Abstract
Alcohols are a rich source of compounds from renewable sources, but they have to be activated in order to allow the modification of their carbon backbone. The latter can be achieved via oxidation to the corresponding aldehydes or ketones. As an alternative to (thermodynamically disfavoured) nicotinamide-dependent alcohol dehydrogenases, alcohol oxidases make use of molecular oxygen but their application is under-represented in synthetic biotransformations. In this review, the mechanism of copper-containing and flavoprotein alcohol oxidases is discussed in view of their ability to accept electronically activated or non-activated alcohols and their propensity towards over-oxidation of aldehydes yielding carboxylic acids. In order to facilitate the selection of the optimal enzyme for a given biocatalytic application, the substrate tolerance of alcohol oxidases is compiled and discussed: Substrates are classified into groups (non-activated prim- and sec-alcohols; activated allylic, cinnamic and benzylic alcohols; hydroxy acids; sugar alcohols; nucleotide alcohols; sterols) together with suitable alcohol oxidases, their microbial source, relative activities and (stereo)selectivities.
Electronic supplementary material
The online version of this article (doi:10.1007/s00253-015-6699-6) contains supplementary material, which is available to authorized users.
Keywords: Oxidation, Biocatalysis, Alcohol oxidase, Substrate tolerance, Flavoprotein, Cu-containing oxidase
Introduction
Oxidation represents a fundamental reaction in nature (Hollmann et al. 2011; Turner 2011), and oxidases are a prominent subclass of redox enzymes, which use oxygen either as oxidant or as electron acceptor. This property made them particularly attractive for the production of chemicals (Vennestrom et al. 2010). In this context, the oxidation of alcohols is an important transformation in synthetic chemistry, which allows to introduce carbonyl groups, which represent excellent acceptors for C-, N-, O- and S-nucleophiles and thereby allows the extension of a given carbon backbone. Consequently, a large number of protocols has been developed, which depend on (i) transition metals in stoichiometric (e.g. Cr, Mn) or catalytic amounts (e.g. Ru, Fe), (ii) metal-free oxidations according to Swern or Pfitzner-Moffat (Pfitzner and Moffatt 1963; Omura and Swern 1978), (iii) molecular oxygen as oxidant (Tojo and Fernández 2006) and more recently, (iv) organocatalysts, such as TEMPO (Wertz and Studer 2013).
In a related fashion, alcohol oxidases convert primary and secondary alcohols to aldehydes and ketones, respectively. During this reaction, molecular oxygen is reduced to hydrogen peroxide. In order to avoid enzyme deactivation, a catalase is usually employed, particularly on preparative scale. For screening purposes, a spectrophotometric assay based on horse radish peroxidase (HRP) together with a suitable artificial electron acceptor, such as 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) may be employed (Scheme 1). The ABTS-radical generated shifts its absorption maximum (Baron et al. 1994; Uwajima and Terada 1980).
Scheme 1.

Biocatalytic oxidation of alcohols using an alcohol oxidase
Although cofactor-lacking oxidases are reported (Fetzner and Steiner 2010), commonly used alcohol oxidases depend on flavin (Macheroux et al. 2011; Dijkman et al. 2013) or a metal (Cu) as a cofactor (Whittaker 2003), which mediates the electron transfer. In flavoprotein oxidases, the oxidation proceeds via two half reactions, where the alcohol is first oxidised by a two-electron transfer during the reductive half reaction, yielding reduced flavin. The oxidised flavin is regenerated by a stepwise single-electron transfer via the oxidative half reaction, which requires triplet oxygen, as it is a spin-forbidden reaction. Hence, di-oxygen acts as single-electron acceptor and forms superoxide (O2−·), stabilised by a positively charged histidine residue (Dijkman et al. 2013; Wongnate et al. 2014). Another single-electron transfer yields a covalent hydroperoxy flavin intermediate, which eliminates hydrogen peroxide and re-forms oxidised flavin (Scheme 2) (Gadda 2012). The highly unstable C4a-hydroperoxyflavin intermediate has only been detected for pyranose oxidase (P2O) (Mattevi 2006; Chaiyen et al. 2012; Wongnate and Chaiyen 2013).
Scheme 2.
Catalytic cycle of flavin-containing alcohol oxidases
The oxidation of primary alcohols catalysed by flavoprotein oxidases does not necessarily stop at the aldehyde stage, but may further proceed to the corresponding carboxylic acid. This second oxidation step is mechanistically less investigated, but it is obvious that the actual substrate is the aldehyde hydrate (gem-diol), rather than its carbonyl form, because hydride abstraction from the former yields a doubly resonance-stabilised oxocarbenium cation, which upon expulsion of H+ furnishes the carboxylic acid. In contrast, hydride abstraction from the carbonyl form would lead to a highly unstable (hypothetical) acylium cation, which would be quenched by a water molecule (Scheme 3).
Scheme 3.
Aldehyde oxidation via the hydrate intermediate
This mechanism for over-oxidation has been proposed for choline oxidase (CHO), whose natural role is the formation of N-trimethylammonium glycine (‘betaine’) from choline via the aldehyde hydrate through a two-step oxidation (Scheme 4) (Rungsrisuriyachai and Gadda 2008).
Scheme 4.

Two-step oxidation of choline by choline oxidase yielding betaine
The over-oxidation of alcohols to carboxylic acids has been observed not only for choline oxidase but also for other flavoprotein oxidases, such as alditol oxidase (AldO), aryl alcohol oxidase (AAO), hydroxymethyl furfuryl oxidase (HMFO), hexose oxidase (HOX, Dbv29), isoamyl alcohol oxidase (IAO) or short- and long-chain alcohol oxidases (SCAOs, LCAOs). Labelling studies proved the existence of the aldehyde hydrate as intermediate (Van Hellemond et al. 2009), and for AAO, which naturally oxidises benzylic alcohols, NMR studies revealed that the gem-diol intermediate was favoured (Ferreira et al. 2010) (Scheme 3).
Structurally, most of the flavoprotein oxidases either belong to the glucose-methanol-choline (GMC) oxidase or the vanillyl alcohol oxidase (VAO) family. Both families have a flavin present in the active site where the binding domain and the binding mode of the flavin differ. In case of VAO, the flavin is covalently linked to a histidine, cysteine or tyrosine residue, while in the GMC family, the majority of the enzymes contain a dissociable flavin adenine dinucleotide (FAD) moiety. In P2O or CHO, a covalent linkage was found. The active sites and consequently the substrate scope of these enzymes show high variance (Fraaije et al. 1998a; Kiess et al. 1998; Leferink et al. 2008; Dijkman et al. 2013).
Another redox cofactor found in alcohol oxidases, such as galactose oxidase (GOase), is the transition metal copper, whose role in catalysis is well described in several reviews (Ridge et al. 2008; Guengerich 2013). Since only a single copper(I) ion is found in the active site, it seems surprising that a two-electron transfer can occur. Detailed investigations revealed that the latter proceeds via two consecutive single-electron transfer steps. Thus, abstraction of the first electron by Cu2+ yields Cu+, which transfers its electron onto a tyrosine residue, which forms a transient radical anion (Monti et al. 2011). The latter is stabilised by a rare covalent thioether bridge with an adjacent cysteine (Ito et al. 1991). The second electron transfer yields a Cu+-tyrosine radical. From this, oxygen accepts two electrons (Wang 1998; Whittaker 2003) (Scheme 5). GOase from Fusarium NRRL 2903 is the most prominent member of Cu-containing alcohol oxidases and belongs to the family of radical copper oxidases, a family with a wide phylogenetic distribution and broad range of functions. The crystal structure of the enzyme revealed that a mononuclear copper ion is centred in a distorted pyramid structure, which is coordinated by two tyrosine residues (Tyr272 and Tyr495) and two histidine side chains (His496 and His581) (Whittaker and Whittaker 2001).
Scheme 5.
Catalytic cycle of copper-containing oxidases
For Cu-containing alcohol oxidases, the oxidation stops at the aldehyde stage and over-oxidation was not observed (Monti et al. 2011).
From a biocatalytic viewpoint, alcohol oxidases are a promising group of enzymes, because they are biochemically well characterised and a broad range of enzymes have been described (Whittaker 2003; Leferink et al. 2008; Dijkman et al. 2013) which were also employed in cascade reactions (Fuchs et al. 2012; Perez-Sanchez et al. 2013; Schrittwieser et al. 2011). Depending on their role in nature, substrates for alcohol oxidases vary to a great extent in terms of substrate size and/or polarity (Turner 2011). In fungi, extracellular alcohol oxidases produce hydrogen peroxide (needed for lignin degradation by peroxidases) by oxidation of cinnamyl alcohols (e.g. coniferyl, coumaryl and sinapyl alcohol). Furthermore, hydrogen peroxide acts as antibiotic in the rhizosphere to protect roots (Monti et al. 2011). As an alternative to alcohol oxidases, NAD(P)+-dependent alcohol dehydrogenases provide a well-investigated enzyme platform for the oxidation of prim- and sec-alcohol functionalities. Although these enzymes are more abundant than alcohol oxidases, the equilibrium for oxidation is strongly disfavored but can be overcome by NAD(P)+ recycling (Hollmann et al. 2011).
In the following, an overview on the current literature of alcohol oxidases is given, by focussing on their substrate tolerance to facilitate the choice of an appropriate enzyme for a given type of alcohol substrate.
Non-activated alcohols
Primary aliphatic alcohols
The enzymatic oxidation of non-activated aliphatic prim-alcohols by alcohol oxidases shows a remarkably broad substrate tolerance (Table 1) and encompasses straight-chain or branched substrates with chain lengths ranging from C1 to C16. In addition, functional groups, such as aromatics, halogens (Cl, Br), non-allylic olefins, alkylamino groups and carboxylates, are tolerated (Scheme 6). Vicinal, 1,3- and α,ω-diols are selectively oxidised at the prim-hydroxy group, while sec-alcohols remain untouched. Depending on the enzyme, the oxidation products are the corresponding aldehydes and carboxylic acids, which are formed by over-oxidation with flavoprotein oxidases.
Table 1.
