Abstract
Background:
Angiogenesis-related corneal blindness includes the spectrum of corneal diseases that are caused by pathological angiogenesis, leading to untoward visual impairment. The purpose of this study was to investigate the antineovascularization effect of topical silica nanoparticles (SiNPs) in inhibiting chemical-burn-induced corneal neovascularization.
Materials and Methods:
A total number of 20 corneas of 10 Wistar Albino rats were included in this study. Silver nitrate cauterization was pressed to the central cornea for 5 s to induce corneal neovascularization. They were randomly allocated to case and control groups (ten eyes in each group). SiNPs were synthesized by the reverse microemulsion method. SiNPs drop 1 mg/ml was started in ten eyes and artificial tear drop was started in the control group (ten eyes) immediately after chemical cauterization. Video-based photography was performed before and after treatment. Corneal image analysis was performed on each cornea using an image analysis software program. All rats were euthanized and the eyes were sent for histopathologic examinations14 days after chemical cauterization.
Results:
Scanning electron microscopy (SEM) images showed spherical-shaped particles. The mean size and polydispersity index of prepared SiNPs were 30.1 ± 5.6 nm and 0.254 ± 0.11, respectively. Fourteen days after chemical cauterization, the mean vascularized corneal area was 21% of total corneal area in the case group and 85% in the control group (P < 0.05). The control group revealed more extensive intrastromal vascularization compared with the case group in histopathologic examinations (P < 0.05).
Conclusions:
SiNPs is an effective modality for inhibiting corneal neovascularization following chemical burn in an experimental model. Further investigations are suggested for evaluation of its safety and efficacy in human eyes.
Keywords: Antineovascularization agent, chemical burn, corneal neovascularization, topical silica nanoparticles
INTRODUCTION
Corneal neovascularization (NV) can be developed in miscellaneous pathological circumstances and can lead to a variety of untoward outcomes; however, most of these pathologies are associated with hypoxia, inflammation, infection, traumatic injury, and malfunction of the limbal stem cells.[1,2,3,4] Currently, the treatments for corneal neovascularization (NV) consist of steroids, nonsteroidal anti-inflammatory agents, photodynamic therapy, photocoagulation, and intravitreal, topical, subconjunctival,[5] and intrastromal injections of antivascular endothelial growth factor-A (VEGF-A).[6,7] Anti-VEGFs directly inhibit the angiogenic factors that cause corneal vascularization. However, they are expensive, and their efficacy is limited by their bioavailability. Nanotechnology may offer a solution, providing effective inhibition of the angiogenic factors that can lead to blindness by increasing drug bioavailability.[8] Targeting treatment to the anterior segment of the eye requires medications with unique features, including the ability to pass through an intact outer epithelium.[9]
Nanomedicine applies small particles with a size less than 100 nm to treat various disorders[10] and offers several advantages over traditional therapies. The unique multifunctional properties of nanomaterials make them suitable for therapeutic use and enable them to overcome many physiologic ocular barriers.[11,12] The versatility in nanomaterial synthesis can also provide opportunities for structural modification to impart properties such as biodegradability, stimuli responsiveness, and attachment of targeting and imaging agents. Nanodrug delivery systems have emerged as promising alternatives to other systems for a wide range of biomedical applications. Among nanoparticles (NPs), silica NPs can be considered as a favorite agent due to their exclusive properties including hydrophilic surface favoring protracted circulation, versatile silane chemistry, excellent biocompatibility, ease of large-scale synthesis, and low cost of NP production.[13,14,15]
Silicate is a compound that is abundant in nature. A wide range of different kinds of transport modalities, including small molecule drugs, photosensitizers for photodynamic therapy (PDT), proteins, peptides, DNAs, and RNAs, have been incorporated into silica NPs to target diseases such as cancer, heart disease,[16,17] and Parkinson's disease.[18] However, there is only one study that confirms its anti-VEGF effect for management of choroidal NV.[19]
Herein, we used topical SiNPs for management of corneal neovascularization as a novel prophylactic anti VEGF modality in an experimental rat model, which to the best of our knowledge has not been yet reported in the literature.
