Abstract
Study Objectives:
Daylight saving time (DST) has been established with the intent to reduce energy expenditure, however unintentional effects on sleep and vigilance have not been consistently measured. The objective of this study was to test the hypothesis that DST adversely affects high school students' sleep and vigilance on the school days following its implementation.
Methods:
A natural experiment design was used to assess baseline and post-DST differences in objective and subjective measures of sleep and vigilance by actigraphy, sleep diary, sleepiness scale, and psychomotor vigilance testing (PVT). Students were tested during school days immediately preceding and following DST.
Results:
A total of 40 high school students were enrolled in this study; 35 completed the protocol. Sleep duration declined by an average of 32 minutes on the weeknights post-DST, reflecting a cumulative sleep loss of 2 h 42 min as compared to the baseline week (p = 0.001). This finding was confirmed by sleep diary analyses, reflecting an average sleep loss of 27 min/night (p = 0.004) post-DST. Vigilance significantly deteriorated, with a decline in PVT performance post-DST, resulting in longer reaction times (p < 0.001) and increased lapses (p < 0.001). Increased daytime sleepiness was also demonstrated (p < 0.001).
Conclusions:
The early March DST onset adversely affected sleep and vigilance in high school students resulting in increased daytime sleepiness. Larger scale evaluations of sleep impairments related to DST are needed to further quantify this problem in the population. If confirmed, measures to attenuate sleep loss post-DST should be implemented.
Citation:
Medina D, Ebben M, Milrad S, Atkinson B, Krieger AC. Adverse effects of daylight saving time on adolescents' sleep and vigilance. J Clin Sleep Med 2015;11(8):879–884.
Keywords: daylight saving time, vigilance, actigraphy, student, sleep, adolescent, sleep deprivation, school
Daylight saving time (DST) has been commonly used in the developed world, first trialed in the United States in 1918 as a temporary wartime measure to save energy expenditure, and formally implemented several decades later through the Uniform Time Act of 1966.1,2 Over the years, the DST period was adjusted based on the potential for added energy savings and other gains, including greater utilization of parks by increasing daylight outdoor playtime for children in the summer.3–6 Currently, the majority of states have adopted DST measures, advancing the clock one hour at 02:00 on the second Sunday of March and delaying it by one hour at 02:00 on the first Sunday of November, with the exception of Arizona, Hawaii, and the U.S. territories.7–10
The biological sleep/wake processes governed by circadian rhythms are entrained to the sun and need time to adapt to DST.11,12 Following the DST advance of clock-time in March, an effective loss of one hour takes place during the night and this transition might result in sleep deprivation. Although its full impact on sleep health has not been consistently studied, there is evidence that this one-hour loss in clock time leads to changes in sleep homeostasis that may take several days to be completed.12–16 Deprivation of sleep may actually occur, leading to behavioral and neurocognitive changes, such as deficits in attention, most commonly measured by psychomotor vigilance testing (PVT).17–21
BRIEF SUMMARY
Current Knowledge/Study Rationale: Daylight Saving Time (DST) is a common practice in the developed world. However, the potential adverse effects of DST on our increasingly sleep deprived young population have not been consistently evaluated using objective measures. The onset of DST occurs early in March in the United States. Despite its proposed economic benefits, detailed analyses of potentially adverse health effects due to DST-related sleep restriction and decreased vigilance on the subsequent days have not been previously reported.
Study Impact: Our study highlights a significant sleep loss associated with DST implementation in a group of high school students leading to decreased vigilance and increased daytime sleepiness on the post-DST weekdays as compared to pre-DST levels. Larger scale evaluations of sleep impairments related to DST are needed to further quantify this problem. If confirmed, measures to attenuate the effects of sleep loss post-DST should be implemented.
Adolescents and high school students are potentially more susceptible to the adverse effects of DST, given their early school hours and a natural tendency to maintain a delayed sleep schedule, which often culminates in a sleep deprived state.22–25 Changes in sleep parameters or vigilance in relation to DST have not been previously evaluated in this population. Therefore, we proposed to study the effects of the spring DST adjustment as a natural experiment in a group of high school students by using objective and subjective measurements of sleep and vigilance during the two weeks surrounding DST (the preceding and following weeks), testing the hypotheses that the onset of DST leads to sleep loss and a measurable decrease in performance in these students.
