Abstract
Published data on the prognostic significance of neutrophil-to-lymphocyte ratio (NLR) in non-small cell lung cancer (NSCLC) are controversial. We performed a meta-analysis to more accurately assess its prognostic value. The analysis was performed based on the data from 14 studies with 3,656 patients to estimate the correlation between NLR and overall survival (OS) and progression-free survival (PFS) in NSCLC. Hazard ratio (HR) with 95% confidence interval (CI) were calculated to estimate the effect. We also conducted subgroup analysis and meta-regression analysis. The results demonstrated that elevated pretreatment NLR predicted poorer OS (HR: 1.70, 95% CI: 1.39–2.09) and PFS (HR: 1.63, 95% CI: 1.27–2.09) in patients with NSCLC. Subgroup analysis indicated that cut-off value of 5 showed consistently prognostic value. There was no significant heterogeneity or publication bias for OS and PFS for included studies. This meta-analysis revealed that elevated pretreatment NLR might be a predicative factor of poor prognosis for NSCLC patients.
Lung cancer is one of the most commonly diagnosed cancers and remains the leading cause of cancer-related death worldwide1. Non-small-cell lung cancer (NSCLC) accounts for approximately 80–85% of all lung cancer cases. Despite diverse treatment methods including surgery, chemotherapy, radiation and targeted therapies are used, the prognosis of NSCLC is disappointing, with 5-year survival rate remains about 17%2. The high mortality rates of NSCLC are partly due to the lack of effective prognostic biomarkers. Therefore, it is urgent for us to identify novel prognostic factors which might enable clinicians to stratify risk patients and further tailor therapeutic strategies.
Up to now, a series of traditional prognostic parameters for NSCLC patients are well known. Several independent prognostic factors for survival in patients with NSCLC have been identified: age, sex, weight loss, smoking status, performance status and TNM stage3. However, these factors are not adequately used in clinical settings for insufficient specificity and sensitivity. In recent years, accumulating evidence demonstrated that systemic inflammatory response is associated with poor prognosis in various solid tumors4,5,6,7. Distinct index or markers of systemic inflammatory response such as Glasgow Prognostic Score(GPS), C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio(NLR) have been evaluated in a series of cancers8,9. These studies demonstrate that tumor cells can recruit neutrophils into the tumor stroma through specific chemokines10. Subsequently, neutrophils exert pro-tumorigenesis effects by inhibiting apoptosis, promoting angiogenesis and metastasis11,12. While, infiltrating lymphocytes which play a role in tumor defence are associated with favorable prognosis13. So the NLR, which is defined as neutrophil counts divided by lymphocyte counts, is particularly noteworthy.
Gathering evidences have indicated that NLR had prognostic significance in patients with breast cancer, colorectal cancer, renal cell carcinoma, gastric cancer and hepatocellular carcinoma14,15,16,17,18. Recent studies suggest a potential prognostic role of NLR in NSCLC patients, however, the majority of the studies had relatively limited sample sizes19,20,21,22,23. Furthermore, some authors presented conflicting data regarding the prognostic significance of NLR in NSCLC24. We thus conducted this meta-analysis to systematically clarify the prognostic value of NLR in NSCLC patients.