MATERIALS AND METHODS
Chemicals
L-arginine and tetraethoxysilane (TEOS, reagent grade, ≥98%) were purchased from Sigma-Aldrich (St. Louis, Missouri). Cyclohexane (99%) was obtained from Merck. Distilled deionized water was used for the preparation of all aqueous solutions.
Devices
Fourier transform infrared spectroscopy (FTIR) was performed to indicate hydrolysis of TEOS to SiO2 in prepared NPs. The mean particle size was determined by dynamic light scattering (DLS, Brookhaven Instruments, USA) and the morphology of the NPs was investigated with the help of scanning electron microscopy (SEM, Hitachi S-4160, Tokyo, Japan). Additionally, inductively coupled plasma atomic emission spectrometry (ICP-AES, Optima 7300 DV, USA) was employed to determine the concentration of topical silicate nanoparticles (SiNPs) suspension.[20]
Methods
Nanoparticles preparation
The two-phase nucleation method[21] was applied as a simple synthetic route for producing ultra-monodisperse silica NPs. In brief, 20 mg L-arginine as catalyst was dissolved in 40 ml of water. To keep an aqueous phase undisturbed, 3 ml cyclohexane and 3 ml of TEOS were added subsequently. To form SiNPs, solutions were slowly stirred for 24 h at 70°C. The size and size distribution of SiNPs were determined by DLS.
Animal model of corneal vascularization
The study was performed using 20 corneas of 10 male Wistar albino rats (Weight: 200-250 g). The animals were treated and maintained in accordance with the tenets of the Association for Research in Vision and Ophthalmology (ARVO) Statement for Use of Animals in Ophthalmic and Vision Research.
Silver nitrate cauterization[22] was used to induce corneal neovascularization under general anesthesia. Chemical cauterization was done by the first author and pressing an applicator stick (diameter 1.8 mm, coated with silver nitrate) to the central cornea for 5 s under the operating microscope.
They were randomly allocated to case and control groups (ten eyes in each group). The SiNPs drop (1 mg/ml sized 30.1 ± 5.6 nm) was started in 10 eyes and artificial tear drop was started in control group (ten eyes) immediately after chemical cauterization. Video-based photography was performed before and after treatment. All rats were euthanized and the eyes were sent for histopathologic exams 14 days after chemical cauterization.
Analysis of corneal neovascularization
Fourteen days after chemical cauterization, all rats were systematically healthy; however, cornea NV was observed in all eyes. All rats were anesthetized as mentioned earlier. Each cornea was evaluated by video-based photography on the 14th day by the same investigator (M.M.). Corneal image analysis was performed on each cornea using imagej® analysis software program (National Institutes of Health (NIH), Bethesda, Maryland, USA). The neovascularization area was measured in terms of pixels, and its ratio to the entire corneal area was determined as the percentage.
Analysis of neovascularization by histological examination
Enucleation was performed after the animals had been euthanized. The eyes were prepared for histological examination using 10% formaldehyde. After fixation for 36 h, they were removed from the fixative and corneas were dehydrated and sectioned. Tissue sections of 10 μm thickness were prepared and stained with hematoxylin and eosin for light microscopy. Light microscopic examination was performed on every section by an examiner who was blinded to the groups. The maximally vascularized area was determined for each histological specimen by counting visible vessels. Photographs of these areas were taken by a light microscope with 40 times magnification.
Statistical analysis
The Kruskal-Wallis test was used for comparison of the two groups concerning the neovascularized area and the average number of vessels. After finding differences among the groups, the Mann-Whitney U test was used to compare each group with the control group and with each other. P< 0.05 was considered statistically significant.
