Abstract
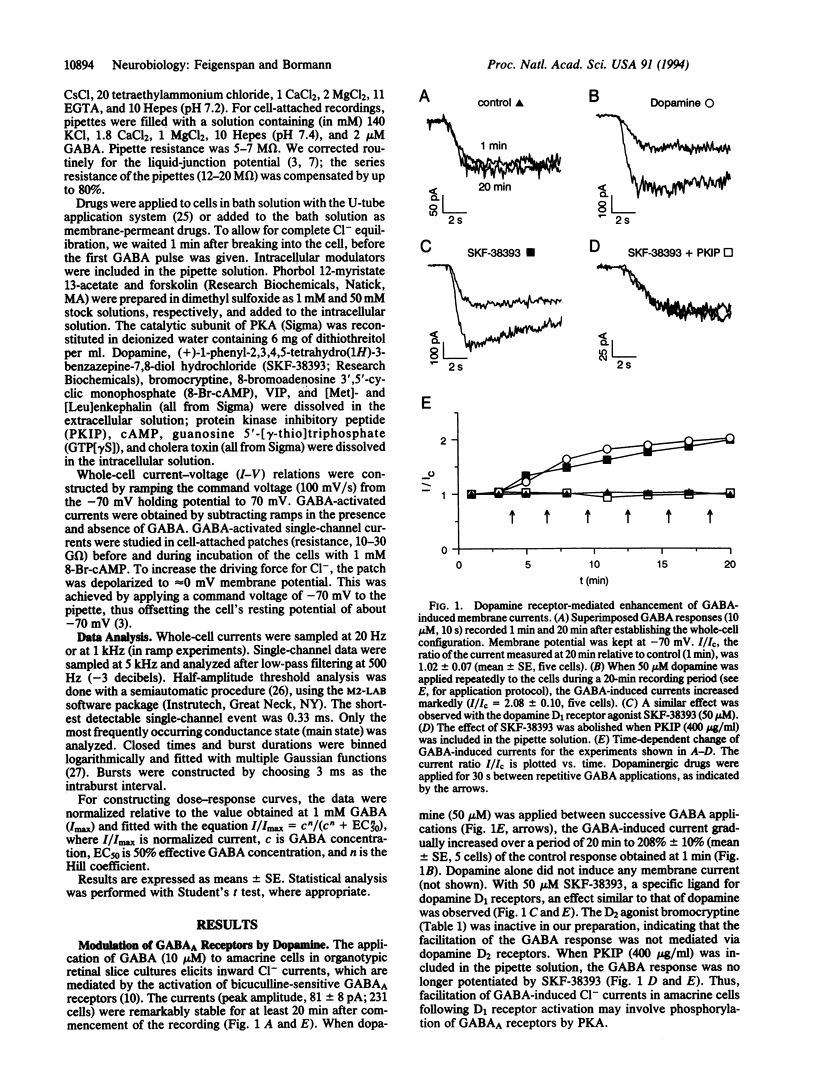
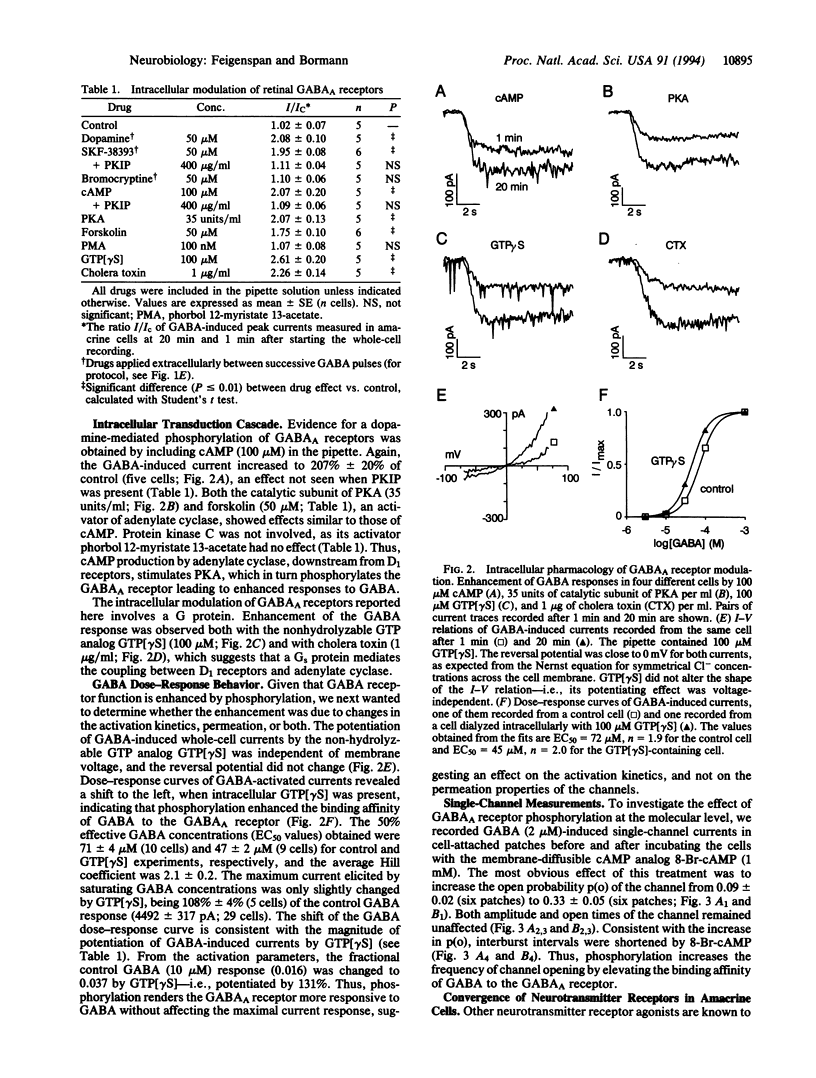
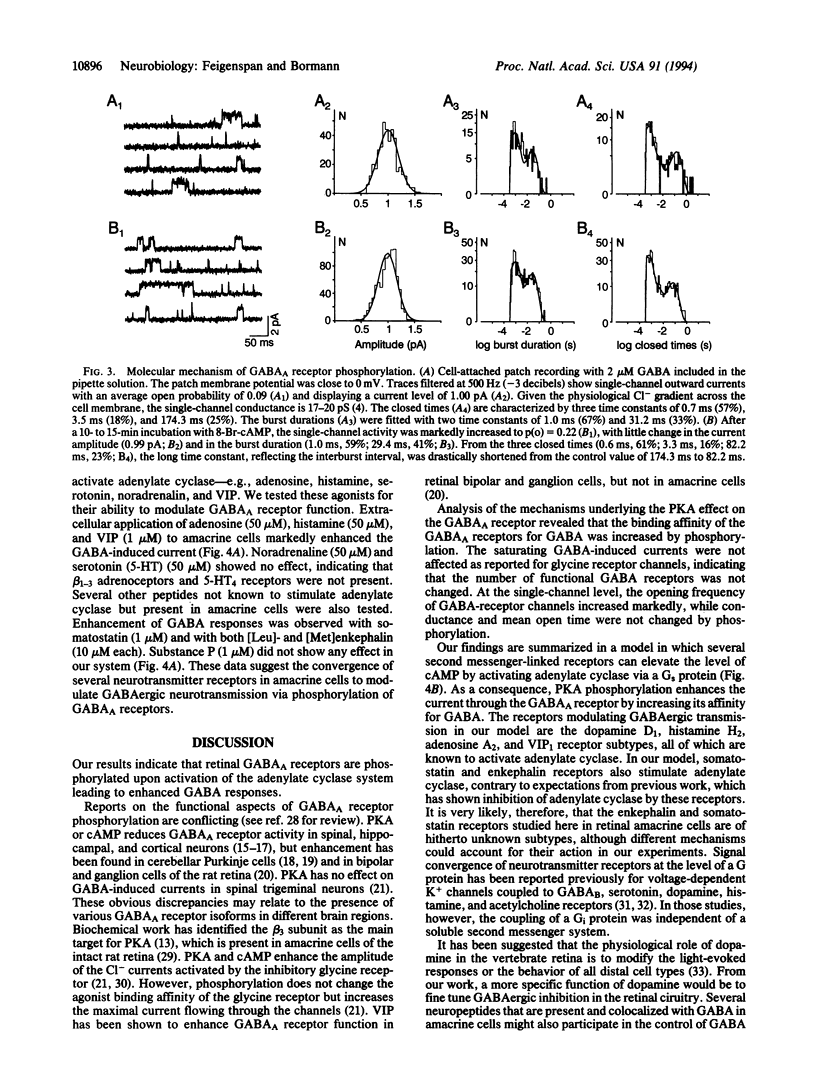
The gamma-aminobutyric acid type A (GABAA) receptor is the predominant Cl(-)-channel protein mediating inhibition in the retina and elsewhere in the mammalian brain. We have observed a time-dependent increase of GABA-induced whole-cell currents when dopamine was applied externally to rat retinal amacrine cells. After 20 min, the peak current was increased to 208% +/- 10% of its initial value. A comparable effect was observed with the dopamine D1 receptor agonist (+)-1-phenyl-2,3,4,5-tetrahydro(1H)-3-benzazepine-7,8-diol hydrochloride (SKF-38393) but not with the D2 agonist bromocryptine. The action of dopamine involved phosphorylation of GABAA receptors by protein kinase A, as evident from intracellular application of protein kinase A, cAMP, and forskolin. Both guanosine 5'-[gamma-thio]triphosphate and cholera toxin augmented the GABA response, indicating a role for the guanosine 5'-triphosphate-binding protein Gs in the transduction cascade. Phosphorylation of GABAA receptors shifted the half-maximally effective GABA concentration from 71 microM to 47 microM without affecting the maximal response amplitude. The elevated binding affinity for GABA was caused by an increase of the open probability of the channels from 0.09 to 0.33 (2 microM GABA); conductance and mean open time did not change. Several other receptor agonists such as adenosine, histamine, somatostatin, enkephalin, and vasoactive intestinal peptide were found to couple to the same intracellular phosphorylation pathway. Since some of these cotransmitters colocalize with GABA in amacrine cells, they may fine-tune GABAergic inhibition in the retina.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agopyan N., Tokutomi N., Akaike N. Protein kinase A-mediated phosphorylation reduces only the fast desensitizing glycine current in acutely dissociated ventromedial hypothalamic neurons. Neuroscience. 1993 Oct;56(3):605–615. doi: 10.1016/0306-4522(93)90360-r. [DOI] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M. D., Endo S., Smith G. B., Dudek E. M., Olsen R. W. Phosphorylation of the GABAA receptor by cAMP-dependent protein kinase and by protein kinase C: analysis of the substrate domain. Neurochem Res. 1993 Jan;18(1):95–100. doi: 10.1007/BF00966927. [DOI] [PubMed] [Google Scholar]
