Abstract
The therapy of relapsed chronic lymphocytic leukemia (CLL) has changed dramatically in the past year with the regulatory approval of idelalisib and ibrutinib, with other therapeutic small molecules likely to become widely available in the next few years. Although durable remissions are being seen in many patients with these agents, it is becoming apparent that some patients with high genomic risk disease will relapse. Next-generation sequencing in patients as well as in vitro models is affording us the opportunity to understand the biology behind these relapses, which is the first step to designing rational therapies to prevent and treat targeted therapy-resistant CLL. These strategies are critical, as these relapses can be very difficult to manage, and a coordinated effort to put these patients on clinical trials will be required to efficiently determine the optimal therapies for these patients. In this review, we will describe mechanisms of resistance, both proven and hypothesized, for idelalisib, ibrutinib, and venetoclax, describe patterns of resistance that have been described with ibrutinib, and discuss potential strategies for management of disease resistant to these drugs as well as potential strategies to prevent resistance.
Introduction
Chronic lymphocytic leukemia (CLL) is undergoing an exciting therapeutic transformation. Prior to 2014, patients with relapsed disease had a generally unfavorable outcome, with standard therapies of chemoimmunotherapy or single-agent immunotherapy achieving response rates and progression-free survival (PFS) rates inferior to what is seen in the treatment-naive setting.1-5 Stem cell transplantation, still the only known curative therapy, produces durable remissions in about 50% of patients (reviewed in Dreger et al6), but morbidity and mortality in this generally older population remain problematic. However, the introduction and subsequent approval of 2 oral kinase inhibitors (idelalisib and ibrutinib) has changed the standard of care for patients with relapsed disease. In addition, other novel small-molecule inhibitors currently in clinical trials have the potential to continue to improve therapy for CLL patients, both in the relapsed and frontline settings.
Despite the outstanding efficacy observed with the novel agents, however, patients do relapse during treatment and some patients fail to respond. Progress has been made to understand the mechanisms by which patients relapse after ibrutinib therapy, and similar strategies are currently being undertaken with other novel therapies. Understanding these distinct mechanisms has the potential to lead to further targeted therapies to combat resistance, as well as the development of rational combinations to prevent the development of resistance in high-risk patients. Additionally, there are patients who do not tolerate these therapies, and work is ongoing to define risk factors for toxicity and outcomes in these patients. In this review, we will discuss the current data with targeted therapies in the treatment of relapsed CLL, mechanisms of resistance that have been identified or hypothesized, and potential strategies to treat and prevent resistance. Although there are many targeted agents coming forward, this review will focus on the currently approved US Food and Drug Administration (FDA) kinase inhibitors ibrutinib and idelalisib as well as the small-molecule venetoclax (ABT-199) which is currently in late-stage clinical trials.
Efficacy of targeted therapies approved for CLL or in late-stage clinical studies
Idelalisib is an orally bioavailable, reversible, p110 δ isoform-specific phosphoinositide-3 kinase (PI3K) inhibitor. Because the δ isoform is specific for B lymphocytes, this agent has selective effects on the CLL cells with relative sparing of other hematopoietic cells. It potently inhibits PI3K signaling and can block the protective effects of the CLL microenvironment in vitro.7 It is currently FDA approved in combination with rituximab for the treatment of relapsed/refractory CLL. In phase 1 study of the single agent, 72% of patients achieved an objective response, with a median progression-free survival (PFS) of 15.8 months. When focusing only on patients receiving a biologically effective dose, the PFS was 32 months. Median overall survival (OS) has not been reached, with a 36-month OS of 75%.8 The definitive phase 3 study of the idelalisib plus rituximab regimen studied this combination vs placebo plus rituximab in 220 patients.9 At the second interim analysis, the addition of idelalisib led to an overall response rate (ORR) of 77% (vs 15% with rituximab plus placebo), and a 12-month PFS of 66%.10
Ibrutinib is an orally bioavailable, selective, irreversible inhibitor of Bruton agammaglobulinemia tyrosine kinase (BTK). Both in vitro and in patients, ibrutinib has been shown to potently inhibit B-cell receptor (BCR) signaling, prevent lymphocyte adhesion and homing, and inhibit protective effects of the microenvironment.11-14 This drug is currently FDA approved for patients with relapsed or refractory CLL and for patients with CLL with del(17p). In the phase 1b/2 study of ibrutinib in patients with relapsed or refractory CLL, the ORR is 91% for those receiving 420 mg daily, with a 26-month estimated PFS of 75% and OS of 83%.15 In the phase 3 RESONATE study, ibrutinib was compared with ofatumumab. With a median follow-up of 9.4 months, ORR was superior with ibrutinib, but more importantly both PFS (median 8.1 months for ofatumumab vs not reached for ibrutinib) and OS (12-month estimates: 81% for ofatumumab, 90% for ibrutinib) were significantly improved with ibrutinib.16 A phase 2 study was also conducted specifically in patients with TP53 aberrations who could have either treatment-naive or relapsed disease. The cumulative incidence of progression at 24 months was 9% for previously untreated and 20% for previously treated patients. Estimated OS at 24 months was 84% for previously untreated and 74% for previously treated patients.17 These data, although not directly comparable to other trials, do suggest that patients with TP53 abnormalities may have inferior prognosis, although the contribution of karyotypic complexity in this series is unknown.
