Abstract
Gastric cancer (GC) is considered as most fourth common cancer in the world. Findings from animal, experimental and epidemiologic studies indicate that diet plays an important role in the etiology of stomach cancer. Among dietary factors, Zinc status has received great attention in recent years. The purpose of the present study was to review the association of serum levels of Zinc, dietary intake of Zinc and GC risk. A complete search was performed about the association of Zinc status and risk of GC was in databases electronic through such as ISI web of science, PubMed, Scopus, IrMedx and SID. Our results of current review suggest that dietary intake of Zinc and serum levels of Zinc are lower in GC patient. In other word, high serum levels of Zinc may be protective in GC risk. However, it seems further studies in particular epidemiological studies with large scale setting are required to reach a definite conclusion.
Keywords: Cancer, gastric, stomach, Zinc
INTRODUCTION
Gastric cancer (GC) is considered as most fourth common cancer in the world.[1] Several factors can increase risk of GC. Helicobacter pylori infection and life-style behaviors are associated with risk of GC. Findings from animal, experimental and epidemiologic studies indicate that diet plays an important role in the etiology of stomach cancer.[2] Among dietary factors, Zinc status has received great attention in recent years. Zinc is a trace element found in blood. Most of Zinc of body is in skin, hair and bones (~70%) and the remainder mainly in muscle, kidneys and liver.[3,4] Zinc as constituent or cofactor or enzymes involved in deoxyribonucleic acid (DNA) repair,[5] metallothionein synthesis, which is considered to suppress free-radical production[6] and also have an important role in transcription factor function.[7] Zinc has been known to be a protective agent of cancer; Because it is an essential component of DNA-binding proteins with Zinc finger[8,9,10] contribute to DNA transcriptions and repairs and copper-zinc superoxide dismutase (SOD) that is considered as important free radical scavenger.[11,12]
In humans, an association between Zinc deficiency and risk of cancer has been reported in several dietary studies.[13,14,15] Findings from animal studies[16] have indicated that dietary Zinc deficiency may increases possibility of carcinogenic compounds formation.[17] Data from epidemiological studies in relation with Zinc level and risk of stomach cancer have been quite limited. A significant inverse relationship was reported between risk of upper-digestive tract cancer and dietary Zinc levels among women in a prospective study.[13,14] Zhang et al. performed a case-control study lower Zinc intake was related with the increased risk of esophagus and gastric cardia cancer.[18,19] However, no association between serum Zinc levels and GC risk was obtained in a nested case-control study.[20] In contrast, a significant elevation of serum copper/Zinc SOD level was observed among GC patients compared with the healthy controls in a case-control study.[21] Despite this documents, we conducted current study to review the association of serum levels of Zinc, dietary intake of Zinc and GC risk.
METHODS
A complete search was performed about the association of Zinc status and risk of GC was in databases electronic through such as ISI web of science, Scopus and PubMed (to access of Latin paper) and also in IrMedx and SID (to access of Persian paper) [Figure 1]. Two authors (SK and AD) independently searched papers published until February 2013 using the following key words: “Zinc,” “SOD,” “SOD,” “Zn,” “gastric,” “stomach,” “tumor,” “cancer,” “carcinoma.” No restriction about time of publication or language or study design was made.
Figure 1.
Study design flowchart
RESULTS
Dietary intake of Zinc and risk of GC
Findings from some studies that have examined the association of Zinc intake and stomach cancer risk are provided in below; also the characteristics of some studies are shown in Table 1.
Table 1.
