Background: Membrane potential (Em) hyperpolarization during sperm capacitation is necessary for acrosome reaction.
Results: cSrc is activated downstream of PKA, regulating the SLO3 K+ channel and promoting membrane hyperpolarization.
Conclusion: Acrosomal responsiveness of mouse sperm depends on cSrc activation of SLO3.
Significance: This represents the first evidence of K+ channel regulation in mouse sperm by a tyrosine kinase, affecting Em.
Keywords: phosphotyrosine signaling, potassium channel, PKA, sperm, Src, acrosome reaction, membrane potential
Abstract
Plasma membrane hyperpolarization is crucial for mammalian sperm to acquire acrosomal responsiveness during capacitation. Among the signaling events leading to mammalian sperm capacitation, the immediate activation of protein kinase A plays a pivotal role, promoting the subsequent stimulation of protein tyrosine phosphorylation that associates with fertilizing capacity. We have shown previously that mice deficient in the tyrosine kinase cSrc are infertile and exhibit improper cauda epididymis development. It is therefore not clear whether lack of sperm functionality is due to problems in epididymal maturation or to the absence of cSrc in sperm. To further address this problem, we investigated the kinetics of cSrc activation using anti-Tyr(P)-416-cSrc antibodies that only recognize active cSrc. Our results provide evidence that cSrc is activated downstream of PKA and that inhibition of its activity blocks the capacitation-induced hyperpolarization of the sperm plasma membrane without blocking the increase in tyrosine phosphorylation that accompanies capacitation. In addition, we show that cSrc inhibition also blocks the agonist-induced acrosome reaction and that this inhibition is overcome by pharmacological hyperpolarization. Considering that capacitation-induced hyperpolarization is mediated by SLO3, we evaluated the action of cSrc inhibitors on the heterologously expressed SLO3 channel. Our results indicate that, similar to SLO1 K+ channels, cSrc blockers significantly decreased SLO3-mediated currents. Together, these results are consistent with findings showing that hyperpolarization of the sperm plasma membrane is necessary and sufficient to prepare the sperm for the acrosome reaction and suggest that changes in sperm membrane potential are mediated by cSrc activation.
Introduction
Mammalian sperm are unable to fertilize the egg immediately after ejaculation. They need to undergo a series of physiological modifications inside the female reproductive tract, collectively known as capacitation, to gain fertilization competence. This capacity to fertilize the egg is the hallmark of capacitation and associates with the onset of a vigorous motility pattern (i.e. hyperactivation) and acrosomal responsiveness (i.e. the capacity to undergo the acrosome reaction upon stimulation) (1). In mammals, the capacitation process can be mimicked in vitro by sperm incubation in standard culture medium containing Ca2+, HCO3−, energy sources, and a cholesterol acceptor that is usually BSA. Upon sperm exposure to these conditions, one of the first signaling events observed is a fast increase of intracellular cAMP concentration with the consequent PKA activation (2, 3). In this regard, cAMP participates either directly or indirectly in many molecular processes, such as membrane lipid remodeling (4), sperm plasma membrane potential (Em)2 hyperpolarization (5–7), alkalinization of the intracellular milieu (8), and the promotion of protein-tyrosine phosphorylation (9). Interestingly, even though intracellular cAMP increases almost instantaneously after sperm exposure to capacitating conditions, the promotion of tyrosine phosphorylation associated with sperm capacitation and, more importantly, the capacity to fertilize the egg are acquired ∼45 min later in mouse sperm (10). Despite major advances achieved in the field of sperm capacitation, the molecular basis of this delay has not yet been resolved.
Considering the relevance of the capacitation-induced increase in tyrosine phosphorylation, it is surprising that very little is known about the tyrosine kinases mediating this process. Work from several laboratories has shown the presence of members of the Src family of kinases (SFK), in mouse sperm: cSrc (3, 11, 12) Fyn (13), and immunocytochemistry-based evidence on Lyn and Hck (11). However, recent work from our group using a combination of pharmacological and genetic approaches has shown that, although SFK inhibitors affect phosphorylation by up-regulation of Ser/Thr phosphatases, SFKs are not directly involved in the increase in tyrosine phosphorylation observed during sperm capacitation that follows PKA activation. In particular, our studies using cSrc KO mouse models have demonstrated that lack of cSrc did not block the onset of tyrosine phosphorylation (3). Despite the normal increase in tyrosine phosphorylation, sperm from these mice are infertile because of a combined reproductive phenotype. First, the cauda epididymis region in these mice does not develop properly, and second, in the absence of cSrc, sperm motility is reduced significantly. Because of the problems observed in the epididymis, the sperm phenotype is difficult to pinpoint. Is the problem in sperm motility the result of a direct role of cSrc in sperm or is it due to problems of these mice in epididymal maturation? Therefore, in this work, to directly address the role of cSrc in sperm, we followed cSrc activation using anti-phospho cSrc (Tyr(P)-416-Src), which only recognized the active form of the SFK. Using this approach, we show that cSrc is activated 10–15 min downstream of PKA activation and that cSrc activation is both necessary and precedes the capacitation-induced Em hyperpolarization. Consequently, considering the need and sufficiency of hyperpolarization for a functional acrosome reaction, we have also shown that cSrc inhibition blocks the acrosomal exocytosis. Finally, we present evidence showing that SLO3 channels expressed in a heterologous system are blocked by cSrc inhibitors.
Experimental Procedures
Materials
Chemicals were obtained from the following sources. BSA (fatty acid-free), dibutyryl-cyclic AMP, and isobutylmethylxanthine were purchased from Sigma. SU6656 was obtained from Calbiochem, and SKI606 (bosutinib) and H-89 were purchased from Cayman Chemicals (Ann Arbor, MI). Anti-Tyr(P) monoclonal antibody (clone 4G10) and anti-Src (clone GD11) were obtained from Upstate Biotechnology (Lake Placid, NY). Rabbit monoclonal anti-phospho-PKA substrates (clone 100G7E), anti-Src and anti-Tyr(P)-416-Src monoclonal antibodies (clones 32G6 and D49G4, respectively), anti-Lck (D88) monoclonal antibodies (clone D88), anti-Lyn monoclonal antibodies (clone C13F9), anti-Yes polyclonal antibodies (catalog no. 32019), and anti-Fyn polyclonal antibodies (catalog no. 4023) were purchased from Cell Signaling Technology (Danvers, MA). Anti-Hck monoclonal antibodies (clone 18/Hck) were obtained from BD Biosciences. Anti-Blk (C-20) and anti-Fgr (D-6) polyclonal antibodies were purchased from Santa Cruz Biotechnology. Anti-β-tubulin monoclonal antibody (clone E7) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of NICHD/National Institutes of Health and maintained by the University of Iowa Department of Biological Sciences, (Iowa City, IA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and Amersham Biosciences (GE Healthcare), respectively.
Mouse Sperm Preparation
Cauda epididymal mouse sperm were collected from BL6 young adult male mice (8–13 weeks old) and sacrificed under supervision of the Animal Care and Use Committee of the Facultad de Ciencias Bioquímicas y Farmacéuticas de Rosario (UNR). Each minced cauda epididymis was placed in 500 μl of a modified Krebs-Ringer medium (Whitten's HEPES-buffered medium containing 5 mg/ml BSA, Ref. 14) containing 100 mm NaCl, 4.4 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 5.4 mm glucose, 0.8 mm pyruvic acid, 4.8 mm lactic acid; 2.4 mm Ca2+, and 20 mm HEPES (pH 7.4). After 10 min, epididymides were removed, and the suspension was adjusted with non-capacitating medium to a final concentration of 1–2 × 107 cells/ml. For capacitation, 15 mm NaHCO3 was added, and sperm was incubated at 37 °C for at least 1 h at 2 × 106 cells/ml. To test the effect of the different inhibitors of capacitation, sperm were preincubated with inhibitors in non-capacitating medium for 15 min prior to the beginning of the capacitating period.
