Abstract
Diabetes mellitus is a heterogeneous complex metabolic disorder with multiple etiology which characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action or both. The widespread occurrence of diabetes throughout the world has increased dramatically over the past few years. For better understanding, appropriate animal models that closely mimic the changes in humans needed, as vital tool for understanding the etiology and pathogenesis of the disease at the cellular/molecular level and for preclinical testing of drugs. This review aims to describe the animal models of type-1 diabetes (T1Ds) and T2Ds to mimic the causes and progression of the disease in humans. And also we highlight patent applications published in the last few years related to animal models in diabetes as an important milestone for future therapies that are aim to treating diabetes with specific symptoms and complications.
Keywords: Alloxan-susceptible/Lt mouse, animal models, diabetes mellitus, dithizone, nonrodent models, patent
INTRODUCTION
Biomedical research involves three facets
Gaining of new knowledge, use of animals, and the testing of compounds, chemicals or devices for better understanding of molecule or disease. Research involving animals contribute significantly in protection and improvement of the health of either humans or animals. In the past few decades animal models have been widely used as tools to develop new therapeutic drugs as well as to study the molecular basis of a disease or disorder because human biology is very much like that of many other animals. Most laboratory animals have the same physiological set of organs which work in the same way as they do in humans with specially relationship to human metabolism. According to the World Health Organization about 347 million people worldwide have diabetics and the number of these diabetic patients have double in the last few years and more than 80% of diabetes deaths occur in low and middle-income countries which are to be double in coming years.[1,2] Advances in drug discovery and drug development have led to better ways to manage diabetes and to treat its complications. Experimental model play an important role in drug discovery for understands the molecular basis and management of diabetes. Over the last few years, several animals model have developed for the studying diabetes mellitus or testing antidiabetic agents.[3] These animal models include drug-induced, diet induced surgical (pancreatectomy) and genetically modified animals.[4] In diabetes research, inappropriate animal models have identified as one of the common problem associated with researchers with choice of animal models is still debatable.
The aim of this review is to summarize the animal models used for diabetic research. The first part of the review concerns with the present status of suitable animal models. In the later part describes the utility and enough disclosure of the invention in the patent applications for commercial research or experimental uses.
ANIMAL MODELS FOR TYPE-1, INSULIN DEPENDENT DIABETES MELLITUS
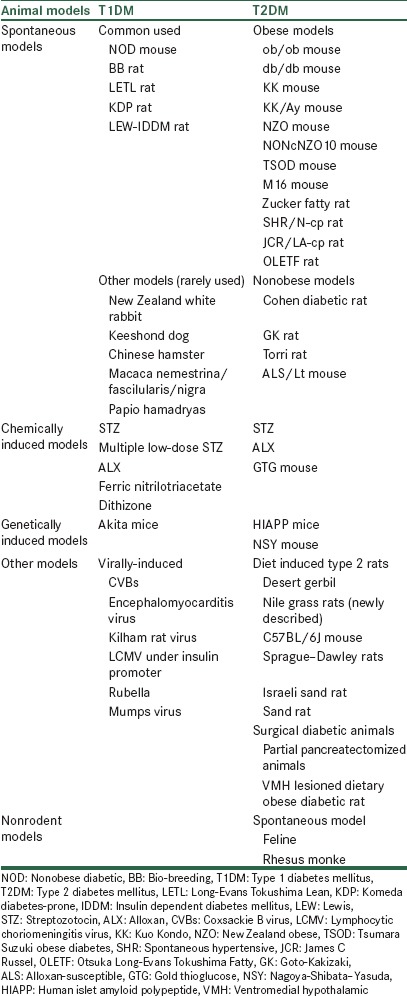
Insulin dependent diabetes mellitus (IDDM) is an autoimmune disease which characterized by destruction of the insulin secreting β-cells results into an absolute loss of endogenous insulin, leads to insulin deficiency and cause hyperglycemia. In this condition, patient required administration of exogenous insulin for survival.[5] In case of animal models, this deficiency in insulin production achieved by different mechanisms, which ranging from chemical ablation of the β-cells to breeding rodents, viral induced; genetically and spontaneously develop autoimmune diabetics animal models (naturally occurring in animals) as summaries in Table 1.[6]
Table 1.
Animals models for T1DM and T2DM
SPONTANEOUS DIABETES TYPE-1 DIABETES MELLITUS
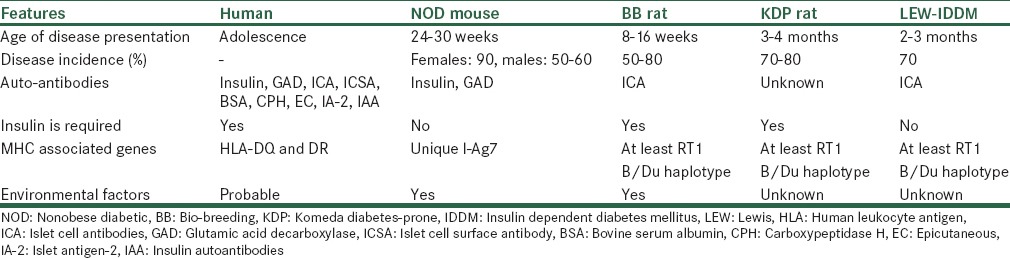
These are the animal models of human disease which utilize naturally occurring genetically variants (mutants) often displaying isomorphic phenotypic similarity between the disease in the animal and the corresponding disease in man.[7] Literature available on spontaneous models showed that majority of research work involves mice and rat models[8,9,10] and comparison of spontaneous diabetes animal models summaries in Table 2.
Table 2.
Comparative features of spontaneous autoimmune diabetes with human and different animal models
COMMONLY USED MODELS
Nonobese diabetic mouse
The nonobese diabetic (NOD) mouse established in 1980 as an animal model of autoimmune type-1 diabetes (T1Ds) by Makino et al. by selectively breeding offspring from a laboratory strain that in fact was first used in the study of cataract development (JcI-Institute of Cancer Research [ICR] mouse).[11] The typical clinical symptoms are like hyperglycemia, glycosuria, polydipsia and polyuria. The mice have larger resistance to ketoacidosis development and can remain alive about 2–4 weeks after the disease establishment without insulin administration and if diabetes is not finally treated death results from dehydration, rather than ketoacidosis.[12,13] Although in comparison with other animal models used in biomedical research, the NOD mouse seems particularly analogous to human T1D and thus a great many studies have performed in the 24–30 years since its development.[14]
Bio-breeding rat
Bio-breeding (BB) rats derived from out-bred Wistar rats and as preferable a small animal model for studying islet transplantation tolerance induction. These rats spontaneous autoimmune diabetes in a Canadian colony was first identified in 1974 and lead to creation of two founder colonies from which all sub-strains have derived, one inbred (BB diabetes-prone [BBdp]/Wor) and one out-bred (BBdp).[15] It develops T-cell dependent autoimmune diabetes, which is also characterised by islet auto-antibodies, as well glutamic acid decarboxylase antibodies.[12] They develop weight loss, polyuria, polydipsia, hyperglycemia, and insulinopenia at about 12 weeks of age, often at the time of puberty,[16] unlike NOD mouse, ketoacidosis is very severe in the BB-rats and as in humans, it become lethal if not treated with insulin.[12,17] Furthermore, the BB-rat is susceptible to subclinical thyroiditis and sialitis.[18,19]
Long-evans tokushima lean rat
The long-evans tokushima lean (LETL) rat is the first discovered rat model that spontaneously develops an autoimmune destruction of the islet β-cells and rapid frank diabetes at a rate of 20%, without being lymphopenic.[20] LETL rat's sub-strain that was finally established as the one that develops the disease at a very good rate is the Komeda diabetes-prone (KDP) rat.[15,21]
Komeda diabetes-prone rat
The KDP rats are characterized by autoimmune destruction of pancreatic β-cells, rapid onset of overt diabetes with no sex difference, and no significant T-cell lymphopenia.[22] Most of the animals show moderate to severe lymphocyte infiltration into pancreatic islets (insulitis), and about 80% of them develop diabetes within 220 days of age. Yokoi et al. performed a genetic analysis of T1D in KDP in which found Cblb is a major susceptibility gene for rat type-1 diabetes mellitus (T1DM). KDP rat could be an animal model for other autoimmune diseases, especially for the autoimmune thyroid disease.[23,24]
Lewis-insulin dependent diabetes mellitus rat
Lewis-IDDM (LEW rats) rats are the T1D animal model characterized by rapid apoptotic pancreatic β-cell destruction in a colony of congenic (LEW rats) with a defined major histocompatibility complex haplotype (LEW.1AR1). These rats exhibit insulitis, and overt diabetes manifests at around 8–9 weeks. Originally, incidence of diabetes was approximately 20%;[25] however, with further inbreeding of diabetic rats, the incidence increased to around 60% with equal incidence in both genders.[26] In contrast to the NOD mouse and BB-rat, the LEW-IDDM rat does not exhibit other autoimmune diseases. It also survives well after the onset of overt diabetes and thus can used to study diabetic complications.[12]
OTHER RARELY USED ANIMAL MODELS
Except from the aforementioned typical models of disease representation, scientists develop and use also many other models for studying T1DM which includes, New Zealand white rabbit, Keeshond dog, Chinese hamster, Macaca nemestrina/fascilularis/nigra and Papio hamadryas.[9]
CHEMICALLY INDUCED DIABETIC ANIMALS
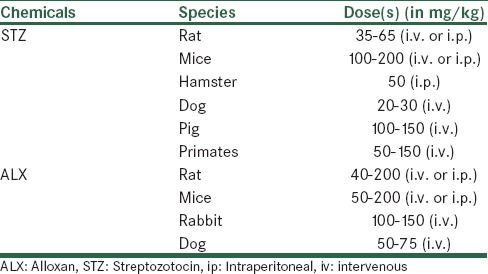
Chemically induced diabetes not only provides a simple and relatively cheap model of diabetes in rodents but can also be used in higher animals[27] by the destruction of endogenous β-cells which leads to decrease in insulin production and leads to hyperglycemia and weight loss. Rarely used diabetogenic agents in rodents listed in Table 3, but streptozotocin (STZ) and alloxan (ALX) are the commonly used compounds for induction of diabetes due to their similarity in structure to glucose.[28] The dose of these agents required for inducing diabetes depends on the animal species, route of administration which summarized in Table 4.[29,30] Chemically induced models are commonly preferred models for testing of new antidiabetic drugs or new formulations of insulin.[31,32,33] This is also an appropriate model for testing transplantation therapies where the end point is lowering of blood glucose.[34] But drugs-inducing diabetes can toxic at other organs of the body. It should also be noted that changes in p450 isozymes in the liver, kidney, lung, intestines, testis and brain have reported after administration of STZ or ALX.[35]
Table 3.