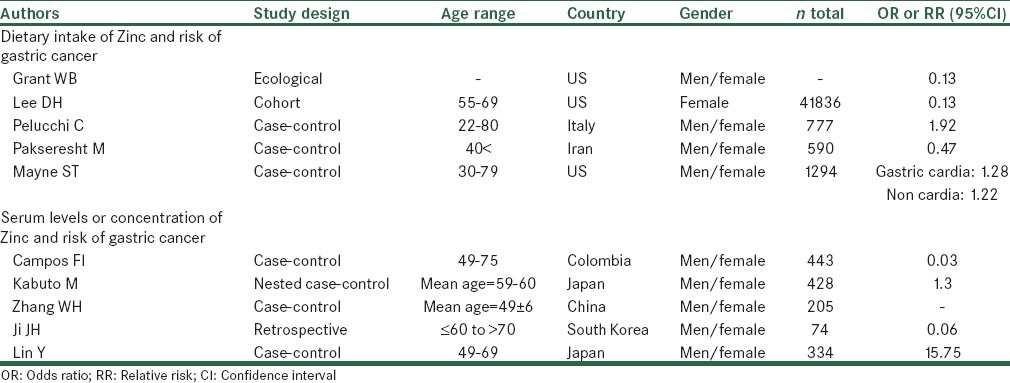
Characteristics of selected studies investigating the correlation between Zinc status and risk of gastric cancer
Grant conducted an ecological study that assessed the relationship between cancer mortality rate and dietary iron and Zinc. The results of the study indicated that dietary Zinc was inversely correlated with bladder, breast, colon, esophageal, gastric, rectal cancer, Hodgkin's lymphoma, laryngeal, nasopharyngeal, oral, skin and vulvar cancers.[22]
The Iowa Women's Health Study was designed to assess risk factors and incidence of cancer in 41,836 postmenopausal women, aged 55-69 years from January 1986 to December 2001. The relationships were examined between dietary Zinc, dietary heme iron and upper digestive tract cancer incidence. Nutrient intake was calculated by multiplying the frequency response by the nutrient content of the specified portion sizes. Nutrient supplements were separately computed from dietary intake. There was a decreased risk in relation to greater intake of Zinc (relative risk: 0.13, 0.03-0.63, P-trend < 0.01). One of Iowa study limitations was lack of consideration more specific tumor sites and histological types due to a small number of cases.[14]
In an Italian case-control study, the association was examined between dietary intake of selected micronutrients and GC risk among 230 patients with GC and 547 controls. Dietary intakes of macro- and micro-nutrients were assessed by using of a reproducible and valid food frequency questionnaire (FFQ) that was considered a major strength of this study. No significant relation obtained between Zinc and stomach cancer (odds ratio [OR]: 1.92, 95% confidence interval [CI]: 0.95-3.88, P: 0.16). There was no information about Helicobacter Pylori infection.[23]
Findings from a case-control study indicated an inverse trend of borderline statistical significance reported between Zinc consumption and risk of GC. For determining dietary Zinc intake was used from FFQ in this study.[19]
A population-based case-control study took place from August 2005 to August 2007. The aim of this study was to examine the relation of Dietary habits and GC risk in north-west Iran. A validated quantitative FFQ included 117 food items was used for assessing dietary intake. Possibility of misclassification of subjects in this study into an incorrect level or category of Zinc was one of these study limitations. An Inverse associations were observed for Zinc intake and risk of GC (OR: 0.47, 95% CI: 0.32-0.70) and its subgroups (cardia, OR: 0.64, 95% CI: 0.33-1.24, non-cardia, OR: 0.46, 95% CI: 0.30-0.70).[24]
Another case-control study examined the relationship of nutrient intakes and risk of subtypes of gastric and esophageal cancer. This paper suggested the association between Zinc intake between risk of gastric cardia and non-cardia GC, separately. Totally, 352 with non-cardia gastric cancer, 255 patients with gastric cardia-adenocarcinoma and 687 controls participated in this study. The investigators observed no significant association between Zinc intake and risk of gastric cardia (OR: 1.28, 95% CI: 0.95-1.72) or non-cardia GC (OR: 1.22, 95% CI: 0.85-0.92-1.60).[25]
There were some limitations in mentioned studies. A possible limitation inherent in case-control study designs is the possibility that cases may have recalled their dietary intakes and supplement-taking practices differently after a cancer diagnosis. Furthermore, there was no information in relation to Helicobacter pylori infection that plays an important role in increasing GC risk.
Serum levels or concentration of Zinc and risk of GC
Results from some studies that have assessed the relationship between serum levels of Zinc and risk of stomach cancer are provided in below:
A hospital-based case-control study was conducted during the period from 2000 to 2002 in Colombia. The aim of this study was to examine the associations of GC risk and the Zinc levels in toenail clippings. The information of dietary habits and Zinc concentrations in toenail clippings were obtained from 156 GC patients and 287 non-cancer patients controls.