Results
Study selection and characteristics
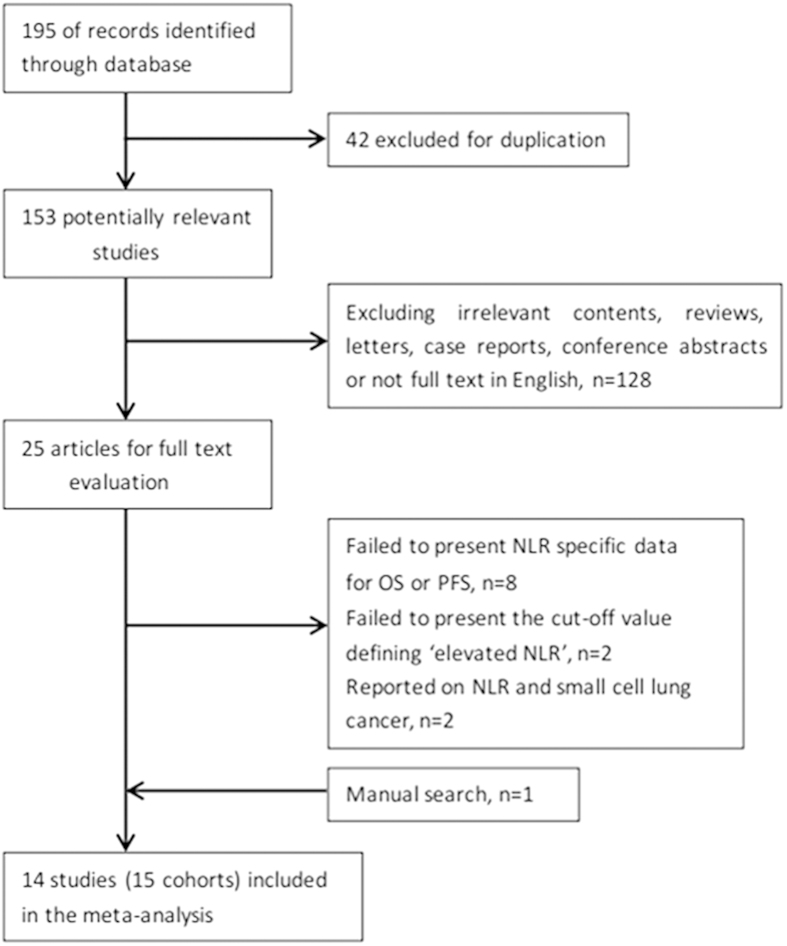
The flow chart of the literature selection was shown in Fig. 1. The initial search strategies retrieved a total of 195 studies. After screening the titles or abstracts, 170 studies were excluded as they were either duplicate reports, conference abstracts, reviews, case reports, reports in language other than English or studies irrelevant to the current analysis. Then, 25 identified studies concerning NLR and the prognosis of NSCLC were further evaluated. Eleven reports of them were discarded because of the following reasons: eight did not provide specific NLR data for OS or PFS, two failed to define cut-off value of “elevated NLR”, two reported on NLR and small cell lung cancer, we also added one article by manual search. Therefore, 14 studies19,20,21,22,23,24,25,26,27,28,29,30,31,32 with 3656 patients published between 2009 and 2015 were included in our meta-analysis finally. As the study by Botta et al.28 included two cohorts and reported the HR and 95%CI respectively, we marked them as Botta1 and Botta2. The main characteristics of these studies are shown in Table 1. Three studies were conducted in USA21,30,32, two studies were performed in Japan25,26, China20,31 and Turkey19,22, respectively, one in Spain27, Italy28, Korea24, Belgium29, and UK23, respectively. One study22 involved all disease stages, six studies19,21,23,26,29,32 included only early stage disease (І/І-ІІ/І-ІІІ/ІІ-ІІІB) and seven studies20,24,25,27,28,30,31 included only late stage disease (ІІІB-ІV/ІV).Thirteen studies19,20,21,22,23,24,25,26,27,29,30,31,32 with 3,544 patients reported the correlations of NLR and OS, while nine studies20,21,24,25,28,29,30,31,32 (ten cohorts) with 2,623 patients reported the correlations of NLR and PFS. NOS scores of the studies ranged from 5 to 8, with a mean value of 6.64.
Figure 1. Flow chart of the included studies.
Table 1. Characteristics of included studies.