SDS-PAGE and Immunoblotting
After treatment, sperm were collected by centrifugation, washed in 1 ml of TBS, resuspended in Laemmli sample buffer (15) without β-mercaptoethanol, and boiled for 3 min. After centrifugation, 5% β-mercaptoethanol was added to the supernatants and boiled again for 5 min. Protein extracts equivalent to 1–2 × 106 sperm/lane were subjected to SDS-PAGE and electrotransferred to PVDF membranes (Bio-Rad) at 250 mA for 60 min on ice. Membranes were blocked with 5% fat-free milk in TBS containing 0.1% Tween 20 (T-TBS). For anti-Tyr(P) and anti-pPKA immunodetections, membranes were blocked with 3% BSA (Sigma) in T-TBS. Antibodies were diluted in T-TBS as follows: 1/10,000 for anti-Tyr(P) (clone 4G10), 1/5000 for anti-pPKA (clone 100G7E), 1/1000 for anti-Fyn, anti-Src, and anti-pSrc antibodies (clones GD11, 32G6, and D49G4, respectively), and 1/10,000 for anti-tubulin (clone E7) and anti-actin. Secondary antibodies were diluted 1/10,000 in T-TBS and developed using an enhanced chemiluminescence detection kit (ECL Plus, Amersham Biosciences, GE Healthcare) according to the instructions of the manufacturer. When necessary, PVDF membranes were stripped at 60 °C for 15 min in 2% SDS, 0.74% β-mercaptoethanol, and 62.5 mm Tris (pH 6.5) and washed six times for 5 min each time in T-TBS. In all experiments, molecular masses were expressed in kilodaltons.
cSrc and Fyn Immunoprecipitation
After sperm incubation under the appropriate conditions, samples were centrifuged at 1700 × g for 1 min. The resulting pellet was resuspended in radioimmune precipitation assay buffer (10 mm Tris-HCl (pH 7.2), 50 mm NaCl, 0.1% SDS, 1% Triton X-100, 1 mm EDTA, 1 mm sodium orthovanadate, and protease inhibitors), incubated on ice for 30 min, and centrifuged at 4 °C for 5 min at 2500 × g. Supernatants were incubated with anti-Src (clone 32G6) or anti-Fyn (Cell Signaling Technology, catalog no. 4023, 1/200 antibody for 1 × 107 cells in a final volume of 500 μl) and anti-tubulin antibodies (clone E7, 1/200) as a negative control for 2 h at room temperature with constant rocking. After adding 20 μl of protein G-Sepharose (GE Healthcare), the reactions were rocked further for 1 h at 4 °C. The immune complex was recovered by centrifugation, washed four times in radioimmune precipitation assay buffer, and subjected to SDS/PAGE and Western blot analysis. To avoid immunoreactive signals from denatured IgGs used for immunoprecipitation, a monoclonal secondary HRP mouse anti-native rabbit IgG was used (clone RabT-50, Sigma).
Acrosomal Status Assays
Sperm were incubated under capacitating conditions for 60 min in the presence or absence of different SFK inhibitor concentrations. Progesterone (20 μm) was then added and incubated for another 30 min. The evaluation of the acrosomal status was performed by two alternative mechanisms. In one of them, sperm from transgenic male mice (BDF1-Tg (CAG-mtDsRed2, Acr-EGFP) RBGS0020sb) displaying acrosomal vesicles expressing green EGFP fluorescence and midpieces (mitochondria) expressing red Ds-Red2 fluorescence were used (16). In this case, sperm were analyzed on a flow cytometer (FACSCanto II flow cytometer, BD Biosciences) after adding propidium iodide to the sperm suspension to discriminate dead cells (6). A 515- to 545-nm band path filter and 650-nm-long path filters were used for GFP and propidium iodide, respectively. For the second mechanism, WT mouse sperm were used. After progesterone stimulation, these cells were seeded on 8-well glass slides. After air-drying, sperm were fixed with 3.7% paraformaldehyde in PBS for 15 min at room temperature, washed with PBS (for times for 5 min each time), and permeabilized with 0.5% Triton X-100 for 5 min. Sperm were then treated with 10% BSA in PBS for 1 h at room temperature and incubated with PBS containing 1% BSA and Alexa Fluor 488-conjugated peanut agglutinin (1:500) for 1 h at room temperature. Before mounting with Slow-Fade Light reagents (Molecular Probes, Eugene, OR), samples were washed with PBS (four times for 5 min each time). Epifluorescence microscopy was performed using a BH2 Olympus microscope. Differential interference contrast images were taken in parallel and served as the control for sperm morphology.
Membrane Potential Assay in Cell Populations
Mature sperm, at a concentration of 4 × 106 sperm in 1.7 ml, from the caudal epididymis were capacitated as described above. After incubation for the indicated period, the potential-sensitive dye DiSC3-(5), which has been successfully used in mammalian sperm, was added to a final concentration of 1 μm, and sperm were incubated for 2 min. No mitochondrial uncouplers were used because their contribution to the resting potential has been determined to be insignificant (17). The sperm were transferred to a gently stirred cuvette at 37 °C, and the fluorescence was monitored with a Varian Cary Eclipse fluorescence spectrophotometer at 620/670 nm excitation/emission wavelength. Recordings were initiated when steady-state fluorescence was reached (approximately 1 min). Calibration was performed by adding 1 μm valinomycin and sequential additions of KCl as described previously (17). The final sperm membrane potential was obtained by linearly interpolating the theoretical Em values against arbitrary fluorescence units of each trace. This internal calibration for each determination compensates for variables that influence the absolute fluorescence values.
Whole Cell Currents from Xenopus Oocytes
Oocytes were harvested from adult female Xenopus laevis as described previously (18). Defolliculated oocytes were injected with 14–20 ng of mouse SLO3 (mSLO3) cRNA using a Nanoject II Drummond Scientific nanoinjector (Broomall, PA). Injected oocytes were incubated at 18 °C in ND96 complete medium (ND96 medium plus 2.5 mm sodium pyruvate and penicillin-streptomycin (100 units/ml to 100 μg/ml). The ND96 medium consisted of 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 5 mm MgCl2, and 5 mm HEPES, adjusted to pH 7.5 with NaOH. Whole cell currents from mock control and injected oocytes were recorded 3–5 days after injection using the two-microelectrode voltage clamp with an Oocyte Clamp OC-725C amplifier (Warner Instrument Corp.). Whole cell currents were recorded in ND96 solution. Recordings were obtained by digitizing at 10 kHz and low-pass filtering at 1 kHz. Electrodes were made with borosilicate glass capillaries (World Precision Instruments), pulled with a Sutter Instrument Co. P-87 pipette puller, and filled with 3 m KCl. Data were analyzed using pClamp 9 (Molecular Devices) and Origin 6.1 (Microcal Software).
Statistical Analysis
Paired Student's t test was used to compare mean values between control and tested groups. The difference between mean values of multiple groups was analyzed by one-way analysis of variance followed by Holm-Šidák test. Statistical significances are indicated in the figure legends.