Primary aliphatic alcohols
| Entry | Substrate | Oxidase | Reference |
|---|---|---|---|
| Mono-alcohols | |||
| 1 | 1-Alkanols C1–C5 | SCAOb from P. pastoris (C1–C4), Hansenula sp. (C1–C5), C. boidinii, T. aurantiacus and A. terreus (C1–C2) | Kato et al. 1976; Ko et al. 2005; Perez-Sanchez et al. 2013; Kumar and Goswami 2006; Siebum et al. 2006; Menon et al. 1995; Couderc and Baratti 1980; Kjellander et al. 2013 |
| 2 | 1-Alkanols C7–C14, C16 | SCAOb from A. terreus (C7); LCAOb from A. terreus (C7–C14), A. thaliana (C12, C16) and C. tropicalis (C8, C10, C12, C14) | Kumar and Goswami 2006; Kumar and Goswami 2008; Kumar and Goswami 2009; Eirich et al. 2004; Cheng et al. 2004 |
| 3 | 2-Bromoethanol 2-Chloroethanol |
SCAO from C. boidinii | Menon et al. 1995 |
| Diols and triols | |||
| 4 | 1,2-Ethanediol | SCAOb from T. aurantiacus and P. pastoris | Ko et al. 2005; Kjellander et al. 2013 |
| 5 | 1,10-Decanediol | LCAOb from C. tropicalis | Eirich et al. 2004 |
| 6 | 1,16-Hexadecanediol | LCAOb from A. terreus, C. tropicalis and A. thaliana | Kumar and Goswami 2006; Eirich et al. 2004; Cheng et al. 2004 |
| 7 | 1,2-Alkanediol C4–C6 | AldOb from S. coelicolor | Van Hellemond et al. 2009 |
| 8 | 1,3,5-Pentanetriol 1,2,4-Butanetriol |
AldOb from S. coelicolor | Van Hellemond et al. 2009 |
| 9 | 1,3-Butanediol | AldOb from S. coelicolor | Van Hellemond et al. 2009 |
| 10 | 3-Chloro-1,2-propanediol 3-Bromo-1,2-propanediol |
GOasea from Fusarium NRRL 2903 | Klibanov et al. 1982 |
| 11 | 1-Phenyl-1,2-ethanediol | AldOb from S. coelicolor and A. cellulolyticus 11B | Van Hellemond et al. 2009; Winter et al. 2012 |
| 12 | ω-Hydroxy-carboxylic acid | LCAOb from A. terreus (C12) and C. tropicalis (C12, C16) | Kumar and Goswami 2006; Eirich et al. 2004; Cheng et al. 2004 |
| Amino alcohols | |||
| 13 | Choline N,N-Dimethyl-ethanolamine N-Methyl-ethanolamine Triethanolamine Diethanolamine |
CHOb from A. globiformis | Gadda et al. 2004; Ikuta et al. 1977 |
| Unsaturated alcohols | |||
| 14 | 3-Buten-1-ol 4-Penten-1-ol |
SCAOb from P. pastoris | Siebum et al. 2006 |
| 15 | 3-Buten-1,2-diol 4-Pentene-1,2-diol |
AldOb from S. coelicolor | Van Hellemond et al. 2009 |
| Branched alcohols | |||
| 16 | 2-Methyl-1-alkanol (C4–C6) | SCAOb from C. boidinii, P. pastoris and Hansenula sp. | Clark et al. 1995 |
| 17 | 3-Methyl-1-butanol | SCAOb from T. aurantiacus; SAOc from A. terreus; IAOb from A. oryzae | Ko et al. 2005; Eirich et al. 2004; Yamashita et al. 1999 |
| 18 | 1-Phenyl-3-propanol | SCAOb from A. terreus | Kumar and Goswami 2009 |
| 19 | 3,3-Dimethylbutan-1-ol | CHOb from A. globiformis | Gadda et al. 2004 |
aCopper containing
bFlavin containing
cNone heme Fe2+ containing
Scheme 6.

Oxidation of primary alcohols by oxidases
Aliphatic alcohols with a chain length of one to seven C atoms were oxidised by short-chain alcohol oxidases (SCAOs) [EC 1.1.3.13] from Pichia pastoris, Hansenula sp. (Table 1, entry 1) and Aspergillus terreus (Table 1, entry 2) while methanol and ethanol were also converted by alcohol oxidases from Candida boidinii, Thermoascus aurantiacus and A. terreus (Table 1, entry 1) (Kato et al. 1976; Siebum et al. 2006; Menon et al. 1995; Couderc and Baratti 1980; Kumar and Goswami 2006; Ko et al. 2005; Perez-Sanchez et al. 2013). In general, the activity decreases with increasing chain length of the fatty alcohol, e.g. 1-pentanol shows 24 % relative activity compared to methanol (Ko et al. 2005). SCAO from Hansenula sp. was employed together with a C–C lyase in a cascade reaction, where short-chain alcohols (methanol, ethanol, 1-propanol and 1-pentanol) were oxidised with excellent conversion to the corresponding aldehydes, which were subjected to cross-acyloin condensation with benzoin generated in situ from benzaldehyde by benzaldehyde lyase to yield 2-hydroxyketones (Shanmuganathan et al. 2012; Perez-Sanchez et al. 2013). prim-Alcohols with a chain length of 7 to 16 carbon atoms were best oxidised by long-chain alcohol oxidases (LCAOs) [EC 1.1.3.20] from A. terreus, Candida tropicalis and Arabidopsis thaliana (Table 1, entry 2). Both, SCAOs and LCAOs, are flavoproteins located in fungal microsomes (Kemp et al. 1988; Eirich et al. 2004; Kumar and Goswami 2006, Cheng et al. 2004). Terminal alcohols bearing a polar functional group, such as α,ω-diols (Table 1, entries 5 and 6) and ω-carboxy fatty alcohols (Table 1, entry 12), with a long hydrocarbon backbone were also oxidised by long-chain alcohol oxidases (Kumar and Goswami 2006).
Short-chain alcohol oxidase from several microorganisms (C. boidinii, Hansenula sp., P. pastoris and T. aurantiacus) was described to convert racemic branched alcohols (Table 1, entries 16–17) in an enantioselective fashion with conversions of 16–76 %, the non-reacted substrate enantiomers showed ees of up to 90 % for SCAO from C. boidinii (Clark et al. 1995). Isoamyl alcohol oxidase (IAO) [EC 1.1.3.x] from Aspergillus oryzae exhibits a narrow substrate range and prefers branched short-chain alcohols, such as 3-methyl-1-butanol (Table 1, entry 17) (Yamashita et al. 1999). Halogen-substituted alcohols, which were oxidised by SCAO, were used as molecular probes for mechanistic studies (Menon et al. 1995).
Saturated and unsaturated vic-1,2-diols were the substrates of choice for alditol oxidase [EC 1.1.3.41] from Streptomyces coelicolor and Acidothermus cellulolyticus (Table 1, entries 7–9, 11 and 15). This enzyme apparently prefers a glycol or 1,3-diol moiety. For rac-1-phenyl-1,2-ethanediol carrying a bulky aryl moiety, the (R)-enantiomer was preferentially oxidised by alditol oxidase (Table 1, entry 11) (Van Hellemond et al. 2009). Short (non-allylic) unsaturated alcohols lacking a second hydroxy group were completely (4-penten-1-ol) or partially (3-buten-1-ol) oxidised by short-chain alcohol oxidase from P. pastoris (Table 1, entry 14) (Siebum et al. 2006).
Another prominent enzyme of this group is choline oxidase from A. globiformis which oxidises choline and analogues, such as N,N-dimethylethanolamine, N-methylethanolamine, triethanolamine, diethanolamine and 3,3-dimethylbutan-1-ol (Table 1, entries 13 and 19) in a two-step oxidation to the corresponding carboxylic acid (Ikuta et al. 1977; Gadda et al. 2004).
Secondary aliphatic alcohols
Racemic secondary aliphatic alcohols are interesting substrates, because enantioselectivities in kinetic resolution are usually much higher than with prim-alcohols bearing a stereogenic centre. In contrast to prim-alcohols, which may undergo over-oxidation to carboxylic acids, the oxidation products derived from sec-alcohols are solely ketones (Scheme 7). Compared to prim-alcohol oxidases, enzymes acting on secondary alcohols are less abundant, but several enzymes were found to be highly active (Table 2). Secondary alcohol oxidase (SAO) [EC 1.1.3.18] from Pseudomonas putida, Pseudomonas vesicularis and A. terreus has shown high activity for the polymeric substrate polyvinyl alcohol (PVA) (Table 2, entry 1) (Sakai et al. 1985; Kawagoshi and Fujita 1997), and it was discovered that one non-heme Fe2+ species is present in the enzyme. To date, it remains unclear whether the iron species serves as a cofactor like the copper in galactose oxidase or if it does not participate in catalysis at all.
Scheme 7.
Oxidation of secondary alcohols by alcohol oxidases
Table 2.
Secondary aliphatic alcohols
| Entry | Substrate | Oxidase | Reference |
|---|---|---|---|
| 1 | Polyvinyl alcohol | SAOa from P. putida and P. vesicularis | Sakai et al. 1985; Kawagoshi and Fujita 1997 |
| 2 | 2-Alkanols C3–C12, C16 | SAOa from A. terreus (C3, C8, C12), P. putida (C3–C7) and P. vesicularis (C4–C8); SCAOb from T. aurantiacus (C3–C4), A. terreus (C8) and P. pastoris (C3); LCAOb from C. tropicalis (C10–C11, C16) | Ko et al. 2005; Kumar and Goswami 2006; Kjellander et al. 2013; Sakai et al. 1985; Kawagoshi and Fujita 1997; Kumar and Goswami 2009; Eirich et al. 2004 |
| 3 | 3-Alkanols C5–C8 | SAOa from P. putida (C5–C8), A. terreus (C8) and P. vesicularis (C6–C8) | Sakai et al. 1985; Kawagoshi and Fujita 1997; Eirich et al. 2004 |
| 4 | 4-Alkanols C7–C10 | SAOa from P. putida (C7–C9) and P. vesicularis (C7, C10) | Sakai et al. 1985; Kawagoshi and Fujita 1997 |
| 5 | 5-Nonanol | SAOa from P. putida | Sakai et al. 1985 |
| 6 | Cycloalkanols C6, C8 | SAOa from P. vesicularis (C6) and A. terreus (C8) | Kawagoshi and Fujita 1997; Kumar and Goswami 2006 |
| 7 | 1,2-Propanediol | SAOa from P. putida | Sakai et al. 1985 |
| 8 | 2,4-Pentanediol | SAOa from P. vesicularis | Kawagoshi and Fujita 1997 |
aNon-heme Fe2+ containing
bFlavin-containing
For monomeric sec-alcohols, the relative activity of SAO from P. putida ranges between 5 and 30 % (compared to PVA). High activity for 2-octanol was found with the enzyme from P. vesicularis (83 % rel. activity), which also accepts cyclohexanol (42 % rel. activity). Its oxidised product (cyclohexanone) is used as a starting material for the synthesis of the polymer building block ε-caprolactam. sec-Alcohols bearing an additional OH group, such as 1,2-propanediol and 2,4-pentanediol, were also accepted as substrates (Table 2, entries 7 and 8); however, no details are reported about the regioselectivity of the oxidation (Sakai et al. 1985; Kawagoshi and Fujita 1997). Additionally, SCAO from T. aurantiacus, A. terreus and P. pastoris as well as LCAO from C. tropicalis showed broad activity on secondary alcohols (Table 2, entry 2) (Eirich et al. 2004; Kumar and Goswami 2009; Kjellander et al. 2013; Ko et al. 2005). Furthermore, 2-methyl-2-propanol was claimed to show 16 % relative activity with SCAO, but this tert-alcohol should be a non-substrate (Ko et al. 2005).
Activated alcohols
Allylic alcohols
In contrast to saturated (non-activated) aliphatic alcohols, allylic and benzylic alcohols are much easier to oxidise, because radicals and carbene ions occurring as intermediates are resonance stabilised (see Electronic Supplementary Material, Scheme S1). Owing to their high intrinsic reactivity, allylic alcohols are easily oxidised by a broad range of alcohol oxidases, such as copper-containing galactose oxidase (GOase) [EC 1.1.3.9], flavoprotein cholesterol oxidase (ChOx) [EC 1.1.3.6], aryl alcohol oxidase (AAO) [EC 1.1.3.7] and hydroxymethylfurfural oxidase (HMFO) [EC 1.1.3.47] (Scheme 8, Table 3) (Guillen et al. 1992; Dieth et al. 1995; Sun et al. 2002; Dijkman and Fraaije 2014).
Scheme 8.