METHODS
High school students from 9th to 12th grades at a High School in Westchester County, NY, were offered participation in this study after written parental consent was obtained. The research study was approved by the White Plains High School's Institutional Review Board and Science Review Committee. Recruitment took place during school hours 2 weeks prior to the DST start date. A total of 40 students volunteered to participate in this study. During the course of 2 consecutive weeks, including the week prior to (baseline) and the week immediately following DST (post-DST), students were asked to record their sleep schedule on a validated sleep questionnaire and wear a wrist actigraph for the entire duration of the study. Students were instructed to maintain their usual routine of physical activity and rest. The school day started at 07:40 EST for all students, and assessment of daytime sleepiness and vigilance took place at a set time each day during the school hours.
Sleep Duration
Actigraphy
Sleep and activity measurements were collected by actigraphy using the Micro-Mini Motionlogger Actigraph (Ambulatory Monitoring, Inc., Ardsley, NY, USA).26,27 Wrist actigraphy is a well-established and validated tool for evaluating sleep patterns, with a high degree of accuracy as compared to overnight polysomnography.28,29 The Micro-Mini Motionlogger Actigraph consists of a piezoelectric accelerometer with a sensitivity of 0.01 g at 2.5 Hz. It records activity and rest periods throughout the day and night, providing data for measuring sleep duration based on sleep onset and final awake time, sleep latency, sleep efficiency, and wake after sleep onset (WASO) using algorithms previously validated against polysomnography.29 Sleep parameters were scored using Action-W software (version 2.7.1, Ambulatory Monitoring, Inc., Ardsley, NY, USA) using a sampling frequency of 16 Hz, filtered 2–3 Hz with data collected in 1-min epochs. Students were informed about the proper use of the actigraph during a research educational session prior to study initiation. They were instructed to wear the device on their non-dominant wrist for the entire duration of the study, including the weekend prior to DST (baseline), the DST weekend, and the weekend post-DST and return the actigraph to the school on the second Monday following DST, for a total recording period of 16 nights (2 full weeks and 3 weekends).
Sleep Diary
The Consensus Sleep Diary for Morning (CSD-M) was used to collect data regarding participants' sleep patterns during the study period. The CSD-M is a standardized, expert consensus diary recommended for research use to facilitate data comparison across studies. It consists of a 2-page landscape diary, containing 15 brief itemized questions regarding sleep behaviors to be completed upon awakening.30 The diary provides information about a number of sleep-related metrics, including nightly sleep onset latency, awakening after initial sleep onset, total sleep time, sleep efficiency, sleep quality, and naps. Each participant was instructed to leave the sleep diary forms pasted to their bathroom mirror, in order to fill the questionnaire every morning upon awakening. A new 2-page diary was used for the post- DST week and all questionnaires were collected from the students at the end of the study.
Daytime Performance
Psychomotor Vigilance Test (PVT-B)
This is a short, validated objective test of vigilance and cognitive performance associated with sleep loss.20,31 Vigilant attention is measured by quantifying the response time to a visual stimuli displaying randomly every 2 to 10 sec during a 3- to 10-min period.31,32 The PVT does not require extensive training, can be quickly learned, and has the advantage of not being memorized after repeated use.32,33 A computerized version of the 3-min PVT (version 2.0.5.9, Pulsar Informatics, Philadelphia, PA, USA) was used, with a random stimulus interval of 2–5 seconds. Students were tested on a laptop under the supervision of the research assistant in a quiet room. Each student was tested at a specific pre-set time which remained the same during the study period in order to avoid potential circadian effect on the PVT results (e.g., student A was always tested at 11:00, student B was always tested at 11:30). Students were instructed to observe a red rectangular box on the screen for 3 min and press the spacebar on the keyboard each time a yellow counter appeared on the monitor.32 Their vigilant attention was measured based on the response time in milliseconds (ms) during each session. The mean reaction time, number of lapses (response time ≥ 355 ms), mean reciprocal response time, and mean slowest 10% response time were recorded for each participant during each session.
