Abstract
Study Objectives:
To describe preoperative and postoperative sleep disruption and its relationship to postoperative delirium.
Design:
Prospective cohort study with 6 time points (3 nights pre-hospitalization and 3 nights post-surgery).
Setting:
University medical center.
Patients:
The sample consisted of 50 English-speaking patients ≥ 40 years of age scheduled for major non-cardiac surgery, with an anticipated hospital stay ≥ 3 days.
Interventions:
None.
Measurements and results:
Sleep was measured before and after surgery for a total of 6 days using a wrist actigraph to quantify movement in a continuous fashion. Postoperative delirium was measured by a structured interview using the Confusion Assessment Method. Sleep variables for patients with (n = 7) and without (n = 43) postoperative delirium were compared using the unpaired Student t-tests or χ2 tests. Repeated measures analysis of variance for the 6 days was used to examine within-subject changes over time and between group differences. The mean age of the patients was 66 ± 11 years (range 43–91 years), and it was not associated with sleep variables or postoperative delirium. The incidence of postoperative delirium observed during any of the 3 postoperative days was 14%. For the 7 patients who subsequently developed postoperative delirium, wake after sleep onset (WASO) as a percentage of total sleep time was significantly higher (44% ± 22%) during the night before surgery compared to the patients who did not subsequently developed delirium (21% ± 20%, p = 0.012). This sleep disruption continued postoperatively, and to a greater extent, for the first 2 nights after surgery. Patients with WASO < 10% did not experience postoperative delirium. Self-reported sleep disturbance did not differ between patients with vs. without postoperative delirium.
Conclusions:
In this pilot study of adults over 40 years of age, sleep disruption was more severe before surgery in the patients who experienced postoperative delirium. A future larger study is necessary to confirm our results and determine if poor sleep is associated with delirium in larger samples and what specific sleep problems best predict postoperative delirium in older surgical patients.
Citation:
Leung JM, Sands LP, Newman S, Meckler G, Xie Y, Gay G, Lee K. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med 2015;11(8):907–913.
Keywords: actigraphy, surgery, postoperative delirium, preoperative sleep disruption
Delirium is a major challenge facing geriatric practice due to its prevalence, complex etiology, and potential severe impact on patients. Postoperative delirium is associated with longer hospital stays, worse functional outcomes, higher healthcare costs, and increased mortality.1 Delirium develops through a complex interaction between the patient's baseline vulnerability (predisposing risk factors before hospitalization) and precipitating factors or insults (events that occur during hospitalization). Some of the vulnerability factors identified include advanced age, cognitive impairment or dementia, and preexisting comorbidities.2,3 Over the last two decades, a large body of literature has focused on the clinical manifestations, risk factors and outcomes of postoperative delirium. Sleep disruption, in particular, has frequently been cited as an important etiological factor associated with the development of delirium.4
Sleep disturbance, especially sleep fragmentation, and poor sleep quality are commonly observed in older adults. In the hospital, environmental factors and health care practices further contribute to sleep disruption.5 These factors include noise, continuous ambient light, and frequent performance of vital sign measures and tests. Although several small studies have demonstrated that patients sleep poorly after surgery,6–8 it is not clear if the sleep disruption began immediately after surgery or if sleep problems were preexisting. Knowing when sleep disruption occurs will inform optimal timing for interventions.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disruption has frequently been cited as an important etiological factor associated with the development of delirium. It is not clear if the sleep disruption began immediately after surgery or if sleep problems were preexisting.
Study Impact: Our novel finding of sleep disruption beginning before surgery provides important guidance for clinical assessment and interventions to improve sleep before surgery. Specifically, future work should target the etiology of nocturnal wake time in the period immediately before the planned surgery.
Therefore we designed a study to examine the temporal relationship between disturbed sleep and postoperative delirium in older patients. We were particularly interested to determine the degree of sleep disruption prior to surgery and its influence on the occurrence of postoperative delirium. We hypothesized that patients with substantial sleep disruption before surgery would be at increased risk of postoperative delirium. Furthermore, we hypothesized that patients with sleep disruption before surgery would have an increased risk of postoperative delirium.