RESULTS
Nanoparticles size and morphology
SEM images showed spherical shapes of particles [Figure 1a and b]. The presence of SiO2 peak (bands appearing at 1109 and 473 cm−1) in nanoparticle FTIR plot demonstrated the appropriate hydrolysis of TEOS in prepared NPs [Diagram 1a–c]. According to DLS results, the mean size and polydispersity index (PDI) of prepared SiNPs were 30.1 ± 5.6 and 0.254 ± 0.11, respectively [Diagram 2].
Figure 1.
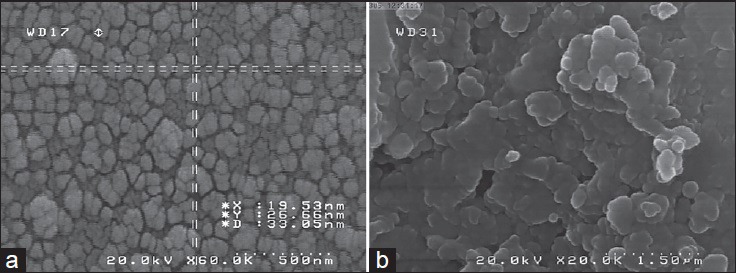
(a and b) Scanning electron microscopy (SEM) images of prepared SiNPs
Diagram 1.
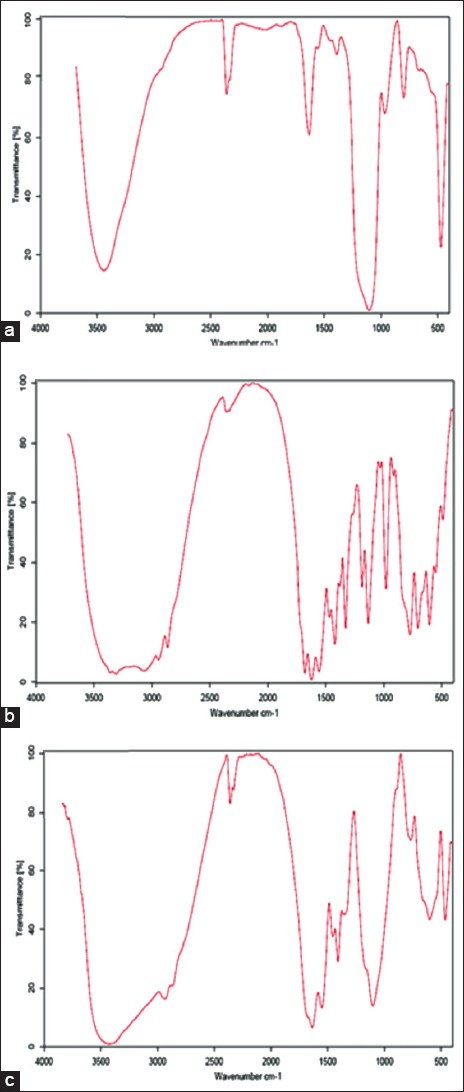
(a) Fourier transform infrared spectroscopy (FTIR) plots of standard SiO2 nanoparticles, (b) arginine, and (c) prepared SiNPs containing arginine as catalyst
Diagram 2.