Results
Inhibition of cSrc Does Not Affect Phosphorylation Cascades Associated with Sperm Capacitation
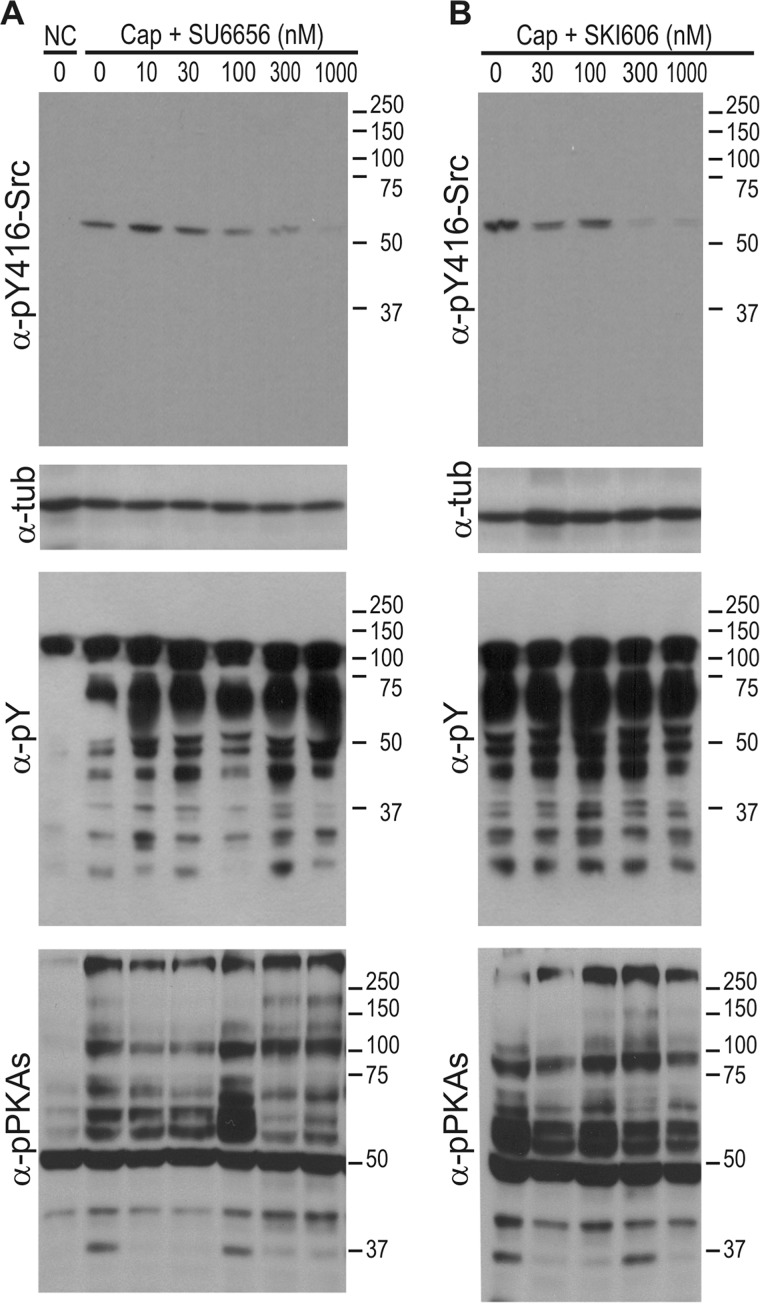
Members of the SFK family require phosphorylation in residue Tyr-416 (known as Tyr-416 for chicken cSrc and corresponding to Tyr-424 in mouse cSrc or its analogue residue in other members of the SFK) to undergo activation and to allow the substrate to gain access to an open-state catalytic site of the active kinase (19, 20). Therefore, antibodies against the phosphorylated state of Tyr-416-SFK (hereafter named Tyr-416-Src) can be used to follow activation of members of the SFK family. As expected, the presence of the cSrc inhibitors SU6656 and SKI606 in the capacitating medium successfully blocked phosphorylation of Tyr-416-Src with an IC50 of ∼100–300 nm and maximum effects of 300 nm and 1 μm for SKI606 and SU6656, respectively (Figs. 1, A and B, first and second panels). Although the SFK detected was clearly inhibited, and consistent with our previously published results (3), these low SKI606 and SU6656 concentrations did not have any effect on either tyrosine phosphorylation of sperm proteins associated with capacitation (Fig. 1, A and B, third panels) or PKA substrate phosphorylation (Fig. 1, A and B, fourth panels).
FIGURE 1.
SFK inhibitors abrogate the phosphorylation of Tyr-416-Src without affecting the phosphorylation pathways associated with sperm capacitation. Mouse sperm were incubated in the absence or presence of increasing concentrations of SU6656 (A) or SKI606 (B) for 60 min in capacitating (Cap, with HCO3−) or non-capacitating medium (NC, without HCO3−). Western blot analyses were performed with anti-Tyr(P)-416-Src antibodies (clone D49G4). Membranes were then stripped and reprobed sequentially with anti-Tyr(P) (clone 4G10), anti-pPKAs (clone 100G7E), and anti-tubulin antibodies. All Western blot analyses are representative of experiments repeated at least three times. α-tub, α-tubulin.
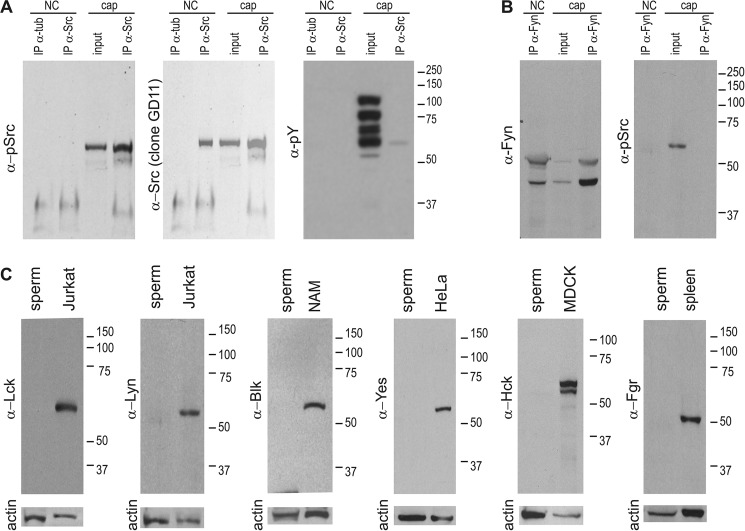
The requirement of Tyr-416 phosphorylation (or the respective conserved site) is common to all members of the SFK family. Therefore, the antibody used to detect Tyr(P)-416-Src might recognize analogue sites in other SFK members. To validate the use of anti Tyr(P)-416-Src for detection of cSrc in its Tyr-416-phosphorylated form in whole sperm cell extracts, immunoprecipitations were carried out using a previously validated monoclonal antibody (clone 32G6) (3). As shown in Fig. 2A, anti Tyr(P)-416-Src antibodies detected an immunoprecipitated protein from capacitated sperm at the expected molecular weight of cSrc. The immunoprecipitation of cSrc from non-capacitated sperm was confirmed by using a second validated specific anti-cSrc antibody (clone GD11) (Fig. 2A, center panel). The use of a secondary antibody that specifically detects native IgGs avoided detection of denatured IgGs used for immunoprecipitation (see “Experimental Procedures”). Regarding the presence of other SFK members in mouse sperm, Fyn has been reported recently (21) to have an active role in spermatogenesis. To analyze the possible activation of Fyn during capacitation, the anti Tyr-416-Src was used because it cross-reacts with all members of the SFK family when phosphorylated at the analogous site (Tyr-420-Fyn). Immunodetections were performed with immunoprecipitation assays using anti-Fyn-specific antibodies. As shown in Fig. 2B, two immunoreactive proteins were obtained. The higher molecular weight protein was of the expected size. However, no signal was obtained when analyzed with anti-Tyr(P)-416-Src, suggesting that Fyn is not phosphorylated on the analogue Tyr-416 residue or that this antibody does not recognize the equivalent position in this SFK family member. The immunoreactive signal in the total extract is expected to correspond to cSrc, as shown above, to be phosphorylated upon capacitation. No other members of the SFK family could be detected by Western blot analysis on non-capacitated mouse sperm extracts (Fig. 2C).