METHODS
Patient Recruitment
The study was approved by the University of California, San Francisco Committee on Human Research, and informed consent was obtained preoperatively from each study patient. The study took place at the University of California, San Francisco Medical Center between May 2012 and September 2013. Inclusion criteria included English-speaking patients ≥ 40 years of age undergoing noncardiac surgery requiring anesthesia, with anticipated hospital stay ≥ 3 days after surgery. Patients were not excluded because of preexistent comorbidities such as hypertension or obstructive sleep apnea. Patients who were unable to provide informed consent were excluded.
Wrist Actigraphy
The gold standard for measuring sleep stages is polysomnography. However, considerable limitations exist for this technology for surgical patients, particularly the limited tolerability by patients over an extended monitoring period. In contrast, a wrist actigraph can be used to estimate sleep and wake time over an extended period. Actigraphy was used to measure preoperative and postoperative sleep in this study because of the feasibility of wearing the device at home and in the hospital. Furthermore, actigraphy has been shown to have good agreement with polysomnography for total sleep time and wake time during the night.9
Study patients wore a Mini Motionlogger Actigraph (Ambulatory Monitoring, Inc., Ardsley, NY) for 72 h at home before the planned surgery. After surgery, before leaving the post-anesthesia recovery unit, the wrist actigraph was reattached to the patient, who continued to wear the device for 72 h after surgery.
Trained research assistants analyzed the actigraph data using the autoscoring Cole–Kripke algorithm program available in Action4 software to reduce any researcher scoring bias (Ambulatory Monitoring, Inc., Ardsley, NY). Activity level was sampled in 1-min epochs. The Cole-Kripke algorithm calculated total sleep time (TST), latency from the time the event marker was pressed to onset of sleep (sleep onset latency) number of awakenings, and percent of TST that the participant spent awake after falling asleep (WASO%) to estimate sleep disruption.
Bedtime and final wake times were determined by the diary entry of clock times that matched with a 50% change in movement during the same 10-min block of time on actigraphy. To estimate daytime sleep, the time after final awakening to the next bedtime was marked, and sleep diary notations of nap times were noted. Daytime minutes of sleep were calculated after excluding any time when the monitor was off the patient's wrist.10
In addition to wearing the actigraph, the patients completed 2 preoperative sleep questionnaires: (1) General Sleep Disturbance Scale (GSDS), consisting of 21 items that query for information about a person's quality and quantity of sleep in the past week, daytime sleepiness and fatigue, and types of sleep medications11; and (2) the Pittsburgh Sleep Quality Index (PSQI) before surgery and at 30 and 90 days after surgery.12 The PSQI asks about perception of sleep quality during the past month and is a valid and reliable measure of habitual sleep patterns. After surgery while in the hospital, patients also completed a sleep diary (St. Mary's Hospital Questionnaire) during the 3 postoperative days, regarding their bedtime, wake time, number of awakenings, and self-perceived sleep latency.13
Cognitive and Delirium Assessments
Each patient was interviewed preoperatively and on each of the first 3 postoperative days. The preoperative interview typically occurred less than one week prior to surgery in the preoperative clinic. Cognitive status was measured before surgery using the Telephone Interview of Cognitive Status. The Telephone Interview of Cognitive Status is an 11-item screening test that was originally developed to assess cognitive function in patients with Alzheimer dementia who were unable to be evaluated in person.14 The Telephone Interview of Cognitive Status has been compared to the Mini Mental State exam and found to have similar scores that allowed for standardized comparison.15 During the preoperative and the 3 postoperative interviews, the presence of delirium was measured using the Confusion Assessment Method (CAM).16 The CAM assessments were performed daily on the first 3 days after surgery between the hours of 09:00 and 12:00, using structured interview. The CAM assessment was developed as a screening instrument based on operationalization of Diagnostic and Statistical Manual of Mental Disorders (DSM)-III-R criteria for use by non-psychiatric clinicians in high-risk settings. Based on a structured interview, the CAM algorithm consists of 4 clinical criteria: acute onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness. The determination of delirium requires that both the first and second criteria be present, and either the third or fourth criterion must also be evident. CAM has a sensitivity of 94% to 100% and a specificity of 90% to 95%, has a high interobserver reliability,16 and has convergent agreement with 4 other mental status tests. During the interviews, trained interviewers determined the presence of delirium using the CAM. All assessments of postoperative delirium were validated by a second investigator (LPS). We defined the occurrence of delirium as the patient meeting CAM criteria for delirium on any of the 3 postoperative day assessments.