| Study | Year | Country | Duration | Sample size | Follow-up(m) (median/range) | Stage | Treat-ment | Cut-off value | Survival analysis | Study design | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Teramukai25 | 2009 | Japan | 2001–2005 | 388 | 18.9(2.3–57) | IIIB-IV | C | 4.74 | OS,PFS | P | 8 |
| Tomita26 | 2011 | Japan | 2000–2005 | 284 | >60 | I-III | S | 2.5 | OS | R | 8 |
| Cedres27 | 2012 | Spain | 2004–2009 | 171 | 9.1(1–70.4) | IV | C | 5 | OS | R | 8 |
| Lee24 | 2012 | Korea | 2005–2007 | 199 | 36 | IIIB-IV | C | 3.17 | OS,PFS | P | 7 |
| Botta128 | 2013 | Italy | 2008–2011 | 73 | 15 | IIIB-IV | C+T | 4 | PFS | R | 7 |
| Botta228 | 2013 | Italy | 2008–2011 | 39 | 15 | IIIB-IV | C | 4 | PFS | R | 7 |
| Forget29 | 2013 | Belgium | 1993–2004 | 255 | 56.1 | I-II | S | 5 | OS,PFS | R | 8 |
| Jafri30 | 2013 | USA | 2000–2011 | 173 | NR | IV | C | 5 | OS,PFS | R | 6 |
| Unal19 | 2013 | Turkey | NR | 94 | NR | II-IIIB | C | 3.44 | OS,PFS | R | 5 |
| Yao20 | 2013 | China | 2007–2010 | 182 | NR | IIIB-IV | C | 2.63 | OS,PFS | R | 6 |
| Kacan22 | 2014 | Turkey | NR | 299 | NR | I-IV | S | 5 | OS | R | 5 |
| Pinato23 | 2014 | UK | 2004–2011 | 220 | 12 | I-III | S | 5 | OS | P | 7 |
| Cannon21 | 2014 | USA | 2006–2012 | 59 | 17 | I | R | 2.98 | OS | R | 6 |
| Lin31 | 2014 | China | 2009–2012 | 81 | 13–40 | IV | T | 3.5 | OS,PFS | R | 6 |
| Choi32 | 2015 | USA | 2004–2010 | 1139 | NR | I-III | S | 5 | OS,PFS | R | 6 |
NR: not reported; Treatment describes whether the patients received surgery (S), chemotherapy (C), radiotherapy (R) or targeted therapy (T); OS: overall survival; PFS: progression-free survival; Study design describes the studies as either prospective (P) or retrospective (R) study.
NLR and OS in NSCLC
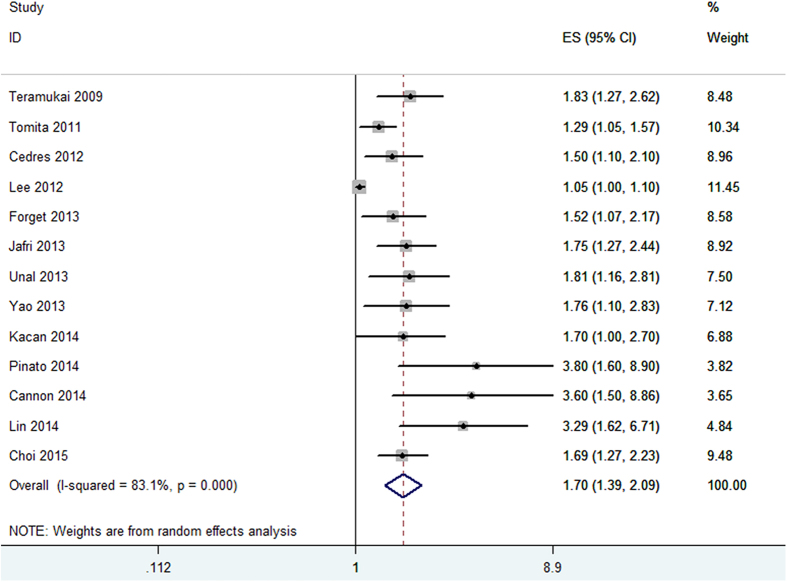
Thirteen cohorts presented the data of pretreatment NLR and OS in NSCLC patients. Meta-analysis of these 13 cohorts showed that patients with elevated NLR were associated with shorter OS (HR obtained from DerSimonian–Laird random-effects model: 1.70 (95% CI: 1.39–2.09, p < 0.001); Fig. 2), although there was heterogeneity between studies (I2 = 83.1%, Ph < 0.001). Then we conducted subgroup analyses according to confounders such as treatment method, study location, tumor stage, sample size, cut-off value defining “elevated NLR” and NOS score.
Figure 2. Forrest plots of studies evaluating hazard ratio (HR) with 95% CI of NLR for overall survival(OS).