Sleepiness Scale
Self-reported indices of sleepiness were collected using the Karolinska Sleepiness Scale (KSS). The KSS is a validated 9-point scale employed to measure the perceived level of sleepiness at a point in time.34–36 It consists of one question, stating: “Please, indicate your sleepiness during the 5 minutes before this rating through circling the appropriate description.” The potential responses are listed underneath, ranging from extremely alert (score = 1) to very sleepy, great effort to keep awake, fighting sleep (score = 9). Students were asked to rate their level of sleepiness at the same time each school day, immediately before completing the PVT under the supervision of the research staff.
Data Analysis
Data analyses were performed using SPSS (version 22, IBM Corporation, Armonk, NY, USA). Data averaged from Monday-Friday of the week preceding the DST shift were compared with data from the school week post-DST. Comparisons were also made between the individual days of the week for all tested measures (e.g., Monday preceding DST was compared to the Monday post-DST, and so on). All subjects acted as their own controls; therefore, comparisons were performed using repeated measures t-tests using a Holm sequential Bonferroni method to control for type I error.37 The error rate for each family was set at α = 0.05. Therefore, if a p value was listed as part of the t-test result, the level of significance would be measured against an adjusted α designated as α1 – αk, where k is equal to the number in the sequence of the pairwise comparisons.
RESULTS
A total of 40 students from 9th to 12th grade volunteered to participate in this research. They were recruited after their own and parental consents were obtained. Each participant was given an ID number, which was assigned to all the study material in order to ensure confidentiality. Five students did not complete the study (one student did not attend school for 5 days following his enrolment in the study and 4 failed to keep ≥ 2 of their scheduled daytime testing appointments). Data from 35 students were analyzed. Their mean age was 16.5 ± 0.89 years (range 15–18), and 43% (n = 15) were boys.
Sleep Duration
Objective measurements of sleep duration revealed a decreased sleep time on the weeknights post-DST. Based on actigraphy, the cumulative sleep loss on the post-DST weeknights was 2 h 42 min, as compared to the pre-DST period. The average sleep duration was 7 h 51 min per night on the baseline weeknights and 7 h 19 min on the weeknights post-DST. This reflects a mean sleep loss of 32 min/night over the weekdays following DST (p = 0.001, α1 = 0.025), as shown on Table 1.
Table 1.
Mean values of sleep parameters and sleepiness during the baseline (pre-DST) week in all students.
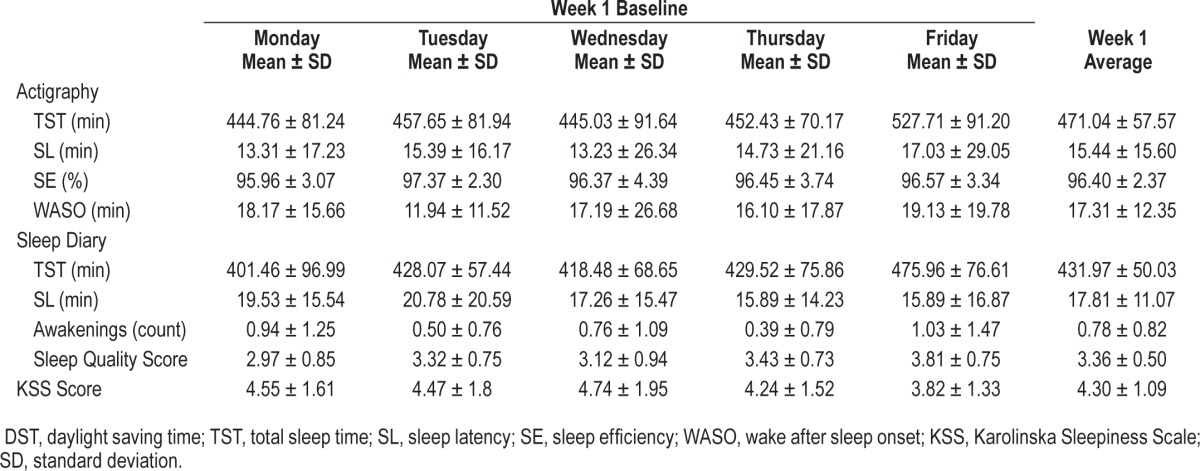
The subjective total sleep time, based on daily sleep diary, showed similar results, with a cumulative sleep loss of 2 h 14 min during the week and a mean sleep loss of 27 min per night post-DST compared to the baseline week (p = 0.004, α1 = 0.05). Subjective weeknight sleep duration documented by sleep diary strongly correlated (r = 0.71, p < 0.001) with total sleep time quantified by actigraphy.