FIGURE 2.
A, immunoprecipitation (IP) reveals autophosphorylation of Tyr-416-Src during capacitation. Capacitated and non-capacitated sperm were extracted with radioimmune precipitation assay buffer and immunoprecipitated with anti-cSrc (clone 32G6) antibodies or anti-tubulin as controls. Immunocomplexes were subjected to 8% SDS/PAGE, transferred to PVDF, and developed by Western blot analysis with anti-Tyr(P)-416-Src (clone D49G4). Immunoprecipitation control was performed after stripping using anti-cSrc (clone GD11) antibodies. Capacitation control was performed using anti-Tyr(P) (clone 4G10) antibodies. Note the signal in the immunoprecipitation performed on the capacitated sample, probably a result of Tyr(P)-416-Src (clone D49G4). NC, non-capacitating; Cap, capacitating. B, capacitated and non-capacitated sperm were extracted with radioimmune precipitation assay buffer and immunoprecipitated with anti-Fyn polyclonal antibodies. Immunocomplexes were subjected to 8% SDS/PAGE, transferred to PVDF, and developed by Western blot analysis with anti-Tyr(P)-416-Src (clone D49G4). No signal was obtained from the immunoprecipitation. Note the positive signal on the input, of a molecular weight comparable with that of cSrc. C, Western blot analysis was unable to detect other members of the SFK family in mouse sperm. Mouse sperm extracts (equivalent to 2E6 cells) were analyzed with different antibodies as indicated. As a control, 20 μg of a positive control was run in parallel. NAM, namalwa cells; MDCK, Madin-Darby canine kidney; spleen, mouse spleen extract. Membranes were reblotted with anti-actin as a loading control.
Src Activation Is Downstream of PKA Activation
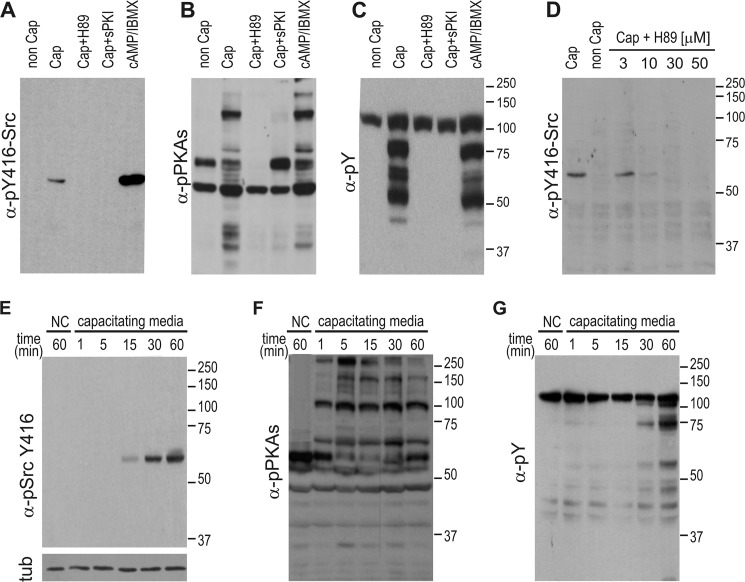
Considering that PKA is fully activated at 1 min (22) and plays a pivotal role in many aspects of sperm capacitation (23), we aimed to analyze the possible regulation of cSrc activity by PKA. Our results indicate that, in medium devoid of HCO3−, which does not support capacitation or PKA activation, phosphorylation of Tyr-416-cSrc was not observed (Fig. 3A). However, when the incubation medium was supplemented with the permeable PKA agonist dibutyryl cyclic AMP and the phosphodiesterase inhibitor isobutylmethylxanthine, cSrc activation was observed (Fig. 3A). Moreover, when sperm were incubated in capacitating medium, phosphorylation of Tyr-416 was blocked by two independent PKA inhibitors. Both the stearated (membrane-permeable) PKA inhibitor PKA inhibitor peptide (which binds to the protein-substrate site, inhibiting the catalytic PKA subunit) and the PKA inhibitor H-89 (which competes for the ATP pocket on the catalytic PKA subunit) (Figs. 3A) abrogated cSrc activation (see Fig. 3, B and C, for pPKAs and Tyr(P) controls). Moreover, the effect of H-89 is expected to be PKA-specific because 10 μm of H-89 effectively blocked phosphorylation of cSrc (Fig. 3D), a concentration shown to specifically affect PKA (3). In a time curve analysis, it was observed that phosphorylation of Tyr-416-cSrc starts 15 min after the beginning of capacitation and reaches a maximum at 30 min, showing-steady levels thereafter (Fig. 3E). This kinetics are clearly delayed compared with phosphorylation of PKA substrates (Fig. 3F) but faster than the onset of tyrosine phosphorylation associated with sperm capacitation (Fig. 3G). Together, these data indicate that PKA activation is upstream of cSrc activation.
FIGURE 3.
Phosphorylation of Tyr-416-cSrc is downstream of activation of PKA. A–C, sperm were incubated for 60 min in non-capacitating (non-cap) or capacitating (cap) medium containing either 30 μm H-89, 10 μm of the cell-permeable PKA inhibitor peptide, or 1 mm dibutyryl cyclic AMP/100 μm isobutylmethylxanthine. Each condition was processed for Western blot analysis with a monoclonal anti-Tyr(P)-416-Src antibody (clone D49G4) (A). As a control, membranes were stripped and analyzed sequentially with anti-pPKAs (clone 100G7E) (B) and anti-Tyr(P) (clone 4G10) (C). D, sperm were incubated for 60 min in non-capacitating or capacitating medium containing different concentrations of H-89. Each condition was processed for Western blot analysis with a monoclonal anti-Tyr(P)-416-Src antibody. The membrane was stripped and analyzed for the presence of cSrc using anti-cSrc (clone 32G6). E, mouse sperm were incubated under conditions that support capacitation during different periods, as indicated, before immunodetection of Tyr(P)-416-Src (clone D49G4). F and G, the PVDF membranes used in C were stripped as described and immunodetected sequentially with p-PKA substrates (clone 100G7E) (F) and anti-Tyr(P) antibodies (clone 4G10) (G). All Western blot analyses are representative of experiments repeated at least three times.
cSrc Activation Is Required for the Acquisition of Acrosomal Responsiveness
Aiming to investigate the biological effect of cSrc blockade on sperm physiology, motility and acrosome reaction were evaluated. Although no effect was observed on hyperactivation, a low concentrations of both SU6656 and SKI606, which effectively blocked activation of cSrc without disturbing tyrosine phosphorylation (Fig. 4A), hampered the acquisition of acrosomal responsiveness to progesterone (Fig. 4B), measured using peanut agglutinin-FITC staining of intact acrosomes. Similar results were obtained by flow cytometry (Fig. 4C) using sperm from Acr-GFP transgenic mice (Fig. 4D), which display green fluorescent acrosomes when intact without staining requirements (16).
FIGURE 4.