- Burt D. R., Kamatchi G. L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991 Nov;5(14):2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Cutting G. R., Curristin S., Zoghbi H., O'Hara B., Seldin M. F., Uhl G. R. Identification of a putative gamma-aminobutyric acid (GABA) receptor subunit rho2 cDNA and colocalization of the genes encoding rho2 (GABRR2) and rho1 (GABRR1) to human chromosome 6q14-q21 and mouse chromosome 4. Genomics. 1992 Apr;12(4):801–806. doi: 10.1016/0888-7543(92)90312-g. [DOI] [PubMed] [Google Scholar]
- Feigenspan A., Bormann J., Wässle H. Organotypic slice culture of the mammalian retina. Vis Neurosci. 1993 Mar-Apr;10(2):203–217. doi: 10.1017/s0952523800003618. [DOI] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Greferath U., Müller F., Wässle H., Shivers B., Seeburg P. Localization of GABAA receptors in the rat retina. Vis Neurosci. 1993 May-Jun;10(3):551–561. doi: 10.1017/s0952523800004764. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lambert N. A. Modification of GABAA receptor function by an analog of cyclic AMP. Neurosci Lett. 1989 Oct 23;105(1-2):137–142. doi: 10.1016/0304-3940(89)90025-6. [DOI] [PubMed] [Google Scholar]
- Heuschneider G., Schwartz R. D. cAMP and forskolin decrease gamma-aminobutyric acid-gated chloride flux in rat brain synaptoneurosomes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2938–2942. doi: 10.1073/pnas.86.8.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Konnerth A. Potentiation of GABA-mediated currents by cAMP-dependent protein kinase. Neuroreport. 1992 Jul;3(7):563–566. doi: 10.1097/00001756-199207000-00004. [DOI] [PubMed] [Google Scholar]
- Leidenheimer N. J., Browning M. D., Harris R. A. GABAA receptor phosphorylation: multiple sites, actions and artifacts. Trends Pharmacol Sci. 1991 Mar;12(3):84–87. doi: 10.1016/0165-6147(91)90509-q. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Parfitt K. D., Hoffer B. J., Bickford-Wimer P. C. Potentiation of gamma-aminobutyric acid-mediated inhibition by isoproterenol in the cerebellar cortex: receptor specificity. Neuropharmacology. 1990 Oct;29(10):909–916. doi: 10.1016/0028-3908(90)90141-d. [DOI] [PubMed] [Google Scholar]