List of diabetogenic agents used in rodents
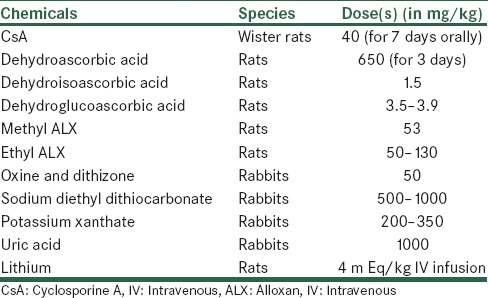
Table 4.
The animal species, dose and route of administration required for chemically inducing diabetes
Streptozotocin
Streptozotocin (streptozocin/izostazin/zanosar/STZ) is a synthetic nitrosoureido glucopyranose derivative isolated from fermentations of Streptomyces achromogenes with broad-spectrum antibiotic and antineoplastic activity.[36] STZ administration by i.p. or intravenous (i.v.) route, it enters into the pancreatic β-cell through the glucose transporter-2 (GLUT-2) transporter and causes alkylation of the DNA. Subsequent activation of poly (adenosine diphosphate ADPc-ribose) polymerase leads to nicotinamide (NAD+) depletion, a reduction in cellular adenosine triphosphate and inhibition of insulin production.[37] A single large dose of STZ can produce diabetes in rodents, probably as a result of direct toxic effects. STZ has longer half-life than ALX, so rat model interestingly exhibits stable, long-lasting hyperglycemia and the symptoms of diabetic complications such as hypertension. But its use in chronic experiments, especially-spontaneous recovery from high blood glucose levels leads to high incidence of kidney and liver tumours.[38,39,40] These problems are due strongly to oncogenic action of STZ.[41] STZ is ineffective in rabbits.[42] Multiple low doses of STZ over 5 days induce insulitis in mice[43,44] or rats.[45] Doses range from 20 to 40 mg/kg/day, depending on the species and strain. Diabetes develops even in the absence of T- and B-cells, and therefore, it does not model the human disease as closely as spontaneous models of autoimmunity.[46]
Alloxan
Alloxan (2, 4, 5, 6-tetraoxypyrimidine; 5, 6-dioxyuracil) is the next most commonly used chemical for induction of diabetes mellitus. It is a well-known diabetogenic agent widely used to induce T2Ds in animals.[47] It used to produce experimental diabetes in animals such as rabbits, rats, mice and dogs. The diabetic effect of ALX is mainly attributed to rapid uptake by the β-cells and formation of free radicals, for which β-cells have poor defense mechanisms[48] and there after highly reactive hydroxyl radicals that cause fragmentation of β-cell DNA.[49] ALX is also taken up by the liver, but it has better protection to reactive oxygen species.[50,51] Other mechanisms of β-cell damage by ALX include oxidation of essential–SH groups, especially that of glucokinase[52] and disturbances in intracellular calcium homeostasis.[53] A dose of 100 mg/kg has used to create a long-term diabetes models in rabbits.[54] It should be noted that ALX has a narrow diabetogenic dose, and even light overdosing can cause general toxicity, especially to the kidney.[49]
Ferric nitrilotriacetate
This is a rarely used model for induction of special type diabetes known as bronze diabetes, in which there are heavy iron deposits, skin pigmentation, and liver cirrhosis due to disturbances in iron metabolism.[55] Rats and rabbits parenterally treated with a large daily dose of ferric nitrilotriacetate manifested diabetic symptoms such as hyperglycemia, glycosuria, ketonemia and ketonuria after approximately 60 days of treatment. Difference from human hemochromatosis was that no fibrotic changes were observed in the liver, pancreas, and other organs.[56]
Dithizone
Dithizone is a ((1E)-3-anilino-1-phenylimino-thiourea) is a sulfur-containing organic compound which used for induction of diabetes in experimental animals.[57] Dithizone injected to cats, rabbits, golden hamsters and mice in a single i.v. dose of 40–100 mg/kg. In rabbits dithizone injection causes a triphasic glycemic reaction. After 2 h initial hyperglycemia detected followed by a normoglycemic phase after 8 h and after 24–72 h a permanent hyperglycemia.[58]
VIRALLY INDUCED DIABETES
Viruses are one environmental factor that implicated in the pathogenesis of T1DM. Different viruses have been reported to associate with development of T1D in humans and animal models. Which include coxsackie B virus,[59,60] encephalomyocarditis virus,[61,62] Kilham rat virus,[63,64] lymphocytic choriomeningitis virus rubella and mumps viruses.[65] Viruses may be involved in the pathogenesis of T1D, by inducing β-cell-specific autoimmunity, by stimulate pre-existing auto reactive T cells that may participate in islet destruction with or without infection of the β-cells[61] and by cytolytic infection and destruction of the β-cells (e.g. encephalomyocarditis virus) in mice.[66]
GENETICALLY INDUCED DIABETES
Genetically modified models, in which diseases or conditions are induced by genetic manipulation with specific either gene removed or replaced. Genetic factors are believed to a major component for the development of diabetes. It is possible now to understand the complex relationship between the gene(s) and the disease.[67] National Heart, Lung and Blood Institute has developed and maintained a database which has information about genetically modified animals of disease or conditions, with a specific gene, type of genetic manipulation, and animal involved in the study.[68]
AKITA mice
The AKITA mouse derived in Akita, Japan from a C57BL/6NSlc mouse with a spontaneous mutation in the insulin-2 gene preventing correct processing of proinsulin. This causes an overload of misfolded proteins and subsequent endoplasmic reticulum (ER) stress, results in severe insulin dependent diabetes starting from 3 to 4 weeks of age, characterize by hyperglycemia, hypoinsulinaemia, polyuria and polydipsia. Untreated homozygotes rarely survive longer than 12 weeks. The lack of β-cell mass in this model makes it an alternative to STZ treated mice in transplantation studies.[69] In addition, this model is commonly used to study potential alleviator of ER stress in the islets.[70]
ANIMAL MODELS FOR TYPE-2, NONINSULIN DEPENDENT DIABETES MELLITUS
Noninsulin dependent diabetes caused by the loss of functional β-cells within the islets of langerhans in the pancreas, resulting in insulin deficiency which resulting hyperglycemia. Under normal condition, there is continual turnover of β-cells with proportion of cells undergoing apoptosis due to senescence and replacement of these dying cells by both β-cell replication and islet neogenesis,[71] but in diabetic condition it is not enough to compensate patients insulin body need and insulin secretion decreased, thus leading to hyperglycemia. However, it is not completely lost, and therefore patients are not insulin dependent, so the body requires exogenous insulin to keep up better metabolic control to prevent chronic complications.[72] Many animal models of T2Ds are obese, reflecting the human condition where obesity is closely linked to T2D development.