Oxidation of allylic alcohols by alcohol oxidases
Table 3.
prim- and sec-Allylic alcohols
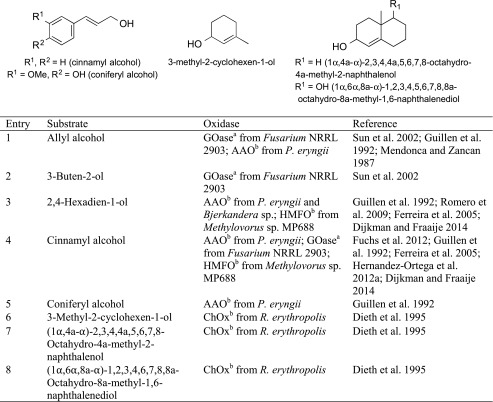
aCopper containing
bFlavin containing
Small allylic alcohol was oxidised poorly by galactose oxidase (Table 3, entries 1 and 2), which prefers large analogues, such as cinnamyl alcohol (Table 3, entry 4). A mutant of galactose oxidase from Fusarium sp. oxidised cinnamyl alcohol with full conversion (Sun et al. 2002; Fuchs et al. 2012). In contrast to galactose oxidase, which does not accept sec-allylic alcohols, cholesterol oxidase from Rhodococcus erythropolis converted sterically demanding secondary allylic alcohols in a complete stereo- and enantioselective fashion with conversions up to 70 % and high to excellent ees. For methyl-substituted bicyclic substrates (Table 3, entries 7 and 8), the relative (cis) position of the hydroxyl group with respect to the methyl group were mandatory to be accepted and non-activated (non-allylic) hydroxy groups were unreactive. Even comparably small monocyclic substrates could be converted (Dieth et al. 1995). Aryl alcohol oxidase exhibits a broad substrate scope and accepts phenyl substituted allylic alcohols such as coniferyl and cinnamyl alcohol (Table 3, entries 4 and 5), as well as slim counterparts, such as 2,4-hexadien-1-ol (Table 3, entry 3), which shows that this enzyme does not necessarily need a cyclic structure, but only a conjugated system (Ferreira et al. 2005; Romero et al. 2009). 5-Hydroxymethylfurfural oxidase exhibited a similar behaviour and appears to be a promising candidate for the oxidation of allylic alcohols, as it showed excellent acceptance of cinnamyl alcohol (Table 3, entry 4) and 2,4-hexadien-1-ol (Table 3, entry 3) (Dijkman and Fraaije 2014). With cinnamyl alcohol and its p-methoxy derivative, AAO shows over-oxidation and forms the corresponding acids (Table 3, entry 4) (Guillen et al. 1992).
Benzylic alcohols
Due to their high degree of electronic activation, benzylic alcohols (Scheme 9, Table 4) are easy to oxidise. In particular, galactose oxidase from Fusarium NRRL 2903 and aryl alcohol oxidase from Pleurotus eryngii are ideally suited for this substrate type, together with a recently discovered flavin-containing oxidase from Bjerkandera sp.
Scheme 9.
Oxidation of benzylic alcohols by alcohol oxidases
Table 4.
prim- and sec-Benzylic alcohols



aCopper containing
bFlavin containing
cFe2+ containing
In the case of benzyl alcohol, two more AAOs (from A. terreus and Pleurotus ostreatus) showed activity, as well as SCAO from C. boidinii and T. aurantiacus, SAO from P. vesicularis and HMFO from Methylovorus sp. (Table 4, entry 2) (Kumar and Rapheal 2011). Although not visible on a non-chiral substrate, AAO acts in a stereoselective fashion by removing the pro-R hydride as shown by deuterium experiments (Hernandez-Ortega et al. 2012b). Various substituents on the aromatic ring system are freely tolerated: Although wild-type galactose oxidase from Fusarium has a broad substrate scope for benzylic prim-alcohols, the activity was considerably increased by mutations. For instance, all regioisomers of pyridine methanol were transformed by a R330K, Q406T-mutant of galactose oxidase, which showed up to 2000-fold enhanced activity towards 2-pyridine methanol compared to the canonical d-galactose (Sun et al. 2002). Meta- and para-substituted substrates (3-F, 3-Br, 3-Cl, 3-NO2, 4-F, 4-Cl, 4-Br, 4-I, 4-NO2, 4-OMe, 4-SMe, 4-Me, 4-CF3) (Table 4, entries 4, 6, 7, 17, 18, 20–24) were converted with up to 20-fold variation of relative rates (Whittaker and Whittaker 2001).
Secondary aryl alcohols undergo kinetic resolution with partly excellent ees using an (R)-selective mutant of galactose oxidase from Fusarium sp. created by directed evolution (Table 4, entries 28–40) (Escalettes and Turner 2008). The same group also reported a rare example of the successful recognition of an atropisomeric pair of enantiomers possessing axial chirality (Table 4, entry 41) (Yuan et al. 2010). Furthermore, an engineered variant of HMFO was able to oxidise phenylethanol in a stereoselective fashion (Dijkman et al. 2015).
Methoxy groups (Table 4, entry 6) were accepted independently from the position on the ring with comparable activities relative to unsubstituted benzyl alcohol, whereas para-substituted analogues reacted more than fivefold faster with aryl alcohol oxidase. Furthermore, dimethoxy benzyl alcohols (Table 4, entries 8 and 9) were converted by aryl alcohol oxidase with high activity (Hernandez-Ortega et al. 2011; Hernandez-Ortega et al. 2012a). In particular, 3,4-dimethoxybenzyl alcohol (veratryl alcohol, Table 4, entry 9) was converted with 326 % activity, while the 2,4-substituted pendant (Table 4, entry 8) was accepted with 178 % activity relative to benzyl alcohol (Guillen et al. 1992). Sterically demanding 3,4,5-trimethoxybenzyl alcohol (Table 4, entry 10) was converted slowly. Besides methoxy groups, also hydroxy groups, combinations thereof and even a meta-substituted phenoxy group were accepted (Table 4, entries 12–16). The hydroxy substrates (Table 4, entries 12 and 13) were poorly converted compared to the 3-phenoxybenzyl alcohol (Table 4, entry 16) which was well accepted (Guillen et al. 1992). Additionally, the name-giving enzyme for the VAO family, vanillyl alcohol oxidase (VAO) [EC1.1.3.38] acts on 4-hydroxy-3-methoxybenzyl alcohol (vanillyl alcohol, Table 4, entry 15) (de Jong et al. 1992; Van den Heuvel et al. 1998; Fraaije et al. 1998b; Van Den Heuvel et al. 2000; Van den Heuvel et al. 2001a; Van den Heuvel et al. 2001b). While the enzyme seems to accept bulky substituents, e.g. bearing a phenoxy group, additional methoxy or especially hydroxy groups (Table 4, entries 12–16) cause unfavourable interactions in the active site. The aryl alcohol oxidase from P. eryngii also acts on 4-hydroxy-substituted α-aryl alcohols (Table 4, entry 13) (Guillen et al. 1992). Piperonyl alcohol (1,3-benzodioxole-5-methanol, Table 4, entry 11), a building block in epinephrine synthesis, was oxidised with full conversion by galactose oxidase from Fusarium sp. (Fuchs et al. 2012). A broad range of chloro- and fluoro-substituted aryl alcohols were accepted by both aryl alcohol oxidase and galactose oxidase (Table 4, entries 20 and 21) (Guillen et al. 1992; Whittaker and Whittaker 2001; Romero et al. 2009). The only exception being meta-chlorobenzyl alcohol, which was not converted at all. A substrate which is sterically demanding and well accepted by AAO is 2-naphthalene methanol (Table 4, entry 26). It showed a relative activity of 746 % compared to the monocyclic substrate analogue (Table 4, entry 2). In conclusion, the position of substituents and their polarity seem to play a crucial role in substrate acceptance. The recently characterised 5-hydroxymethylfurfural oxidase from Methylovorus sp. MP688 showed a broad substrate acceptance of various furfuryl alcohols (Table 4, entry 42), but it also showed activity on benzylic alcohols with substituents in para-position (Table 4, entries 3 and 5) and vanillyl alcohol (Table 4, entry 15) (Dijkman and Fraaije 2014). In view of the growing importance of furan derivatives, such as hydroxymethyl furfural, which can easily be obtained via double elimination of H2O from hexoses or pentoses and hence constitute a promising C source for organic synthesis (Schwartz et al. 2014), HMFO has a considerable potential to be used in large-scale applications. In a recent study, site-directed mutagenesis allowed to boost the activity of HMFO on 5-formyl-2-furancarboxylic acid leading to improved yields of 2,5-furandicarboxylic acid, which is a promising monomer for polyester production from renewable resources (Dijkman et al. 2015).
α-Hydroxy acids
Owing to the negative charge of α-hydroxy acids at neutral pH, the latter are oxidised by a subgroup of flavoprotein oxidases, which are specific for this type of polar substrate and furnish the corresponding α-ketoacids (Scheme 10). On a first glimpse, this transformation appears to have little value, because it goes in hand with the destruction of a chiral centre. However, α-hydroxy acids are usually more easily accessible than the corresponding sensitive α-keto-analogues, which are prone to decarboxylation; this transformation is of practical value, and in addition, racemic α-hydroxy acids undergo kinetic resolution with a preference for the (S)-enantiomer (Turner 2011).
Scheme 10.

Enzymatic oxidation of hydroxy acids by hydroxy acid oxidases
A broad range of α-hydroxy acids were studied as substrates for FMN-depending glycolate oxidase (GlyO), l-lactate oxidase (LLO) or long-chain 2-hydroxyacid oxidase (LHAO), which belongs to the group of (S)-2-hydroxy acid oxidases (HAOX) [EC 1.1.3.15]. For GlyO, the natural substrate is glycolic acid (Table 5, entry 1) and the most prominent GlyO originates from spinach (Spinacia oleracea) (Zelitch and Ochoa 1953). The name-giving substrate can be over-oxidised to oxalic acid, although the second step is less efficient (Richardson and Tolbert 1961). Short- and medium-chain 2-hydroxy acids with up to ten carbon atoms (Table 5, entries 10 and 11), unsaturated cis- and trans-2-hydroxydec-4-enoic acid (Table 5, entry 14), bulky phenyllactic acid (Table 5, entry 9) and the oxa-analogue 2-hydroxy-4-pentoxybutyric acid (Table 4, entry 13) were well accepted as substrates in kinetic resolution with good to excellent ees. Furthermore, 3-chlorolactic acid (110 % rel. activity), 2-hydroxybutanoic acid (120 % rel. activity), 3-indolelactic acid (18 % rel. activity) (Table 5, entries 9 and 10), 3,3,3-trifluorolactic acid (11 % rel. activity) (Table 5, entry 15) and 2-hydroxydecanoic acid (40 % rel. activity) (Table 4, entry 12) were nicely converted relative to lactic acid (Adam et al. 1997; Adam et al. 1998; Das et al. 2009; Stenberg et al. 1995). The substrate spectrum of FMN-containing l-lactate oxidase from Aerococcus viridans and a mutant thereof encompasses also sterically demanding α-hydroxy acids, such as para-substituted mandelic acid derivatives (Table 5, entries 5–7) and was analysed in a quantitative structure analysis (Duncan et al. 1989; Maeda-Yorita et al. 1995; Yorita et al. 1997). Additionally, an enzyme originating from Pseudomonas stutzeri was used to oxidise lactic acid to pyruvate enantioselectively (Table 5, entry 2, Gao et al. 2009). LHAO, on the contrary, originating from mammalian sources, such as pig kidney, rat kidney or hog renal cortex, oxidises 2-hydroxy acids with a carbon chain length of at least three C atoms (Blanchard et al. 1946; Robinson et al. 1962). 2-Hydroxy-4-methylpentanoic acid, 2-hydroxybutyric acid and also mandelic acid (Table 5, entries 8–10, 12) were oxidised with moderate conversions (Urban et al. 1988).
Table 5.
α-Hydroxy acids

aCopper containing
bFlavin containing
Sterols
The bioactivity of steroids strongly depends on their substitutional pattern, which is dominated by secondary hydroxy groups in α- or β-positions, which upon oxidation furnish keto-steroids. This transformation can be achieved in a regio- and stereoselective fashion by alcohol oxidases. Owing to the spacious molecular framework, it is conceivable that alcohol oxidases acting on steroids have a strong preference for large substrates and are generally not ideally suited for small alcohols (Scheme 11).
Scheme 11.