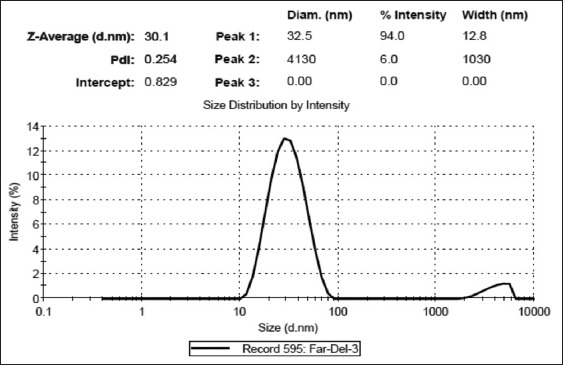
Scattered intensity of the nanoparticles in the aqueous phase after 16 h of reaction times in a two-phase reactor at 60°C and by dynamic light scattering (DLS)
Analysis of corneal neovascularization
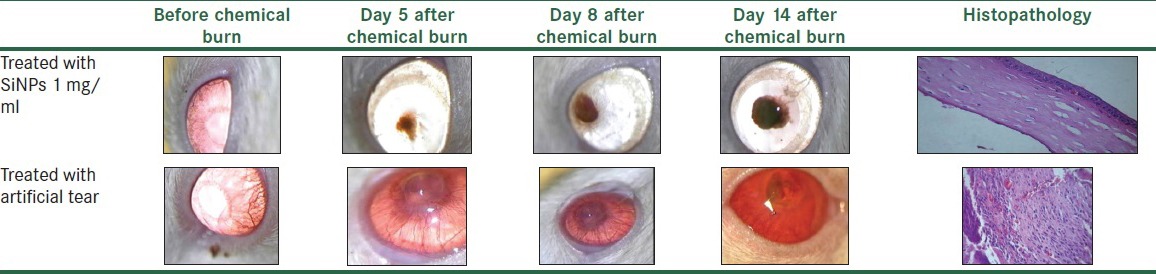
All rats were healthy except corneal neovascularization through the study. There was no missing case during evaluations. Fourteen days after chemical cauterization the mean vascularized corneal area in case group was 21% while in control group was 85% of total corneal area (P < 0.05). This indicates the remarkable antivascularization effect of the SiNPs drop. There was significantly more extensive intrastromal vascularization in control rather than case group in histopathologic examination (P < 0.05) [Table 1].
Table 1.
Corneal photograph and histopathology specimens of treated (case group) versus untreated (control group) immediately following chemical cauterization shows significant more corneal intrastromal vascularization in control group rather than case group
DISCUSSION
In this study, we evaluated the antineovascularization effect of topical SiNPs as a novel anti-VEGF for management of corneal neovascularization. We analyzed the corneal surface involved with corneal neovascularization with imagej® software which is a standard software for calculating the neovascular area that increases the validity of our results. The mean vascularized corneal area in case group was 21% of total corneal area, and 85% in control group 14 days after chemical cauterization that showed remarkable antineovascularization effect of the SiNPs drop and is higher than relevant studies with other anti VEGF studies.[22,23,24,25,26,27]
Nanotechnology help enhanced drug permeation, controlled drug release, and drug targeting.[28] The bioavailability of a drug in the eye is one of the major limitations for effective drug therapy.[29,30] An ideal nanocarrier for ocular delivery should have multiple criteria in order to achieve an optimal bioavailability. The previous known role of SiNPs in the literature in the field of drug delivery is to transport the intended drug or agent into its target;[12,13,14,15] however, in our study we used SiNPs as a sole agent to block the VEGF receptors and not a carrier for other drugs.
Nanoparticles have been conventionally applied via intravitreal injections into the eye. However, this procedure is invasive and has a high degree of risk. An alternative that satisfies the need for less invasive and safer delivery methods is to combine nanotechnology with contact lenses.[31] The goal in using contact lenses for drug delivery is to increase the amount of time drugs reside on the corneal surface without being cleared through the lacrimal system.[32] Topical SiNPs were neither used as an eye drop nor as an injection form for management of corneal NV before. The novelty of our study is to apply topical form of SiNPs for the first time in inhibiting corneal NV. One of the major advantages of topical SiNPs is its simple and noninvasive route of administration that exempts the physician and patient from other invasive modalities such as intravitreal injection of anti-VEGF agents and its possible complications such as postinjection infections and intraocular injuries.