SPONTANEOUS DIABETES TYPE-2 DIABETIC MODELS
Obese models
Obese mouse
obese mouse (ob/ob mouse), is an obese rodent model of spontaneous T2DM, at the age of 3–4 weeks these mice observed mild hyperglycemia due to compensatory hyperinsulinemia or insulin resistance. This mice model inherited as (monogenic) autosomal recessive mutation in C57BL/6J mouse strain which is now identified in leptin gene.[73] The first C57BL/6J-ob/ob mouse originated from the Jackson Laboratory in Bar Harbor, ME, USA.[74] Another mouse model C57BL/KS comes in this category. When ob gene expressed on C57BL/KS background, mice become severely diabetic with regression of islets, hepatic glucose overproduction, and increased lipogenesis in the liver and early death.[75]
db/db mouse
This is intensively studied mice model of obesity and T2D with a defect in the leptin receptor (db) These mice are spontaneously hyperphagicinsulin over secretors becoming obese, hyperglycaemic, hyperinsulinaemic and insulin resistant within 1st month of age and develop hypoinsulinaemia, hyperglycemia later with a peak between 3 and 4 months of age but later (4–8 weeks) develops hyperglycemia, due to β-cell failure[76] and does not live longer than 8–10 months. Female diabetic db/db mice survive longer than male due to protective effect of oestrogen on the pancreatic β-cells.[77]
Kuo Kondo mouse
Kuo Kondo (KK) mouse is polygenic model of obesity and T2D produced by selective inbreeding for the large body size in Japan, also named as Japanese KK mouse.[78] These animals are hyperphagic, hyperinsulinaemic, diabetic nephropathy insulin resistant and show moderate obesity by 2 months of age, which attains maximum at 4–5 months.[79] Normally Insulin resistance precedes the onset of obesity. The increase in pancreatic insulin content associated with increase in number and size of pancreatic islets but histological degranulation of β-cells and hypertrophy of islets are found.[80] KK mouse mimics human obesity (the Goto–Kakizaki [GK] rat being relatively slim) and easier production of transgenic variants from mice rather than rats.[81]
Kuo Kondo/Ay mouse
The KK/Ay are polygenic mouse and serves as a good model for obesity and T2D maintained at Upjohn colony (KK/Upj-Ay/J) are now available from Jackson Laboratory, Bar Harbor, USA, These mice were created by introducing the yellow obese AY gene into the KK strain, which turns the hair color from black to yellow Kuo Kondo Yellow (KKY).[82,83,84] This model develops maturity-obesity and has more severe hyper insulinaemia and more prominent changes in the pancreatic islets. So it used for screening various classes of antidiabetic agents.[85]
New Zealand obese mouse
The New Zealand obese (NZO) mouse is a polygenic model of obesity, glucose intolerance, and metabolic syndrome which exhibits hepatic and peripheral leptin insensitivity, insulin resistance, impaired insulin secretion, hypercholesteremia, and hypertension, which worsens with age and about 50% of males develop diabetes and islets are hyperplastic and hypertrophic at 3–6 months of age.[86] NZO mouse is a rarely preferred model, because they are resistant to peripheral leptin administration but sensitive to centrally administrated leptin[87] indicating a defect in leptin transport across the blood brain barrier. New recombinant congenic strains that have developed by entering NZO loci into other strain genomes, e.g. the nonobese and nondiabetic mouse (NON/Lt) for studying “diabesity” and its treatment.[88]
NONc/New Zealand obese10 mouse
NONc/NZO10 model is suitable for studies in diabetic wound healing created by combining independent diabetes risk-conferring quantitative trait loci from two unrelated strains of NZO mice with NON/Lt.[89,90] NONc/NZO 10 females are diabetes resistant and can serve as normoglycaemic control, but males are not hyperphagic develop more moderate level of obesity, and reproduce normally. They are more in weigh than NON males, but significantly less than the NZO males. Despite the reduced rate of weight gain compared to NZO.[88]
Tsumara Suzuki obese diabetes mouse
Tsumara Suzuki obese diabetes (TSOD) mouse is a polygenic origin model which established by Miura et al. through repeatedly selective inbreeding of obese male mice of ddY strain.[91] Their symptoms, like polydipsia polyuria followed by hyperglycemia and hyperinsulinaemia obesity gradually develops only in male mice about 12 months. It has shown that the reduced insulin sensitivity in diabetic TSOD mice is due, to the impaired GLUT-4 translocation by insulin in both skeletal muscle and adipocytes.[92] Pancreatic islets of TSOD male mice are found hypertrophic without any signs of insulitis or fibrous formation.[91]
M16 mouse
M16 mouse is an out bred animal model to facilitate gene discovery and pathway regulation controlling early onset polygenic obesity and T2DM phenotypes. This results from long-term selection for 3–6 weeks weight gain from an ICR, London, UK base population. These mice characterized by increased body fat percentage, fat cell size; fat cell numbers, organ weights and mice also exhibit hyperphagia, accompanied by moderate obesity and are hyperinsulinaemic, hyperleptinaemic and hypercholesterolemia relative to ICR.[93]
Zucker fatty rat
The Zucker fatty rats discovered from the simple autosomal recessive (fa) gene on chromosome after a cross of Merck M-strain and Sherman rats in 1961 as a model for human obesity and T2D.[6] This rat has attributed to hypothalamic defect in leptin receptor signaling.[94] It associated with type IV hyperlipidaemia (increased very low density lipoprotein and triglyceride levels in the circulating blood) and hypertension; with increasing age of obese Zucker rats spontaneously develop proteinuria and focal segmental glomerulosclerosis, ultimately leading to renal failure.[95] In some cases, it is also reported that abnormal glucose tolerance found in these rats due to the metabolic defects in hepatic organ.[96]
Spontaneously hypertensive rat/National Institute of Health-corpulent
Spontaneously hypertensive rat/National Institute of Health (NIH)-corpulent (SHR/N-cp) rat a genetic model of obesity and T2DM with hypertension. It is derived by inbreeding of SHR/N strains at the NIH, Bethesda, Maryland, USA, associated with hyperplasia and early onset of normal or slight hyperglycemia, dyslipidaemia, profound hyperinsulinaemia, hyperleptinaemia, insulin resistance, impaired glucose tolerance and essential hypertension. It is highly useful for investigating obesity associated T2D and also for studying influence of dietary carbohydrate on development of diabetes in certain genetically predisposed carbohydrate sensitive individuals.[97]
James C Russel/LA-corpulent rat
The JCR: LA-corpulent rat model is extensively studied for the pharmacological and dietary intervention prior to the onset of cardiovascular lesions or hyperinsulinaemia to determine the efficacy for preventing or slowing occurrence of cardiovascular lesions. The major drawback of this rat as model of diabetes, it is normoglycaemic when fasted. However, the distinct feature of this animal is the vasculopathy progresses inherently without any dietary cholesterol and high-fat diet interventions.[98]
Otsuka long-evans tokushima fatty rat
The otsuka long-evans tokushima fatty (OLETF) rat was originates from an out bred colony of long-evans rats strain by selective breeding, maintained at the otsuka pharmaceutical and named OLETF.[99] Development of diabetes in OLETF rats is due to defects in the β-cell proliferation and sustained hyperglycemia due to poor capacity of pancreatic islet regeneration after surgery and is the treated with NAD which corrects hyperglycemia by increasing β-cell proliferation.[100] Male's rats are more likely to develop diabetes in adult life than females.[17] This rat model has been extremely used in pharmacological research while testing for a number of antidiabetic and antihypertensive drugs.[101]
Nonobese models
Cohen diabetic rat
The Cohen diabetic rat is an exceptional genetically derived experimental model of diet induced T2D that having many similar features of the disease in humans. It exhibit retinopathy and nephropathy, reduced fertility and testicular degeneration.[102] Chief complications were nephropathy with mesangial expansion and thickening of the glomerular basement membrane, proliferative retinopathy, testicular atrophy and gastrointestinal disorders. The diabetes is due to cell dysfunction and reduced insulin secretion. The hyperglycemia was reversible by diet adjustment.[103]
Goto–Kakizaki rat
Goto–Kakizaki rats were created by a Japanese group by repetitive breeding of Wistar rats with the poorest glucose tolerance as T2Ds model. The GK rat model may result from inadequacy of pancreatic growth factors with the defects in β-cells and impaired insulin sensitivity in the liver, skeletal muscle and adipose tissues has reported.[104] Impaired insulin secretion and hepatic glucose over production are early events in diabetic. GK rats are mostly contributing to development of hyperglycemia rather than the peripheral (muscle and adipose tissue) insulin resistance. The renal alterations found only in 2 years old GK rats at a later stage.[105]
Torri rat
Torri rat is a new spontaneously diabetic nonobese rat from the Sprague–Dawley rat strain established recently in 1997 at Torri Pharmaceutical Co., Japan. The distinct characteristics are ocular complications, cataract and retinopathy with fractional, retinal detachment, fibrous proliferation and massive haemorrhage at 70–77 weeks of age. Torri rats are able to survive for longer duration without insulin treatment and hence more useful for studies on diabetic complications.[106,107]
Alloxan-susceptible/Lt mouse
Alloxan-susceptible (ALS) new mouse model implicated in the pathogenesis and complications of both T1DM and T2DM is the sub-strain maintained at Jackson Laboratory, Bar Habor. It characterized by hyperinsulinaemia and impaired glucose tolerance develops spontaneously between 6 and 8 weeks of age in ALX-untreated males.[108] The T2Ds predisposition of ALS mouse recognized by congenic analysis of the yellow mutation (Ay) at the agouti locus on chromosome-2. Indeed, in ALS/Lt (a) mice, this mouse model with reduced ability to diffuse free radical stress.[109]
CHEMICALLY INDUCED TYPE-2 DIABETIC MODELS
Streptozotocin
Streptozotocin is the most widely used for screening the compounds for their insulin mimetic, insulin tropic and other hypoglycemic/antihyperglycaemic activities.[110] It cause breakage of DNA strands and results the increase activity of poly-ADP-ribose synthetase, an enzyme depleting NAD in β-cells, which finally leading to energy deficiency and death of β-cells reported. Recently, a new animal model of T2Ds has produced by combination of STZ and NAD administration in adult rats. In which NAD (230 mg/kg, i.p.) administered to rats 15 min before STZ (65 mg/kg, i.v.) has shown to develop moderate and stable nonfasting hyperglycemia without any significant change in plasma insulin level. NAD play a role as an antioxidant which exerts protective effect on the cytotoxic action of STZ by scavenging free radicals and causes only minor damage to pancreatic β-cell mass producing T2DM.[111] This provides good opportunity to investigate diabetes in much closely similar path physiological situation as in human with fewer incidences of ketosis as well as mortality.[112]
Alloxan
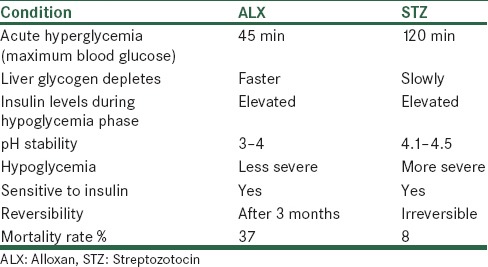
Alloxan which is a uric acid derivative causes diabetes in many rodent and nonrodent animals and highly unstable in water at neutral pH. ALX acts by selectively destroying the pancreatic β-islets leading to insulin deficiency, hyperglycemia and ketosis.[113] Most preferably use of ALX in case of rabbit because of the relative ineffectiveness of STZ) in rabbits for induction of diabetes and development of well characterized diabetic complications.[114] Now days ALX is almost replaced by STZ, due to development of various complications such as neuropathy, cardiomyopathy, has well-marked retinopathy and the percentage incidence of diabetes is quite variable and is not proportionately related to increasing doses of ALX. Incidence of ketosis resulting high mortality and the reversal of hyperglycemia due to pancreatic regeneration are common in case of ALX treated animals as shown in Table 5.[75]
Table 5.