Enzymatic oxidation and C=C isomerisation of cholesterol derivatives by cholesterol oxidase
Cholesterol oxidase (ChOx) [EC 1.1.3.6] found in Streptomyces hygroscopicus, Rhodococcus and Brevibacterium sterolicum is the enzyme of choice for the oxidation of the secondary alcohol function at C3, which leads to rare keto-steroids (Table 6). From a biochemical point of view, it is remarkable that cholesterol oxidases are strictly FAD containing, although they belong to two different families: Cholesterol oxidase from Streptomyces is a member of the GMC oxidase family, whereas B. sterolicum ChOx belongs to the VAO family. Remarkably, most cholesterol oxidases are bifunctional enzymes (Pollegioni et al. 1999; Gadda et al. 1997; Pollegioni et al. 2009; Vrielink and Ghisla 2009), as they not only oxidise the alcohol functionality at C3 yielding 5-cholesten-3-one but also mediate the isomerisation of the C5–C6 double bond of the latter into conjugation with the newly formed keto-function by assistance of an active-site glutamate residue to furnish the corresponding 4-en-3-one, as demonstrated in detail with ChOx from B. sterolicum (Kass and Sampson 1995) (Scheme 11). The enzyme exhibited a surprisingly broad substrate scope, and a variant from R. erythropolis even lacks enantiospecificity at the C3 position (Dieth et al. 1995; Biellmann 2001). For the enzyme from Rhodococcus sp., moderate activities (relative to the natural substrate cholesterol) on β-sitosterol (80 % rel. activity) and stigmasterol (78 % rel. activity) were found by Wang et al. (2008) (Table 6, entries 6 and 7). Furthermore, the enzyme was active on cholestanol, 7-dehydrocholesterol and dehydroepiandrosterone (15–37 % rel. activity) (Table 6, entries 2, 4 and 8), and 5 % relative activity was found on 5α-androstane-3α,17β-diol (Table 6, entry 11) (Labaree et al. 1997; Toyama et al. 2002; Wang et al. 2008, Fujishiro et al. 2002; Xiang and Sampson 2004). Moreover, cholesterol oxidase from B. sterolicum was employed for the oxidation of 7α- and 7β-hydroxycholesterol (90 % conv.) (Table 6, entry 3) in a chemoenzymatic multistep synthesis (Alexander and Fisher 1995).
Table 6.
Sterols

aCopper containing
bFlavin containing
Sugar-related alcohols
Sugars
Although sugars constitute the most abundant group of renewable compounds/materials (Straathof 2014), their polyhydroxy structure imposes several unsolved problems in view of their utility as starting materials in organic synthesis: (i) they possess only a single type of functional group—the hydroxy group, and (ii) there are too many of them with similar reactivity (Scheme 12). This causes a selectivity problem, which is usually circumvented by tedious and inefficient protection-deprotection chemistry. (iii) Furthermore, except for the anomeric carbon, the carbon framework is inaccessible to C–C extension/modification, because the [CH–OH] moiety cannot be directly accessed without prior activation of the hydroxy group. In this context, regioselective oxidation of OH groups in sugars at the expense of O2 offers an elegant method to introduce a carbonyl group, which is an ideal acceptor for C nucleophiles in C–C bond forming reactions.
Scheme 12.
Regioselectivity of alcohol oxidases on a hexose framework
Due to the presence of numerous hydroxy groups, carbohydrates are usually bound in the active site of proteins via a tight hydrogen-bonding network, which is not possible for lipophilic mono-alcohols or diols. Consequently, one might surmise, that alcohol oxidases acting on lipophilic (mono) alcohols would not accept polar carbohydrates, and vice versa. However, comparison of Tables 1 and 3 shows that many sugar alcohol oxidases are also surprisingly active on small non-polar alcohols, in particular galactose oxidase and alditol oxidase.
The relative reactivity of hydroxy groups in sugars can be associated with different subgroups of alcohol oxidases, most of which possess a strong regio-preference for a specific hydroxyl group, which is exemplified on a schematic hexose (Scheme 12). With its hemiacetal structure, the anomeric OH is most reactive, which can be oxidised by glucose oxidase (GOX), hexose oxidase (HOX) and oligosaccharide oxidases forming the corresponding sugar lactone. Next, the terminal prim-OH is sterically least hindered among the non-activated hydroxy groups; it can be selectively oxidised by GOase to yield the aldehyde; no over-oxidation to the acid is observed in this case. Due to small steric and electronic differences, internal secondary hydroxy groups show very similar reactivities, they are oxidised by P2O with mixed regioselectivities with a prevalence of C2 > C3 yielding ketoses. C3-Oxidation products are only formed on 2-deoxy and methylated sugars.
(i) The most reactive anomeric hydroxy group in sugars can be selectively oxidised by a range of well-studied oxidases (Scheme 12): d-Glucose (Table 7, entry 1) is the natural substrate of the flavoenzyme GOX [EC 1.1.3.4], well studied from Aspergillus niger, which displayed a very narrow substrate spectrum and oxidises glucose at the C1 position (Nakamura and Ogura 1968). Furthermore, chitooligosaccharide oxidase (ChitO) [EC 1.1.3.x] from Fusarium graminearum catalyses the oxidation of C1 of d-glucose. The catalytic activity was improved by mutation (Heuts et al. 2007a), and the wild-type and mutant enzymes also accepted cellulose degradation products like cellobiose, cellotriose and cellotetraose (Table 7, entry 18). Mutants of chitooligosaccharide oxidase also accepted d-lactose and d-maltose besides the before mentioned d-glucose oligomers (Table 7, entries 9 and 10) (Heuts et al. 2007a). Variants obtained by further mutagenesis studies showed a switch in the preference for the oligosugar preference as well as improved activities on d-lactose, d-maltose and d-glucose (Ferrari et al. 2015).
Table 7.
Sugars




aCopper containing
bFlavin containing
Furthermore, also glucooligosaccharide oxidase (GOO) [EC 1.1.3.x] from various sources oxidised d-glucose and its oligomers at C1 (Huang et al. 2005). Lactose oxidase (LAO) [EC 1.1.3.x] from Microdochium nivale displayed a similar substrate preference. Cellobiose (Table 7, entry 18) with 100 % relative activity was the preferred substrate, whereas di-sugars as d-maltose (84 % rel. activity) and d-lactose (52 % rel. activity) were also well accepted (Table 7, entries 9,10). Furthermore, the monosugars d-glucose (69 % rel. activity) and d-galactose (31 % rel. activity) were both oxidised at C1 (Xu et al. 2001) (Table 7, entries 1 and 2). Moreover, Pezzotti and Therisod synthesised aldonic acids starting with C6 sugars (d-galactose, d-xylose, d-mannose and 2-deoxy-d-glucose) employing glucose oxidase for the oxidation of the C1 hydroxy group (2006). HOX [EC 1.1.3.5] from Chondrus crispus is an enzyme with a fairly broad substrate scope for the oxidation of sugars at C1. Hexose oxidase accepted d-xylose, d-arabinose and d-glucose containing di-sugars, like d-lactose and d-cellobiose (Table 7, entries 4, 5, 10 and 18) (Poulsen and Hostrup 1998; Savary et al. 2001; Rand et al. 2006).
(ii) The sterically least hindered prim-OH group of sugars can be selectively oxidised by copper-containing galactose oxidase (Scheme 12). Relative activities were measured in relation to the reactivity of the C6-hydroxy group of d-galactose as the canonical substrate. The most prominent galactose oxidase from Fusarium converted d-galactose containing substrates d-lactose (10 % conv.), lactitol (20 % conv.), lactobionic acid and the synthetic disaccharide and laxativum d-lactulose completely (Table 7, entries 8, 10 and 17) (Siebum et al. 2006). For substrate acceptance of GOase, the axial position of the C4 position is crucial. The di-sugars d-melibiose, d-raffinose and d-stachyose were good substrates for galactose oxidase (83 % rel. activity for d-melibiose, up to 161 % rel. activity for d-stachyose) (Table 7, entries 14–16) (Mendonca and Zancan 1987). For d-fructose (Table 7, entry 7), a GOase mutant from Fusarium seems to be an appropriate biocatalyst (Deacon et al. 2004). Recently, a FAD-containing hexose oxidase was discovered. The so-called Dbv29 oxidised a glycopeptide at C6 to the corresponding carboxylic acid in a two-step reaction (Li et al. 2007; Liu et al. 2011).
(iii) d-Glucose (Table 7, entry 1) was also oxidised by the flavoenzyme pyranose oxidase (P2O) [EC 1.1.3.10] (Giffhorn 2000), which was obtained from several fungi (Peniophora sp., Trametes sp., Tricholoma matsutake and Gloeophyllum sepiarium). It oxidises hydroxyl groups on the C2 position, but also oxidation at C3 can occur (Scheme 12) (Kujawa et al. 2006). The process based on C2 oxidation of d-glucose followed by catalytic hydrogenation yielding d-fructose is known as ‘Cetus process’, which was also utilised for the synthesis of d-tagatose (Geigert et al. 1983; Freimund et al. 1996). d-Galactose was a rather poor substrate for pyranose oxidase from P. gigantea (Table 7, entry 2) (Freimund et al. 1998; Cook and Thygesen 2003; Bastian et al. 2005). Furthermore, the configuration on C4 played an important role in substrate acceptance. d-Allose (94 % overall yield), d-xylose (100 % overall yield) and d-mannose (only moderate rel. activity of 23 %) were all oxidised by pyranose oxidase originating from several microorganisms (Table 7, entries 3, 4 and 6) (Danneel et al. 1993; Freimund et al. 1998; Takakura and Kuwata 2003; Bannwarth et al. 2006; Machida and Nakanishi 1984). Pyranose oxidase accepted the di-sugars d-trehalose (54 % rel. activity), d-gentiobiose (1 % conversion) and d-maltose (8–56 % rel. activity) as substrates (Table 7, entries 9, 13 and 19) (Danneel et al. 1993; Freimund et al. 1998; Takakura and Kuwata 2003). Moreover, P2O was used as a biocatalyst for the C2 oxidation of disaccharides to obtain 2-keto-aldopyranose intermediates (Leitner et al. 2001) and the di-sugar d-sucrose (Table 7, entry 11) was fully converted by P2O in a multistep process (Seto et al. 2008).
Deoxy sugars were often employed in kinetic studies to investigate the catalytic mechanism of enzymes. 1-, 2-, 3- and 6-deoxy-d-glucose and 2-deoxy-d-galactose (Table 7, entries 20–24) were used for this purpose showing full conversions. The enzymes exhibited their expected regioselectivity. For pyranose oxidase, activity was observed for 2-deoxy-d-glucose (52 % rel. activity) for oxidation at C3 (Table 7, entry 21). 1-Deoxy-d-glucose (Table 7, entry 20) was converted by pyranose oxidase (8 % rel. conversion P2O from Phanerochaete gigantea, 22 % from Trametes versicolor and 69 % from T. matsutake). The substrate 3-deoxy-d-glucose was almost as good for pyranose oxidase as the natural one, but 6-deoxy-d-glucose showed significantly diminished relative conversion rate of 15 % (Table 7, entries 22 and 23). Glucose oxidase also shows activity for 2-deoxy-d-glucose and 6-deoxy-d-glucose (Table 7, entries 21 and 23). Galactose oxidase showed 74 % relative activity for 2-deoxy-d-galactose (Table 7, entry 24) (Danneel et al. 1993; Freimund et al. 1998; Takakura and Kuwata 2003; Leskovac et al. 2005; Siebum et al. 2006; Masuda-Nishimura et al. 1999).