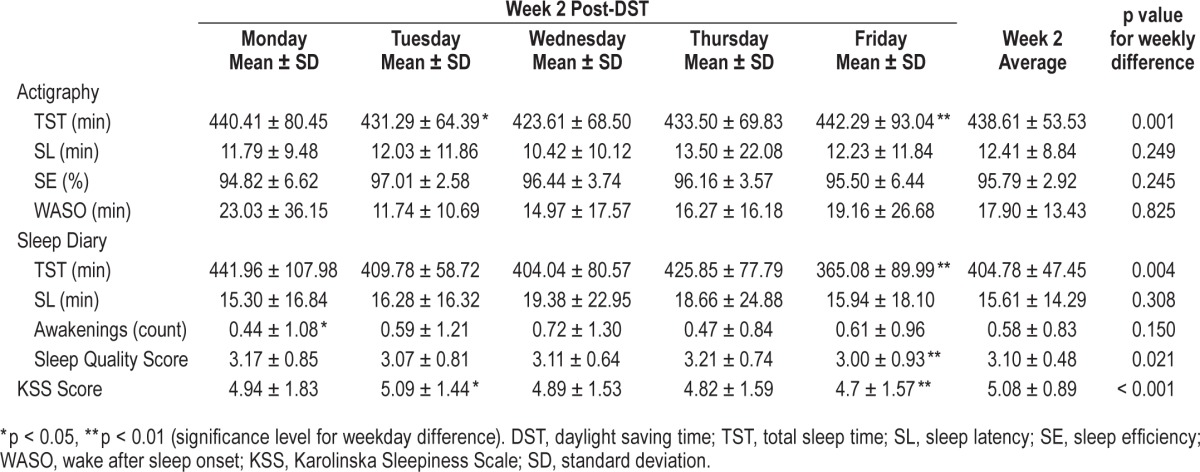
There were no statistically significant changes in sleep efficiency, sleep latency, or WASO post-DST. Mean sleep efficiency levels by actigraphy were high during the entire study period, ranging from 95% to 97%, with no measurable change in relation to DST. Detailed data from actigraphy and sleep log variables for each week are shown on Tables 1 and 2.
Table 2.
Mean ± standard deviation values of sleep parameters and sleepiness during the post-DST week in all students.
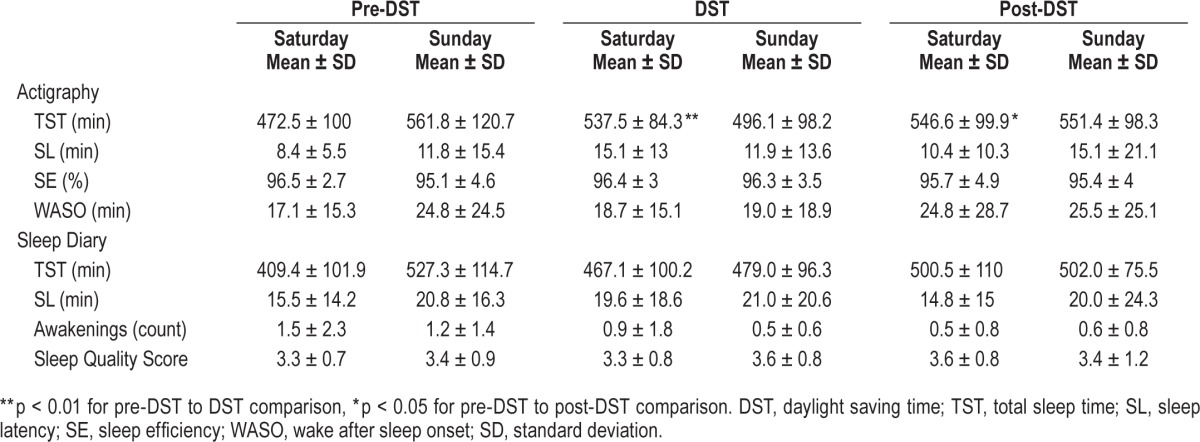
Objective weekend sleep duration by actigraphy showed an increase in sleep duration on the Saturdays during DST and post-DST, as compared to the pre-DST weekend. Despite a trend towards longer sleep duration on the post-DST weekend by actigraphy, there was no statistically difference in the average sleep duration for the 3 weekends. The average sleep duration on the weekend prior to DST was 8 h 36 min ± 15 min, on the DST weekend was 8 h 35 min ± 16 min and the post- DST was 9 h 9 min ± 16, min (p = 0.21) as shown on Table 3.