Stratification by treatment methods, we found the pooled HRs were 1.70 (95%CI: 1.39–2.10) for patients treated by surgery and 1.76 (95%CI: 1.30–2.39) for patients treated by non-surgery methods. Subgroup analyses by countries indicated that elevated NLR predicted poor prognosis for patients both in western countries (HR = 1.74, 95%CI: 1.44–2.12) and in eastern countries(HR = 1.58, 95%CI: 1.22–2.04). Stratification by cutoff value = 5 and cut-off value ≠ 5, the data showed that the pooled HR was 1.67 (95%CI:1.44–1.94) for cutoff value = 5 and 1.67 (95%CI:1.26–2.23) for cut-off value ≠ 5. Notably, when cut-off value = 5 was used, there was no heterogeneity (I2 = 0, Ph = 0.506), which may indicate NLR = 5 is more stable in prognosis prediction. In addition, subgroup analyses showed the elevated NLR predicted prognosis for NSCLC regardless of tumor stage (early stage vs. late stage), sample size(≥200 vs.<200) and NOS score(≥7 vs.<7) (Table 2).
Table 2. Summary of the meta analysis results.
| Outcome | Stratified analysis | No. of studies | No. of patients | Random-effects model |
Fixed-effects model |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | P | HR(95%CI) | p | I2(%) | Ph | ||||
| OS | Treatment | ||||||||
| Surgery | 5 | 2197 | 1.70(1.39–2.10) | <0.001 | 1.10(1.05–1.15) | <0.001 | 85.2 | <0.001 | |
| Non-surgery | 8 | 1347 | 1.76(1.30–2.39) | <0.001 | 1.49(1.30–2.72) | <0.001 | 46.8 | 0.111 | |
| Country | |||||||||
| Western | 6 | 2017 | 1.74(1.44–2.12) | 0.001 | 1.70(1.46–1.99) | <0.001 | 82.4 | <0.001 | |
| Eastern | 7 | 1527 | 1.58(1.22–2.04) | <0.001 | 1.09(1.04–1.14) | <0.001 | 29.7 | 0.212 | |
| Tumor stage | |||||||||
| Early stage | 7 | 2350 | 1.69(1.37–2.10) | <0.001 | 1.55(1.36–1.77) | <0.001 | 48.8 | 0.068 | |
| Late stage | 6 | 1194 | 1.64(1.19–2.27) | 0.003 | 1.09(1.04–1.14) | <0.001 | 85.8 | <0.001 | |
| Sample size | |||||||||
| ≥200 | 6 | 2585 | 1.61(1.33–1.95) | <0.001 | 1.53(1.34–1.75) | <0.001 | 41.7 | 0.127 | |
| <200 | 7 | 959 | 1.76(1.26–2.44) | 0.001 | 1.09(1.04–1.14) | <0.001 | 84.8 | <0.001 | |
| Cut–off value | |||||||||
| =5 | 6 | 2257 | 1.67(1.44–1.94) | <0.001 | 1.67(1.44–1.94) | <0.001 | 0 | 0.506 | |
| ≠5 | 7 | 1287 | 1.67(1.26–2.23) | <0.001 | 1.09(1.04–1.14) | <0.001 | 84.2 | <0.001 | |
| NOS score | |||||||||
| ≥7 | 6 | 1517 | 1.46(1.14–1.86) | 0.002 | 1.09(1.04–1.14) | <0.001 | 82.2 | <0.001 | |
| <7 | 7 | 2027 | 1.83(1.56–2.15) | <0.001 | 1.83(1.56–2.15) | <0.001 | 0 | 0.498 | |
| PFS | Country | ||||||||
| Western | 5 | 1679 | 1.56(1.31–1.86) | <0.001 | 1.56(1.31–1.86) | <0.001 | 0 | 0.791 | |
| Eastern | 5 | 944 | 1.68(1.12–2.52) | 0.012 | 1.05(1.00–1.11) | 0.049 | 86.9 | <0.001 | |
| Sample size | |||||||||
| ≥200 | 3 | 1782 | 1.46(1.21–1.77) | <0.001 | 1.46(1.21–1.77) | <0.001 | 0 | 0.643 | |
| <200 | 7 | 841 | 1.72(1.20–2.46) | 0.003 | 1.06(1.01–1.12) | 0.019 | 84.5 | <0.001 | |
| Cut-off value | |||||||||
| =5 | 3 | 1567 | 1.54(1.27–1.86) | <0.001 | 1.54(1.27–1.86) | <0.001 | 0 | 0.453 | |
| ≠5 | 7 | 1056 | 1.67(1.19–2.35) | 0.003 | 1.06(1.01–1.12) | 0.027 | 82.7 | <0.001 | |
| NOS score | |||||||||
| ≥7 | 5 | 954 | 1.40(1.03–1.91) | 0.032 | 1.04(0.99–1.10) | 0.115 | 71 | 0.008 | |
| <7 | 5 | 1669 | 1.79(1.38–2.33) | <0.001 | 1.67(1.41–1.98) | <0.001 | 52.3 | 0.079 | |
Ph: p value of Q test for heterogeneity test; N: number of studies (cohorts); HR: hazard ratio; 95% CI: 95% confidence interval; For OS and PFS, subgroup analyses were performed by treatment (surgery vs. non-surgery), study location (Western vs. Eastern countries), sample size (≥200 vs.<200) ,cut-off value of NLR (5 vs. not 5) and NOS score(≥7 vs.<7).