Venetoclax is an orally bioavailable, selective inhibitor of BCL2. It is a BH3 mimetic which acts similarly to the BH3-only proteins which antagonize BCL2 and its prosurvival family members (reviewed in Anderson et al18). It is not currently FDA approved, but is being tested in late-stage clinical studies in CLL. In phase 1 study with a median follow-up of 15 months, the ORR was 77%, with a high complete response (CR) rate of 23%. Estimated median PFS is 18 months.19 In combination with rituximab, at a median follow-up of about 7 months, the ORR was 88%, with 6 of 49 patients experiencing disease progression. This drug has been shown in clinical studies to induce minimal residual disease–negative CRs, which are generally not seen with PI3K and BTK inhibitors.20
Mechanism of resistance to targeted therapies
As targeted therapies have become more established in cancer therapy, so has our understanding that these agents have unique mechanisms of resistance compared with chemotherapy. In hematologic malignancies, imatinib for chronic myelogenous leukemia was the first kinase inhibitor in widespread use.21 Mechanisms of resistance to this drug are well characterized and include primarily point mutations in the kinase domain of ABL, where >100 resistance mutations have been identified that prevent imatinib binding through binding site configuration or destabilization of the inactive conformation of ABL (reviewed in Quintas-Cardama et al22). This mechanism of resistance, with mutations altering drug binding, is also seen in lung cancer with EGFR inhibitors23,24 and ALK inhibitors,25-27 in gastrointestinal stromal tumors with KIT inhibitors,28 and in acute myeloid leukemia with FLT3 inhibitors.29,30
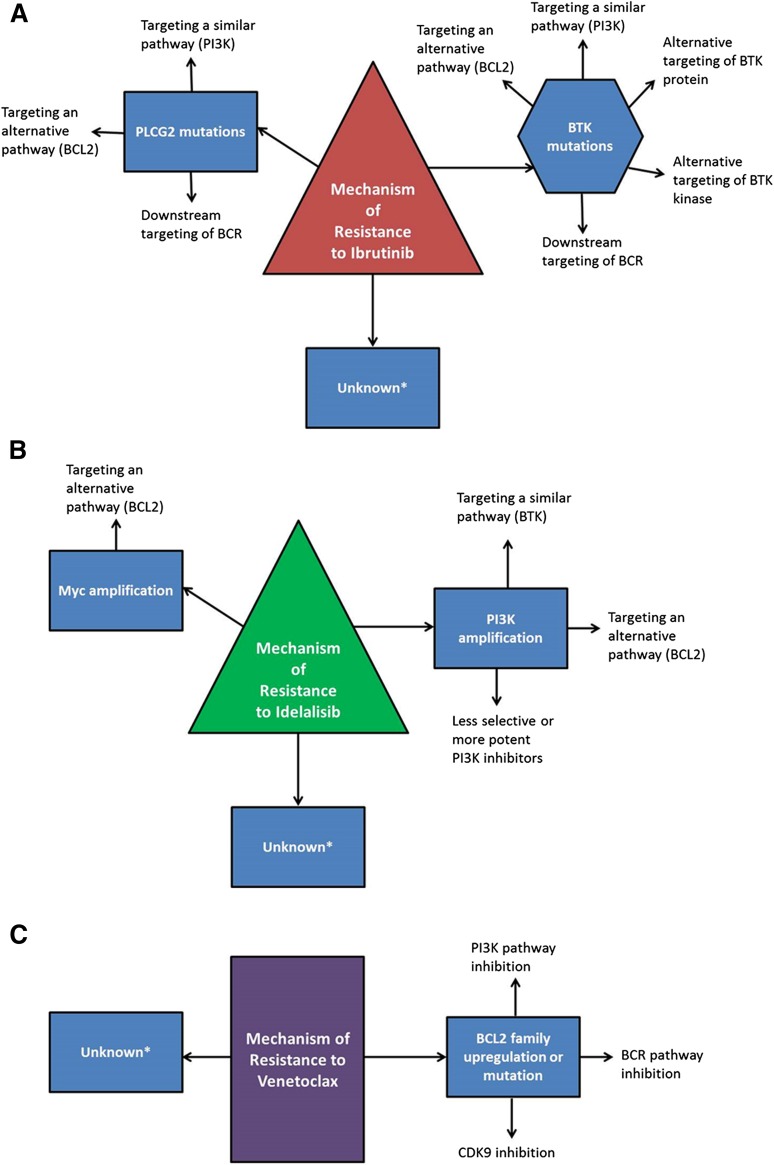
Ibrutinib is currently the only targeted agent in CLL therapy where resistance mechanisms have been confirmed in multiple patients. From whole-exome sequencing of 6 patients with late relapses, acquired mutations were found in BTK at the binding site of ibrutinib (C481) with a cysteine to serine mutation, and in phospholipase Cγ2 (PLCγ2), the kinase immediately downstream of BTK, where multiple mutations were identified.31 Functional characterization of these mutations demonstrated that BTK C481S reduces the binding affinity of ibrutinib for BTK and allows only reversible BTK inhibition, rather than irreversible. Because of the relatively short half-life of ibrutinib, this results in only transient inhibition of BTK, and in multiple patients, it has been confirmed that patients who relapse with the C481S mutation have expression of phosphorylated BTK which is not inhibited by the administration of ibrutinib.31,32 The mutations identified in PLCγ2 have all been demonstrated to be potentially gain of function, allowing activation in the presence of inactive BTK.31,33 More recently, 8 additional patients with CLL who relapsed after ibrutinib were genetically characterized using targeted deep sequencing at baseline and relapse, and all were found to have mutations in BTK at C481 or in PLCγ2.34 Importantly, there was not a clear association of BTK or PLCγ2 mutations with Richter transformation, only CLL progression. As well, another group has reported 3 patients who relapsed on ibrutinib and did not identify BTK mutations in any patient, although 1 had a mutation in PLCγ2.35 Suggesting that there likely are relapses that are not the result of mutations in the BCR pathway and may reflect a difference between early relapses and late relapses. Primary resistance to ibrutinib has been observed only rarely and no mechanism has yet been identified, although it is intuitive that activating mutations distal to BTK (eg, mitogen-activated protein kinase [MAPK] pathway or PI3K pathway) might render a patient less sensitive to ibrutinib early in therapy. Known and predicted resistance mechanisms and potential alternative targets can be found in Figure 1A.