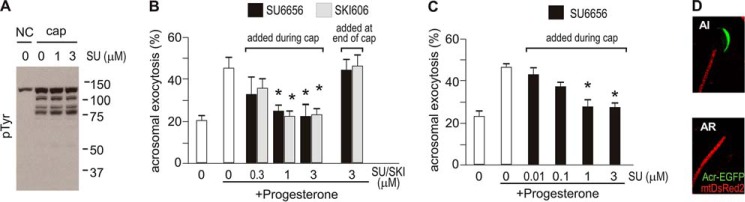
cSrc activation is required for acrosomal exocytosis. A, sperm were capacitated in the presence or absence of SU6656 (SU). Samples were prepared for Western blot analyses performed with anti-Tyr(P) (clone 4G10) antibodies. NC, non-capacitating; Cap, capacitating. B, sperm were incubated under capacitating conditions in the presence of different concentration of SU6656 (SU) or SKI606 (SKI) for 60 min. When indicated, sperm were challenged with 20 μm progesterone and incubated further for 30 min before assessment of the acrosomal status by fluorescence microscopy. Alternatively, SU6656 or SKI606 was added after 60 min of capacitation, followed 3 min later by 20 μm progesterone, and incubated further for 30 min. C and D, sperm were incubated under capacitating conditions in the presence of different concentrations of SU6656 for 60 min. When indicated, sperm were challenged with 20 μm progesterone and incubated further for 30 min before assessment of the acrosomal status by flow cytometry using sperm expressing EGFP under the acr promoter, as shown in D. AI, acrosome intact; AR, acrosome reacted. Data represent mean ± S.E. of at least three independent experiments. *, p < 0.01.
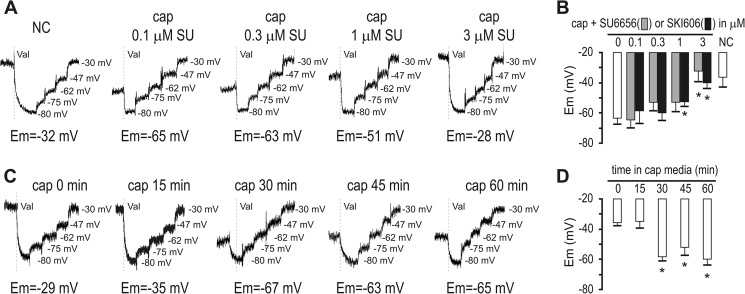
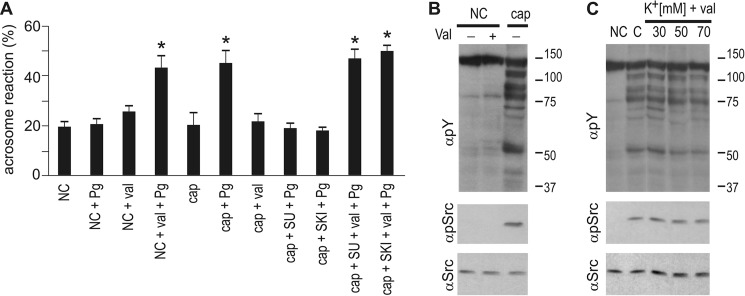
We have shown previously that capacitation-induced Em hyperpolarization is necessary and sufficient to promote acrosomal responsiveness (5). Therefore, we evaluated whether the effect of cSrc inhibitors on acrosomal responsiveness was a result of blocking hyperpolarization. To test this possibility, sperm Em was analyzed in a population assay using the carboxicyanine dye DISC(3)5. Both SKI606 and SU6656 effectively blocked capacitation-induced hyperpolarization at concentrations that have no effect on tyrosine phosphorylation (Fig. 4A). Consistent with a role of cSrc in this event, both cSrc activation and hyperpolarization present similar kinetics (between 15 and 30 min after incubation under capacitating conditions) (Fig. 5, C and D). These results suggest that the role of cSrc in acrosomal responsiveness is mediated by changes in sperm membrane Em. Supporting this hypothesis, when in the presence of cSrc inhibitors, hyperpolarization is pharmacologically induced with the K+ ionophore valinomycin, and then the acrosomal responsiveness toward progesterone is restored (Fig. 6). As a control, treatment of non-capacitated sperm with valinomycin did not promote phosphorylation of Tyr-416-Src (Fig. 6B), further substantiating that cSrc activation is upstream of Em hyperpolarization. Conversely, clamping of sperm Em to different potentials during capacitation, as done before (5), did not impair normal activation of cSrc (Fig. 6C). Together, these data indicate that cSrc activation is upstream the Em hyperpolarization observed during sperm capacitation and that this signaling pathway is required to prepare the sperm for an agonist-induced acrosome reaction.
FIGURE 5.
Src activation is upstream of and required for hyperpolarization. A, fluorescence traces showing the values of the sperm Em obtained after sperm incubation under capacitating conditions containing different concentrations of SU6656 (SU). Each experiment displays its calibration curve. NC, non-capacitating; Cap, capacitating. B, summary of Em measurements of sperm incubated under capacitating conditions containing different concentrations of either SU6656 or SKI606. Data are mean ± S.E., n ≥ 3. *, p < 0.01. C, fluorescence traces and values of the sperm Em obtained after sperm incubation under capacitating conditions for different periods of time. D, summary of Em measurements obtained as indicated in C. Data are mean ± S.E., n = 3. *, p < 0.01.
FIGURE 6.
Pharmacological hyperpolarization of sperm bypasses the blockade performed by the presence of SFK inhibitors. A–C, sperm were incubated for 60 min in the presence of 3 μm of SU6656 (SU) or SKI606 (SKI). When indicated, 1 μm valinomycin (Val) was added 20 min after the beginning of capacitation. The stimulation of the acrosome reaction was performed with 20 μm progesterone (Pg) and evaluation of the acrosomal status after 30 additional minutes by lectin staining. Controls are also shown for non-capacitated sperm treated with valinomycin and/or progesterone. NC, non-capacitating; Cap, capacitating.