Demographic and Other Information
The patient's past health history and other demographic information were abstracted from the medical chart or in-person interview. Current preoperative and postoperative pain at rest was measured prospectively by a visual analog scale (range 0 = no pain, to 10 = maximum pain). Activities of daily living and instrumental activities of daily living17 and instrumental activities of daily living were assessed preoperatively,18 typically within one week of the planned surgery. If a patient could not independently perform without assistance in one or more of the activities, that patient would be considered to have dependence on the activities listed. Preoperative symptoms of depression was measured using the 15-item Geriatric Depression Scale (GDS).19 Perioperative risk was estimated using the Charlson Comorbidity index,20 and American Society of Anesthesiologists classification,21 and surgical risk was estimated using the guidelines from the American College of Cardiology and American Heart Association update for the perioperative cardiovascular evaluation for noncardiac surgery which took into consideration of the type of surgery, intraoperative blood loss, and surgical duration.22
Statistical Analysis
The amount of wake after sleep onset (WASO) in minutes after falling asleep obtained from wrist actigraphy was standardized by the participant's total sleep time and also expressed as WASO%. Sleep variables between the patients with and without postoperative delirium were compared using unpaired Student t-tests. Bivariate analysis for categorical variables were conducted using either χ2 or Fisher exact tests. All analyses were conducted in SAS 9.3. Normally distributed data are reported as mean ± SD and compared using t-test. Continuous variables that were not normally distributed were compared using the Wilcoxon rank sum test and the data are reported as median (IQR) that includes the 25th and 75th percentile.
RESULTS
This analysis includes a total of 50 patients with complete data. An additional 10 patients were excluded because of equipment failure (n = 4) or surgical cancellation after preoperative monitoring (n = 6). The mean age of the patients was 66 ± 11 years (range 43–91 years). Approximately half (48%) were women. Additional preoperative demographic data are shown in Table 1.
Table 1.
Preoperative demographic data of the study patients.
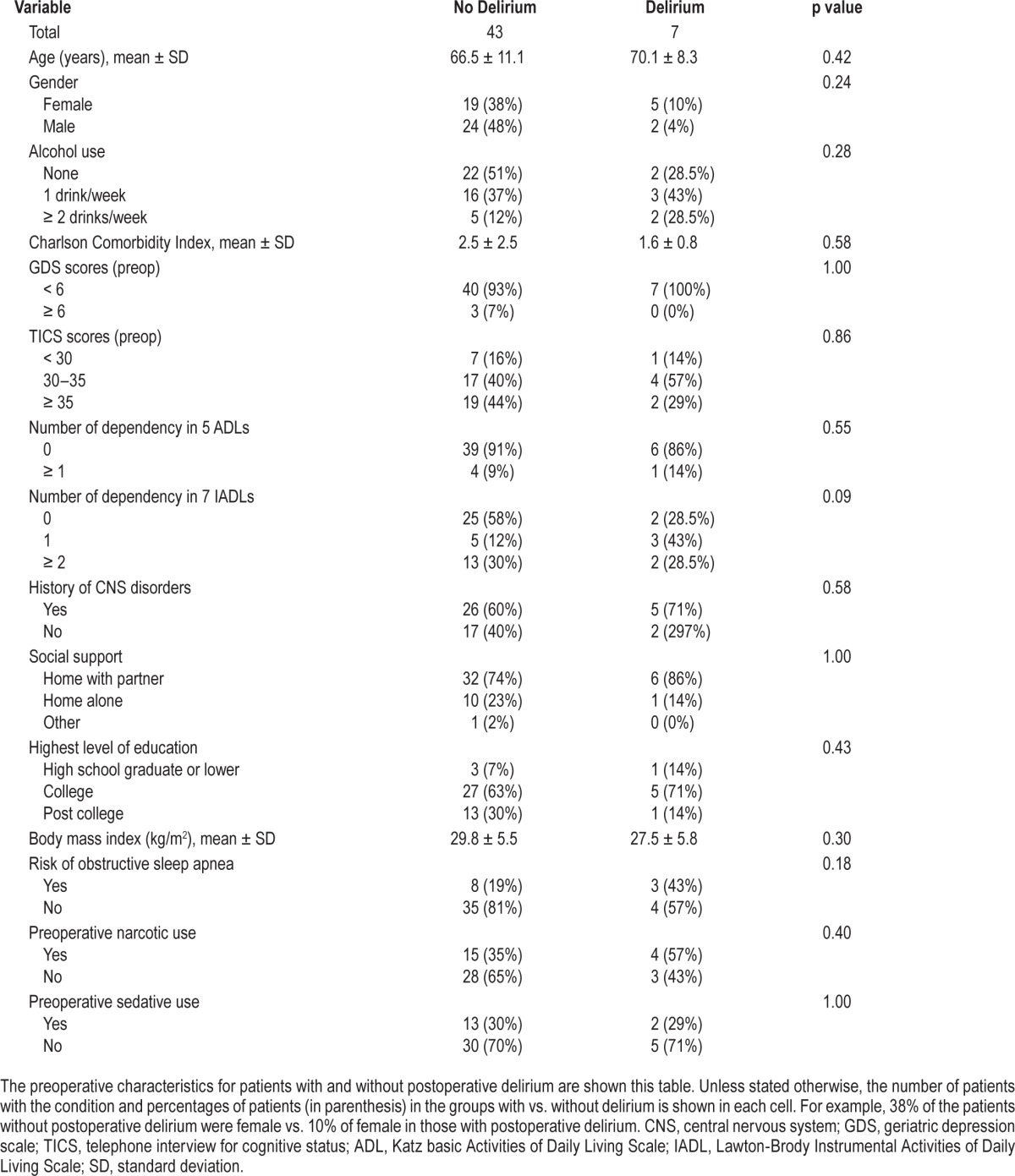
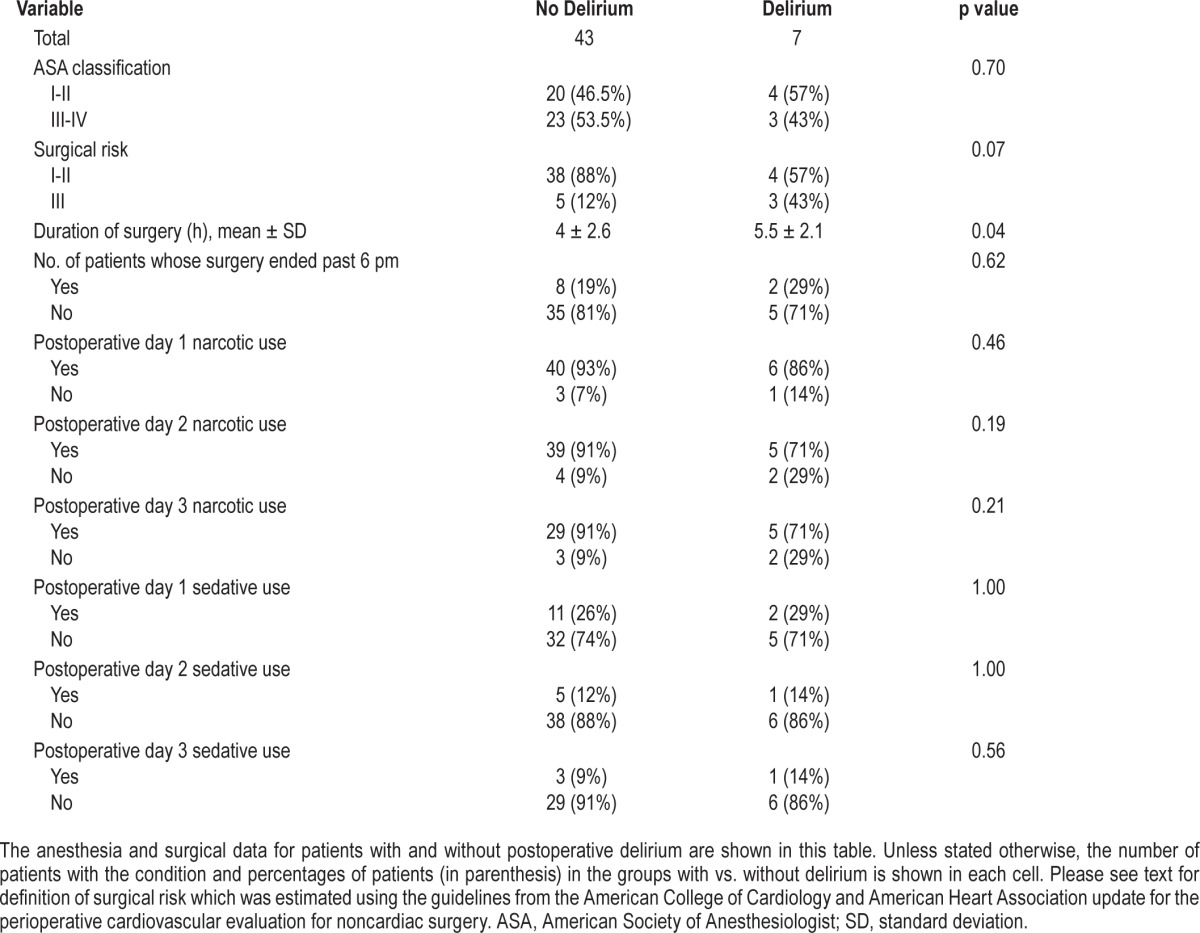
No patient had preoperative delirium. The overall incidence of postoperative delirium observed during any of the 3 postoperative days was 14% (n = 7 of the 50 patients). The incidence of postoperative delirium in patients who reported no pain before surgery (15.4%) was not significantly different from patients who reported preoperative pain (13.5%). By bivariate analyses, patients who developed postoperative delirium had longer surgical duration but when surgery ended was not different between those with vs. without delirium (Table 2).