Figure 1.
Mechanisms of resistance to targeted therapies and potential bypass strategies. Known and hypothesized mechanisms of resistance to ibrutinib (A), idelalisib (B), and venetoclax (C) are outlined along with potential mechanisms to overcome resistance. *Unknown mechanisms which may have the potential to respond to other targeted therapies or chemoimmunotherapies, but may also be considered for salvage with reduced-intensity allogeneic stem cell transplantation or CAR T-cell therapies.
Because it appears that most CLL progressions on ibrutinib are the result of mutations in BTK or PLCγ2, a critical question is whether these mutations are present at baseline or acquired during therapy. Computational evolutionary models suggest that resistance mutations should be present prior to ibrutinib administration,36 however, this has not yet been proven in patients. In both reports of ibrutinib resistance due to BTK or PLCγ2 mutations, deep sequencing of the peripheral blood was performed at baseline, and no mutations were identified.31,34 In addition, a separate group performed deep sequencing in a cohort of 613 ibrutinib-naive patients and found no mutations in BTK at C481.37 Although these data suggest that BTK C481 mutations are not commonly found in the peripheral blood, further study will need to be performed to determine whether these mutations are present in very small clones or in alternative niches.
Ibrutinib resistance has also been explored in hematologic malignancies other than CLL. In mantle cell lymphoma, BTK C481S mutations have been identified in patients with late progressions, whereas early progressions and primary resistance have not been demonstrated to be associated with this mutation.38 In Waldenstrom macroglobulinemia, mutations in CXCR4 have been shown in vitro and in vivo to lead to ibrutinib resistance,39,40 however, these mutations have not been described in CLL. Additionally, ibrutinib has shown some activity in activated B-cell-like subtype diffuse large B-cell lymphoma, and preclinical data suggest that the activity is due to NF-κB blockade and is limited to those tumors with chronic active BCR signaling and wild-type CARD11.41,42 Recurrent mutations in CARD11 have not been observed in CLL.43,44
Mechanisms of resistance to idelalisib have not yet been described in CLL, although work is ongoing in this area, and predicted mechanisms of resistance and potential alternative targets can be found in Figure 1B. Because idelalisib selectively targets p110δ (encoded by PIK3CD), 1 likely resistance mechanism is upregulation of either PIK3CD or an alternative class 1A PI3K, either PIK3CA or PIK3CB. In mantle cell lymphoma, it has been shown in vitro that a higher ratio of PIK3CA to PIK3CD messenger RNA in cell lines predicts resistance to selective p110δ inhibition.45 In a breast cancer cell line where PIK3CA was mutated, resistance to a PI3Kα inhibitor was mediated by upregulation of PIK3CA, so that PI3K function was maintained even with normally saturating concentrations of the inhibitor.46 Other preclinical studies primarily with p110α inhibitors in breast cancer models have noted other potential resistance mechanisms outside of the drug target, including MYC amplification.47 Whether these mechanisms will be relevant to CLL remains to be seen.