Mouse SLO3 Currents Are Blocked by cSrc Inhibitors in a Heterologous System
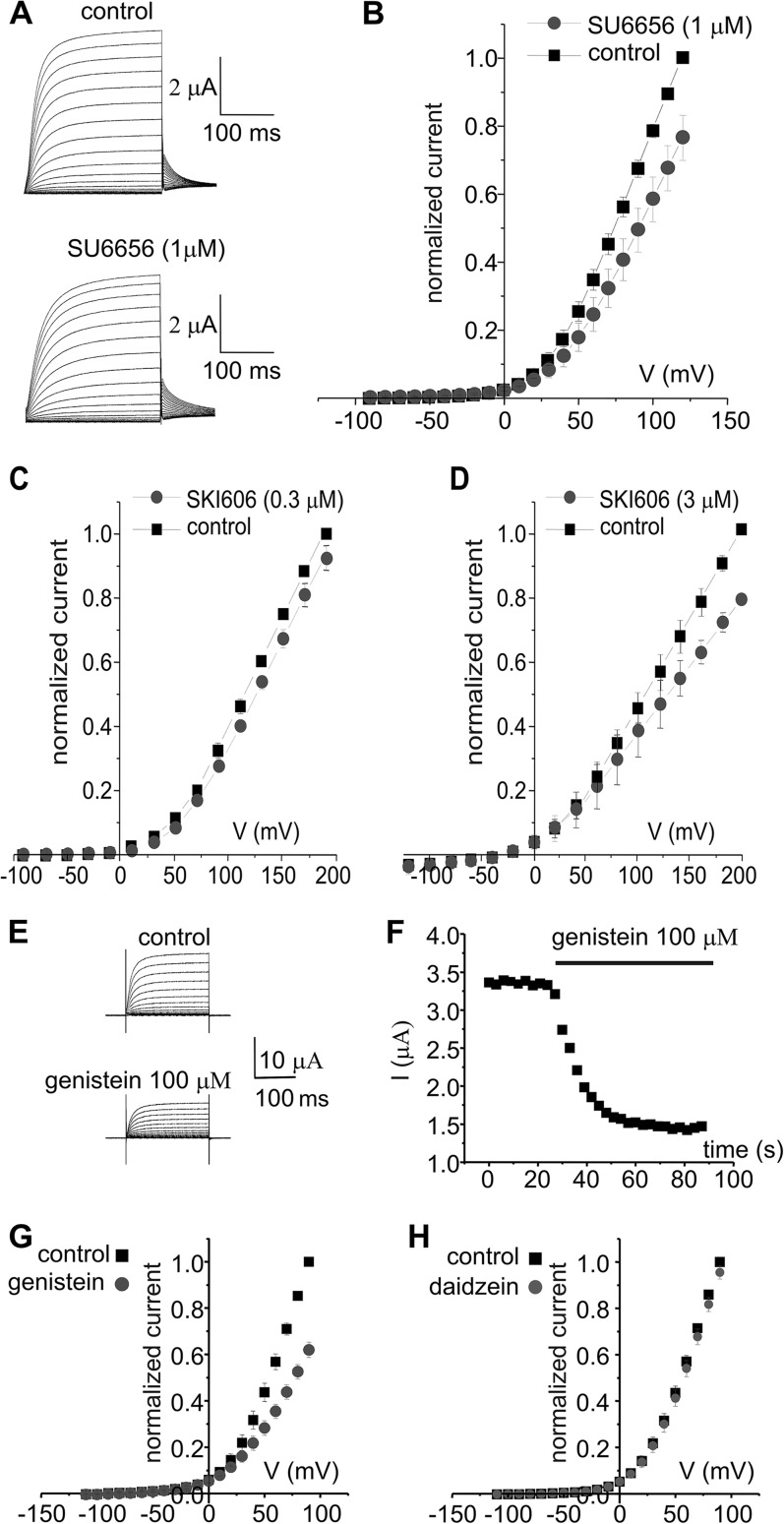
Experiments using SLO3 knockout mouse models indicate that this K+ channel plays an essential role in the regulation of sperm hyperpolarization and, consequently, on the sperm preparation for the acrosome reaction. Interestingly, SLO3 is closely related to the SLO1 channel, which is known to be activated by cSrc. The site of regulation appears to be a tyrosine located at the COOH terminus of the channel (24), within a highly conserved region compared with SLO3. Aiming to determine whether SLO3 channel activity could be modulated by tyrosine phosphorylation, we tested the effect of the Src inhibitors SU6656 and SKI606 on heterologously expressed SLO3 channels. Our results showed that whole cell SLO3 K+ currents expressed in Xenopus oocytes were inhibited significantly by 1 μm SU6656 at all voltages tested (Fig. 7, A and B) and by low concentrations of SKI606 (Fig. 7, C and D). The inhibitory effect was established in 2 min and was partially reversible. Because fast inhibition was observed, and to discard a direct block of the channel from the extracellular side, a third inhibitor was tested. The following reasons supported the use of genistein on this experiment. It is a tyrosine kinase broad-spectrum inhibitor (25), it has been shown to act rapidly (within 1–5 min) on both ion channels and receptors through tyrosine phosphorylation-dependent mechanisms (26, 27), and it has an inactive analogue. Although genistein and SU6656 or SKI606 are structurally different and present different mechanisms of action from each other, we found that SLO3 currents were also inhibited significantly by 100 μm genistein at all voltages tested (Fig. 7E). In this case, the maximum effect was achieved in 1 min and was partially reversible. On the other hand, the genistein inactive analogue daidzein had no effect on mSLO3 currents even at 100 μm concentration (Fig. 7, G and H).
FIGURE 7.
SFK control mammalian SLO3 currents expressed in Xenopus oocytes. SLO3 current traces obtained from a holding potential (Vh) of −70 mV, applying 10-mV steps from −90 to +150 mV and repolarizing to 0 mV. A, under control conditions (ND96 solution in the bath) and after the application of 1 μm SU6656. B, mean current-voltage relationships under control conditions (black squares) and in the presence of 1 μm SU6656 (gray dots) (n = 5 oocytes). C, mean current-voltage relationships under control conditions (black squares) and in the presence of 0.3 μm SKI606 (gray dots) (n = 9 oocytes). mSLO3 current traces were obtained from a Vh of −70 mV, applying 20-mV steps from −90 to +190 mV and repolarizing to −40 mV. Mean currents were significantly different (p = 0.00196). D, mean current-voltage relationships under control conditions (black squares) and in the presence of 3 μm SKI606 (gray dots) (n = 3 oocytes). mSLO3 current traces were obtained from a Vh of −120 mV, applying 20-mV steps from −100 to +200 mV and repolarizing to −40 mV. E, mSLO3 currents obtained from a Vh of −80 mV, applying 10-mV steps from −80 to +90 mV and repolarizing to −80 mV under control conditions (normal saline solution in the bath) and after application of genistein (100 μm). F, time course of genistein inhibition. Pulses to +30 mV were applied every 3 s. The current was maximally inhibited in ∼60 s. G, mean I-V plots under control conditions and during the application of genistein (100 μm) (n = 5 oocytes). H, current-voltage plots obtained under control conditions and after application of daidzein (100 μm) (an inactive analogue of genistein) (n = 4 oocytes). The currents in C and D were obtained with the same voltage protocol used in A.
Discussion
After ejaculation, mammalian sperm are morphologically mature, but they are not able to fertilize. More than 50 years ago, Chang (28) and Austin (29) reported independently that mammalian sperm should reside in the female tract for an obligatory period of time to acquire “fertilizing capacity” in a process called capacitation. At the molecular level, capacitation is initiated as soon as sperm are ejaculated in the female tract. One of the first signaling pathways observed are an HCO3−-induced activation of Adcy10, increased cAMP intracellular concentrations, and the consequent activation of PKA. This fast increase in cAMP occurs immediately, and maximum levels of PKA activation are observed in 1 min (3, 22). It has been shown that PKA activation is needed for many other signaling events associated with capacitation. Among these events, and particularly important for the findings in this manuscript, is the PKA requirement for both the hyperpolarization of the sperm plasma membrane (6, 30) and the increase in tyrosine phosphorylation (31). However, the molecules that mediate the connection between PKA and other pathways have not been identified. Noteworthy is that the activation kinetics of PKA (∼1 min), Refs. 3, 22), hyperpolarization (30 min, Ref. 17 and this work), and tyrosine phosphorylation (∼45 min) suggest that the role of PKA in the regulation of these other pathways is indirect. Regarding tyrosine phosphorylation, considering that PKA is a Ser/Thr kinase, the activation of a tyrosine kinase downstream in the pathway is predicted.
The identity of this tyrosine kinase has remained elusive. Recent data demonstrating that the FAK (focal adhesion kinase) family inhibitor PF-431396 blocks tyrosine phosphorylation in horse and human sperm without blocking PKA activation suggest that either FAK or PYK2 are the kinases involved in this process (32, 33). cSrc has also been proposed as the kinase involved in tyrosine phosphorylation. However, cSrc KO mice present normal levels of tyrosine phosphorylation (34). Although these data can be explained by compensation with other SFKs, we showed that, although high concentrations of SFK inhibitors (SKI-606 and SU6656 50 μm) block tyrosine phosphorylation, these concentrations also blocked PKA activation (3). Interestingly, this inhibition is completely overcome with very low concentrations of the Ser/Thr phosphatase inhibitors okadaic acid and calyculin (3). Together, these data suggest that a kinase inhibited by high concentration of Src inhibitors is involved in the down-regulation of Ser/Thr phosphatases (most likely PP2A) and that its involvement in the regulation of phosphorylation pathways is indirect. The fact that cSrc KO undergoes tyrosine phosphorylation added to the high SKI-606 and SU6656 concentrations required to inhibit this event indicate that cSrc is not directly involved in the regulation of this signaling pathway during sperm capacitation.