In addition, various sugar derivatives were tested: 4-O-Methylated sugars were accepted by pyranose oxidase and galactose oxidase (Schoevaart and Kieboom 2004). With pyranose oxidase, oxidation occurred at C3. Phenyl- and hexyl-glucosides were well accepted, but underwent a glycosyl transfer reaction forming a disaccharide (Table 7, entry 28). These bulky substrates indicate that the size of the active site is not a limiting factor. Nitro sugars were tested with pyranose oxidase, and glycosyl transfer occurred yielding a 4:1 ratio of 1-6 vs. 1-3 di-sugar at C2 position in 15 % overall yield. At C4 position, a 2:1 mixture of 1-6 vs. 1-3 di-sugar was obtained in 24 % yield. α-d-Glucosyl fluoride (Table 7, entry 31) was a moderate substrate for pyranose oxidase from P. gigantea (40 % yield, Danneel et al. 1993; Freimund et al. 1998). Pyranose oxidase also converted the unnaturall-sugar l-sorbose completely (Table 7 entry 34). Mono- and poly-fluorinated galactose analogues were oxidised by galactose oxidase (Table 7, entry 33) (Ioannou et al. 2011), and also hydroxyacetone derivatives represented excellent substrates. Dihydroxyacetone (Table 7, entry 36) was also oxidised at a fair rate by glycerol oxidase (GlycOx) from Aspergillus japonicus (59 % rel. activity) (Uwajima and Terada 1980). Furthermore, galactose oxidase was active on guaran, a galactomannan (Table 7, entry 38) (47 % rel. activity) (Mendonca and Zancan 1987). This enzyme was also applied for the oxidation of the nucleotide sugars uridine 5′-diphospho-α-d-galactose and uridine 5′-diphospho-N-acetyl-α-d-galactosamine (Table 7, entry 39) for subsequent biotinylation (Bülter et al. 2001; Namdjou et al. 2007).
Sugar alcohols and amino sugar alcohols
Several enzymes were reported to oxidise sugar alcohols to the corresponding aldoses, and in case of flavoprotein oxidases, aldonic acids were obtained via over-oxidation. FAD-containing alditol oxidase (AldO) [EC 1.1.3.41] has shown a broad acceptance for sugar alcohols: AldO from Streptomyces sp. and thermophilic A. cellulolyticus acted on several d- and even l-sugar alcohols (Table 8) and oxidised them to the corresponding aldoses or even further to carboxylic acids. d-Galactitol, d-xylitol, d-sorbitol, d-mannitol, l-threitol and prochiral glycerol (Table 8, entries 1–5, 9) were tested as substrates in kinetic studies (Heuts et al. 2007b; Forneris et al. 2008; Van Hellemond et al. 2009; Murooka and Yamashita 2001; Drueckhammer et al. 1991; Yamashita et al. 2000). Glycerol was oxidised to l-glyceraldehyde as a building block for a follow-up aldolase reaction in a multienzyme cascade (Franke et al. 2003). The latter is also oxidised by the Cu-containing glycerol oxidase which exhibited excellent activity towards glycerol, which was selected as a name-giving substrate (Uwajima and Terada 1980; Uwajima et al. 1984). The building block dihydroxyacetone phosphate (DHAP), which is a popular C donor in asymmetric aldol reactions, can be obtained using flavoprotein glycerol 3-phosphate oxidase (GPO) [EC 1.1.3.21] for the oxidation of l-glycerol 3-phosphate (Table 8, entry 10) at the sec-OH (Babich et al. 2011). Furthermore, also copper-containing galactose oxidase from Fusarium exhibited a broad acceptance of sugar alcohols without acid formation (Table 8, entries 1, 2, 5, 6 and 8).
Table 8.
Sugar alcohols and amino sugars

aCopper containing
bFlavin containing
For the oxidation of amino sugars, N-acyl-d-hexosamine oxidase [EC 1.1.3.29] from Pseudomonas sp. is the enzyme of choice, although also galactose oxidase showed activities on this substrate class (Mendonca and Zancan 1987; Takahashi and Kawamura 2000). N-Acetyl-d-galactosamine (Table 8, entry 12) was converted almost as fast as the natural substrate (98–99 % rel. activity) by N-acyl-d-hexosamine oxidase. It seems that (in contrast to other enzymes) the configuration of C4 is not relevant for substrate acceptance of N-acyl-d-hexosamine oxidase. Amino sugars without N-acyl function, such as d-glucosamine (26 % rel. activity) and d-galactosamine (81 % rel. activity), were moderate substrates (Table 8, entry 11), like N,N′-diacetylchitobiose (31–49 % rel. activity) and N-acetylmuramic acid (44 % rel. activity) (Table 8, entry 14) with respect to the natural substrate N-acetyl-d-glucosamine (Horiuchi 1989; Takahashi and Kawamura 2000). The diamino sugar N,N′-diacetyllactosamine and oligomers thereof were successfully oxidised by galactose oxidase (Kupper et al. 2012). Another enzyme which was found to be active on C1 of N-acetyl-d-glucosamine and its oligomers N,N′-diacetylchitobiose, N,N′,N″-triacetylchitotriose and N,N′,N″,N‴-tetraacetylchitotetraose (Table 8, entry 16) is chitooligosaccharide oxidase (ChitO) (Heuts et al. 2007a) (Table 9).
Table 9.
Cofactor presence, substrate scope and propensity for over-oxidation of alcohol oxidases
| Enzyme | Cofactor | Substrate (major activities) | Over-oxidation |
|---|---|---|---|
| Alditol oxidase (AldOx) | FAD | Primary alcohols, sugar alcohols | Yes |
| Aryl alcohol oxidase (AAO) | FAD | Benzylic alcohols, allylic alcohols | Yes |
| Chitooligosaccharide oxidase (ChitO) | FAD | Sugars | No |
| Cholesterol oxidase (ChOx) | FAD | Sterols, allylic alcohols | No |
| Choline Oxidase (CHO) | FAD | Amino alcohols | Yes |
| Galactose oxidase (GOase) | Cu2+ | Benzylic alcohols, sugars | No |
| Glucooligosaccharide oxidase (GOO) | FAD | Sugars | No |
| Glucose oxidase (GOX) | FAD | Sugars | No |
| Glycerol Oxidase (GlycOx) | Cu2+ | Sugar alcohols | No |
| Glycerol 3-phosphate oxidase (GPO) | FAD | Secondary alcohols | No |
| Glycolate oxidase (GlyO) | FMN | α-Hydroxy acids | No |
| Hexose oxidase (HOX) | FAD | Sugars | No |
| Hydroxymethylfurfural oxidase (HMFO) | FAD | Benzylic alcohols, allylic alcohols | Yes |
| (S)-2-Hydroxy acid oxidase (HAOX) | FMN | α-Hydroxy acids | No |
| Isoamyl alcohol oxidase (IAO) | FAD | Branched aliphatic alcohols | Yes |
| L-lactate oxidase (LLO) | FMN | α-Hydroxy acids | No |
| Lactose oxidase (LAO) | FAD | Sugars | No |
| Long-chain alcohol oxidase (LCAO) | FAD | Aliphatic alcohols | No |
| Secondary alcohol oxidase (SAO) | Fe2+ | Secondary aliphatic alcohols | No |
| Short-chain alcohol oxidase (SCAO) | FAD | Aliphatic alcohols | Yes |
| Pyranose oxidase (P2O) | FAD | Sugars | No |
| Vanillyl alcohol oxidase (VAO) | FAD | Benzylic alcohols | Yes |
Summary and outlook
The broad substrate scope coupled with high regio- and stereoselectivity makes alcohol oxidases a fantastic tool for the oxidation of primary and secondary alcohols using molecular oxygen as an alternative to traditional chemical methods. Owing to their mechanism, copper-depending oxidases selectively yield aldehydes from primary alcohols, while over-oxidation to furnish carboxylic acids may take place to a varying degree with flavin-depending oxidases. For a broad range of alcohols—non-activated prim- and sec-alcohols, activated allylic, cinnamic and benzylic alcohols, hydroxy acids, hydroxy steroids, carbohydrates and derivatives thereof—alcohol oxidases are available from various microbial sources, which are reviewed with respect to their substrate tolerance to facilitate the choice of the optimal enzyme for a given alcohol substrate.
Electronic supplementary material
(PDF 32 kb)
Acknowledgments
Funding by the Austrian Science Fund (FWF), within the DK Molecular Enzymology (project W9), an Erwin-Schrödinger fellowship (J3466) and the Austrian BMWFW, BMVIT, SFG, Standortagentur Tirol, Government of Lower Austria and ZIT through the Austrian FFG-COMET-Funding Program is gratefully acknowledged.
Conflict of interest
The authors declare that they have no competing interests.