Resistance mechanisms to venetoclax also have not yet been described in CLL patients. Predicted mechanisms and potential alternative targets can be found in Figure 1C. The most likely mechanism of resistance would be upregulation of alternative antiapoptotic BCL2 family members, such as BCL-XL, BCL-W, MCL1, and BCL2A1.48 ABT-737, an inhibitor of BCL2 and BCL-XL, was shown in vitro to induce resistance in a lymph node model by upregulation of BCL-XL and BCL2A1.49 Using another lymph node model, CLL cells which upregulated BCL-XL after stimulation with CD40 and IL4 were resistant to high doses of venetoclax.50 Similarly, in follicular lymphoma cell lines and primary cells with induced resistance to venetoclax, increased levels of MCL-1 as well as increased autophagy were observed.51 Acquired resistance in vitro after venetoclax continuous exposure has also been described and resulted in mutations in the BCL2 BH3 domain and in BAX.52 These in vitro studies will be helpful to guide the analysis of patients who relapse after venetoclax therapy.
Strategies to treat resistance
With these exciting agents so early in clinical use, the optimal therapy to treat patients with resistant disease has not yet been established. Fortunately, at this juncture we have 3 distinct mechanisms of actions with these therapies, and 2 are FDA approved for patients with relapsed CLL, so 1 strategy would be to switch among these therapies. At this time, many patients are receiving ibrutinib in first relapse unless they are on a clinical trial with venetoclax, and then idelalisib or venetoclax on a trial is reserved for ibrutinib failures. As ibrutinib moves into frontline therapy, this sequence may become even more standard. Anecdotally, there are patients who have relapsed on idelalisib that have been successfully salvaged with ibrutinib, and studies are ongoing to evaluate efficacy of PI3K inhibitors and venetoclax in kinase inhibitor–resistant disease. Scientifically, there does not appear to be a reason why patients who relapse with venetoclax cannot go on to be treated with idelalisib or ibrutinib, although this remains an open question. In vitro data suggest that distinct mechanisms of resistance to venetoclax may be successfully targeted by dasatinib,50 ibrutinib,50 or inhibition of CDK9,53 and recent data suggest that inhibition of PI3K can overcome resistance mediated by MCL-1 or BCL-XL.54 At this juncture, resistance to ibrutinib appears to be the predominant clinical challenge for a subgroup of patients.
CLL progressions on ibrutinib tend to occur late in therapy (after 12 months) in patients who previously had attained a response, in contrast to Richter transformations, which tend to occur during the first 1 to 2 years of treatment.34 In 2 single-center studies, it has been demonstrated that patients do poorly following relapse from ibrutinib34,55; median survival following CLL relapse in our experience is 17.6 months. Most patients require rapid onset of salvage therapy within 2 weeks of discontinuing ibrutinib, and relapse tends to be rapidly progressing after ibrutinib discontinuation, so it is important that ibrutinib not be discontinued until alternative treatment is planned and available. In addition, for patients with Richter transformation receiving standard chemoimmunotherapy, the current practice at our institution is to continue ibrutinib during Richter-directed therapy. Because the standard of care for patients who progress on ibrutinib is unknown and clinical consequences of progression are severe, identifying treatment options for these patients is a priority.
Data in lymphoma suggest that upregulation of PI3K is commonly seen in ibrutinib resistance,38 which suggests that idelalisib plus rituximab combination therapy may be successful in this scenario. Additionally, preclinical data with the PI3K p110 γ/δ inhibitor IPI-145 suggest that this agent may be effective in patients with C481S BTK mutations.56 Preliminary results with IPI-145 have not shown striking efficacy,57 however, patients were not necessarily stratified based on BTK mutational status and there may be differences in postibrutinib response among PI3K inhibitors.
In patients with BTK and PLCγ2 mutations, it appears that the BCR pathway remains intact and therefore potentially targetable. One possible strategy, therefore, is an agent which targets the BCR downstream of BTK, such as PKCβ.58 Agents which target upstream of BTK, such as SYK inhibitors, also appear interesting. In diffuse large B-cell lymphoma cell lines with either BTK or PLCγ2 mutations, cells could be resensitized to ibrutinib in combination with SYK inhibitors or venetoclax.59
Specifically in patients with BTK C481 mutations, alternative targeting of BTK may be an option to treat resistance, and BTK inhibitors which bind BTK distinct from C481 may be of clinical utility. Agents that affect BTK in a manner distinct from kinase inhibition are also interesting. One such class of agents is the HSP90 inhibitors. BTK is a client protein of HSP90, and in vitro, HSP90 inhibitors have been shown to overcome ibrutinib resistance in a mantle cell line driven by alternative NF-κB signaling.60 Similarly, the XPO1/CRM1 inhibitor selinexor has been shown to suppress BTK gene expression and to be effective in cell lines with BTK C481S mutations, as well as an in vivo model of ibrutinib resistance.61 Other agents that deplete BTK through alternative mechanisms are under active investigation for these patients.