Despite these conclusions, sperm from cSrc KO mice are infertile, and their motility is compromised (34). In addition to the sperm phenotype, these mice have deficiencies in cauda epididymis development because of the lower number and size of clear cells. Clear cells in the cauda epididymis are responsible for H+ secretion through the V-ATPase pump. This function is essential for epididymal fluid acidification. Therefore, the mechanisms by which lack of cSrc affects sperm function are not clear. Whether cSrc has an essential function is sperm or cSrc affects epididymal fluid and, consequently, alters sperm epididymal maturation, or even a combination of both problems, remained unknown. To directly investigate the role of cSrc in sperm, in this work, we followed cSrc activation using antibodies against phosphorylated Tyr-416. Phosphorylation of this site is required for cSrc activity, and Western blots using these antibodies are routinely used to follow cSrc activation (35, 36). Using this approach, we showed that cSrc is activated during capacitation in about 15 min and that this activation is blocked with much lower SKI606 and SU6656 (cSrc inhibitors) concentrations than those required for the inhibition of capacitation-induced phosphorylation pathways. Taking into consideration that the anti-Tyr(P)-416 antibody could recognize other SFKs, we investigated the presence of other members of this family in mouse sperm and found only cSrc and Fyn present in sperm of the eight SFK family members investigated. Moreover, when cSrc and Fyn were immunoprecipitated, the anti-Tyr(P)-416 recognized cSrc but not Fyn in capacitated sperm. Considering the organizing role of PKA in most capacitation-associated processes, we then investigated whether PKA was required for cSrc activation, and we found a positive correlation. First, cSrc is not activated when the sperm are incubated in the absence of HCO3−. Second, under the same conditions (absence of HCO3−), cAMP agonists induced cSrc activation. Finally, PKA inhibition with either H-89 or PKI obliterated cSrc activation.
The previous experiments indicate that cSrc is activated downstream of PKA activation, similar to other capacitation-dependent processes. However, these experiments were silent regarding the role of cSrc in other capacitation-dependent functions. Because of the cSrc KO phenotype, we first investigated the extent by which cSrc was involved in the regulation of sperm motility. To our surprise, low concentrations of the cSrc inhibitors SKI606 and SU6656, which were effective in the inhibition of cSrc, did not have an effect on motility parameters. These experiments suggest that the motility phenotype observed in cSrc KO sperm could be due to problems in epididymal maturation. This hypothesis is consistent with the role of epididymal maturation on the ability of the sperm to move (37). On the other hand, cSrc inhibition completely blocked the progesterone-induced acrosome reaction. We have shown that agonist-induced acrosome reaction is downstream of the hyperpolarization changes of the sperm plasma membrane observed during capacitation (5). Therefore, we investigated whether the role of cSrc in the acrosome reaction was connected with the changes in sperm plasma membrane potential. First, we found that the kinetics of both processes, cSrc activation and hyperpolarization, were similar. Second, low SKI606 and SU6656 concentrations blocked the capacitation-induced hyperpolarization. Third, the inhibition of the progesterone-induced acrosome reaction observed when cSrc is blocked was overcome when hyperpolarization was induced with the K+ ionophore valinomycin.
At what level does cSrc participate in the regulation of sperm membrane potential? Recent evidence is consistent with the hypothesis that the capacitation-induced hyperpolarization depends on the activation of the sperm-specific K+ channel SLO3 (17, 38, 39). Sperm from SLO3 KO mice are infertile, do not undergo hyperpolarization, and present serious deficiencies in their ability to undergo the acrosome reaction (38). Interestingly, the closest SLO3 homologue is SLO1, which is known to be regulated by cSrc activation in other cell types through phosphorylation of a tyrosine located at the highly conserved carboxyl terminus of the channel (24). Likewise, the majority of voltage-gated, ligand-gated, and second messenger-gated channels are regulated to some degree by tyrosine phosphorylation. Non-receptor tyrosine kinases, and cSrc in particular, have also been shown to regulate a variety of membrane ion channels, including ligand-gated ion channels, voltage-gated calcium channels, and potassium channels (for a review, see Ref. 40). Whether there is a direct effect of cSrc over SLO3 is currently unknown. However, our electrophysiological recording of currents derived from Xenopus oocytes expressing mammalian SLO3 suggests that inhibition of SLO3 currents is related to altered tyrosine phosphorylation. SFK family members have been identified in Xenopus oocytes (41, 42), making the regulation of the channel by these kinases possible when SLO3 channels are heterologously expressed. The inhibition of SLO3 currents observed, although consistent with the in vivo inhibition of Em hyperpolarization in mouse sperm, awaits further work to elucidate the mechanism operating under cSrc control.
Author Contributions
C. S. and D. K. designed, performed, and analyzed the experiments shown in Figs. 1–6. M. G. B., F. A. L., and D. K. designed, performed, and analyzed the experiments shown in Figs 4 and 6. C. B. G. and S. E. A. provided technical assistance and contributed to the preparation of the figures. E. A. performed and analyzed the experiments shown in Fig. 2. J. J. F., S. L. G., V. A. D., and C. M. S. designed, performed, and analyzed the experiments shown in Fig. 7. P. E. V. and C. M. S. revised the manuscript for important intellectual content. D. K. conceived and coordinated the study and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Note Added in Proof
Victor A. Dzikunu's contributions to this article fulfill the JBC authorship criteria, but his authorship was inadvertently omitted from the version of the article that was published on June 9, 2015 as a Paper in Press.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1TW008662 (to M. G. B.), RO1 HD38082 and HD440044 (to P. E. V.), and RO1 HD069631 (to C. M. S.). This work was also supported by Agencia Nacional de Promoción Cientifica y Tecnológica Grants PICT 2011-0540 and PICT 2014-2702 (to D. K.) and PICT 2013-1175 (to M. G. B.) and CONICET Grant PIP 112-201101-00740 (to D. K. and M. G. B.). The authors declare that they have no conflicts of interest with the contents of this article.
- Em
- membrane potential
- SFK
- Src family of protein-tyrosine kinases
- EGFP
- enhanced GFP.