Comparison of ALX and STZ
Gold thioglucose mouse
Gold thioglucose (GTG obese diabetic) mouse induced T2DM with obesity at the dose of (~200 mg/kg, i.p.) in mice. It exhibits many molecular defects in relations to insulin signaling pathways.[115] Which subsequently is responsible for development of hyperphagia and obesity. It also shows increased body lipid and hepatic lipogenesis and triglyceride secretion, increased adipose tissue lipogenesis and decreased glucose metabolism in muscle with gradually develop obesity, hyperinsulinaemia, hyperglycemia, insulin resistance over a period of 16–20 weeks.[116] GTG limited in their uses because it takes very long time to develop obesity/diabetes and the number of mortalities.[117]
SURGICAL TYPE-2 DIABETIC MODELS
This method consists of complete or partial pancreatectomy in animals used for induction of T1D or T2D. This finding from these partial pancreatectomized animals supports the notion that simply reduction in pancreatic β-cell mass itself not be responsible for the glucose intolerance as seen in neonatal STZ rats.[118] These partial pancreatectomized animals reported to develop hyperglycemia and insulin resistance.[119] Diabetic dog model discovered by Oskar Minkowski through surgical complete pancreatectomy has considered to the first animal model of diabetes[120] and is rarely now used for the investigation. It does not cause severe form of diabetes and characterized by moderate hyperglycemia with neither reduction in body weight nor reduction in plasma insulin levels. The 90% partially pancreatectomized rats also show defect or selective impairment to glucose stimulated insulin release but remain intact to other insulin secretogogues viz., arginine, isoproterenol like neonatal STZ rats.[118] Recently, an another model of stable form of T2D has produced by combination of 50% partial pancreatectomy along with NAD (350 mg/kg) and STZ (200 mg/kg) treatment in BALB/c mice.[121] There are some advantages in combination procedure as it minimizes the risk of unnecessary adverse effect of chemicals on body.
DIET INDUCED TYPE-2 RATS
Desert gerbil
The desert gerbil (Psammomys obesus) was originally discovered to develop diabetes in captivity in the 1960s. The diabetes ranged from mild hyperglycemia with hyperinsulinaemia to severe hyperglycemia with hypoinsulinaemiaand ketoacidosis.[122] Due to its poor adaption to excess nutrition, it has suggested that the Psammomys representsan ideal model of the “thrifty gene” effect.[6]
Nile grass rat
The nile grass rat (Arvicanthis niloticus) has recently been suggested as a model for metabolic syndrome. Most of these animals spontaneously develop obesity, dyslipidaemia and hyperglycemia by 1-year of age when kept on a normal chow diet in captivity. They show other signs of diabetes and metabolic syndrome such as reduced β-cell mass, atherosclerosis and liver steatosis.[123]
C57BL/6J mouse
C57BL/6J mice are susceptible to obesity-linked diabetes when maintained on a high-fat diet. They also present abnormalities in the autonomic nervous function, β-cells, and expression of uncoupling protein-2 in adipocytes, but it is itself prone to nutritionally induced diabetes and obesity as well as hypertension.[124] Excess food intake leads to tissue deposition, primarily in a dipocytes, resulting in untoward changes in metabolism. Several animals without genetic mutations undergoing diet-induced obesity and develop T2Ds reviewed by Coscun et al.[125]
Israeli sand rat
The Israeli sand rat (Psammomys obesus) model is particularly useful when studying the effects of diet and exercise on development of T2DM.[126] In its natural habitat rat that has an essentially vegetarian diet. However, when fed laboratory chow, the animals become obese, insulin resistant and hyperglycemic.[127] If a cholesterol-rich diet used, hyperlipidaemia and atherosclerosis develop.[128]
GENETIC MODELS FOR TYPE-2 DIABETES
Human islet amyloid polypeptide mice
A variety of human islet amyloid polypeptide (hIAPP) models have created and it has demonstrated that increasing expression of hIAPP increases β-cell toxicity.[129] In addition, replicating β-cells are more susceptible to hIAPP toxicity, and thus, β-cell adaption to increased insulin demand in this model restricted with characteristic of formation of amyloid within the islet tissue, which derives from IAPP.[130]
Nagoya–Shibata–Yasuda mouse
Nagoya–Shibata–Yasuda (NSY) developed by selective inbreeding using a laboratory strain of mouse termed Jc1: ICR with impaired insulin secretion in the face of mild insulin resistance. Obesity is not a major feature of these animals and marked gender difference with almost all males developing hyperglycemia, but less than females, being affected.[131] The NSY mouse is particularly useful when considering age related phenotypes (e.g. decline in β-cell function).