Table 3.
Mean values of sleep parameters by actigraphy and sleep diary on the weekends pre-DST, during and post-DST in all students.
Daytime Performance
Subjective and objective measurements of daytime sleepiness and vigilance, using the KSS questionnaire and PVT performed during the school days preceding and following DST implementation were analyzed. Increased daytime sleepiness during the weekdays post-DST was seen, with an average increase in mean KSS score during the post-DST week of 0.78 points (p < 0.001), as compared to baseline.
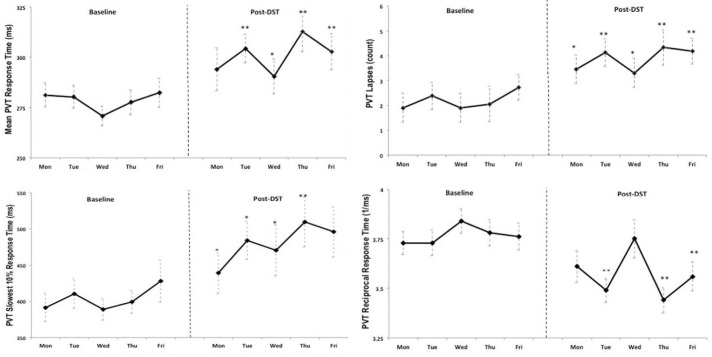
A decline in psychomotor vigilance was seen during the week post-DST as determined by students' mean reaction time, number of lapses, and reciprocal reaction time. These post-DST changes were all highly significant (p < 0.001, α1 = 0.05 - α4 = 0.0125). There was also an increase in the slowest 10% of reaction times, with a statistically significant increase of 68 ms post-DST (p = 0.003, α5 = 0.01). This effect persisted during the entire week; in fact, it increased in magnitude toward the end of the week (Thursday and Friday post-DST) as shown in Figure 1. A mixed model analysis including gender and age as between subject variables failed to reveal a statistically significant relationship with PVT in this group (eta square 0.116, p = 0.167).
Figure 1. Mean response time on PVT tests performed by all students at baseline and post-DST.
PVT, psychomotor vigilance testing; DST, daylight saving time.
DISCUSSION
To our knowledge, this is the first study to quantify the detrimental effects of DST implementation using objective measurements of sleep duration and vigilance in students attending high school. Maintenance of sleep homeostasis after this one-hour time loss can be particularly difficult for adolescents and younger adults.10,12,22–24 Our analyses demonstrated a decline in sleep duration with a cumulative sleep loss of more than two hours on the week following DST implementation. Furthermore, an increase in subjective daytime somnolence and a drop in vigilance test performance were seen in these high school students during the school days following DST, as compared to baseline. These are critical findings for our young population, who already are exposed to sleep insufficiency given their propensity for a delayed sleep phase, and chronic partial sleep deprivation related to school hours, homework, sports, electronics use, and social activities.25,38,39
For several years now, researchers have been concerned about the potential acute effects of the DST on sleep, given the evidence that a circadian rhythm adaptive response to this one-hour time change may take several days to be completed.11–16 In our study, the effects of DST on total sleep time, subjective daytime sleepiness, and vigilance in adolescents were statistically significant during the week following DST as compared to the week prior to DST implementation. This decline in sleep duration and vigilance post-DST may lead to serious public health concerns, as this population includes inexperienced drivers and adolescents often trusted in the care of younger children.