References
- Adam W, Lazarus M, Boss B, Saha-Möller CR, Humpf H-U, Schreier P. Enzymatic resolution of chiral 2-hydroxy carboxylic acids by enantioselective oxidation with molecular oxygen catalyzed by the glycolate oxidase from spinach (Spinacia oleracea) J Org Chem. 1997;62:7841–7843. [Google Scholar]
- Adam W, Lazarus M, Saha-Möller CR, Schreier P. Quantitative transformation of racemic 2-hydroxy acids into (R)-2-hydroxy acids by enantioselective oxidation with glycolate oxidase and subsequent reduction of 2-keto acids with D-lactate dehydrogenase. Tetrahedron Asymmetry. 1998;9:351–355. [Google Scholar]
- Alexander DL, Fisher JF. A convenient synthesis of 7α-hydroxycholest-4-en-3-one by the hydroxypropyl- 3-cyclodextrin-facilitated cholesterol oxidase oxidation of 3β,7α-cholest-5-ene-3,7-diol. Steroids. 1995;60:290–294. doi: 10.1016/0039-128x(95)93851-o. [DOI] [PubMed] [Google Scholar]
- Babich L, Van Hemert LJC, Bury A, Hartog AF, Falcicchio P, Van der Oost J, Van Herk T, Wever R, Rutjes FPJT. Synthesis of non-natural carbohydrates from glycerol and aldehydes in a one-pot four-enzyme cascade reaction. Green Chem. 2011;13:2895–2900. [Google Scholar]
- Bannwarth M, Bastian S, Giffhorn F, Schulz GE. Reaction geometry and thermostable variant of pyranose 2-oxidase from the white-rot fungus Peniophora sp. Biochemistry. 2006;45:6587–6595. doi: 10.1021/bi052465d. [DOI] [PubMed] [Google Scholar]
- Baron AJ, Stevens C, Wilmot C, Seneviratne KD, Blakeley V, Dooley DM, Phillips SEV, Knowles PF, McPherson MJ. Structure and mechanism of galactose oxidase: the free radical site. J Biol Chem. 1994;269:25095–25105. [PubMed] [Google Scholar]
- Bastian S, Rekowski MJ, Witte K, Heckmann-Pohl DM, Giffhorn F. Engineering of pyranose 2-oxidase from Peniophora gigantea towards improved thermostability and catalytic efficiency. Appl Microbiol Biotechnol. 2005;67:654–663. doi: 10.1007/s00253-004-1813-1. [DOI] [PubMed] [Google Scholar]
- Biellmann J. Resolution of alcohols by cholesterol oxidase from Rhodococcus erythropolis: lack of enantiospecificity for the steroids. Chirality. 2001;13:34–39. doi: 10.1002/1520-636X(2001)13:1<34::AID-CHIR7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Blanchard M, Green DE, Nocito-Carroll V, Ratner S. L-Hydroxy acid oxidase. J Biol Chem. 1946;163:137–144. [PubMed] [Google Scholar]
- Bülter T, Schumacher T, Namdjou D, Gallego R, Clausen H, Elling L, Gutie R. Chemoenzymatic synthesis of biotinylated nucleotide sugars as substrates for glycosyltransferases. ChemBioChem. 2001;2:884–894. doi: 10.1002/1439-7633(20011203)2:12<884::AID-CBIC884>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Chaiyen P, Fraaije MW, Mattevi A. The enigmatic reaction of flavins with oxygen. Trends Biochem Sci. 2012;37:373–380. doi: 10.1016/j.tibs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Liu H-T, Bombelli P, Smith A, Slabas AR. Functional identification of AtFao3, a membrane bound long chain alcohol oxidase in Arabidopsis thaliana. FEBS Lett. 2004;574:62–68. doi: 10.1016/j.febslet.2004.07.086. [DOI] [PubMed] [Google Scholar]
- Clark DS, Geresh S, DiCosimo R. Enantioselective oxidation of 2-methyl-l-alkanols by alcohol oxidase from methylotrophic yeasts. Bioorg Med Chem Lett. 1995;5:1383–1388. [Google Scholar]
- Cook MW, Thygesen HV. Safety evaluation of a hexose oxidase expressed in Hansenula polymorpha. Food Chem Toxicol. 2003;41:523–529. doi: 10.1016/s0278-6915(02)00280-6. [DOI] [PubMed] [Google Scholar]
- Couderc R, Baratti J. Oxidation of methanol by the yeast, Pichia pastoris. purification and properties of the alcohol oxidase. Agric Biol Chem. 1980;44:2278–2289. [Google Scholar]
- Danneel H-J, Rössner E, Axel Z, Giffhorn F. Purification and characterization of a pyranose oxidase from the basidiomycete Peniophora gigantea and chemical analyses of its reaction products. Eur J Biochem. 1993;214:795–802. doi: 10.1111/j.1432-1033.1993.tb17982.x. [DOI] [PubMed] [Google Scholar]
- Das S, Glenn JH, Subramanian M. Enantioselective oxidation of 2-hydroxycarboxylic acids by glycolate oxidase and catalase coexpressed in methylotrophic Pichia pastoris. Biotechnol Prog. 2009;26:607–615. doi: 10.1002/btpr.363. [DOI] [PubMed] [Google Scholar]
- De Jong E, Van Berkel WJH, Van der Zwan R, de Bont JAM. Purification and characterization of vanillyl-alcohol oxidase from Penicillium simplicissimum. Eur J Biochem. 1992;208:651–657. doi: 10.1111/j.1432-1033.1992.tb17231.x. [DOI] [PubMed] [Google Scholar]
- Deacon SE, Mahmoud K, Spooner RK, Firbank SJ, Knowles PF, Phillips SEV, McPherson MJ. Enhanced fructose oxidase activity in a galactose oxidase variant. ChemBioChem. 2004;5:972–979. doi: 10.1002/cbic.200300810. [DOI] [PubMed] [Google Scholar]
- Dieth S, Tritsch D, Biellmann J-F. Resolution of allylic alcohols by cholesterol oxidase isolated from Rhodococcus erythropolis. Tetrahedron Lett. 1995;36:2243–2246. [Google Scholar]
- Dijkman WP, Fraaije MW. Discovery and characterization of a 5-hydroxymethylfurfural oxidase from Methylovorus sp. strain MP688. Appl Environ Microbiol. 2014;80:1082–1090. doi: 10.1128/AEM.03740-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkman WP, de Gonzalo G, Mattevi A, Fraaije MW. Flavoprotein oxidases: classification and applications. Appl Microbiol Biotechnol. 2013;97:5177–5188. doi: 10.1007/s00253-013-4925-7. [DOI] [PubMed] [Google Scholar]
- Dijkman WP, Binda C, Fraaije MW, Mattevi A. Structure-based enzyme tailoring of 5-hydroxymethylfurfural oxidase. ACS Catal. 2015;5:1833–1839. [Google Scholar]
- Drueckhammer DG, Hennen WJ, Pederson RL, Barbas ICF, Gautheron CM, Krach T, Wong C-H. Enzyme catalysis in synthetic carbohydrate chemistry. Synthesis. 1991;1991:499–525. [Google Scholar]
- Duncan D, Wallis JO, Torrey N, Azari MR. Purification and properties of Aerococcus viridans lactate oxidase. Biochem Biophys Res Commun. 1989;164:919–926. doi: 10.1016/0006-291x(89)91546-5. [DOI] [PubMed] [Google Scholar]
- Eirich LD, Craft DL, Steinberg L, Asif A, Eschenfeldt WH, Stols L, Donnelly MI, Wilson CR. Cloning and characterization of three fatty alcohol oxidase genes from Candida tropicalis strain ATCC 20336. Appl Environ Microbiol. 2004;70:4872–4879. doi: 10.1128/AEM.70.8.4872-4879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalettes F, Turner NJ. Directed evolution of galactose oxidase: generation of enantioselective secondary alcohol oxidases. ChemBioChem. 2008;9:857–860. doi: 10.1002/cbic.200700689. [DOI] [PubMed] [Google Scholar]
- Ferrari AR, Lee M, Fraaije MW. Expanding the substrate scope of chitooligosaccharide oxidase from Fusarium graminearum by structure-inspired mutagenesis. Biotechnol Bioeng. 2015;112:1074–1080. doi: 10.1002/bit.25532. [DOI] [PubMed] [Google Scholar]
- Ferreira P, Medina M, Guillen F, Martinez MJ, Van Berkel WJH, Martinez AT. Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem J. 2005;389:731–738. doi: 10.1042/BJ20041903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P, Hernandez-Ortega A, Herguedas B, Rencoret J, Gutierrez A, Martinez MJ, Jimenez-Barbero J, Medina M, Martinez AT. Kinetic and chemical characterization of aldehyde oxidation by fungal aryl-alcohol oxidase. Biochem J. 2010;425:585–593. doi: 10.1042/BJ20091499. [DOI] [PubMed] [Google Scholar]
- Fetzner S, Steiner RA. Cofactor-independent oxidases and oxygenases. Appl Microbiol Biotechnol. 2010;86:791–804. doi: 10.1007/s00253-010-2455-0. [DOI] [PubMed] [Google Scholar]
- Forneris F, Heuts DPHM, Delvecchio M, Rovida S, Fraaije MW, Mattevi A. Structural analysis of the catalytic mechanism and stereoselectivity in Streptomyces coelicolor alditol oxidase. Biochemistry. 2008;47:978–985. doi: 10.1021/bi701886t. [DOI] [PubMed] [Google Scholar]
- Fraaije MW, Van Berkel WJH, Benen JAE, Visser J, Mattevi A. A novel oxidoreductase family sharing a conserved FAD-binding domain. Trends Biochem Sci. 1998;23:206–207. doi: 10.1016/s0968-0004(98)01210-9. [DOI] [PubMed] [Google Scholar]
- Fraaije MW, Van den Heuvel RH, Roelofs JC, Van Berkel WJH. Kinetic mechanism of vanillyl-alcohol oxidase with short-chain 4-alkylphenols. Eur J Biochem. 1998;253:712–719. doi: 10.1046/j.1432-1327.1998.2530712.x. [DOI] [PubMed] [Google Scholar]
- Franke D, Machajewski T, Hsu C-C, Wong C-H. One-pot synthesis of L-fructose using coupled multienzyme systems based on rhamnulose-1-phosphate aldolase. J Org Chem. 2003;68:6828–6831. doi: 10.1021/jo030021m. [DOI] [PubMed] [Google Scholar]
- Freimund S, Huwig A, Giffhorn F, Köpper S. Convenient chemo-enzymatic synthesis of d-tagatose. J Carbohydr Chem. 1996;15:115–120. [Google Scholar]
- Freimund S, Huwig A, Giffhorn F, Köpper S. Rare keto-aldoses from enzymatic oxidation: substrates and oxidation products of pyranose 2-oxidase. Chem Eur J. 1998;4:2442–2455. [Google Scholar]
- Fuchs M, Tauber K, Sattler J, Lechner H, Pfeffer J, Kroutil W, Faber K. Amination of benzylic and cinnamic alcohols via a biocatalytic, aerobic, oxidation–transamination cascade. RSC Adv. 2012;2:6262–6265. [Google Scholar]
- Fujishiro K, Uchida H, Shimokawa K, Nakano M, Sano F, Ohta T, Kayahara N, Aisaka K, Uwajima T. Purification and properties of a new Brevibacterium sterolicum cholesterol oxidase produced by E. coli MM294/pnH10. FEMS Microbiol Lett. 2002;215:243–248. doi: 10.1111/j.1574-6968.2002.tb11397.x. [DOI] [PubMed] [Google Scholar]
- Gadda G. Oxygen activation in flavoprotein oxidases: the importance of being positive. Biochemistry. 2012;51:2662–2669. doi: 10.1021/bi300227d. [DOI] [PubMed] [Google Scholar]
- Gadda G, Powell NLN, Menon P. The trimethylammonium headgroup of choline is a major determinant for substrate binding and specificity in choline oxidase. Arch Biochem Biophys. 2004;430:264–273. doi: 10.1016/j.abb.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Gadda G, Wels G, Pollegioni L, Zucchelli S, Ambrosius D, Pilone MS, Ghisla S (1997) Characterization of cholesterol oxidase from Streptomyces hygroscopicus and Brevibacterium sterolicum. Eur J Biochem 250:369–376 [DOI] [PubMed]
- Gao C, Qiu J, Li J, Ma C, Tang H, Xu P. Enantioselective oxidation of racemic lactic acid to D-lactic acid and pyruvic acid by Pseudomonas stutzeri SDM. Bioresour Technol. 2009;100:1878–1880. doi: 10.1016/j.biortech.2008.09.053. [DOI] [PubMed] [Google Scholar]
- Geigert J, Neidleman SL, Hirano DS. Convenient, laboratory procedure for reducing D-glucosone to D-fructose. Carbohydr Res. 1983;113:159–162. [Google Scholar]