Gold and silver NPs (Au-NPs and Ag-NPs, respectively) conjugated with a heparin derivative have demonstrated efficacy as antiangiogenesis agents. Studies of ocular-therapy applications have shown that Au-NPs and Ag-NPs conjugated with a heparin derivative are effectively delivered to their target, where they bind VEGF receptors to inhibit the actions of VEGF via different signaling pathways and ultimately inhibit angiogenesis. Thus, both Au-NPs and Ag-NPs conjugated with antiangiogenic factors clearly have antiangiogenic properties that make them excellent candidates for biomedical use.[33,34] We found that topical silica NPs offer a promising alternative to other anti-VEGF modalities with lower cost and higher availability. We also found that topical SiNPs was biocompatible with corneal tissues and did not irritate the corneal surface in terms of clinical and histopathologic examinations.
In a recent study using polymeric NPs for antiangiogenesis therapy, Poly(D,L-lactic-co-glycolic acid) (PLGA)-based NPs were used for targeted nonviral retinal gene delivery for the management of choroidal NV. The NPs were then coated with linear sequences of arginine-glycine-aspartic acid peptides, transferrin or both, and tested in a rat model of choroidal NV. Upon intravenous administration, NPs exhibited targeted delivery to the neovascular eye but not the control eye. This study demonstrates that therapies based on nanotechnology have the capacity to target unique components of the angiogenesis cycle in a very different manner than conventional therapies.[35] The exact mechanism of inhibiting corneal neovascularization by SiNPs is not clear; however, the most possible way is direct blocking of VEGF receptors by these NPs. It was showed that SiNPs could be considered in the treatment of retinal neovascularization without acute toxicity. SiNPs effectively inhibited VEGF-induced retinal neovascularization and suppressed ERK 1/2 activation via inhibition of VEGFR-2 phosphorylation;[19] however, application of topical SiNPs and its ability for inhibiting corneal NV has not been studied before.
Other studies confirmed SiNPs organic drug delivery systems and exhibit many unique properties, such as highly controllable size and shape. Brunner et al. used amorphous silica NPs as a nontoxic control in their studies.[36] Using the Comet assay, Barnes et al. found no significant genotoxicity for the tested silica NPs on 3T3-L1 fibroblasts after incubation for 3, 6, and 24 h.[37] Teng and co-workers recently reported that mesoporous hollow silica NPs show low and dose-dependent toxicity when intravenously injected at single or repeated doses.[38] For a single dose, the median lethal dose (LD50) of 110-nm NPs exceeded 1,000 mg/kg. For repeated doses, no deaths were observed after mice were exposed to NPs at 20, 40, and 80 mg/kg by continuous intravenous administration for 14 days. The intravenously injected NPs accumulate mainly in mononuclear phagocytic cells in the liver and spleen, and the total clearance time of the particles is estimated to exceed 4 weeks.[39] Another study evaluated the in vivo toxicity of silica NPs in immunocompetent mice when administered intravenously and found that in vivo toxicity of silica NPs was mainly influenced by the porosity and surface charge.[40] Many in vitro evaluations of silica NP toxicity have been done.[41] The toxicity of silica NPs depends strongly on physicochemical properties such as particle size, shape, porosity, chemical purity, surface chemistry, and solubility.[42] The surface chemistry of silica NPs also plays an important role in their cytotoxicity.[43] We used topical SiNPs for treatment of corneal neovascularization and noticed no corneal or conjunctival irritation in our experimental series; however, its definite safety evaluation needs further in vitro and in vivo examinations.