NLR and PFS in NSCLC
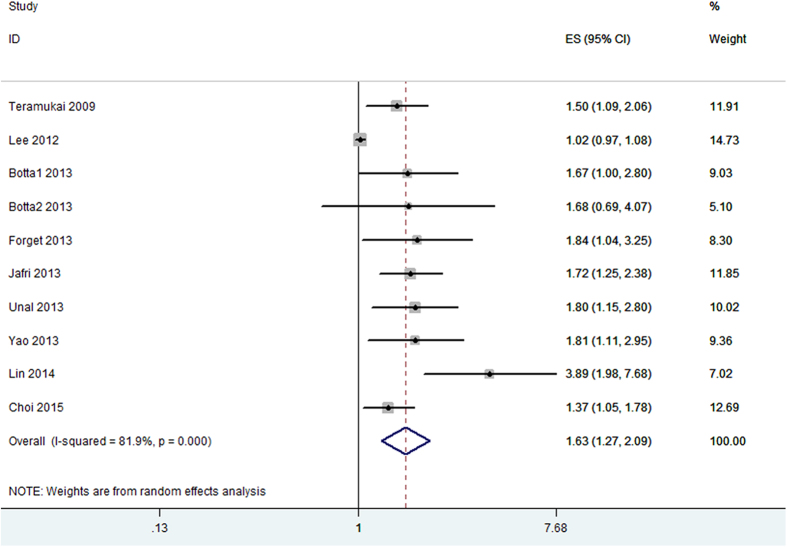
Ten cohorts with 2,623 cases reported the data of pretreatment NLR and PFS in NSCLC patients. Combined data from the ten cohorts suggested that elevated pretreatment NLR were significantly correlated with PFS with a pooled HR estimate of 1.63 (95% CI: 1.27–2.09, p < 0.001; Fig. 3), with heterogeneity (I2 = 81.9%, Ph < 0.001). Subgroup analysis indicated that elevated pretreatment NLR were significantly associated with PFS in weastern countries (HR: 1.56, 95% CI: 1.31–1.86, p < 0.001), without significant heterogeneity in the data (I2 = 0, Ph = 0.791). We did not perform subgroup analysis for PFS based on treatment method as majority therapeutic regimen in the studies was chemotherapy. Moreover, elevated pretreatment NLR was also associated significantly with PFS in NSCLC patients with a cut-off value of 5 (HR: 1.54, 95% CI: 1.27–1.86, p < 0.001), without significant heterogeneity in the data (I2 = 0, Ph = 0.453).
Figure 3. Forrest plots of studies evaluating hazard ratio (HR) with 95% CI of NLR for progression-free survival(PFS).