- Giffhorn F. Fungal pyranose oxidases: occurrence, properties and biotechnical applications in carbohydrate chemistry. Appl Microbiol Biotechnol. 2000;54:727–740. doi: 10.1007/s002530000446. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Thematic minireview series: metals in biology 2013. J Biol Chem. 2013;288:13164. doi: 10.1074/jbc.R113.467712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen F, Martinez AT, Martinez MJ. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem. 1992;209:603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ortega A, Lucas F, Ferreira P, Medina M, Guallar V, Martinez AT. Modulating O2 reactivity in a fungal flavoenzyme: involvement of aryl-alcohol oxidase Phe-501 contiguous to catalytic histidine. J Biol Chem. 2011;286:41105–41114. doi: 10.1074/jbc.M111.282467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ortega A, Ferreira P, Martinez AT. Fungal aryl-alcohol oxidase: a peroxide-producing flavoenzyme involved in lignin degradation. Appl Microbiol Biotechnol. 2012;93:1395–1410. doi: 10.1007/s00253-011-3836-8. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ortega A, Ferreira P, Merino P, Medina M, Guallar V, Martinez AT. Stereoselective hydride transfer by aryl-alcohol oxidase, a member of the GMC superfamily. ChemBioChem. 2012;13:427–435. doi: 10.1002/cbic.201100709. [DOI] [PubMed] [Google Scholar]
- Heuts DPHM, Janssen DB, Fraaije MW. Changing the substrate specificity of a chitooligosaccharide oxidase from Fusarium graminearum by model-inspired site-directed mutagenesis. FEBS Lett. 2007;581:4905–4909. doi: 10.1016/j.febslet.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Heuts DPHM, Van Hellemond EW, Janssen DB, Fraaije MW. Discovery, characterization, and kinetic analysis of an alditol oxidase from Streptomyces coelicolor. J Biol Chem. 2007;282:20283–20291. doi: 10.1074/jbc.M610849200. [DOI] [PubMed] [Google Scholar]
- Hollmann F, Arends IWCE, Buehler K, Schallmey A, Bühler B. Enzyme-mediated oxidations for the chemist. Green Chem. 2011;13:226–265. [Google Scholar]
- Horiuchi T. Purification and properties of N-Acyl-D-hexosamine oxidase from Pseudomonas sp. 15-1. Agric Biol Chem. 1989;53:361–368. [Google Scholar]
- Huang C-H, Lai W-L, Lee M-H, Chen C-J, Vasella A, Tsai Y-C, Liaw S-H (2005) Crystal structure of glucooligosaccharide oxidase from Acremonium strictum: a novel flavinylation of 6-S-cysteinyl, 8alpha-N1-histidyl FAD. J Biol Chem 280:38831–38838 [DOI] [PubMed]
- Ikuta S, Imamura S, Misaki H, Horiuti Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J Biochem. 1977;82:1741–1749. doi: 10.1093/oxfordjournals.jbchem.a131872. [DOI] [PubMed] [Google Scholar]
- Ioannou A, Cini E, Timofte RS, Flitsch SL, Turner NJ, Linclau B. Heavily fluorinated carbohydrates as enzyme substrates: oxidation of tetrafluorinated galactose by galactose oxidase. Chem Commun. 2011;47:11228–11230. doi: 10.1039/c1cc13956h. [DOI] [PubMed] [Google Scholar]
- Ito N, Phillips SEV, Stevens C, Ogel ZB, McPherson MJ, Keen JN, Yadav KDS, Knowles PF. Novel thioether bond revealed by a 1.7 Å crystal structure of galactose oxidase. Nature. 1991;350:87–90. doi: 10.1038/350087a0. [DOI] [PubMed] [Google Scholar]
- Kass IJ, Sampson NS. The isomerization catalyzed by Brevibacterium sterolicum cholesterol oxidase proceeds stereospecifically with one base. Biochem Biophys Res Commun. 1995;206:688–693. doi: 10.1006/bbrc.1995.1097. [DOI] [PubMed] [Google Scholar]
- Kato N, Omori Y, Tani Y, Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Eur J Biochem. 1976;350:341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Kawagoshi Y, Fujita M. Purification and properties of polyvinyl alcohol oxidase with broad substrate range obtained from Pseudomonas vesicularis var. povalolyticus PH. World J Microbiol Biotechnol. 1997;13:273–277. [Google Scholar]
- Kemp GD, Dickinson FM, Ratledge C. Inducible long chain alcohol oxidase from alkane-grown Candida tropicalis. Appl Microbiol Biotechnol. 1988;29:370–374. [Google Scholar]
- Kiess M, Hecht HJ, Kalisz HM. Glucose oxidase from Penicillium amagasakiense. Primary structure and comparison with other glucose-methanol-choline (GMC) oxidoreductases. Eur J Biochem. 1998;252:90–99. doi: 10.1046/j.1432-1327.1998.2520090.x. [DOI] [PubMed] [Google Scholar]
- Kjellander M, Go K, Liljeruhm J, Boman M, Johansson G. Steady-state generation of hydrogen peroxide: kinetics and stability of alcohol oxidase immobilized on nanoporous alumina. Biotechnol Lett. 2013;35:585–590. doi: 10.1007/s10529-012-1110-5. [DOI] [PubMed] [Google Scholar]
- Klibanov AM, Alberti BN, Marletta MA. Stereospecific oxidation of aliphatic alcohols catalyzed by galactose oxidase. Biochem Biophys Res Commun. 1982;108:804–808. doi: 10.1016/0006-291x(82)90900-7. [DOI] [PubMed] [Google Scholar]
- Ko H-S, Yokoyama Y, Ohno N, Okadome M, Amachi S, Shinoyama H, Fujii T. Purification and characterization of intracellular and extracellular, thermostable and alkali-tolerant alcohol oxidases produced by a thermophilic fungus, Thermoascus aurantiacus NBRC 31693. J Biosci Bioeng. 2005;99:348–353. doi: 10.1263/jbb.99.348. [DOI] [PubMed] [Google Scholar]
- Kujawa M, Ebner H, Leitner C, Hallberg BM, Prongjit M, Sucharitakul J, Ludwig R, Rudsander U, Peterbauer C, Chaiyen P, Haltrich D, Divne C. Structural basis for substrate binding and regioselective oxidation of monosaccharides at C3 by pyranose 2-oxidase. J Biol Chem. 2006;281:35104–35115. doi: 10.1074/jbc.M604718200. [DOI] [PubMed] [Google Scholar]
- Kumar AK, Goswami P. Functional characterization of alcohol oxidases from Aspergillus terreus MTCC 6324. Appl Microbiol Biotechnol. 2006;72:906–911. doi: 10.1007/s00253-006-0381-y. [DOI] [PubMed] [Google Scholar]
- Kumar AK, Goswami P. Purification and properties of a novel broad substrate specific alcohol oxidase from Aspergillus terreus MTCC 6324. Biochim Biophys Acta. 2008;1784:1552–1559. doi: 10.1016/j.bbapap.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Kumar AK, Goswami P. Dissociation and reconstitution studies of a broad substrate specific multimeric alcohol oxidase protein produced by Aspergillus terreus. J Biochem. 2009;145:259–265. doi: 10.1093/jb/mvn163. [DOI] [PubMed] [Google Scholar]
- Kumar VV, Rapheal VS. Induction and purification by three-phase partitioning of aryl alcohol oxidase (AAO) from Pleurotus ostreatus. Appl Biochem Biotechnol. 2011;163:423–432. doi: 10.1007/s12010-010-9050-9. [DOI] [PubMed] [Google Scholar]
- Kupper CE, Rosencrantz RR, Henßen B, Pelantova H, Thönes S, Drozdova A, Kren V, Elling L. Chemo-enzymatic modification of poly-N-acetyllactosamine (LacNAc) oligomers and N, N-diacetyllactosamine (LacDiNAc) based on galactose oxidase treatment. J Org Chem. 2012;8:712–725. doi: 10.3762/bjoc.8.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaree D, Hoyte RM, Hochberg RB. A direct stereoselective synthesis of 7β-hydroxytestosterone. Steroids. 1997;62:482–486. doi: 10.1016/s0039-128x(97)00018-4. [DOI] [PubMed] [Google Scholar]
- Leferink NGH, Heuts DPHM, Fraaije MW, Van Berkel WJH. The growing VAO flavoprotein family. Arch Biochem Biophys. 2008;474:292–301. doi: 10.1016/j.abb.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Leitner C, Mayr P, Riva S, Volc J, Kulbe KD, Nidetzky B, Haltrich D. Enzymatic redox isomerization of 1,6-disaccharides by pyranose oxidase and NADH-dependent aldose reductase. J Mol Catal B Enzym. 2001;11:407–414. [Google Scholar]
- Leskovac V, Trivić S, Wohlfahrt G, Kandrac J, Pericin D. Glucose oxidase from Aspergillus niger: the mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int J Biochem Cell Biol. 2005;37:731–750. doi: 10.1016/j.biocel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Li Y-S, Ho J-Y, Huang C-C, Lyu S-Y, Lee C-Y, Huang Y-T, Wu C-J, Chan H-C, Huang C-J, Hsu N-S, Tsai M-D, Li T-L. A unique flavin mononucleotide-linked primary alcohol oxidase for glycopeptide A40926 maturation. J Am Chem Soc. 2007;129:13384–13385. doi: 10.1021/ja075748x. [DOI] [PubMed] [Google Scholar]
- Liu Y-C, Li Y-S, Lyu S-Y, Hsu L-J, Chen Y-H, Huang Y-T, Chan H-C, Huang C-J, Chen G-H, Chou C-C, Tsai M-D, Li T-L. Interception of teicoplanin oxidation intermediates yields new antimicrobial scaffolds. Nat Chem Biol. 2011;7:304–309. doi: 10.1038/nchembio.556. [DOI] [PubMed] [Google Scholar]
- Macheroux P, Kappes B, Ealick SE. Flavogenomics—a genomic and structural view of flavin-dependent proteins. FEBS J. 2011;278:2625–2634. doi: 10.1111/j.1742-4658.2011.08202.x. [DOI] [PubMed] [Google Scholar]
- Machida Y, Nakanishi T. Purification and properties of pyranose oxidase from Coriolus versicolor. Agric Biol Chem. 1984;48:2463–2470. [Google Scholar]
- Maeda-Yorita K, Aki K, Sagai H, Misaki H, Massey V. L-Lactate oxidase and L-lactate monooxygenase: mechanistic variations on a common structural theme. Biochimie. 1995;77:631–642. doi: 10.1016/0300-9084(96)88178-8. [DOI] [PubMed] [Google Scholar]
- Masuda-Nishimura I, Minamihara T, Koyama Y. Improvement in thermal stability and reactivity of pyranose oxidase from Coriolus versicolor by random mutagenesis. Biotechnol Lett. 1999;21:203–207. [Google Scholar]
- Mattevi A. To be or not to be an oxidase: challenging the oxygen reactivity of flavoenzymes. Trends Biochem Sci. 2006;31:276–283. doi: 10.1016/j.tibs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Mendonca MH, Zancan GT. Purification and characterization of intracellular oxidase from Dactylium dendroides. Arch Biochem Biophys. 1987;252:507–514. doi: 10.1016/0003-9861(87)90058-0. [DOI] [PubMed] [Google Scholar]
- Menon V, Hsieh CT, Fitzpatrick PF. Substituted alcohols as mechanistic probes of alcohol oxidase. Bioorg Chem. 1995;23:42–53. [Google Scholar]
- Monti D, Ottolina G, Carrea G, Riva S. Redox reactions catalyzed by isolated enzymes. Chem Rev. 2011;111:4111–4140. doi: 10.1021/cr100334x. [DOI] [PubMed] [Google Scholar]
- Murooka Y, Yamashita M. Genetic and protein engineering of diagnostic enzymes, cholesterol oxidase and xylitol oxidase. J Biosci Bioeng. 2001;91:433–441. doi: 10.1263/jbb.91.433. [DOI] [PubMed] [Google Scholar]
- Nakamura BS, Ogura Y. Action mechanism of glucose oxidase of Aspergillus niger. J Biochem. 1968;63:308–316. [PubMed] [Google Scholar]
- Namdjou D-J, Sauerzapfe B, Schmiedel J, Dräger G, Bernatchez S, Wakarchuk WW, Elling L. Combination of UDP-Glc(NAc) 4′-epimerase and galactose oxidase in a one-pot synthesis of biotinylated nucleotide sugars. Adv Synth Catal. 2007;349:314–318. [Google Scholar]
- Omura K, Swern D. Oxidation of alcohols by “activated” dimethyl sulfoxide. A preparative, steric and mechanistic study. Tetrahedron. 1978;34:1651–1660. [Google Scholar]
- Perez-Sanchez M, Müller CR, Dominguez de Maria P. Multistep oxidase-lyase reactions: synthesis of optically active 2-hydroxyketones by using biobased aliphatic alcohols. ChemCatChem. 2013;5:2512–2516. [Google Scholar]
- Pezzotti F, Therisod M. Enzymatic synthesis of aldonic acids. Carbohydr Res. 2006;341:2290–2292. doi: 10.1016/j.carres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Pfitzner KE, Moffatt JG. A new and selective oxidation of alcohols. J Am Chem Soc. 1963;85:3027–3028. [Google Scholar]
- Pollegioni L, Wels G, Pilone MS, Ghisla S. Kinetic mechanisms of cholesterol oxidase from Streptomyces hygroscopicus and Brevibacterium sterolicum. Eur J Biochem. 1999;264:140–151. doi: 10.1046/j.1432-1327.1999.00586.x. [DOI] [PubMed] [Google Scholar]