Corneal angiogenic factors include VEGF for promoting neovascularization. This factor promotes vascular endothelial cell proliferation, migration, and tube formation.[23] It also increases vascular leakage and promotes monocyte chemotaxis and B-cell production in mice, indicating the key role of VEGF in inflammation.[24] VEGF and VEGF-dependent signaling pathways are known to be involved in pathological corneal NV. In particular, VEGFR-2 is considered to play a pivotal role in developmental angiogenesis.[44] This theory assumes that SiNP seffectively suppressed phosphorylation of VEGFR-2. The requirement of VEGF for corneal NV was first demonstrated in a rat model where NV was later subsequently blocked by anti-VEGF antibodies.[25,45] Indeed, anti-VEGF antibodies have shown initial therapeutic success. Bevacizumab is a humanized murine monoclonal antibody that recognizes all isoforms of VEGF. Bevacizumab was initially approved to treat metastatic colon cancer,[26] but has also shown efficacy in partial reduction of corneal NV through topical, subconjunctival and intraocular applications of bevacizumab.[6,27,46] Nanoparticulate systems improve the delivery of poorly water-soluble drugs while significantly reducing toxicity compared to the free drug.[47] There are several different modalities for ocular drug administration. The most common include liquids topically applied onto the front of the eye in the form of eye drops, subconjunctival or sub-Tenon's injection in the conjunctival tissue or below the Tenon's capsule and intravitreal injection. Dong and his co-workers demonstrated that SiNPs had negligible acute toxicity to retinal neuronal cells, retinal endothelial cells and the retinal tissue at concentrations 100 times the effective therapeutic dosage. In addition, they reported that SiNPs had antiangiogenic effects.[19] Other investigators declared AgNPs had the potential of inhibiting the VEGF in a model employing bovine retinal endothelial cells in vitro.[48] These results suggest that the concentration, shape, or size of NPs could be key factors exerting their antiangiogenic effects. According to tight junctions between corneal epithelial cells, the small size of NPs (20-50 nm) will be effective for increasing drug penetration into deep corneal stromal layers that warrants NP efficacy. The physicochemical characteristics of SiNPs are critical for cellular uptake, intracellular trafficking and interaction with plasma proteins. Nonetheless, the issue of biodistribution of NPs should be addressed in their biomedical application. Therefore, biodegradation and biodistribution of NPs might be investigated before their clinical application. Since this study is the first to evaluate efficacy of topical SiNPs, we suggest evaluating the above-mentioned factors for SiNPs in future investigations. The major treatment for corneal neovascularization is anti-VEGF and steroids. Topical, subconjunctival, and intrastromal injection of bevacizumab are reported for management of corneal neovascularization.[6,25,26,27,45,46,49,50] However, there is no report of applying SiNps for decreasing effect of corneal neovascularization. SiNPs mass product may be more feasible and cost-effective than monoclonal antibodies such as avastin or bevacizumab. Also, SiNPs could have modifiable size and concentration. Using topical SiNPs as an anti-VEGF therapy seems effective in suppressing new vessel-formation and vascular leakage, which can improve visual function. It seems reasonable that topical administration of SiNPs can be a novel treatment for corneal NV. It may also be investigated for noninvasive management of choroidal neovascularization with minimal side effects in future studies.
In conclusion, our study showed, for the first time, that topical SiNPs can effectively decrease the extent of corneal neovascularization after chemical burn. We suggest further investigations with different SiNPs concentrations to determine its minimal antineovascularization concentration and evaluating biodegradation, biodistribution, and safety issues of topical SiNPs.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Mohammadpour M, Mohajernezhadfard Z, Khodabande A, Vahedi P. Antibiotic susceptibility patterns of pseudomonas corneal ulcers in contact lens wearers. Middle East Afr J Ophthalmol. 