Table 2.
Anesthesia and surgical data of the study patients.
Self-reported sleep disturbance scores for the past week (GSDS) did not differ between patients with delirium (45.7 ± 16.2) and without delirium (40.5 ± 11.1, p = 0.86). Importantly, patients with < 90 min of WASO or who had < 10% WASO preoperatively did not experience postoperative delirium.
By actigraphy, the preoperative sleep for patients who ultimately developed postoperative delirium showed disruption beginning two days before surgery. For patients who subsequently developed postoperative delirium, wake time (221 ± 93 min; 43% ± 22%) was significantly higher on the night before surgery than in patients who did not develop delirium (109 ± 87 min; 21% ± 20%; Table 3). This pattern of sleep disruption as represented by mean % time WASO continued for the first 2 nights after surgery (Figure 1).
Table 3.
Sleep characteristics (by actigraphy) the night immediately before surgery stratified by postoperative delirium status.
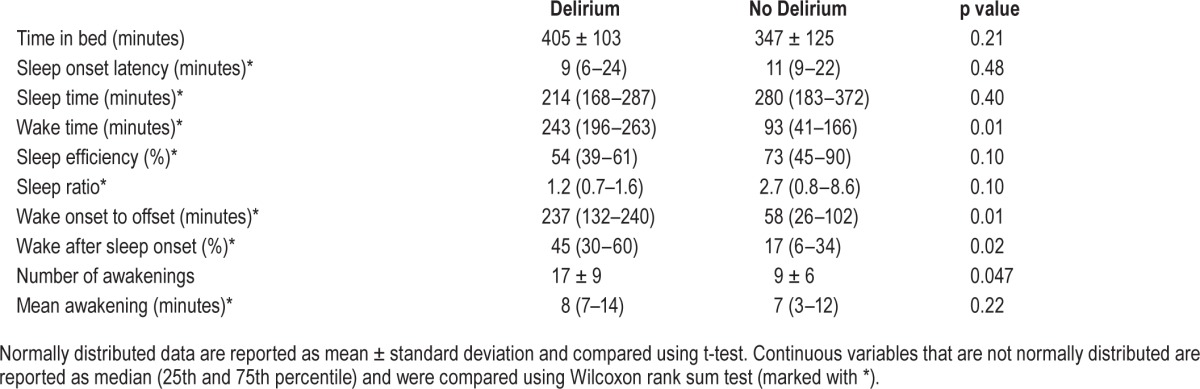
Figure 1. The mean number of awakenings as recorded by actigraphy is shown here for patients with no delirium (solid line) vs. those with delirium (dotted line) for the 6 monitoring periods (nightly for three nights before surgery and three nights after surgery).