Heterogeneity
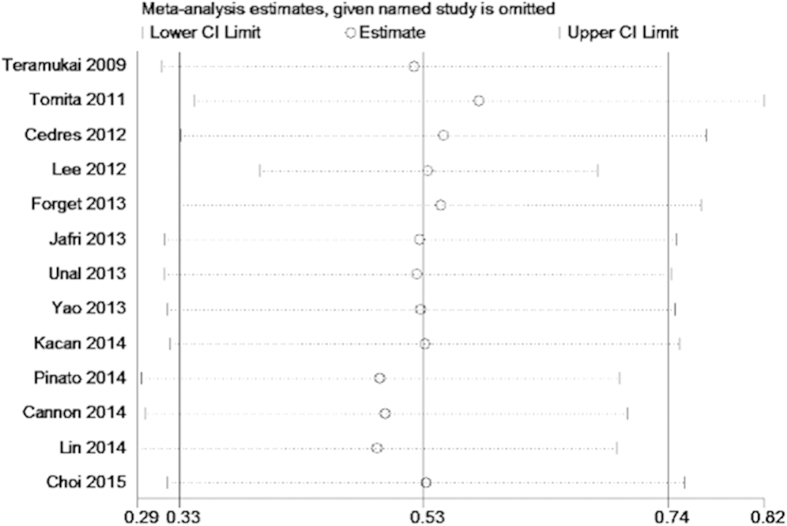
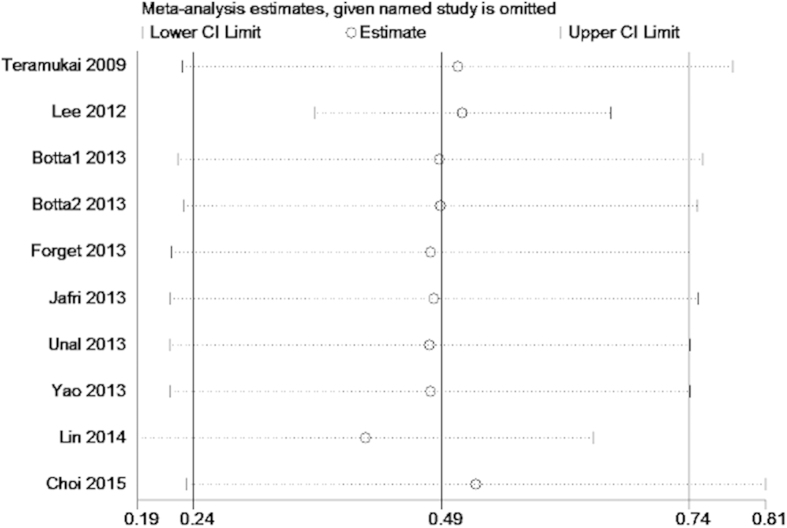
We conducted meta-regression analysis to investigate the potential source of heterogeneity among studies for OS and PFS. The results showed that treatment method (p = 0.891), study location(p = 0.387), tumor stage(p = 0.625), sample size(p = 0.97), cut-off value (p = 0.693) and NOS score (p = 0.084) did not contribute to the source of heterogeneity for OS. Moreover, the data demonstrated that study location(p = 0.944), sample size(p = 0.733) and NOS score (p = 0.202) did not contribute to the source of heterogeneity for PFS. Sensitivity analysis indicated that removing any single study by turn did not significantly affect the pooled HRs for OS and PFS (Figs 4 and 5).
Figure 4. Sensitivity analysis on the relationship between NLR and OS in NSCLC.
Figure 5. Sensitivity analysis on the relationship between NLR and PFS in NSCLC.
Publication bias
Publication bias estimate was mainly used to evaluate the reliability of meta-analysis results, especially which showed statistical significance33. Assessment of publication bias by using Begg’s test (statistical significance was set at p < 0.05) suggested that were no significant publication bias in OS and PFS studies (p = 0.2 and p = 0.721, respectively).
Discussion
This meta-analysis aimed to examine the associations between elevated pretreatment NLR and OS and PFS of NSCLC. Our analysis combined the outcomes of 3,656 NSCLC patients from 14 individual studies, demonstrating that elevated pretreatment NLR significantly predicted poor OS (HR: 1.70, 95% CI 1.39–2.09), and PFS (HR: 1.63, 95% CI 1.27–2.09) of NSCLC cancer patients. Although heterogeneity exists, most of the prognostic significance is not weakened by subgroup analysis stratified by treatment method, study location, tumor stage, sample size, cut-off value of NLR and NOS score. Furthermore, subgroup analysis indicated that NLR had consistent prognostic value for NSCLC populations of OS with a cut-off value of 5. Whereas, NLR could better predicted poor PFS for NSCLC patients in western countries with a cut-off value of 5. This finding suggested that dichotomized NLR cut-off value of 5 could help guide clinical decision-making in regard of therapeutic strategies and outcomes for NSCLC patients both for OS and PFS. To the best of our knowledge, this is the first meta-analysis on the association between elevated pretreatment NLR and clinical outcomes in NSCLC.