Finally, patients who relapse on ibrutinib or other targeted agents may warrant consideration for an alternative therapeutic strategy such as reduced-intensity allogeneic stem cell transplant or chimeric antigen receptor (CAR) T-cell therapy. Transplant may be an ideal consideration in patients who have a donor and are able to enter a remission with salvage therapy, but is limited to younger patients with preserved performance status. CAR T cells administered within the context of a clinical trial may be a very realistic option for some patients and may offer the potential for long-term disease control.
At this time, although there is not yet definitive data, reasonable options for patients with resistance secondary to BTK mutations would be idelalisib or clinical trials with venetoclax, novel BTK inhibitors, selinexor, or HSP90 inhibitors. For PLCγ2 mutations, clinical trials with venetoclax or downstream BCR inhibitors would be reasonable. Clinical trials of CAR T cells or stem cell transplantation would be reasonable for all patients.
Strategies to prevent resistance
As we have seen that targeted therapy resistance presents a clinical challenge, it would certainly be preferable to prevent resistance from developing. For single-agent idelalisib, large data sets are not yet available, but it appears that patients with del(17p) and/or TP53 mutations do not have as durable responses as patients with intact TP53.8 Data are not yet available regarding clinical characteristics which predict relapse in patients treated with venetoclax. For ibrutinib, complex karyotype and presence of BCL6 abnormalities on pretreatment fluorescent in situ hybridization are independently associated with a risk of relapse. Del(17p) or del(11q) appear to be significant in univariable analysis, however, likely because of coassociation with complex karyotype, they have not been shown to be an independent risk for relapse.34,55 As more patients are treated with these drugs, it will be critically important to determine who is at risk for relapse, as these would be patients for consideration of combination therapy upfront.
Besides potential prevention of resistance, combination strategies also offer the possibility of earlier induction of deep responses with potential to discontinue therapy. Venetoclax has this potential as a single agent, however, both idelalisib and ibrutinib have only infrequently been associated with CRs as a single agent, and minimal residual disease (MRD)-negative CRs have been rare. Although the potential for intermittent therapy discontinuation has not yet been tested in clinical trials, this approach may be effective in some patients, and combinations which promote deeper and quicker responses may facilitate this. Drug discontinuation may discourage the development of resistance by eliminating the selective pressure of the drug, and would be very appealing in terms of toxicity and cost.
For idelalisib, ibrutinib, and venetoclax, combination studies in CLL are completed or ongoing, and presented and published outcomes from these trials can be found in Table 1.9,10,20,62-65 With idelalisib, it seems that combination therapy may increase response rates over those published for single-agent therapy. Increases in PFS are difficult to determine given the relatively short follow-up in these trials. For ibrutinib, combination studies have been encouraging, but response durations at this point do not appear to be different from those seen with single-agent ibrutinib, potentially because only certain patients benefit from combinations.
Table 1.
Published/presented combination studies with idelalisib, ibrutinib, and venetoclax in CLL
| Agents | Reference | No. of patients | Response rate | PFS | OS |
|---|---|---|---|---|---|
| Idelalisib + rituximab | 9, 10 | 220 | 77% ORR | 12-mo PFS = 66% | Not reached |
| Idelalisib + bendamustine, bendamustine/rituximab, fludarabine, chlorambucil, or chlorambucil/rituximab | 62 | IB = 18 | 82% ORR; 10% CR | 24-mo PFS = 57% | Not reported |
| IBR = 15 | |||||
| IF = 12 | |||||
| ICh = 15 | |||||
| IChR = 14 | |||||
| Ibrutinib + rituximab | 63 | 40 (36 previously treated) | 95% ORR; 8% CR | 18-mo PFS = 78% | 18-mo OS = 84% |
| Ibrutinib + ofatumumab | 64 | 71 | 83% ORR | 12-mo PFS 85%-90% depending on schedule |
Not reported |
| Ibrutinib + lenalidomide | 65 | 11 | 100% ORR | 91% at 263 d | 100% at 263 d |
| Venetoclax + rituximab | 20 | 49 (34 evaluable for response) | 88% ORR; 32% CR/CRi | Not reported | Not reported |
CR, complete response; CRi, complete response with incomplete marrow recovery; IB, idelalisib + bendamustine; IBR, idelalisib + bendamustine + rituximan; ICh, idelalisib + chlorambucil; IChR, idelalisib + chlorambucil + rituximab; IF, idelalisib + fludarabine; OR, overall response; ORR, overall response rate.