References
- 1. Visconti P. E., Krapf D., de la Vega-Beltrán J. L., Acevedo J. J., Darszon A. (2011) Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 13, 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison R. A., Miller N. G. (2000) cAMP-dependent protein kinase control of plasma membrane lipid architecture in boar sperm. Mol. Reprod. Dev. 55, 220–228 [DOI] [PubMed] [Google Scholar]
- 3. Krapf D., Arcelay E., Wertheimer E. V., Sanjay A., Pilder S. H., Salicioni A. M., Visconti P. E. (2010) Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J. Biol. Chem. 285, 7977–7985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gadella B. M., Harrison R. A. (2000) The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 127, 2407–2420 [DOI] [PubMed] [Google Scholar]
- 5. De La Vega-Beltran J. L., Sánchez-Cárdenas C., Krapf D., Hernandez-González E. O., Wertheimer E., Treviño C. L., Visconti P. E., Darszon A. (2012) Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 287, 44384–44393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Escoffier J., Krapf D., Navarrete F., Darszon A., Visconti P. E. (2012) Flow cytometry analysis reveals a decrease in intracellular sodium during sperm capacitation. J. Cell Sci. 125, 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng Y., Clark E. N., Florman H. M. (1995) Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 171, 554–563 [DOI] [PubMed] [Google Scholar]
- 8. Zeng Y., Oberdorf J. A., Florman H. M. (1996) pH regulation in mouse sperm: identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 173, 510–520 [DOI] [PubMed] [Google Scholar]
- 9. Visconti P. E., Bailey J. L., Moore G. D., Pan D., Olds-Clarke P., Kopf G. S. (1995) Capacitation of mouse spermatozoa: I: correlation between the capacitation state and protein tyrosine phosphorylation. Development 121, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 10. Visconti P. E. (2009) Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. U.S.A. 106, 667–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goupil S., La Salle S., Trasler J. M., Bordeleau L. J., Leclerc P. (2011) Developmental expression of SRC-related tyrosine kinases in the mouse testis. J. Androl. 32, 95–110 [DOI] [PubMed] [Google Scholar]
- 12. Baker M. A., Hetherington L., Aitken R. J. (2006) Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J. Cell Sci. 119, 3182–3192 [DOI] [PubMed] [Google Scholar]
- 13. Luo J., Gupta V., Kern B., Tash J. S., Sanchez G., Blanco G., Kinsey W. H. (2012) Role of FYN Kinase in Spermatogenesis: defects characteristic of Fyn-null sperm in mice. Biol. Reprod. 86, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Visconti P. E., Johnson L. R., Oyaski M., Fornés M., Moss S. B., Gerton G. L., Kopf G. S. (1997) Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev. Biol. 192, 351–363 [DOI] [PubMed] [Google Scholar]
- 15. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 16. Hasuwa H., Muro Y., Ikawa M., Kato N., Tsujimoto Y., Okabe M. (2010) Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp. Anim. 59, 105–107 [DOI] [PubMed] [Google Scholar]
- 17. Chávez J. C., de la Vega-Beltrán J. L., Escoffier J., Visconti P. E., Treviño C. L., Darszon A., Salkoff L., Santi C. M. (2013) Ion permeabilities in mouse sperm reveal an external trigger for SLO3-dependent hyperpolarization. PloS ONE 8, e60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan A., Dourado M., Butler A., Walton N., Wei A., Salkoff L. (2000) SLO-2, a K+ channel with an unusual Cl− dependence. Nat. Neurosci. 3, 771–779 [DOI] [PubMed] [Google Scholar]
- 19. Bjorge J. D., Jakymiw A., Fujita D. J. (2000) Selected glimpses into the activation and function of Src kinase. Oncogene 19, 5620–5635 [DOI] [PubMed] [Google Scholar]
- 20. Roskoski R., Jr. (2005) Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 331, 1–14 [DOI] [PubMed] [Google Scholar]
- 21. Luo J., Gupta V., Kern B., Tash J. S., Sanchez G., Blanco G., Kinsey W. H. (2012) Role of FYN kinase in spermatogenesis: defects characteristic of Fyn-null sperm in mice. Biol. Reprod. 86, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison R. A. (2004) Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol. Reprod. Dev. 67, 337–352 [DOI] [PubMed] [Google Scholar]
- 23. Buffone M. G., Wertheimer E. V., Visconti P. E., Krapf D. (2014) Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochim. Biophys. Acta 1842, 2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ling S., Woronuk G., Sy L., Lev S., Braun A. P. (2000) Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J. Biol. Chem. 275, 30683–30689 [DOI] [PubMed] [Google Scholar]
- 25. Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262, 5592–5595 [PubMed] [Google Scholar]
- 26. Wischmeyer E., Döring F., Karschin A. (1998) Acute suppression of inwardly rectifying Kir2.1 channels by direct tyrosine kinase phosphorylation. J. Biol. Chem. 273, 34063–34068 [DOI] [PubMed] [Google Scholar]
- 27. Cho C. H., Song W., Leitzell K., Teo E., Meleth A. D., Quick M. W., Lester R. A. (2005) Rapid upregulation of α7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J. Neurosci. 25, 3712–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang M. C. (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698 [DOI] [PubMed] [Google Scholar]
- 29. Austin C. R. (1952) The capacitation of the mammalian sperm. Nature 170, 326. [DOI] [PubMed] [Google Scholar]
- 30. Hernández-González E. O., Sosnik J., Edwards J., Acevedo J. J., Mendoza-Lujambio I., López-González I., Demarco I., Wertheimer E., Darszon A., Visconti P. E. (2006) Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J. Biol. Chem. 281, 5623–5633 [DOI] [PubMed] [Google Scholar]
- 31. Morgan D. J., Weisenhaus M., Shum S., Su T., Zheng R., Zhang C., Shokat K. M., Hille B., Babcock D. F., McKnight G. S. (2008) Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation. Proc. Natl. Acad. Sci. U.S.A. 105, 20740–20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Battistone M. A., Alvau A., Salicioni A. M., Visconti P. E., Da Ros V. G., Cuasnicú P. S. (2014) Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Mol. Hum. Reprod. 20, 1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. González-Fernández L., Macías-García B., Loux S. C., Varner D. D., Hinrichs K. (2013) Focal adhesion kinases and calcium/calmodulin-dependent protein kinases regulate protein tyrosine phosphorylation in stallion sperm. Biol. Reprod. 88, 138. [DOI] [PubMed] [Google Scholar]
- 34. Krapf D., Ruan Y. C., Wertheimer E. V., Battistone M. A., Pawlak J. B., Sanjay A., Pilder S. H., Cuasnicu P., Breton S., Visconti P. E. (2012) cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev. Biol. 369, 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Michels S., Trautmann M., Sievers E., Kindler D., Huss S., Renner M., Friedrichs N., Kirfel J., Steiner S., Endl E., Wurst P., Heukamp L., Penzel R., Larsson O., Kawai A., Tanaka S., Sonobe H., Schirmacher P., Mechtersheimer G., Wardelmann E., Büttner R., Hartmann W. (2013) SRC signaling is crucial in the growth of synovial sarcoma cells. Cancer Res. 73, 2518–2528 [DOI] [PubMed] [Google Scholar]
- 36. Nikolic D. S., Lehmann M., Felts R., Garcia E., Blanchet F. P., Subramaniam S., Piguet V. (2011) HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood 118, 4841–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornwall G. A. (2009) New insights into epididymal biology and function. Hum. Reprod. Update. 15, 213–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santi C. M., Martínez-López P., de la Vega-Beltrán J. L., Butler A., Alisio A., Darszon A., Salkoff L. (2010) The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584, 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeng X. H., Yang C., Kim S. T., Lingle C. J., Xia X. M. (2011) Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. U.S.A. 108, 5879–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis M. J., Wu X., Nurkiewicz T. R., Kawasaki J., Gui P., Hill M. A., Wilson E. (2001) Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol 281, H1835–H1862 [DOI] [PubMed] [Google Scholar]
- 41. Steele R. E. (1985) Two divergent cellular src genes are expressed in Xenopus laevis. Nucleic Acids Res. 13, 1747–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato K., Aoto M., Mori K., Akasofu S., Tokmakov A. A., Sahara S., Fukami Y. (1996) Purification and characterization of a Src-related p57 protein-tyrosine kinase from Xenopus oocytes: isolation of an inactive form of the enzyme and its activation and translocation upon fertilization. J. Biol. Chem. 271, 13250–13257 [DOI] [PubMed] [Google Scholar]