Accumulating evidence showed the connection between inflammation and cancer and mechanistic studies have presented solid evidence to support the biological and prognostic importance of a pro-inflammatory tumor microenvironment in cancer progression7,34. An elevated NLR implies an increased neutrophil count and/or a decreased lymphocyte count, as well as a relative lymphopenia. Lymphocytes have an important role in tumor defence, which inhibits tumor cell proliferation and migration7,35. However, a large amount of neutrophils had been indicated to influence cytolytic activity of lymphocytes or natural killer cells, as well as suppress T-cell proliferation36. Thus, neutrophils in the tumor microenvironment could have negative impact on tumor growth. Therefore, NLR could concisely reflect the imbalance of pro-tumor and anti-tumor activity of the hosts in respect of inflammatory response. Thus, the relative value of a combined neutrophil and lymphocyte counts index in form of a neutrophil to lymphocyte (N/L) ratio can reflect the protumor efficacy and antitumor capacity of the host more accurately. IL-17 and peritumoral CD163 may exert important roles in the inflammatory tumor microenvironment and facilitate tumor progression and recurrence37. Additionally, it is convenient and cost-effective to measure the parameter of NLR in clinical practice, which makes NLR an attractive biomarker for NSCLC prognostication.
More recently, several meta-analyses reported the prognostic value of NLR in a variety of cancers, including colorectal cancer, hepatocellular carcinoma, gastric cancer, renal cell carcinoma, pancreatic cancer and esophageal cancer17,18,38,39,40,41. Our study was the first study investigating the prognostic significance of NLR for NSCLC patients and the results were in line with previous reports, indicating that elevated NLR gained prognostic values for solid tumors and NLR could be widely used in clinical settings, especially for cancer patients. In addition, the value of NLR was easy to obtain because it is a routine test and more importantly, it does not add extra cost. So NLR is a promising biomarker for clinical use.
In spite of the intrinsic defects associated with meta-analysis, there are a number of other limitations in our study. First, significant heterogeneity was observed in the results due to confounding factors, such as the baseline characteristics of the patients, treatment methods, follow-up period, sample size and cut-off value of NLR. However, subgroup analysis, meta-regression analysis and sensitivity analysis showed that none of the above-mentioned confounders could completely explain the heterogeneity. Thus, we supposed that the heterogeneity could be a result of combined effect of the above-mentioned confounders and the genotypic diversity of lung cancer in these studies. Second, we did not analyze the correlation between the elevated NLR and clinicopathological parameters of patients, such as lymph node metastasis, grade of differentiation and tumor stage, because only two studies reported the relevant information. The data is insufficient to analyze. Third, some primary studies evaluated the prognostic role of NLR in univariate analysis, whereas others used multivariate analysis, which may contribute to some bias when the data were pooled. Forth, most of the original studies showed that high NLR predicted poor prognosis due to positive results tend to be published, although two studies24,28 gained negative results for PFS, more controversial papers could not be searched.
Despite several limitations, our meta-analysis also had some advantages. First, we got similar results when the data were analyzed neither in random-effects model nor in fixed-effects model, which indicated that robustness of the statistic results. Second, the results of sensitivity analysis did not significantly altered, indicating that our results were stable. At last, all the scores of study quality assessed by NOS were ≥5, which demonstrated the creditability of our meta-analysis results.
In conclusion, our results indicated that elevated pretreatment NLR might be an unfavorable prognostic factor for patients with NSCLC, which could be useful in stratifying patients and in determining individual treatment plans. However, these findings need to be interpreted cautiously when used in clinical practice because of the limitations listed above. More well-designed and large-scale investigations are warranted to better understand the value of NLR in the prognosis of NSCLC.