- Polenzani L., Woodward R. M., Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N. M., Twyman R. E., Uhler M. D., Macdonald R. L. Cyclic AMP-dependent protein kinase decreases GABAA receptor current in mouse spinal neurons. Neuron. 1990 Dec;5(6):789–796. doi: 10.1016/0896-6273(90)90338-g. [DOI] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993 Jan 14;361(6408):162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sato M. A single GTP-binding protein regulates K+-channels coupled with dopamine, histamine and acetylcholine receptors. Nature. 1987 Jan 15;325(6101):259–262. doi: 10.1038/325259a0. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Song Y. M., Huang L. Y. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990 Nov 15;348(6298):242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- Tehrani M. H., Hablitz J. J., Barnes E. M., Jr cAMP increases the rate of GABAA receptor desensitization in chick cortical neurons. Synapse. 1989;4(2):126–131. doi: 10.1002/syn.890040206. [DOI] [PubMed] [Google Scholar]
- Veruki M. L., Yeh H. H. Vasoactive intestinal polypeptide modulates GABAA receptor function in bipolar cells and ganglion cells of the rat retina. J Neurophysiol. 1992 Apr;67(4):791–797. doi: 10.1152/jn.1992.67.4.791. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Schütte M. The organization of dopaminergic neurons in vertebrate retinas. Vis Neurosci. 1991 Jul-Aug;7(1-2):113–124. doi: 10.1017/s0952523800010981. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Polenzani L., Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists. Mol Pharmacol. 1993 Apr;43(4):609–625. [PubMed] [Google Scholar]
- Woodward R. M., Polenzani L., Miledi R. Characterization of bicuculline/baclofen-insensitive gamma-aminobutyric acid receptors expressed in Xenopus oocytes. I. Effects of Cl- channel inhibitors. Mol Pharmacol. 1992 Jul;42(1):165–173. [PubMed] [Google Scholar]