An inverse association was obtained between toenail Zinc concentrations and risk of stomach cancer (P for trend = 0.039). When, this relationship was assessed separately for current and former smokers and non-smokers, only a significant inverse association was observed in current-smokers (P for trend = 0.035).[26]
A nested case-control study was conducted in cohort of bomb atomic survivors in Hiroshima and Nakazoki. This study examined the relation of pre diagnostic serum selenium and Zinc levels and subsequent risk of lung and stomach cancer. Serum samples were collected in Hiroshima and Nagasaki from 1970 to 1972 for 208 persons who in 1973-1983 developed GC and for controls matched for sex, age, city and season of blood collection. No significant association was observed between serum levels of Zinc and risk of GC.[20]
Zhang et al. carried out a case-control study in 45 patients of GC and 44 matched (age and sex) patients of peptic ulcer, 52 matched patients of gastritis, 64 matched healthy control. The serum levels of Zinc were measured with flame atomic absorption spectrophotometer. This study reported that serum levels of Zinc were significantly lower in stomach cancer patients than in health controls. Furthermore, this study suggested that enough Zinc intake can protect the destruction of H. pylori to the gastric membrane individuals with H. pylori infection.[27,28,29]
A retrospective study was performed to examine the clinical correlation between the characteristics of GC and serum selenium and Zinc levels among 74 GC patients. Serum Zn level was measured by inductive coupled plasma mass spectrometry technique. The results of this study suggested that serum Zn concentrations was lower in the early GC patients group (33 cases, 78.7 ± 29.6 mcg/dl) than the advanced GC patients group (41 cases, 66.9 ± 17.8 mcg/dl) (P = 0.064).[30]
A case-control study was performed to evaluate the relationship between serum levels of copper/Zinc SOD and stomach cancer risk. There were 214 cases with GC and 120 controls. Serum levels of Cu/Zn SOD were assessed by enzyme-linked immunosorbent assay. The results of this study indicated that serum levels of Cu/Zn SOD were significantly elevated in GC patients compared with apparently healthy controls and higher Cu/Zn SOD levels can be related with an increased GC risk.[21]
Major limitation of studies was the lack of information about Helicobacter pylori infection.
SUMMARY
Zinc is known as a key constituent or cofactor for more than 300 mammalian proteins or enzymes that play an important role in DNA repair.[5] Zinc is considered to inhibit free-radical production[6] and also involved in transcription factor function, antioxidant defense and DNA repair.[5,7] Thus, Zinc deficiencies may contribute to DNA breaks and oxidative modifications to DNA that may increase the cancer risk[5] As regards, papers searching has been made of Persian sources and Latin sources, it can be distorted the final conclusion and this is a limitation for this study. It seems further studies in particular epidemiological studies with large scale setting are required to reach a definite conclusion. Furthermore, meta-analysis studies can be useful in achieving more accurate results.
Footnotes
Source of Support: The study was supported by a grant from Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM. International variation. Oncogene. 2004;23:6329–40. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Russell RM. Nutrition and gastric cancer risk: An update. Nutr Rev. 2008;66:237–49. doi: 10.1111/j.1753-4887.2008.00029.x. [DOI] [PubMed] [Google Scholar]
- 3.Biesalski HK, Grimm P. 3rd ed. Stuttgart: Georg Thieme Verlag; 2005. Pocket Atlas of Nutrition. [Google Scholar]
- 4.Fukazawa K. Biological defense systems in oxygen stress and new antioxidant drugs based on these systems. In: Kondo M, editor. Free Radicals in Clinical Medicine. Vol. 3. Tokyo: Nihon-Igakukan; 1988. [Google Scholar]
- 5.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–8. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid Redox Signal. 2006;8:1419–41. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 7.Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475:7–20. doi: 10.1016/s0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar B. Metal replacement in DNA-binding zinc finger proteins and its relevance to mutagenicity and carcinogenicity through free radical generation. Nutrition. 1995;11:646–9. [PubMed] [Google Scholar]
- 9.Witkiewicz-Kucharczyk A, Bal W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol Lett. 2006;162:29–42. doi: 10.1016/j.toxlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002;21:291–5. doi: 10.1023/a:1021215111729. [DOI] [PubMed] [Google Scholar]