NONRODENT MODELS OF SPONTANEOUS TYPE-2 DIABETE
Feline
Feline diabetes mellitus closely resembles human T2DM in many respects including clinical, physiological, and development of islet amyloid deposits, loss of approximately 50% of cell mass, and development of complications in several organ systems including peripheral polyneuropathy and retinopathy.[132]
Rhesus monkey
Rhesus monkey (Macaca mulatta) is a nonrodent model with symptoms of T2DM that provides the most human-like model of metabolic disorders in diabetes representative of other monkey species prone to diabetes.[133] It develops obesity, hyperinsulinaemia and insulin resistance when maintained on ad libitum laboratory diet, which gradually progresses to necrosis of β-cells, severe fall in insulin levels and overt hyperglycemia over a period of several years.[134]
PATENTS REPORTED IN DIABETIC ANIMAL MODELS
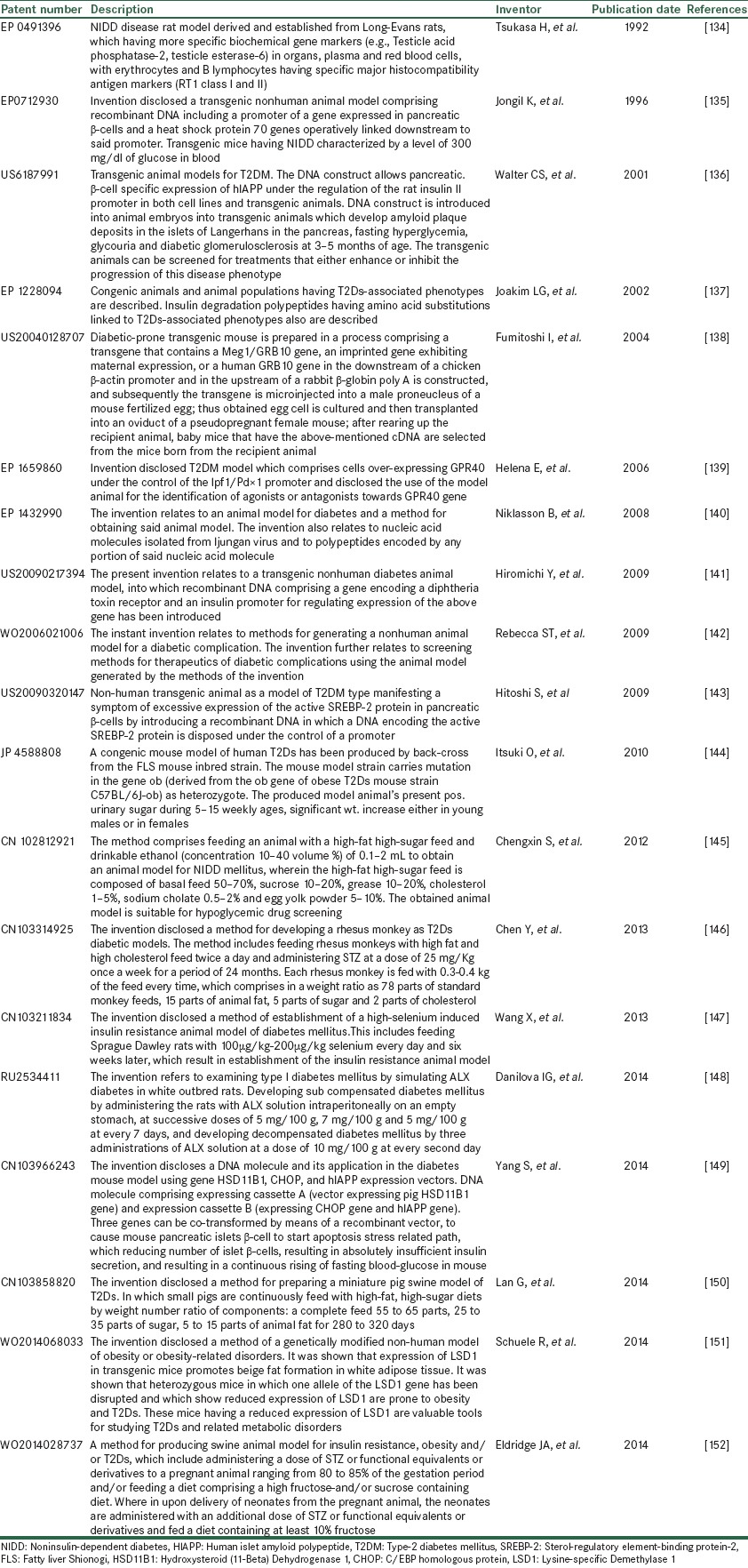
These days, animal models are created by insertion of a particular human DNA into fertilized mouse oocytes which are then allowed to develop by implantation into the oviducts of pseudo pregnant females. Many researchers, companies, and academic institutions try to publish such work as patents not just the methods used to make animals suffer from a disease but also the animals themselves. Patents on such animals could be very rewarding if they become in demand for biomedical and testing laboratories as describe in Table 6.
Table 6.
List of reported patents as animal models of diabetes with specific feature
CONCLUSION
Animal models of diabetes mellitus are very useful tools for studying the pathophysiology and the clinical aspects of the disease because that would be impossible in humans due to lack of toxic profile and unclear mechanism action of tested compounds. In animals none of the known single species is exactly equivalent to human diabetes, but each model act as essential tool for investigating genetic, endocrine, metabolic, morphologic changes and underlying aetiopathogenic mechanisms. So, it is important to note that some animal models are better suited to screen particular class of antidiabetic compounds like use of smaller animal models such as mice, will reduce the expense of producing test materials while some advanced efficacy studies or toxicological examinations which require invasive procedures and large blood and tissue samples, may facilitated by using animals with large body size such as rat or other nonrodents. Chemical induction appears to the most popularly used procedure in inducing diabetes mellitus in experimental animals. They are capable of inducing both T1DM and T2DM with proper dosage selection. But these experimental animals’ models must put to use within 7 days after induction of diabetes mellitus or maintain with appropriate doses of insulin to prevent animal death and in surgical and genetic methods require highly technical skills, may be associated with a high percentage of animal death and thus are rarely used. But some limitations are with large nonrodent animal models like expensiveness, practical difficulties, and extreme care and ethical considerations associated with their use (viz., pigs, dogs and nonhuman primates). In transgenic technology, that can impact at many points in the discovery process, including target identification and target validation. It also provides models designed to alert researchers early to the potential problems with drug metabolism and toxicity which will help in providing better models for human diseases. Drugs from transgenic animals can minimize the attrition rate in clinical trials by increasing quality of the target and compound combinations making transition from discovery into development. The regulatory aspects and ethics should give due consideration while using transgenic animals. From research, pigs and transgenic animals derived products like milk, eggs seems to promising in developments of therapeutic strategies. Therefore, the continuing effort for inventing new models has always positive critics and animal models will continue to have a major and meaningful place in diabetes research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X. USA: Agennix Incorporated; 2012. Translational Animal Models in Drug Discovery and Development. [Google Scholar]
- 4.Etuk EU. Animals models for studying diabetes mellitus. Agr Biol J N Am. 2010;1:130–4. [Google Scholar]
- 5.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:62–9. [Google Scholar]
- 6.King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–94. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hau J. Animal models for human diseases. In: Conn PM, editor. Sourcebook of Models for Biomedical Research. New Jersey: Human Press; 2008. pp. 3–8. [Google Scholar]
- 8.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–80. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper E. The use of animal models in the study of diabetes mellitus. In Vivo. 2009;23:245–58. [PubMed] [Google Scholar]
- 10.Polychronakos C. Animal models of spontaneous autoimmune diabetes: notes on their relevance to the human disease. Curr Diab Rep. 2004;4:151–4. doi: 10.1007/s11892-004-0071-z. [DOI] [PubMed] [Google Scholar]
- 11.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 12.Mathews CE. Utility of murine models for the study of spontaneous autoimmune type 1 diabetes. Pediatr Diabetes. 2005;6:165–77. doi: 10.1111/j.1399-543X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–4. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 14.Giarratana N, Penna G, Adorini L. Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse. Methods Mol Biol. 2007;380:285–311. doi: 10.1007/978-1-59745-395-0_17. [DOI] [PubMed] [Google Scholar]
- 15.Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 2004;45:278–91. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- 16.Nakhooda AF, Like AA, Chappel CI, Murray FT, Marliss EB. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977;26:100–12. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- 17.Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359–70. doi: 10.1111/j.1464-5491.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 18.Crisá L, Mordes JP, Rossini AA. Autoimmune diabetes mellitus in the BB rat. Diabetes Metab Rev. 1992;8:4–37. [PubMed] [Google Scholar]
- 19.Jun HS, Yoon JW. A new look at viruses in type 1 diabetes. Diabetes Metab Res Rev. 2003;19:8–31. doi: 10.1002/dmrr.337. [DOI] [PubMed] [Google Scholar]
- 20.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. New inbred strain of Long-Evans Tokushima lean rats with IDDM without lymphopenia. Diabetes. 1991;40:1375–81. doi: 10.2337/diab.40.11.1375. [DOI] [PubMed] [Google Scholar]
- 21.Komeda K, Noda M, Terao K, Kuzuya N, Kanazawa M, Kanazawa Y. Establishment of two substrains, diabetes-prone and non-diabetic, from Long-Evans Tokushima Lean (LETL) rats. Endocr J. 1998;45:737–44. doi: 10.1507/endocrj.45.737. [DOI] [PubMed] [Google Scholar]
- 22.Yokoi N, Namae M, Fuse M, Wang HY, Hirata T, Seino S, et al. Establishment and characterization of the Komeda diabetes-prone rat as a segregating inbred strain. Exp Anim. 2003;52:295–301. doi: 10.1538/expanim.52.295. [DOI] [PubMed] [Google Scholar]
- 23.Yokoi N, Komeda K, Wang HY, Yano H, Kitada K, Saitoh Y, et al. Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nat Genet. 2002;31:391–4. doi: 10.1038/ng927. [DOI] [PubMed] [Google Scholar]
- 24.Yokoi N, Hayashi C, Fujiwara Y, Wang HY, Seino S. Genetic reconstitution of autoimmune type 1 diabetes with two major susceptibility genes in the rat. Diabetes. 2007;56:506–12. doi: 10.2337/db06-1027. [DOI] [PubMed] [Google Scholar]
- 25.Lenzen S, Tiedge M, Elsner M, Lortz S, Weiss H, Jörns A, et al. The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44:1189–96. doi: 10.1007/s001250100625. [DOI] [PubMed] [Google Scholar]
- 26.Jörns A, Günther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S. Immune cell infiltration, cytokine expression, and beta-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes. 2005;54:2041–52. doi: 10.2337/diabetes.54.7.2041. [DOI] [PubMed] [Google Scholar]