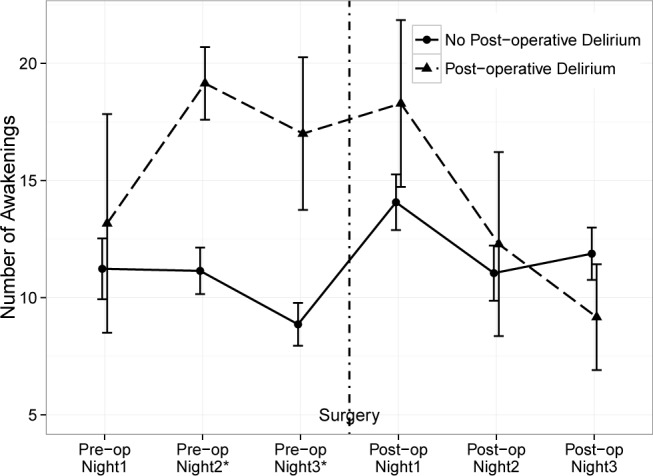
The error bars are mean ± standard error. *p value of Mann-Whitney U test at that monitoring period is less than 0.05.
In addition to WASO, we also examined the number of awakenings as measured by actigraphy for patients with postoperative delirium vs. patients without delirium (Figure 2). This plot shows that the mean number of awakenings began to increase 2 nights before surgery and became significantly higher only on the night before surgery for the group who subsequently developed postoperative delirium.
Figure 2. The mean percent time of time spent awake after falling asleep (WASO) is plotted for patients with no delirium (solid line) vs. those with delirium (dotted line) for the 6 monitoring periods (nightly for three nights before surgery and three nights after surgery).
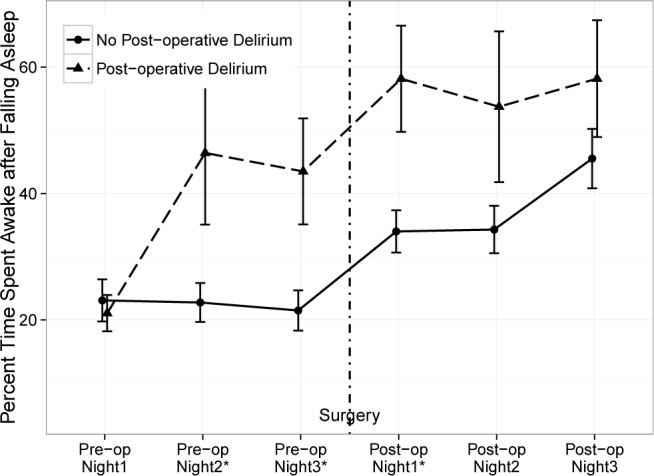
The error bars are mean ± standard error. *p value of Mann-Whitney U test at that monitoring period is less than 0.05.
Overall, the PSQI score at baseline was not a predictor, and GSDS scores did not differ between patients with delirium (45.7 ± 16.2) and without delirium (40.5 ± 11.1, p = 0.86).
DISCUSSION
Our study demonstrates that sleep disruption in older surgical patients in fact began in the period before hospital admission and surgery, and continued for the first two nights after surgery while still hospitalized. Objectively measured preoperative sleep disruption was associated with postoperative delirium. This novel finding of sleep disruption beginning before surgery provides important guidance for clinical assessment and interventions to improve sleep before surgery by assessing the baseline sleep characteristics of patients and considering the etiology of nocturnal wake time in the period immediately before the planned surgery.
