. Cell Res. 127, 351–359 [DOI] [PubMed] [Google Scholar]
- 57. Kanduc D., Grazia di Corcia M., Lucchese A., Natale C. (1997) Enhanced expression of initiator tRNA(Met) in human gastric and colorectal carcinoma. Biochem. Mol. Biol. Int. 43, 1323–1329 [DOI] [PubMed] [Google Scholar]
- 58. Pang Y. L., Abo R., Levine S. S., Dedon P. C. (2014) Diverse cell stresses induce unique patterns of tRNA up- and down-regulation: tRNA-seq for quantifying changes in tRNA copy number. Nucleic Acids Res. 42, e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pan T. (2013) Adaptive translation as a mechanism of stress response and adaptation. Annu. Rev. Genet 47, 121–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gingold H., Tehler D., Christoffersen N. R., Nielsen M. M., Asmar F., Kooistra S. M., Christophersen N. S., Christensen L. L., Borre M., Sørensen K. D., Andersen L. D., Andersen C. L., Hulleman E., Wurdinger T., Ralfkiær E., Helin K., Grønbæk K., Orntoft T., Waszak S. M., Dahan O., Pedersen J. S., Lund A. H., Pilpel Y. (2014) A dual program for translation regulation in cellular proliferation and differentiation. Cell 158, 1281–1292 [DOI] [PubMed] [Google Scholar]
- 61. Neufeld T. P. (2004) Genetic analysis of TOR signaling in Drosophila. Curr. Top. Microbiol. Immunol. 279, 139–152 [DOI] [PubMed] [Google Scholar]
- 62. Stocker H., Radimerski T., Schindelholz B., Wittwer F., Belawat P., Daram P., Breuer S., Thomas G., Hafen E. (2003) Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 5, 559–565 [DOI] [PubMed] [Google Scholar]
- 63. Zinzalla V., Stracka D., Oppliger W., Hall M. N. (2011) Activation of mTORC2 by association with the ribosome. Cell 144, 757–768 [DOI] [PubMed] [Google Scholar]
- 64. Rojas-Benítez D., Ibar C., Glavic Á. (2013) The Drosophila EKC/KEOPS complex: roles in protein synthesis homeostasis and animal growth. Fly 7, 168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scheidt V. J., Bär C., Klassen R., Schaffrath R. (2015) Loss of woble uridine modification in tRNA anticodons interferes with TOR pathway signaling. Microbial. Cell 1, 416–424 [DOI] [PMC free article] [PubMed] [Google Scholar]