- 27.Dufrane D, van Steenberghe M, Guiot Y, Goebbels RM, Saliez A, Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81:36–45. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 28.Bansal R, Ahmad N, Kidwai JR. Alloxan-glucose interaction: effect on incorporation of 14C-leucine into pancreatic islets of rat. Acta Diabetol Lat. 1980;17:135–43. doi: 10.1007/BF02580995. [DOI] [PubMed] [Google Scholar]
- 29.Federiuk IF, Casey HM, Quinn MJ, Wood MD, Ward WK. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatment. Comp Med. 2004;54:252–7. [PubMed] [Google Scholar]
- 30.Jederström G, Gråsjö, Nordin A, Sjöholm I, Andersson A. Blood glucose-lowering activity of a hyaluronan-insulin complex after oral administration to rats with diabetes. Diabetes Technol Ther. 2005;7:948–57. doi: 10.1089/dia.2005.7.948. [DOI] [PubMed] [Google Scholar]
- 31.Sheshala R, Peh KK, Darwis Y. Preparation, characterization, and in vivo evaluation of insulin-loaded PLA-PEG microspheres for controlled parenteral drug delivery. Drug Dev Ind Pharm. 2009;35:1364–74. doi: 10.3109/03639040902939213. [DOI] [PubMed] [Google Scholar]
- 32.Jansson L, Eizirik DL, Pipeleers DG, Borg LA, Hellerström C, Andersson A. Impairment of glucose-induced insulin secretion in human pancreatic islets transplanted to diabetic nude mice. J Clin Invest. 1995;96:721–6. doi: 10.1172/JCI118115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61:356–60. [PMC free article] [PubMed] [Google Scholar]
- 34.Makhlouf L, Duvivier-Kali VF, Bonner-Weir S, Dieperink H, Weir GC, Sayegh MH. Importance of hyperglycemia on the primary function of allogeneic islet transplants. Transplantation. 2003;76:657–64. doi: 10.1097/01.TP.0000080881.75767.0E. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Yang SH, Oh JM, Lee MG. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol. 2010;62:1–23. doi: 10.1211/jpp.62.01.0001. [DOI] [PubMed] [Google Scholar]
- 36.Bono VH., Jr Review of mechanism of action studies of the nitrosoureas. Cancer Treat Rep. 1976;60:699–702. [PubMed] [Google Scholar]
- 37.Sandler S, Swenne I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia. 1983;25:444–7. doi: 10.1007/BF00282526. [DOI] [PubMed] [Google Scholar]
- 38.Steiner H, Oelz O, Zahnd G, Foresch ER. Studies on islet cell regeneration, hyperplasia and intrainsular cellular interrelations in long lasting streptozotocin diabetes in rats. Diabetologia. 1970;6:558–64. doi: 10.1007/BF00418221. [DOI] [PubMed] [Google Scholar]
- 39.Yamagami T, Miwa A, Takasawa S, Yamamoto H, Okamoto H. Induction of rat pancreatic B-cell tumors by the combined administration of streptozotocin or alloxan and poly (adenosine diphosphate ribose) synthetase inhibitors. Cancer Res. 1985;45:1845–9. [PubMed] [Google Scholar]
- 40.Iwase M, Nunoi K, Wakisaka M, Kikuchi M, Maki Y, Sadoshima S, et al. Spontaneous recovery from non-insulin-dependent diabetes mellitus induced by neonatal streptozotocin treatment in spontaneously hypertensive rats. Metabolism. 1991;40:10–4. doi: 10.1016/0026-0495(91)90184-x. [DOI] [PubMed] [Google Scholar]
- 41.Kazumi T, Yoshino G, Fujii S, Baba S. Tumorigenic action of streptozotocin on the pancreas and kidney in male Wistar rats. Cancer Res. 1978;38:2144–7. [PubMed] [Google Scholar]
- 42.Rerup CC. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970;22:485–518. [PubMed] [Google Scholar]
- 43.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–7. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47:50–6. doi: 10.2337/diab.47.1.50. [DOI] [PubMed] [Google Scholar]
- 45.Lukic ML, Stosic-Grujicic S, Shahin A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol. 1998;6:119–28. doi: 10.1155/1998/92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy S, Wu D, Elliott RB. Low dose streptozotocin causes diabetes in severe combined immunodeficient (SCID) mice without immune cell infiltration of the pancreatic islets. Autoimmunity. 1995;20:83–92. doi: 10.3109/08916939509001931. [DOI] [PubMed] [Google Scholar]
- 47.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–26. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 48.Nerup J, Mandrup-Poulsen T, Helqvist S, Andersen HU, Pociot F, Reimers JI, et al. On the pathogenesis of IDDM. Diabetologia. 1994;37 Suppl 2:S82–9. doi: 10.1007/BF00400830. [DOI] [PubMed] [Google Scholar]
- 49.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 50.Malaisse WJ, Malaisse-Lagae F, Sener A, Pipeleers DG. Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci U S A. 1982;79:927–30. doi: 10.1073/pnas.79.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathews CE, Leiter EH. Constitutive differences in antioxidant defense status distinguish alloxan-resistant and alloxan-susceptible mice. Free Radic Biol Med. 1999;27:449–55. doi: 10.1016/s0891-5849(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 52.im Walde SS, Dohle C, Schott-Ohly P, Gleichmann H. Molecular target structures in alloxan-induced diabetes in mice. Life Sci. 2002;71:1681–94. doi: 10.1016/s0024-3205(02)01918-5. [DOI] [PubMed] [Google Scholar]
- 53.Kim HR, Rho HW, Park BH, Park JW, Kim JS, Kim UH, et al. Role of Ca2+in alloxan-induced pancreatic beta-cell damage. Biochim Biophys Acta. 1994;1227:87–91. doi: 10.1016/0925-4439(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Wan R, Mo Y, Zhang Q, Sherwood LC, Chien S. Creating a long-term diabetic rabbit model. Exp Diabetes Res 2010. 2010:289614. doi: 10.1155/2010/289614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finch SC, Finch CA. Idiopathic hemochromatosis, an iron storage disease. A. Iron metabolism in hemochromatosis. Medicine (Baltimore) 1955;34:381–430. doi: 10.1097/00005792-195512000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am J Pathol. 1979;95:663–73. [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen WA, Christie MR, Kahn R, Norgaard A, Abel I, Petersen AM, et al. Supravital dithizone staining in the isolation of human and rat pancreatic islets. Diabetes Res. 1989;10:53–7. [PubMed] [Google Scholar]
- 58.Goldberg ED, Eshchenko VA, Bovt VD. The diabetogenic and acidotropic effects of chelators. Exp Pathol. 1991;42:59–64. doi: 10.1016/s0232-1513(11)80038-1. [DOI] [PubMed] [Google Scholar]
- 59.Yoon JW, London WT, Curfman BL, Brown RL, Notkins AL. Coxsackie virus B4 produces transient diabetes in nonhuman primates. Diabetes. 1986;35:712–6. doi: 10.2337/diab.35.6.712. [DOI] [PubMed] [Google Scholar]
- 60.Jaïdane H, Sané F, Gharbi J, Aouni M, Romond MB, Hober D. Coxsackievirus B4 and type 1 diabetes pathogenesis: contribution of animal models. Diabetes Metab Res Rev. 2009;25:591–603. doi: 10.1002/dmrr.995. [DOI] [PubMed] [Google Scholar]
- 61.Craighead JE, McLane MF. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968;162:913–4. doi: 10.1126/science.162.3856.913. [DOI] [PubMed] [Google Scholar]
- 62.Baek HS, Yoon JW. Direct involvement of macrophages in destruction of beta-cells leading to development of diabetes in virus-infected mice. Diabetes. 1991;40:1586–97. doi: 10.2337/diab.40.12.1586. [DOI] [PubMed] [Google Scholar]
- 63.Guberski DL, Thomas VA, Shek WR, Like AA, Handler ES, Rossini AA, et al. Induction of type I diabetes by Kilham's rat virus in diabetes-resistant BB/Wor rats. Science. 1991;254:1010–3. doi: 10.1126/science.1658938. [DOI] [PubMed] [Google Scholar]
- 64.Ellerman KE, Richards CA, Guberski DL, Shek WR, Like AA. Kilham rat triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996;45:557–62. doi: 10.2337/diab.45.5.557. [DOI] [PubMed] [Google Scholar]
- 65.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–31. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 66.Yoon JW, Onodera T, Notkins AL. Virus-induced diabetes mellitus. XV. Beta cell damage and insulin-dependent hyperglycemia in mice infected with coxsackie virus B4. J Exp Med. 1978;148:1068–80. doi: 10.1084/jem.148.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin JH. Applications and limitations of genetically modified mouse models in drug discovery and development. Curr Drug Metab. 2008;9:419–38. doi: 10.2174/138920008784746355. [DOI] [PubMed] [Google Scholar]
- 68. [Last accessed on 2013 Mar 15]. Available from: http://www.apps.nhlbi.nih.gov/transgenic/default.htm .