A recent review of actigraphy for measurement of sleep in relation to surgery found 32 studies with relevant data, and reported that total sleep time and sleep efficiency was reduced after surgery, and number of awakenings was increased in patients undergoing major surgery.23 However, in this review, no mention was made of whether sleep was already disturbed before surgery. A systematic review of the literature also shows no previous study that has evaluated the trajectory of sleep patterns in the perioperative period in patients awaiting major surgery.
If sleep disruption occurs before the onset of surgery, what are the potential reasons for such changes? In a small study of women who were scheduled for surgery for diagnosis or treatment of breast cancer, Wright et al. monitored these patients with actigraphy the night before surgery and reported that intrusive thoughts, anxiety and emotional well-being were each related to sleep duration the night before surgery,7 but these investigators did not continue actigraphy monitoring after surgery. Kain et al. studied 92 patients undergoing outpatient elective surgery and found no significant differences in sleep measures according to wrist actigraphy when compared with 35 community controls.6 However, their sample had a mean age of 39 years.
In our study, pain at preoperative and postoperative assessments did not differ between patients with and without delirium. Fielden et al. reported that hip pain was the most common explanation for frequent nocturnal awakenings before surgery in patients awaiting total hip arthroplasty.8 Another potential cause for sleep disruption is obstructive sleep apnea (OSA). In fact, OSA has been reported to be associated with postoperative delirium.24
In our study, there was a high occurrence of awakenings and sleep inefficiency across the study group. It appears that sleep disruption is common preoperatively, but more so in patients who develop delirium postoperatively. Furthermore, the mean percent time spent awake after falling asleep and the mean number of awakenings were similar on the first day of preoperative recordings, and the differences in these variables between patients with and without subsequent postoperative delirium became larger on the subsequent days of preoperative monitoring as the surgical dates approached, suggesting that anxiety about the upcoming surgery; this needs to be investigated in future studies that include a tool to measure preoperative anxiety.
Taken together, limited data from several previous small studies show that sleep disruption from any cause is a potential risk factor for postoperative delirium. However, no study has investigated when sleep disruption begins, whether patients with sleep disruption can be identified early, and what interventions may be effective to minimize the risk of postoperative delirium. Furthermore, the evidence for interventions to improve sleep continuity in order to reduce postoperative delirium is scarce. In fact, the Institute of Medicine characterizes sleep disorders and sleep deprivation to be “an unmet public health problem” and suggests taking action to “Increase the investment in interdisciplinary sleep programs in academic health centers that emphasize long-term clinical care, training, and research.”25
There are some potential limitations of our study. First, we did not measure the preoperative and postoperative state of anxiety; therefore, we were unable to determine whether it was anxiety that led to sleep disruption before surgery. Second, there were only 7 cases of delirium, and while our 14% incidence is within the expected range for this population, the delirium clinical assessment we employed did not include a measure of the severity of delirium, and this needs to be considered in future studies of sleep and delirium. Thirdly, we measured delirium only once daily, given the fluctuating nature of delirium; we might have underestimated its incidence. Fourthly, this is a small study, which does not allow the consideration of all potential covariates of postoperative delirium, which need to be included in future studies of sleep disruption and postoperative delirium.
Clinical Implications
The preponderance of studies in the literature describes how sleep is disrupted for hospitalized patients. In particular, the emphasis has been on describing how hospital practices and environmental factors create not conducive conditions for achieving a good night's sleep. Our results provide additional evidence to suggest that sleep disruption occurs even before hospitalization for some patients, and this persists in the postoperative period. This result has potential clinical implications to target the preoperative period and risk factors for sleep disruption. These may include targeting patient-specific symptoms such as pain, anxiety, and sleep disordered breathing. In addition, since self-report sleep disturbance did not differ between patients with vs. without subsequent postoperative delirium, there is a need to include more objective measures of sleep disturbance in the preoperative clinical evaluation of at risk patients.