The major strengths of our study include the fact that the design involved a natural experiment and objective measurements of sleep parameters and vigilance were used to evaluate students in their usual environment. Our results cannot be explained by a tendency to decrease sleep duration and worsen performance during the school year, given the magnitude of the findings. Despite our small sample size, clear evidence of acute sleep loss with measurable effects on daytime performance was demonstrated in this group, which was not explained by chronotype differences among the students using sleep midpoint by actigraphy as a surrogate marker.
Several limitations of this study need to be taken into consideration. These include the small sample size and the homogenous characteristics of this group regarding age and geographic location in Westchester County in the Northeast U.S., which may introduce a sample bias and affect the generalizability of the results. In addition, the timing of vigilance test, which was intentionally performed at the same clock time on the weeks pre- and post-DST, may have introduced a measurement bias given the potential underlying circadian effect of the one-hour change on students' performance. Furthermore, our study only monitored students for one week post-DST, and it is possible that a longer period is needed to fully quantify the duration of DST effects on sleep and vigilance.12 Potential modifiers of the sleep loss effect associated with DST in adolescents include genetic and intrinsic sleep patterns, including changes in circadian rhythm and a delay in the sleep phase, which were beyond the scope of this study.22 More detailed approaches to determine the relationship of intrinsic sleep phase in high school students, such as dimmed-light melatonin onset may be an interesting approach to determine the modulatory effect of circadian rhythm on performance after DST changes.
Intrinsic sleep characteristics and external factors have, over the years, contributed to generating a large group of sleep deprived youngsters.40,41 Insufficient sleep and increased daytime sleepiness in adolescents has been extensively documented25,42; therefore, as a society, measures to preserve sleep instead of hinder it should become a health priority. Concerns regarding the immediate effect of DST implementation have been raised by several researchers; however, up to now, no clear evidence was present to advocate for changes in DST or in the school schedule on the week following DST. Our study opens the door for discussions regarding the unintended effects of DST in a susceptible population and consideration for measures that may attenuate these effects. Adapting the school and testing schedule during the post-DST week should be considered along with reassessing the true value for society, beyond energy savings, of advancing the clock at this time of the year.6,24,43
In conclusion, our study results show that sleep in adolescents may be hindered by the early spring DST adjustment, leading to a decline in cognitive function. Given that DST has been implemented in many countries as an economic measure, its impact on sleep health deserves further investigation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors sincerely thank the students and their families for consenting to participate in this research and the kind support received from the school and the science research faculty. Authors' contributions: Diana Medina—literature search, study design, recruitment, data collection, data analysis, manuscript review. Dr. Ebben—study design, data analysis, data interpretation, manuscript writing. Sara Milrad—literature search, data collection, data interpretation, manuscript writing. Brianna Atkinson—literature search, data collection, manuscript review. Dr. Krieger—literature search, study design, data collection, data analysis, data interpretation, manuscript writing.
ABBREVIATIONS
- CSD-M
Consensus Sleep Diary for Morning
- DST
daylight saving time
- KSS
Karolinska Sleepiness Scale
- PVT
psychomotor vigilance testing
- SE
sleep efficiency
- SL
sleep latency
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.United States Statutes at Large. The Calder Act of 1918. Pub. L. 65-106, 1918; ch. 24, § 2, 19 Mar 1918, 40 Stat. 451.
- 2.United States Statutes at Large. Uniform Time Act of 1966. Pub. L. 89-387, 1966; §2, 13 April 1966, 80 Stat. 107.
- 3.United States Statutes at Large. Uniform Time Act of 1966. Pub. L. 99-359, 1986; §2(b), 8 July 1986, 100 Stat. 764.
- 4.Goodman A, Paskins J, Mackett R. Day length and weather effects on children's physical activity and participation in play, sports, and active travel. J Phys Act Health. 2012;9:1105–16. doi: 10.1123/jpah.9.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Statutes at Large. Energy Policy Act of 2005. Pub. L. 109-58, 2005; title I, §110(a), 8 Aug 2005, 119 Stat. 615.
- 6.Belzer DB, Hadley SW, Chin S-M. Impact of extended daylight saving time on national energy consumption: technical report. US Department of Energy Office of Energy Efficiency and Renewable Energy. 2008 [Google Scholar]
- 7.Statutes of Hawaii, United States. L 1947, c 161, §1; RL 1955, §1-42; am L. 1967, c 4, §2; HRS §1-31.