- 69.Mathews CE, Langley SH, Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation. 2002;73:1333–6. doi: 10.1097/00007890-200204270-00024. [DOI] [PubMed] [Google Scholar]
- 70.Chen H, Zheng C, Zhang X, Li J, Li J, Zheng L, et al. Apelin alleviates diabetes-associated endoplasmic reticulum stress in the pancreas of Akita mice. Peptides. 2011;32:1634–9. doi: 10.1016/j.peptides.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 71.Jun H, Bae HY, Lee BR, Koh KS, Kim YS, Lee KW, et al. Pathogenesis of non-insulin-dependent (type II) diabetes mellitus (NIDDM) – Genetic predisposition and metabolic abnormalities. Adv Drug Deliv Rev. 1999;35:157–77. doi: 10.1016/s0169-409x(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 72.Yap IS, Giddings G, Pocock E, Chantler JK. Lack of islet neogenesis plays a key role in beta-cell depletion in mice infected with a diabetogenic variant of coxsackievirus B4. J Gen Virol. 2003;84:3051–68. doi: 10.1099/vir.0.19150-0. [DOI] [PubMed] [Google Scholar]
- 73.Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–43. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 74.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097–9. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 75.Bell RH, Jr, Hye RJ. Animal models of diabetes mellitus: physiology and pathology. J Surg Res. 1983;35:433–60. doi: 10.1016/0022-4804(83)90034-3. [DOI] [PubMed] [Google Scholar]
- 76.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–78. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Berglund O, Frankel BJ, Hellman B. Development of the insulin secretory defect in genetically diabetic (db/db) mouse. Acta Endocrinol (Copenh) 1978;87:543–51. doi: 10.1530/acta.0.0870543. [DOI] [PubMed] [Google Scholar]
- 78.Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia. 1967;3:212–21. doi: 10.1007/BF01222198. [DOI] [PubMed] [Google Scholar]
- 79.Ikeda H. KK mouse. Diabetes Res Clin Pract. 1994;24 Suppl:S313–6. doi: 10.1016/0168-8227(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 80.Reddi AS, Camerini-Davalos RA. Hereditary diabetes in the KK mouse: an overview. Adv Exp Med Biol. 1988;246:7–15. doi: 10.1007/978-1-4684-5616-5_2. [DOI] [PubMed] [Google Scholar]
- 81.Lenzen S, Panten U. Alloxan: history and mechanism of action. Diabetologia. 1988;31:337–42. doi: 10.1007/BF02341500. [DOI] [PubMed] [Google Scholar]
- 82.Ramarao P, Kaul CL. Insulin resistance: current therapeutic approaches. Drugs Today (Barc) 1999;35:895–911. [PubMed] [Google Scholar]
- 83.Kato H, Ohue M, Kato K, Nomura A, Toyosawa K, Furutani Y, et al. Mechanism of amelioration of insulin resistance by beta3-adrenoceptor agonist AJ-9677 in the KK-Ay/Ta diabetic obese mouse model. Diabetes. 2001;50:113–22. doi: 10.2337/diabetes.50.1.113. [DOI] [PubMed] [Google Scholar]
- 84.Diani AR, Sawada G, Wyse B, Murray FT, Khan M. Pioglitazone preserves pancreatic islet structure and insulin secretory function in three murine models of type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;286:E116–22. doi: 10.1152/ajpendo.00331.2003. [DOI] [PubMed] [Google Scholar]
- 85.Shafrir E, Ziv E. A useful list of spontaneously arising animal models of obesity and diabetes. Am J Physiol Endocrinol Metab. 2009;296:E1450–2. doi: 10.1152/ajpendo.00113.2009. [DOI] [PubMed] [Google Scholar]
- 86.Haskell BD, Flurkey K, Duffy TM, Sargent EE, Leiter EH. The diabetes-prone NZO/HlLt strain. I. Immunophenotypic comparison to the related NZB/BlNJ and NZW/LacJ strains. Lab Invest. 2002;82:833–42. doi: 10.1097/01.lab.0000018915.53257.00. [DOI] [PubMed] [Google Scholar]
- 87.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leiter EH, Reifsnyder PC. Differential levels of diabetogenic stress in two new mouse models of obesity and type 2 diabetes. Diabetes. 2004;53 Suppl 1:S4–11. doi: 10.2337/diabetes.53.2007.s4. [DOI] [PubMed] [Google Scholar]
- 89.Fang RC, Kryger ZB, Buck DW, 2nd, De la Garza M, Galiano RD, Mustoe TA. Limitations of the db/db mouse in translational wound healing research: Is the NONcNZO10 polygenic mouse model superior? Wound Repair Regen. 2010;18:605–13. doi: 10.1111/j.1524-475X.2010.00634.x. [DOI] [PubMed] [Google Scholar]
- 90.Cho YR, Kim HJ, Park SY, Ko HJ, Hong EG, Higashimori T, et al. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;293:E327–36. doi: 10.1152/ajpendo.00376.2006. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki W, Iizuka S, Tabuchi M, Funo S, Yanagisawa T, Kimura M, et al. A new mouse model of spontaneous diabetes derived from ddY strain. Exp Anim. 1999;48:181–9. doi: 10.1538/expanim.48.181. [DOI] [PubMed] [Google Scholar]
- 92.Miura T, Suzuki W, Ishihara E, Arai I, Ishida H, Seino Y, et al. Impairment of insulin-stimulated GLUT4 translocation in skeletal muscle and adipose tissue in the Tsumura Suzuki obese diabetic mouse: a new genetic animal model of type 2 diabetes. Eur J Endocrinol. 2001;145:785–90. doi: 10.1530/eje.0.1450785. [DOI] [PubMed] [Google Scholar]
- 93.Allan MF, Eisen EJ, Pomp D. The M16 mouse: an outbred animal model of early onset polygenic obesity and diabesity. Obes Res. 2004;12:1397–407. doi: 10.1038/oby.2004.176. [DOI] [PubMed] [Google Scholar]
- 94.Augstein P, Salzsieder E. Morphology of pancreatic islets: a time course of pre-diabetes in Zucker fatty rats. Methods Mol Biol. 2009;560:159–89. doi: 10.1007/978-1-59745-448-3_12. [DOI] [PubMed] [Google Scholar]
- 95.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12:383–92. doi: 10.1080/10739680590960241. [DOI] [PubMed] [Google Scholar]
- 96.McIntosh CH, Pederson RA. Non-insulin dependent animalmodels of diabetes mellitus. In: McNeil JH, editor. Experimental Models of Diabetes. Florida, USA: CRC Press LLC; 1999. pp. 337–98. [Google Scholar]
- 97.Michaelis OE, 4th, Patrick DH, Hansen CT, Canary JJ, Werner RM, Carswell N. Insulin-independent diabetes mellitus (type II). Spontaneous hypertensive/NIH-corpulent rat. Am J Pathol. 1986;123:398–400. [PMC free article] [PubMed] [Google Scholar]
- 98.Russel JC, Kelly SE, Proctor S. The JCR: LA-cp rat: animal model of the metabolic syndrome exhibiting micro-and macromolecular disease. In: Shafrir E, editor. Animal Models of Diabetes, Frontiers in Research. Boca Raton, FL: CRC Press; 2007. pp. 159–83. [Google Scholar]
- 99.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–8. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 100.Zhu M, Noma Y, Mizuno A, Sano T, Shima K. Poor capacity for proliferation of pancreatic beta-cells in Otsuka-Long-Evans-Tokushima Fatty rat: a model of spontaneous NIDDM. Diabetes. 1996;45:941–6. doi: 10.2337/diab.45.7.941. [DOI] [PubMed] [Google Scholar]
- 101.Kosegawa I, Chen S, Awata T, Negishi K, Katayama S. Troglitazone and metformin, but not glibenclamide, decrease blood pressure in Otsuka Long Evans Tokushima Fatty rats. Clin Exp Hypertens. 1999;21:199–211. doi: 10.3109/10641969909068661. [DOI] [PubMed] [Google Scholar]
- 102.Weksler-Zangen S, Yagil C, Zangen DH, Ornoy A, Jacob HJ, Yagil Y. The newly inbred cohen diabetic rat: a nonobese normolipidemic genetic model of diet-induced type 2 diabetes expressing sex differences. Diabetes. 2001;50:2521–9. doi: 10.2337/diabetes.50.11.2521. [DOI] [PubMed] [Google Scholar]
- 103.Wechsler-Zangen S, Orlanski E, Zangen DH. Shafrir E. Animal Models of Diabetes, Frontiers in Research. Boca Raton, FL: CRC Press; 2007. Cohen diabetic rat; pp. 323–34. [Google Scholar]
- 104.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 105.Picarel-Blanchot F, Berthelier C, Bailbé D, Portha B. Impaired insulin secretion and excessive hepatic glucose production are both early events in the diabetic GK rat. Am J Physiol. 1996;271:E755–62. doi: 10.1152/ajpendo.1996.271.4.E755. [DOI] [PubMed] [Google Scholar]
- 106.Shinohara M, Masuyama T, Shoda T, Takahashi T, Katsuda Y, Komeda K, et al. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int J Exp Diabetes Res. 2000;1:89–100. doi: 10.1155/EDR.2000.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinohara M. Establishment and clinical features in spontaneously diabetic Torii rat. Open Diabetes J. 2011;4:18–20. [Google Scholar]