Combinations with chemotherapy or chemoimmunotherapy are appealing because of the prospect of attainment of deeper remissions. However, it has not yet been shown with idelalisib, ibrutinib, or venetoclax that response duration or survival are correlated with attainment of a CR. In the CLL population, which is predominantly elderly, it may be prudent to explore additional combinations with less toxic therapies until it is determined that there is a benefit to CR or MRD-negative status attainment. Combinations of novel therapies with antibodies have been of great interest, as CD20 antibodies added to standard therapies have prolonged survival in previous trials.66 Idelalisib in particular seems to be well suited for antibody combinations, and clinical evidence of this success has been observed.9 As well as the CD20 antibodies, CD37 targeting agents would be interesting to combine with idelalisib, as CD37 has been shown to induce PI3K activity, and in vitro combination of a CD37 targeted protein therapeutic and PI3K inhibitors have shown synergy.67 For ibrutinib, however, inhibition of interleukin-2-inducible T-cell kinase in addition to BTK68 leads to an in vitro antagonism of NK-cell antibody-dependent cell-mediated cytotoxicity,69 which may account for the lack of demonstrable improvements when CD20 antibodies have been added to ibrutinib. It may be that for BTK inhibitors, a more specific inhibitor that lacks interleukin-2-inducible T-cell kinase activity would be beneficial in combinations with antibodies. Like idelalisib, venetoclax is a very reasonable combination partner for CD20 antibody therapy, and clinical trials are ongoing.
Combinations of idelalisib and ibrutinib with lenalidomide or other immune-modulating agents are also appealing. Lenalidomide plus idelalisib has the potential to alleviate lenalidomide-induced tumor flare, as lenalidomide-induced immune activation and cytokine release have been shown to be PI3K dependent.7 The combination of ibrutinib with lenalidomide as well has a number of potential advantages. Besides alternative mechanisms of action and the potential for both agents to impact the CLL microenvironment,11,70 both lenalidomide and ibrutinib have the potential to restore normal T-cell function. Ibrutinib has been shown to induce CD4 cell Th1 skewing which is predominantly a cytotoxic phenotype and may help in both antitumor immunity and recovery from infectious toxicity.68 Lenalidomide has been shown to induce T-cell activation and results in increases sensitivity of CLL cells to apoptosis.71 The combination of the 2, therefore, has the potential to restore normal T-cell function and induce antitumor immunity. These novel agents also have the potential to synergize with immune checkpoint inhibitors. Ibrutinib specifically has been shown to reduce PD-1 expression on CD8+ T cells,72 and combinations of ibrutinib and an anti-PD-L1 antibody are effective in a mouse model of lymphoma that is insensitive to either agent alone. Although more preclinical work is necessary to fully understand the effects of the combinations of checkpoint inhibitors and kinase inhibitors, the prospect is exciting.
Conclusions
With the advent of many exciting and effective novel therapies for CLL comes the challenge of managing resistant disease. We expect that these drugs are improving the lifespan (and quality of life) of our CLL patients, however, resistant disease has been difficult to treat with standardly available therapies. With ibrutinib, despite the relatively low number of patients who have relapsed, a mechanism of relapse has been identified which makes it easier to determine rational combinations and salvage therapies. As more patients are treated and more relapse after idelalisib and venetoclax, it will be important to study mechanisms of resistance associated with these agents in patients as well. In addition, as these agents move into frontline therapy, it will be important to determine whether similar mechanisms and trends of resistance exist.
It is tempting to believe that the challenge of refractory CLL has been effectively managed with the introduction of these oral agents, however, the disease remains incurable. As patients are treated for longer periods of time with these agents, and as increasing numbers of patients are being treated outside of clinical trials, we are likely to see the incidence of relapse increase. Disease and patient-related characteristics that predict relapse to these agents remain an area of active investigation, and as it becomes more clear which patients are likely to relapse, and conversely, which patients are not likely to relapse, we will be better able to determine which patients should be preferentially referred for clinical trials investigating combinations and novel agents. Clinicians and scientists will need to continue to work together to develop rational salvage and combination therapies with these agents that can then be tested in clinical studies of patients at high risk to fail standard therapy with kinase inhibitors.
Despite these challenges, this remains a hopeful time in CLL therapy, with novel agents recently FDA approved and additional promising agents on the horizon. Unprecedented advances in our understanding of disease biology are uncovering other potential targets in this disease and others. Moving from the era of chemotherapy toward an era of patient-friendly novel agents represents a dramatic improvement in the life of our CLL patients, and brings us a step closer to cure of this disease.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Cancer Institute (K23 CA178183, R01 CA177292, R01 CA183444, P01 CA081534, P50 CA140158, and P30 CA016058).