2011;18:228–31. doi: 10.4103/0974-9233.84053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadpour M, Jabbarvand M, Karimi N. Therapeutic possibilities of ceftazidime nanoparticles in devasting pseudomonas ophthalmic infections; keratitis and endophthalmitis. Med Hypothesis Discov Innov Ophthalmol. 2012;1:6–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Menzel-Severing J. Emerging techniques to treat corneal neovascularisation. Eye (Lond) 2012;26:2–12. doi: 10.1038/eye.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tshionyi M, Shay E, Lunde E, Lin A, Han KY, Jain S, et al. Hemangiogenesis and lymphangiogenesis in corneal pathology. Cornea. 2012;31:74–80. doi: 10.1097/ICO.0b013e31821dd986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FallahTafti MR, Khosravifard K, Mohammadpour M, Hashemian MN, Kiarudi MY. Efficacy of Intralesional bevacizumab injection in decreasing pterygium size. Cornea. 2011;30:127–9. doi: 10.1097/ICO.0b013e3181e16d67. [DOI] [PubMed] [Google Scholar]
- 6.Hashemian MN, Zare MA, Rahimi F, Mohammadpour M. Deep intrastromalbevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011;30:215–8. doi: 10.1097/ICO.0b013e3181e291a6. [DOI] [PubMed] [Google Scholar]
- 7.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. CurrOpinOphthalmol. 2001;12:242–9. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Öner V, Küçükerdönmez C, Akova YA, Çolak A, Karalezli A. Topical and subconjunctivalbevacizumab for corneal neovascularization in an experimental rat model. Ophthalmic Res. 2012;48:118–23. doi: 10.1159/000337139. [DOI] [PubMed] [Google Scholar]
- 9.Shakiba Y, Mansouri K, Arshadi D, Rezaei N. Corneal neovascularization: Molecular events and therapeutic options. Recent Pat Inflamm Allergy Drug Discov. 2009;3:221–31. doi: 10.2174/187221309789257450. [DOI] [PubMed] [Google Scholar]
- 10.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadpour M, Jabbarvand M, Delrish E, Khoshzaban A. Antiangiogenic effect of silicate nanoparticles on corneal neovascularization induced by vascular endothelial growth factor. Journal of Medical Hypotheses and Ideas. 2014;8:14–20. [Google Scholar]
- 12.Mohammadpour M, Hashemi H, Jabbarvand M, Delrish E. Penetration of silicate nanoparticles into the corneal stroma and intraocular fluids. Cornea. 2014;33:738–43. doi: 10.1097/ICO.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 13.Barbé C, Bartlett J, Kong LG, Finnie K, Lin HQ, Larkin M, et al. Silica particles: A novel drug-delivery system. Adv Mater. 2004:1959–66. [Google Scholar]
- 14.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest. 2011;121:2768–80. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, et al. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. IntegrBiol (Camb) 2013;5:74–86. doi: 10.1039/c2ib20174g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galagudza MM, Korolev DV, Sonin DL, Postnov VN, Papayan GV, Uskov IS, et al. Targeted drug delivery into reversibly injured myocardium with silica nanoparticles: Surface functionalization, natural biodistribution, and acute toxicity. Int J Nanomed. 2010;5:231–7. doi: 10.2147/ijn.s8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borak B, Arkowski J, Skrzypiec M, Zió³kowski P, Krajewska B, Wawrzyñska M, et al. Behavior of silica particles introduced into an isolated rat heart as potential drug carriers. Biomed Mater. 2007;2:220–3. doi: 10.1088/1748-6041/2/4/003. [DOI] [PubMed] [Google Scholar]
- 18.López T, Bata-García JL, Esquivel D, Ortiz-Islas E, Gonzalez R, Ascencio J, et al. Treatment of Parkinson's disease: Nanostructured sol-gel silica-dopamine reservoirs for controlled drug release in the central nervous system. Int J Nanomedicine. 2010;6:19–31. doi: 10.2147/IJN.S13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo DH, Kim JH, Yu YS, Lee TG, Kim JH. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomedicine. 2012;8:784–91. doi: 10.1016/j.nano.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Kazuo AB, Watanabe Y. Determination of silicate in seawater by inductively coupled plasma atomic emission spectrometry. J Oceanogr. 1992;48:283–92. [Google Scholar]
- 21.Hartlen KD, Athanasopoulos AP, Kitaev V. Facile preparation of highly monodisperse small silica spheres (15 to >200 nm) suitable for colloidal templating and formation of ordered arrays. Langmuir. 2008;24:1714–20. doi: 10.1021/la7025285. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney JM, Waterbury LD. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr Eye Res. 1985;4:531–5. doi: 10.3109/02713688508999984. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 24.Klettner A, Roider J. Treating age-related macular degeneration - interaction of VEGF-antagonists with their target. Mini Rev Med Chem. 2009;9:1127–35. doi: 10.2174/138955709788922665. [DOI] [PubMed] [Google Scholar]