- 108.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–72. [PubMed] [Google Scholar]
- 109.Mathews CE, Bagley R, Leiter EH. ALS/Lt: a new type 2 diabetes mouse model associated with low free radical scavenging potential. Diabetes. 2004;53 Suppl 1:S125–9. doi: 10.2337/diabetes.53.2007.s125. [DOI] [PubMed] [Google Scholar]
- 110.Reed MJ, Scribner KA. In-vivo and in-vitro models of type 2 diabetes in pharmaceutical drug discovery. Diabetes Obes Metab. 1999;1:75–86. doi: 10.1046/j.1463-1326.1999.00014.x. [DOI] [PubMed] [Google Scholar]
- 111.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–9. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 112.Larsen MO, Wilken M, Gotfredsen CF, Carr RD, Svendsen O, Rolin B. Mild streptozotocin diabetes in the Göttingen minipig. A novel model of moderate insulindeficiency and diabetes. Am J Physiol Endocrinol Metab. 2002;282:E1342–51. doi: 10.1152/ajpendo.00564.2001. [DOI] [PubMed] [Google Scholar]
- 113.Kasiviswanath R, Ramesh A, Kumar KE. Hypoglycemic and antihyperglycemic effect of Gmelina asiatica LINN. in normal and in alloxan induced diabetic rats. Biol Pharm Bull. 2005;28:729–32. doi: 10.1248/bpb.28.729. [DOI] [PubMed] [Google Scholar]
- 114.Le Marchand-Brustel Y. Molecular mechanisms of insulin action in normal and insulin-resistant states. Exp Clin Endocrinol Diabetes. 1999;107:126–32. doi: 10.1055/s-0029-1212087. [DOI] [PubMed] [Google Scholar]
- 115.Le Marchand-Brustel Y, Jeanrenaud B, Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978;234:E348–58. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- 116.Karasawa H, Takaishi K, Kumagae Y. Obesity-induced diabetes in mouse strains treated with gold thioglucose: a novel animal model for studying ß-cell dysfunction. Obesity (Silver Spring) 2011;19:514–21. doi: 10.1038/oby.2010.171. [DOI] [PubMed] [Google Scholar]
- 117.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71:1544–53. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi SB, Park CH, Choi MK, Jun DW, Park S. Improvement of insulin resistance and insulin secretion by water extracts of Cordyceps militaris, Phellinus linteus, and Paecilomyces tenuipes in 90% pancreatectomized rats. Biosci Biotechnol Biochem. 2004;68:2257–64. doi: 10.1271/bbb.68.2257. [DOI] [PubMed] [Google Scholar]
- 119.Luft R. Oskar Minkowski: discovery of the pancreatic origin of diabetes, 1889. Diabetologia. 1989;32:399–401. doi: 10.1007/BF00271257. [DOI] [PubMed] [Google Scholar]
- 120.Kurup S, Bhonde RR. Combined effect of nicotinamide and streptozotocin on diabetic status in partially pancreatectomized adult BALB/c mice. Horm Metab Res. 2000;32:330–4. doi: 10.1055/s-2007-978646. [DOI] [PubMed] [Google Scholar]
- 121.Shafrir E, Ziv E, Kalman R. Nutritionally induced diabetes in desert rodents as models of type 2 diabetes: Acomys cahirinus (spiny mice) and Psammomys obesus (desert gerbil) ILAR J. 2006;47:212–24. doi: 10.1093/ilar.47.3.212. [DOI] [PubMed] [Google Scholar]
- 122.Noda K, Melhorn MI, Zandi S, Frimmel S, Tayyari F, Hisatomi T, et al. An animal model of spontaneous metabolic syndrome: Nile grass rat. FASEB J. 2010;24:2443–53. doi: 10.1096/fj.09-152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–7. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 124.Heled Y, Shapiro Y, Shani Y, Moran DS, Langzam L, Braiman L, et al. Physical exercise prevents the development of type 2 diabetes mellitus in Psammomys obesus. Am J Physiol Endocrinol Metab. 2002;282:E370–5. doi: 10.1152/ajpendo.00296.2001. [DOI] [PubMed] [Google Scholar]
- 125.Coscun T, Chen Y, Sindelar D, Heiman M. Animal models to study obesity and type 2 diabetes induced by diet. In: Shafrir E, editor. Animal Models of Diabetes, Frontiers of Research. Boca Raton, FL: CRC Press; 2007. pp. 349–57. [Google Scholar]
- 126.Ziv E, Shafrir E, Kalman R, Galer S, Bar-On H. Changing pattern of prevalence of insulin resistance in Psammomys obesus, a model of nutritionally induced type 2 diabetes. Metabolism. 1999;48:1549–54. doi: 10.1016/s0026-0495(99)90244-5. [DOI] [PubMed] [Google Scholar]
- 127.Marquié G, Hadjiisky P, Arnaud O, Duhault J. Development of macroangiopathy in sand rats (Psammomys obesus), an animal model of non-insulin-dependent diabetes mellitus: effect of gliclazide. Am J Med. 1991;24;90:55S–61. doi: 10.1016/0002-9343(91)90419-x. [DOI] [PubMed] [Google Scholar]
- 128.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–33. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 129.Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes. 2009;58:906–16. doi: 10.2337/db08-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ueda H, Ikegami H, Yamato E, Fu J, Fukuda M, Shen G, et al. The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia. 1995;38:503–8. doi: 10.1007/BF00400717. [DOI] [PubMed] [Google Scholar]
- 131.Henson MS, O’Brien TD. Feline models of type 2 diabetes mellitus. ILAR J. 2006;47:234–42. doi: 10.1093/ilar.47.3.234. [DOI] [PubMed] [Google Scholar]
- 132.Hansen BC, Tigno XF. The rhesus monkey (Macaca mulatta) manifestsall the features of human type 2 diabetes. In: Shafrir E, editor. Animal Models of Diabetes, Frontiers in Research. Boca Raton, FL: CRC Press; 2007. pp. 251–70. [Google Scholar]
- 133.Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA. Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes. 2005;54:1534–42. doi: 10.2337/diabetes.54.5.1534. [DOI] [PubMed] [Google Scholar]
- 134.Tsukasa H, Kazuya K, Masao K, Yuichi S. Non-insulin-dependent diabetic rat. EP0491396. 1992 [Google Scholar]
- 135.Jongil K, Soonhee K, Jeongsun S. A transgenic nonhuman animal model for diabetes. EP 0712930. 1996 [Google Scholar]
- 136.Walter CS, Maynard DC, David KK. Transgenic animal models for type II diabetes mellitus. US6187991. 2001 [Google Scholar]
- 137.Luthman LH, Joakim LG. Congenic animal models of non-insulin dependent diabetes mellitus. EP1228094. 2002 [Google Scholar]
- 138.Fumitoshi I, Naoki M, Tomoko I, Minesuke Y, Shigeharu W. Mammalian model for diabetes. US 20040128707. 2004 [Google Scholar]
- 139.Helena E, Rubins N, Steneberg P, Michael DW. New diabetes type 2 animal model. EP1659860. 2006 [Google Scholar]
- 140.Rebecca ST, Edward HK, Joan FF, Dennis LG. Non-human animal models for diabetic complications and their uses. WO2006021006. 2006 [Google Scholar]
- 141.Lernmark A, Niklasson B. Diabetes model. EP1432990. 2008 [Google Scholar]
- 142.Hiromichi Y, Kunie M, Hiroshi S, Kenji K. Diabetes model animal. US20090217394. 2009 [Google Scholar]
- 143.Hitoshi S, Nobuhiro Y. Nonhuman transgenic animal as type 2 diabetes model. US20090320147. 2009 [Google Scholar]
- 144.Itsuki O, Masahiko S, Susumu M. Production of congenic mouse model of human type 2 diabetes. JP4588808. 2010 [Google Scholar]
- 145.Chengxin S, Yuying F, Xinzhi L. A vertical type 2 diabetes mellitus the animal model claims a method for the screening of reducing blood sugar application in the medicine. CN102812921. 2012 [Google Scholar]
- 146.Chen Y, Cheng J, Lu Y, Liu J, Li Xi, Yang G, et al. A method for preparing rhesus non insulin dependent diabetes mellitus model. CN103314925. 2013 [Google Scholar]
- 147.Wang X, Hai C. High selenium-induced insulin resistance animal models, its application and construction method. CN103211834. 2013 [Google Scholar]
- 148.Danilova IG, Gette IF, Bulavintseva TS. Method for simulating alloxan diabetes. RU2534411. 2014 [Google Scholar]
- 149.Yang S, Kong S, Ruan J, Xin L, Li K. Diabetes mouse model using gene HSD11B1, CHOP and IAPP expression vectors. CN103966243. 2014 [Google Scholar]
- 150.Lan G, Yang H, Jiang Q, Guo Y, Chen J, Liang J. A method for preparing model of mini pig with non insulin dependent diabetes mellitus. CN103858820. 2014 [Google Scholar]
- 151.Schuele R, Duteil D, Metzger E, Guenther T. Animal models for type 2 diabetes and obesity having reduced LSD1 expression as well as transgenic animals over expressing human LSD1 gene. WO2014068033. 2014 [Google Scholar]
- 152.Eldridge JA, Campaigne WC. Diabetic animal model for diabetes research. WO 2014028737. 2014 [Google Scholar]