Authorship
Contribution: J.A.W. and A.J.J. designed, organized, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer A. Woyach, Department of Internal Medicine, Division of Hematology, The Ohio State University, 455A OSU CCC, 410 West 12th Ave, Columbus, OH 43210; e-mail: jennifer.woyach@osumc.edu.
References
- 1.Catovsky D, Richards S, Maututes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukemia (the LRF CLL4 trial): a randomised controlled trial. Lancet. 2007;370(9583):230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 2.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29(26):3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 3.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 4.Wierda WG, Kipps TJ, Mayer J, et al. Hx-CD20-406 Study Investigators. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartron G, de Guibert S, Dilhuydy MS, et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124(14):2196–2202. doi: 10.1182/blood-2014-07-586610. [DOI] [PubMed] [Google Scholar]
- 6.Dreger P, Schetelig J, Andersen N, et al. European Research Initiative on CLL (ERIC) and the European Society for Blood and Marrow Transplantation (EBMT) Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124(26):3841–3849. doi: 10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): efficacy analysis in patient subpopulations with Del(17p) and other adverse prognostic factors [abstract].; Blood; 2014. Abstract 330. [Google Scholar]
- 11.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-κB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123(21):3286–3295. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd JC, Brown JR, O’Brien S, et al. RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol. 2014;51(3):219–227. doi: 10.1053/j.seminhematol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Seymour JF, Davids MS, Pagel JM, et al. ABT-199 (GDC-0199) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): high complete-response rate and durable disease control [abstract]. J Clin Oncol. 2014;32(suppl 5s) Abstract 7015. [Google Scholar]
- 20.Roberts AW, Ma S, Brander DM, et al. Determination of recommended phase 2 dose of ABT-199 (GDC-0199) combined with rituximab (R) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) [abstract].; Blood; 2014. Abstract 325. [Google Scholar]
- 21.O’Brien SG, Guilhot F, Larson RA, et al. IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 22.Quintas-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16(2):122–131. doi: 10.1177/107327480901600204. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 24.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi YL, Soda M, Yamashita Y, et al. ALK Lung Cancer Study Group. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 26.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120) doi: 10.1126/scitranslmed.3003316. 120ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11(11):4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 29.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 31.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng S, Guo A, Lu P, Ma J, Coleman M, Wang YL. Functional characterization of BTK(C481S) mutation that confers ibrutinib resistance: exploration of alternative kinase inhibitors. Leukemia. 2015;29(4):895–900. doi: 10.1038/leu.2014.263. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Lee GS, Brady J, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012;91(4):713–720. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burger JA, Landau D, Hoellenriegel J, et al. Clonal evolution in patients with chronic lymphocytic leukemia (CLL) developing resistance to BTK inhibition [abstract]. Blood. 2013;122(21) doi: 10.1038/ncomms11589. Abstract 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komarova NL, Burger JA, Wodarz D. Evolution of ibrutinib resistance in chronic lymphocytic leukemia (CLL). Proc Natl Acad Sci USA. 2014;111(38):13906–13911. doi: 10.1073/pnas.1409362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Famà R, Bomben R, Rasi S, et al. Ibrutinib-naïve chronic lymphocytic leukemia lacks Bruton tyrosine kinase mutations associated with treatment resistance. Blood. 2014;124(25):3831–3833. doi: 10.1182/blood-2014-08-592725. [DOI] [PubMed] [Google Scholar]
- 38.Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Disc. 2014;4(9):1022–1035. doi: 10.1158/2159-8290.CD-14-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y, Hunter ZR, Liu X, et al. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom’s macroglobulinemia. Leukemia. 2015;29(1):169–176. doi: 10.1038/leu.2014.187. [DOI] [PubMed] [Google Scholar]
- 40.Treon SP, Tripsas CK, Yang G, et al. A prospective multicenter study of the Bruton’s tyrosine kinase inhibitor ibrutinib in patients with relapsed or refractory Waldenstrom’s macroglobulinemia [abstract]. Blood. 2013;122(21) Abstract 251. [Google Scholar]