- 11.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130(5S Suppl):1447S–54. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi N. Clinical significances of superoxide dismutases: Changes in aging, diabetes, ischemia, and cancer. Adv Clin Chem. 1992;29:1–59. doi: 10.1016/s0065-2423(08)60221-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Folsom AR, Jacobs DR., Jr Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: The Iowa women's health study. Am J Clin Nutr. 2005;81:787–91. doi: 10.1093/ajcn/81.4.787. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Anderson KE, Folsom AR, Jacobs DR., Jr Heme iron, zinc and upper digestive tract cancer: The Iowa women's health study. Int J Cancer. 2005;117:643–7. doi: 10.1002/ijc.21215. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Park S, Liu G, Miller DP, Wang LI, Pothier L, et al. Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology. 2005;16:772–9. doi: 10.1097/01.ede.0000181311.11585.59. [DOI] [PubMed] [Google Scholar]
- 16.Fong LY, Li JX, Farber JL, Magee PN. Cell proliferation and esophageal carcinogenesis in the zinc-deficient rat. Carcinogenesis. 1996;17:1841–8. doi: 10.1093/carcin/17.9.1841. [DOI] [PubMed] [Google Scholar]
- 17.Fong LY, Lau KM, Huebner K, Magee PN. Induction of esophageal tumors in zinc-deficient rats by single low doses of N-nitrosomethylbenzylamine (NMBA): Analysis of cell proliferation, and mutations in H-ras and p53 genes. Carcinogenesis. 1997;18:1477–84. doi: 10.1093/carcin/18.8.1477. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Dawodu JB, Etolhi G, Husain A, Gemmell CG, Russell RI. Relationship between the mucosal production of reactive oxygen radicals and density of Helicobacter pylori in patients with duodenal ulcer. Eur J Gastroenterol Hepatol. 1997;9:261–5. doi: 10.1097/00042737-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZF, Kurtz RC, Yu GP, Sun M, Gargon N, Karpeh M, Jr, et al. Adenocarcinomas of the esophagus and gastric cardia: The role of diet. Nutr Cancer. 1997;27:298–309. doi: 10.1080/01635589709514541. [DOI] [PubMed] [Google Scholar]
- 20.Kabuto M, Imai H, Yonezawa C, Neriishi K, Akiba S, Kato H, et al. Prediagnostic serum selenium and zinc levels and subsequent risk of lung and stomach cancer in Japan. Cancer Epidemiol Biomarkers Prev. 1994;3:465–9. [PubMed] [Google Scholar]
- 21.Lin Y, Kikuchi S, Obata Y, Yagyu K, Tokyo Research Group on Prevention of Gastric Cancer Serum copper/zinc superoxide dismutase (Cu/Zn SOD) and gastric cancer risk: A case-control study. Jpn J Cancer Res. 2002;93:1071–5. doi: 10.1111/j.1349-7006.2002.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant WB. An ecological study of cancer mortality rates including indices for dietary iron and zinc. Anticancer Res. 2008;28:1955–63. [PubMed] [Google Scholar]
- 23.Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann Oncol. 2009;20:160–5. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 24.Pakseresht M, Forman D, Malekzadeh R, Yazdanbod A, West RM, Greenwood DC, et al. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 2011;22:725–36. doi: 10.1007/s10552-011-9744-5. [DOI] [PubMed] [Google Scholar]
- 25.Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055–62. [PubMed] [Google Scholar]
- 26.Campos FI, Koriyama C, Akiba S, Carrasquilla G, Serra M, Carrascal E, et al. Toenail zinc level and gastric cancer risk in Cali, Colombia. J Cancer Res Clin Oncol. 2008;134:169–78. doi: 10.1007/s00432-007-0266-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang WH, Wu XJ, Niu JX, Yan H, Wang XZ, Yin XD, et al. Serum zinc status and Helicobacter pylori infection in gastric disease patients. Asian Pac J Cancer Prev. 2012;13:5043–6. doi: 10.7314/apjcp.2012.13.10.5043. [DOI] [PubMed] [Google Scholar]
- 28.Alipour B, Ghaffari A, Ostadrahimi A, Safaiyan A, Modaresi J, Vaghef Mehrabany E. Relationship between serum zinc, iron and copper level and apoptosis in human gastric mucosa: A cross-sectional study. Pak J Nutr. 2011;10:919–24. [Google Scholar]
- 29.Janjetic MA, Goldman CG, Balcarce NE, Rua EC, González AB, Fuda JA, et al. Iron, zinc, and copper nutritional status in children infected with Helicobacter pylori. J Pediatr Gastroenterol Nutr. 2010;51:85–9. doi: 10.1097/MPG.0b013e3181c2c2cd. [DOI] [PubMed] [Google Scholar]
- 30.Ji JH, Shin DG, Kwon Y, Cho DH, Lee KB, Park SS, et al. Clinical correlation between gastric cancer type and serum selenium and zinc levels. J Gastric Cancer. 2012;12:217–22. doi: 10.5230/jgc.2012.12.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]