- Pollegioni L, Piubelli L, Molla G. Cholesterol oxidase: biotechnological applications. FEBS J. 2009;276:6857–6870. doi: 10.1111/j.1742-4658.2009.07379.x. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Hostrup PB. Purification and characterization of a hexose oxidase with excellent strengthening effects in bread. Cereal Chem J. 1998;75:51–57. [Google Scholar]
- Rand T, Qvist KB, Walter CP, Poulsen CH. Characterization of the flavin association in hexose oxidase from Chondrus crispus. FEBS J. 2006;273:2693–2703. doi: 10.1111/j.1742-4658.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- Richardson KE, Tolbert NE. Oxidation of glyoxylic acid to oxalic acid by glycolic acid oxidase. J Biol Chem. 1961;236:1280–1284. [PubMed] [Google Scholar]
- Ridge PG, Zhang Y, Gladyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS One. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JC, Keay L, Molinari R, Sizer IW. Cortex L-α-hydroxy acid oxidases of hog renal cortex. J Biochem. 1962;237:2001–2010. [PubMed] [Google Scholar]
- Romero E, Ferreira P, Martínez AT, Martínez MJ. New oxidase from Bjerkandera arthroconidial anamorph that oxidizes both phenolic and nonphenolic benzyl alcohols. Biochim Biophys Acta. 2009;1794:689–697. doi: 10.1016/j.bbapap.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Rungsrisuriyachai K, Gadda G. On the role of histidine 351 in the reaction of alcohol oxidation catalyzed by choline oxidase. Biochemistry. 2008;47:6762–6769. doi: 10.1021/bi800650w. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hamada N, Watanabe Y. Purification and properties of secondary alcohol oxidase with an acidic isoelectric point. Agric Biol Chem. 1985;49:817–825. [Google Scholar]
- Savary BJ, Hicks KB, O’Connor JV. Hexose oxidase from Chondrus crispus: improved purification using perfusion chromatography. Enzym Microb Technol. 2001;29:42–51. doi: 10.1016/s0141-0229(01)00351-9. [DOI] [PubMed] [Google Scholar]
- Schoevaart R, Kieboom T. Application of galactose oxidase in chemoenzymatic one-pot cascade reactions without intermediate recovery steps. Top Catal. 2004;27:3–9. [Google Scholar]
- Schrittwieser JH, Sattler J, Resch V, Mutti FG, Kroutil W. Recent biocatalytic oxidation-reduction cascades. Curr Opin Chem Biol. 2011;15:249–256. doi: 10.1016/j.cbpa.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TJ, O’Neill BJ, Shanks BH, Dumesic JA. Bridging the chemical and biological catalysis gap: challenges and outlooks for producing sustainable chemicals. ACS Catal. 2014;4:2060–2069. [Google Scholar]
- Seto H, Kawakita H, Ohto K, Harada H, Inoue K. Novel carbonyl-group-containing dextran synthesis by pyranose-2-oxidase and dextransucrase. Carbohydr Res. 2008;343:2417–2421. doi: 10.1016/j.carres.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan S, Natalia D, Greiner L, Domínguez de María P. Oxidation-hydroxymethylation-reduction: a one-pot three-step biocatalytic synthesis of optically active α-aryl vicinal diols. Green Chem. 2012;14:94–97. [Google Scholar]
- Siebum A, Van Wijk A, Schoevaart R, Kieboom T. Galactose oxidase and alcohol oxidase: scope and limitations for the enzymatic synthesis of aldehydes. J Mol Catal B Enzym. 2006;41:141–145. [Google Scholar]
- Stenberg K, Clausen T, Lindqvist Y, Macheroux P. Involvement of Tyr24 and Trp108 in substrate binding and substrate specificity of glycolate oxidase. Eur J Biochem. 1995;228:408–416. [PubMed] [Google Scholar]
- Straathof AJJ. Transformation of biomass into commodity chemicals using enzymes or cells. Chem Rev. 2014;114:1871–1908. doi: 10.1021/cr400309c. [DOI] [PubMed] [Google Scholar]
- Sun L, Bulter T, Alcalde M, Petrounia IP, Arnold FH. Modification of galactose oxidase to introduce glucose 6-oxidase activity. ChemBioChem. 2002;3:781–783. doi: 10.1002/1439-7633(20020802)3:8<781::AID-CBIC781>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kawamura Y. Renin inhibits N-acetyl-D-glucosamine 2 epimerase (Renin-Binding Protein) J Biochem. 2000;128:951–956. doi: 10.1093/oxfordjournals.jbchem.a022846. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Kuwata S. Purification, characterization, and molecular cloning of a pyranose oxidase from the fruit body of the Basidiomycete, Tricholoma matsutake. Biosci Biotechnol Biochem. 2003;67:2598–2607. doi: 10.1271/bbb.67.2598. [DOI] [PubMed] [Google Scholar]
- Tojo G, Fernández M. Oxidation of alcohols to aldehydes and ketones: a guide to current common practice. New York: Springer; 2006. [Google Scholar]
- Toyama M, Yamashita M, Yoneda M, Zaborowski A, Nagato M, Ono H, Hirayama N, Murooka Y. Alteration of substrate specificity of cholesterol oxidase from Streptomyces sp. by site-directed mutagenesis. Protein Eng. 2002;15:477–484. doi: 10.1093/protein/15.6.477. [DOI] [PubMed] [Google Scholar]
- Turner NJ. Enantioselective oxidation of C-O and C-N bonds using oxidases. Chem Rev. 2011;111:4073–4087. doi: 10.1021/cr200111v. [DOI] [PubMed] [Google Scholar]
- Urban P, Chirat I, Lederer F. Rat kidney L-2-hydroxyacid oxidase. Structural and mechanistic comparison with flavocytochrome b2 from Baker’s yeast. Biochemistry. 1988;27:7365–7371. doi: 10.1021/bi00419a029. [DOI] [PubMed] [Google Scholar]
- Uwajima T, Terada O. Properties of new enzyme glycerol oxidase from Aspergillus japonicus AT 008. Agric Biol Chem. 1980;44:2039–2045. [Google Scholar]
- Uwajima T, Shimizu Y, Terada O. Glycerol oxidase, a novel copper hemoprotein from Aspergillus japonicus. Molecular and catalytic properties of the enzyme and its application to the analysis of serum triglycerides. J Biol Chem. 1984;259:2748–2753. [PubMed] [Google Scholar]
- Van den Heuvel RHH, Fraaije MW, Van Berkel WJH. Regio- and stereospecific conversion of 4-alkylphenols by the covalent flavoprotein vanillyl-alcohol oxidase. J Bacteriol. 1998;180:5646–5651. doi: 10.1128/jb.180.21.5646-5651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel RHH, Fraaije MW, Ferrer M, Mattevi A, Van Berkel WJH. Inversion of stereospecificity of vanillyl-alcohol oxidase. Proc Natl Acad Sci U S A. 2000;97:9455–9460. doi: 10.1073/pnas.160175897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel RHH, Fraaije MW, Laane C, Van Berkel WJH. Enzymatic synthesis of vanillin. J Agric Food Chem. 2001;49:2954–2958. doi: 10.1021/jf010093j. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel RHH, Laane C, Van Berkel WJH. Exploring the biocatalytic potential of vanillyl-alcohol oxidase by site-directed mutagenesis. Adv Synth Catal. 2001;343:515–520. [Google Scholar]
- Van Hellemond EW, Vermote L, Koolen W, Sonke T, Zandvoort E, Heuts DPHM, Janssen DB, Fraaije MW. Exploring the biocatalytic scope of alditol oxidase from Streptomyces coelicolor. Adv Synth Catal. 2009;351:1523–1530. [Google Scholar]
- Vennestrom PNR, Christensen CH, Pedersen S, Grunwaldt J-D, Woodley JM. Next-generation catalysis for renewables: combining enzymatic with inorganic heterogeneous catalysis for bulk chemical production. ChemCatChem. 2010;2:249–258. [Google Scholar]
- Vrielink A, Ghisla S. Cholesterol oxidase: biochemistry and structural features. FEBS J. 2009;276:6826–6843. doi: 10.1111/j.1742-4658.2009.07377.x. [DOI] [PubMed] [Google Scholar]
- Wang Y. Catalytic galactose oxidase models: biomimetic Cu(II)-phenoxyl-radical reactivity. Science. 1998;279:537–540. doi: 10.1126/science.279.5350.537. [DOI] [PubMed] [Google Scholar]
- Wang C, Cao Y, Sun B, Ji B, Nout MJR, Wang J, Zhao Y. Preparation and some properties of cholesterol oxidase from Rhodococcus sp. R14-2. World J Microbiol Biotechnol. 2008;24:2149–2157. [Google Scholar]
- Wertz S, Studer A. Nitroxide-catalyzed transition-metal-free aerobic oxidation processes. Green Chem. 2013;15:3116–3134. [Google Scholar]
- Whittaker JW. Free radical catalysis by galactose oxidase. Chem Rev. 2003;103:2347–2363. doi: 10.1021/cr020425z. [DOI] [PubMed] [Google Scholar]
- Whittaker MM, Whittaker JW. Catalytic reaction profile for alcohol oxidation by galactose oxidase. Biochemistry. 2001;40:7140–7148. doi: 10.1021/bi010303l. [DOI] [PubMed] [Google Scholar]
- Winter RT, Heuts DPHM, Rijpkema EMA, Van Bloois E, Wijma HJ, Fraaije MW. Hot or not? Discovery and characterization of a thermostable alditol oxidase from Acidothermus cellulolyticus 11B. Appl Microbiol Biotechnol. 2012;95:389–403. doi: 10.1007/s00253-011-3750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongnate T, Chaiyen P. The substrate oxidation mechanism of pyranose 2-oxidase and other related enzymes in the glucose-methanol-choline superfamily. FEBS J. 2013;280:3009–3027. doi: 10.1111/febs.12280. [DOI] [PubMed] [Google Scholar]
- Wongnate T, Surawatanawong P, Visitsatthawong S, Sucharitakul J, Scrutton NS, Chaiyen P. Proton-coupled electron transfer and adduct configuration are important for C4a-hydroperoxyflavin formation and stabilization in a flavoenzyme. J Am Chem Soc. 2014;136:241–253. doi: 10.1021/ja4088055. [DOI] [PubMed] [Google Scholar]
- Xiang J, Sampson NS. Library screening studies to investigate substrate specificity in the reaction catalyzed by cholesterol oxidase. Protein Eng Des Sel. 2004;17:341–448. doi: 10.1093/protein/gzh041. [DOI] [PubMed] [Google Scholar]
- Xu F, Golightly EJ, Fuglsang CC, Schneider P, Duke KR, Lam L, Christensen S, Brown KM, Jorgensen CT, Brown SH. A novel carbohydrate: acceptor oxidoreductase from Microdochium nivale. Eur J Biochem. 2001;1142:1136–1142. doi: 10.1046/j.1432-1327.2001.01982.x. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Motoyoshi T, Nishimura A. Purification and characterization of isoamyl alcohol oxidase (“Mureka”-forming enzyme) Biosci Biotechnol Biochem. 1999;63:1216–1222. doi: 10.1271/bbb.63.1216. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Omura H, Okamoto E, Furuya Y, Yabuuchi M. Isolation, characterization and molecular cloning of a thermostable xylitol oxidase from Streptomyces sp. IKD472. J Biosci Bioeng. 2000;89:350–360. doi: 10.1016/s1389-1723(00)88958-6. [DOI] [PubMed] [Google Scholar]
- Yorita K, Janko K, Aki K, Ghisla S, Palfey BA, Massey V. On the reaction mechanism of L-lactate oxidase: quantitative structure-activity analysis of the reaction with para-substituted L-mandelates. Proc Natl Acad Sci U S A. 1997;94:9590–9595. doi: 10.1073/pnas.94.18.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Page A, Worrall CP, Escalettes F, Willies SC, McDouall JJW, Turner NJ, Clayden J. Biocatalytic desymmetrization of an atropisomer with both an enantioselective oxidase and ketoreductases. Angew Chem Int Ed. 2010;49:7010–7013. doi: 10.1002/anie.201002580. [DOI] [PubMed] [Google Scholar]
- Zelitch I, Ochoa S. Oxidation and reduction of glyoxylic acids in plants: I. glycolic acid oxidase. J Biol Chem. 1953;201:707–718. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 32 kb)