- 8.United States Statutes at Large. Uniform Time Act of 1966. Pub. L. 106-564, §1(c), 23 Dec 2000, 114 Stat. 2811.
- 9.Statutes of Arizona, United States. §1-242, 1967.
- 10.Gaski JF, Sagarin J. Detrimental effects of daylight-saving time on SAT scores. J Neurosci Psychol Econ. 2011;4:44–53. [Google Scholar]
- 11.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Cur Biol. 2007;17:R44–5. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock's seasonal adjustment is disrupted by daylight saving time. Curr Biol. 2007;17:1996–2000. doi: 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Monk TH, Folkard S. Adjusting to the changes to and from Daylight Saving Time. Nature. 1976;261:688–9. doi: 10.1038/261688a0. [DOI] [PubMed] [Google Scholar]
- 14.Monk TH, Aplin L. Spring and autumn daylight saving time changes: studies of adjustment in sleep timings, mood, and efficiency. Ergonomics. 1980;23:167–78. doi: 10.1080/00140138008924730. [DOI] [PubMed] [Google Scholar]
- 15.Lahti TA, Leppamaki S, Lonnqvist J, Partonen T. Transitions into and out of daylight saving time compromise sleep and the rest-activity cycles. BMC Physiol. 2008;8:3. doi: 10.1186/1472-6793-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison Y. The impact of daylight saving time on sleep and related behaviours. Sleep Med Rev. 2013;17:285–92. doi: 10.1016/j.smrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 19.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep deprivation. New York: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 20.Basner M, Dinges DF. An adaptive-duration version of the PVT accurately tracks changes in psychomotor vigilance induced by sleep restriction. Sleep. 2012;35:193–202. doi: 10.5665/sleep.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson RT. After Effect of Sleep Deprivation. J Exp Psychol. 1963;66:439–42. doi: 10.1037/h0047363. [DOI] [PubMed] [Google Scholar]
- 22.Schneider AM, Randler C. Daytime sleepiness during transition into daylight saving time in adolescents: are owls higher at risk? Sleep Med. 2009;10:1047–50. doi: 10.1016/j.sleep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Tonetti L, Erbacci A, Fabbri M, Martoni M, Natale V. Effects of transitions into and out of daylight saving time on the quality of the sleep/wake cycle: an actigraphic study in healthy university students. Chronobiol Int. 2013;30:1218–22. doi: 10.3109/07420528.2013.812651. [DOI] [PubMed] [Google Scholar]
- 24.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31:175–84. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 25.Wolfson AR, Carskadon MA. Understanding adolescents' sleep patterns and school performance: a critical appraisal. Sleep Med Rev. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- 26.Rupp TL, Balkin TJ. Comparison of Motionlogger Watch and Actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav Res Methods. 2011;43:1152–60. doi: 10.3758/s13428-011-0098-4. [DOI] [PubMed] [Google Scholar]
- 27.Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–66. doi: 10.5665/sleep.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 29.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–55. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Methods Instrum Comput. 2004;36:339–46. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- 32.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut. 2011;69:949–59. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 35.Kecklund G, Akerstedt T. Sleepiness in long distance truck driving: an ambulatory EEG study of night driving. Ergonomics. 1993;36:1007–17. doi: 10.1080/00140139308967973. [DOI] [PubMed] [Google Scholar]
- 36.Kaida K, Takahashi M, Akerstedt T, et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117:1574–81. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Holm S. A simple sequential rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 38.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–84. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46:124–32. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Carskadon MA, Dement WC. Sleepiness in the normal adolescent. In: Guilleminault C, editor. Sleep and its disorders in children. New York: Raven Press; 1987. pp. 53–66. [Google Scholar]
- 41.Thorpy MJ, Korman E, Spielman AJ, Glovinsky PB. Delayed sleep phase syndrome in adolescents. J Adolesc Health Care. 1988;9:22–7. doi: 10.1016/0197-0070(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 42.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 43.Bartky I, Harrison E. Standard and Daylight-Saving Time. Sci Am. 1979;240:46–53. [Google Scholar]