- 41.Yang Y, Shaffer AL, III, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21(6):723–737. doi: 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landau DA, Carter SL, Getz G, Wu CJ. Clonal evolution in hematological malignancies and therapeutic implications. Leukemia. 2014;28(1):34–43. doi: 10.1038/leu.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyengar S, Clear A, Bödör C, et al. P110α-mediated constitutive PI3K signaling limits the efficacy of p110δ-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121(12):2274–2284. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huw LY, O’Brien C, Pandita A, et al. Acquired PIK3CA amplification causes resistance to selective phosphoinositide 3-kinase inhibitors in breast cancer. Oncogenesis. 2013;2:e83. doi: 10.1038/oncsis.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muellner MK, Uras IZ, Gapp BV, et al. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol. 2011;7(11):787–793. doi: 10.1038/nchembio.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 49.Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 50.Thijssen R, Geest CR, de Rooij MFM, et al. Possible mechanisms of resistance to the novel BH3-mimetic ABT-199 in in vitro lymph node models of CLL–the role of Abl and Btk [abstract]. Blood. 2013;122(21) Abstract 4188. [Google Scholar]
- 51.Bodo J, Zhao X, Smith MR, Hsi ED. Activity of ABT-199 and acquired resistance in follicular lymphoma cells [abstract]. Blood. 2014;124(21) Abstract 3635. [Google Scholar]
- 52.Fresquet V, Rieger M, Carolis C, García-Barchino MJ, Martinez-Climent JA. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood. 2014;123(26):4111–4119. doi: 10.1182/blood-2014-03-560284. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Jin S, Tapang P, et al. CDK9 inhibition reverses resistance to ABT-199 (GDC-0199) by down-regulating MCL-1 [abstract]. Blood. 2014;124(21) Abstract 2161. [Google Scholar]
- 54.Choudhary GS, Al-Harbi S, Mazumder S, et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015 doi: 10.1038/cddis.2014.525. 6:e1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson PA, Wierda WG, Ferrajoli A, et al. Complex karyotype, rather than Del(17p), is associated with inferior outcomes in relapsed or refractory CLL patients treated with ibrutinib-based regimens [abstract]. Blood. 2014;124(21) Abstract 22. [Google Scholar]
- 56.Dong S, Guinn D, Dubovsky JA, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124(24):3583–3586. doi: 10.1182/blood-2014-07-587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porcu P, Flinn I, Kahl BS, et al. Clinical activity of duvelisib (IPI-145), a phosphoinositide-3-kinase-δ,γ inhibitor, in patients previously treated with ibrutinib [abstract]. Blood. 2014;124(21) Abstract 3335. [Google Scholar]
- 58.El-Gamal D, Williams K, LaFollette TD, et al. PKC-β as a therapeutic target in CLL: PKC inhibitor AEB071 demonstrates preclinical activity in CLL. Blood. 2014;124(9):1481–1491. doi: 10.1182/blood-2014-05-574830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo H, Crowley R, Xue L, et al. Combination of ibrutinib and BCL-2 or SYK inhibitors in ibrutinib resistant ABC-subtype of diffuse large B-cell lymphoma [abstract]. Blood. 2014;124(21) Abstract 505. [Google Scholar]
- 60.Kopp N, Tschuri S, Haebe S, et al. Newer-generation HSP90 inhibitors can overcome ibrutinib resistance and suppress proliferation in human mantle cell lymphoma in vitro and in vivo [abstract].; Blood; 2014. Abstract 1686. [Google Scholar]
- 61.Hing ZA, Mantel R, Beckwith KA, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood. 2015;125(20):3128–3132. doi: 10.1182/blood-2015-01-621391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrientos JC, Coutre SE, De Vos S, et al. Long-term follow-up of a phase 1 trial of idelalisib (ZYDELIG®) in combination with bendamustine (B), bendamustine/rituximab (BR), fludarabine (F), chlorambucil (Chl), or chlorambucil/rituximab (ChlR) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) [abstract]. Blood. 2014;124(21) Abstract 3343. [Google Scholar]
- 63.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaglowski S, Jones J, Flynn J, et al. A phase 1b/2 study evaluating activity and tolerability of the BTK inhibitor ibrutinib in combination with ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases [abstract]. J Clin Oncol. 2014;32(suppl 5s) Abstract 7009. [Google Scholar]
- 65.Pollyea DA, Coutre S, Gore L, et al. A dose escalation study of ibrutinib with lenalidomide for relapsed and refractory chronic lymphocytic leukemia/small lymphocytic lymphoma [abstract]. Blood. 2014;124(21) Abstract 1987. [Google Scholar]
- 66.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 67.Lapalombella R, Yeh YY, Wang L, et al. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21(5):694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kohrt HE, Sagiv-Barfi I, Rafiq S, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123(12):1957–1960. doi: 10.1182/blood-2014-01-547869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee BN, Gao H, Cohen EN, et al. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117(17):3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClanahan F, Miller S, Riches JC, et al. T-cell dysfunction in CLL is mediated not only by PD-1/PD-L1 but also by PD-1/PD-L2 interactions - partial functionality is maintained in PD-1 defined CD8 subsets and this can be further promoted by ibrutinib treatment [abstract]. Blood. 2013;122(21) Abstract 4120. [Google Scholar]