- 25.Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound-and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- 26.Oh JY, Kim MK, Wee WR. Subconjunctival and Intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea. 2009;28:1070–3. doi: 10.1097/ICO.0b013e31819839f9. [DOI] [PubMed] [Google Scholar]
- 27.Yeung SN, Lichtinger A, Kim P, Amiran MD, Slomovic AR. Combined use of subconjunctival and Intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011;30:1110–4. doi: 10.1097/ICO.0b013e31821379aa. [DOI] [PubMed] [Google Scholar]
- 28.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 29.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–51. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int J Pharm. 2012;432:105–12. doi: 10.1016/j.ijpharm.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Jones L, Gu FX. Nanomaterials for ocular drug delivery. MacromolBiosci. 2012;12:608–20. doi: 10.1002/mabi.201100419. [DOI] [PubMed] [Google Scholar]
- 32.Loo SC, Tan ZY, Chow YJ, Lin SL. Drug release from irradiated PLGA and PLLA multi-layered films. J Pharm Sci. 2010;99:3060–71. doi: 10.1002/jps.22079. [DOI] [PubMed] [Google Scholar]
- 33.Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, Ajayan P, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20:455104. doi: 10.1088/0957-4484/20/45/455104. [DOI] [PubMed] [Google Scholar]
- 34.Basavaraj KH. Nanotechnology in medicine and relevance to dermatology: Present concepts. Indian J Dermatol. 2012;57:169–74. doi: 10.4103/0019-5154.96186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–59. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, et al. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ SciTechnol. 2006;40:4374–81. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 37.Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Lesniak A, et al. Reproducible comet assay of amorphous silica nanoparticles detects no genotoxicity. Nano Lett. 2008;8:3069–74. doi: 10.1021/nl801661w. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, Li L, Teng X, Huang X, Liu H, Chen D, et al. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials. 2011;32:1657–68. doi: 10.1016/j.biomaterials.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Cho M, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, et al. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. ToxicolLett. 2009;189:177–83. doi: 10.1016/j.toxlet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Yu T, Hubbard D, Ray A, Ghandehari H. In vivobiodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J Control Release. 2012;163:46–54. doi: 10.1016/j.jconrel.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: Another variable entity. Part FibreToxicol. 2010;7:39. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberman A, Martinez HP, Ta CN, Barback CV, Mattrey RF, Kono Y, et al. Hollow silica and silica-boron nano/microparticles for contrast-enhanced ultrasound to detect small tumors. Biomaterials. 2012;33:5124–9. doi: 10.1016/j.biomaterials.2012.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu T, Malugin A, Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5:5717–28. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–78. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. BiochemBiophys Res Commun. 2005;333:328–35. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 46.Hosseini H, Nejabat M, Khalili MR. Bevacizumab (Avastin) as a potential novel adjunct in the management of pterygia. Med Hypotheses. 2007;69:925–7. doi: 10.1016/j.mehy.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 47.Wood RW, Li VH, Kreuter J, Robinson JR. Ocular disposition of poly-hexyl-2-cyano[3-14C] acrylate nanoparticles in the albino rabbit. Int J Pharm. 1985;23:175–83. [Google Scholar]
- 48.Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30:6341–50. doi: 10.1016/j.biomaterials.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Mohammadpour M. Managements for corneal neovascularization. Cornea. 2013;32:e190. doi: 10.1097/ICO.0b013e3182a710a8. [DOI] [PubMed] [Google Scholar]
- 50.Mohammadpour M. Deep intrastromal injection of bevacizumab for the management of corneal neovascularization. Cornea. 2013;32:109–10. doi: 10.1097/ICO.0b013e318262e872. [DOI] [PubMed] [Google Scholar]