In conclusion, we used minimally invasive wrist actigraphy devices in a group of older patients to estimate sleep before and after major noncardiac surgery. Sleep disruption was evident before surgery, while patients were still residing in their home environment. Moreover, preoperative sleep disruption was associated with postoperative delirium. A larger study with adequate power to include all other potential predictors of postoperative delirium is indicated to validate our results.
DISCLOSURE STATEMENT
This was not an industry supported study. This project was supported in part by the National Institute of Aging, National Institutes of Health, Bethesda, MD, Grant # NIH 1RO1AG031795-05 to Dr. Leung. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- ADL
Katz basic Activities of Daily Living Scale
- CAM
Confusion Assessment Method
- CNS
central nervous system
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- GDS
Geriatric Depression Scale
- GSDS
General Sleep Disturbance Scale
- IADL
Lawton-Brody Instrumental Activities of Daily Living Scale
- OSA
obstructive sleep apnea
- PSQI
Pittsburgh Sleep Quality Index
- SD
standard deviation
- TICS
telephone interview for cognitive status
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Shim J, Leung J. An update on delirium in the postoperative setting: prevention, diagnosis and management. Best Pract Res Clin Anaesthesiol. 2012;3:327–43. doi: 10.1016/j.bpa.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Inouye S, Charpentier P. Precipitating factors for delirium in hospitalized elderly persons: predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- 3.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54:1578–89. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 4.Drouot X, Cabello B, d'Ortho MP, Brochard L. Sleep in the intensive care unit. Sleep Med Rev. 2008;12:391–403. doi: 10.1016/j.smrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol. 2014;29:e2014006. doi: 10.5620/eht.2014.29.e2014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kain ZN, Caldwell-Andrews AA. Sleeping characteristics of adults undergoing outpatient elective surgery: a cohort study. J Clin Anesth. 2003;15:505–9. doi: 10.1016/j.jclinane.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Wright CE, Schnur JB, Montgomery GH, Bovbjerg DH. Psychological factors associated with poor sleep prior to breast surgery: an exploratory study. Behav Med. 2010;36:85–91. doi: 10.1080/08964280903521305. [DOI] [PubMed] [Google Scholar]
- 8.Fielden JM, Gander PH, Horne JG, Lewer BM, Green RM, Devane PA. An assessment of sleep disturbance in patients before and after total hip arthroplasty. J Arthroplasty. 2003;18:371–6. doi: 10.1054/arth.2003.50056. [DOI] [PubMed] [Google Scholar]
- 9.Shinkoda H, Matsumoto K, Hamasaki J, Seo YJ, Park YM, Park KP. Evaluation of human activities and sleep-wake identification using wrist actigraphy. Psychiatry Clin Neurosci. 1998;52:157–9. doi: 10.1111/j.1440-1819.1998.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee KA, Gay CL. Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Res Nurs Health. 2011;34:7–19. doi: 10.1002/nur.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary's Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4:93–7. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Desmond D, Tatemichi TK, Hanzawa L. The Telephone Interview for Cognitive Status (TICS): reliabiility and validity in a stroke sample. Int J Geriatr Psychiatry. 1994;9:803–7. [Google Scholar]
- 15.Fong TG, Fearing MA, Jones RN, et al. Telephone interview for cognitive status: creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement. 2009;5:492–7. doi: 10.1016/j.jalz.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye S, van Dyke C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 17.Katz W, Ford A, Moskokwitz R, Jackson B, Jaffe M. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 18.Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 19.Brink T, Yesavage J, Lum O, Heersema P, Adey M, Rose T. Screening tests for geriatric depression. Clin Gerontol. 1982;1:37–43. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 22.ACC/AHA guideline update for the perioperative cardiovascular evaluation for noncardiac surgery - executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Anesth Analg. 2002;94:1052–64. doi: 10.1097/00000539-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Madsen MT, Rosenberg J, Gogenur I. Actigraphy for measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. 2013;9:387–94. doi: 10.5664/jcsm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116:788–96. doi: 10.1097/ALN.0b013e31824b94fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Academy of Sciences. Washington, DC: National Academy of Sciences; 2006. Sleep disorders and sleep deprivation - an unmet public health problem. [PubMed] [Google Scholar]