Methods
Publication search
A literature search was conducted via Pubmed, Embase, and Web of Science databases for articles that assessed NLR as a prognostic factor for survival of patients with NSCLC (last search was updated on May 6, 2015). The search strategy used key words such as “neutrophil-to-lymphocyte ratio” , “neutrophil lymphocyte ratio” , “NLR” , “lung cancer” , ‘lung carcinoma’, ‘NSCLC”, “non small cell lung cancer”, “non-small cell lung cancer”, “prognosis”, “prognostic” and “survival”. Article language was restrained to English. The references in the identified articles were also retrieved to find other relevant studies.
Study selection criteria
Two reviewers (X.B.G. and X.J.T.) reviewed all candidate articles independently. Discrepancies were resolved by discussion. Studies were eligible for inclusion in the meta-analysis if they met the following criteria: (a) patients with NSCLC in the studies were confirmed histopathologically; (b) investigated the association of pre-treatment NLR with overall survival (OS) or progression-free survival (PFS); (c) reported a hazard ratio (HR) and 95% confidence intervals (CIs) or the data sufficient to estimate the HR and 95% CIs; (d) to be published as full texts in the English language. Small-cell lung cancer was not included in our study because it is a highly undifferentiated cancer with distinct biological behaviors from NSCLC.
Data extraction and quality assessment
Two investigators (X.B.G. and T.T.) reviewed each eligible study and extracted data. The extracted data including: first author’s name, study location, publication year, duration of the studies, follow-up period, sample size, tumor stage, predominant treatment methods, study design, cut-off value of “elevated NLR” and HRs with 95% CIs. If not available, data were extracted to calculate HR by the method of Tierney et al.42. Quality assessment was independently conducted in all the included studies by three investigators (X.B.G., X.J.Z. and X.J.T.) using the Newcastle–Ottawa Quality Assessment Scale (NOS). Disagreements were resolved by discussion. The NOS comprised of three parameters of quality: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). The maximum possible score is 9 points and NOS scores ≥7 are considered as high-quality studies.
Statistical analysis
We directly obtained hazard ratio (HR) and 95% confidence intervals(95% CI) from each article or estimated these data according to the methods illustrated by Tierney et al.42. A test of heterogeneity of pooled results was performed using Cochran’s Q test and Higgins I-squared statistic. I2 > 50% is considered as a measure of significant heterogeneity. Both random effects (DerSimonian–Laird method) and fixed-effects (Mantel–Haenszel method) models were used to generate the pooled HRs and 95%CIs. Owing to a tendency of possible heterogeneity between primary studies, the random-effects model was chosen because it was usually more conservative. We also investigated reasons for inter-study heterogeneity using subgroup analysis and meta-regression analysis. Sensitivity analyses were conducted to evaluate the stability of the results. Publication bias of literatures was evaluated using Begg’s funnel plot. All statistical tests were two sided and the significance level was set at 5%. All analyses were carried out using STATA 12.0 software (STATA, College Station, TX).
Additional Information
How to cite this article: Gu, X.-B. et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci. Rep. 5, 12493; doi: 10.1038/srep12493 (2015).
Acknowledgments
This work was supported by Special Fund for Clinical Research of Wu Jieping Medical Foundation (Grant No. 320.6750.1284)
Footnotes
Author Contributions Conceived and designed the experiments: X.B.G., T.T. and X.J.Z. Performed the experiments: X.B.G., T.T., X.J.T. and X.J.Z. Analyzed the data: X.B.G, T.T. and X.J.T. Contributed reagents/materials/analysis tools: T.T., X.J.Z and X.J.Z. Wrote the paper: X.B.G., T.T., X.J.T. and X.J.Z. Access to full-text articles: X.J.Z.
References
- Jemal A. et al. Global Cancer Statistics. CA-Cancer J. Clin. 61, 69–90, 10.3322/caac.20107 (2011). [DOI] [PubMed] [Google Scholar]
- Siegel R. et al. Cancer treatment and survivorship statistics, 2012. CA-Cancer J. Clin. 62, 220–241, 10.3322/caac.21149 (2012). [DOI] [PubMed] [Google Scholar]
- Paesmans M. et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 13, 1221–1230 (1995). [DOI] [PubMed] [Google Scholar]
- Proctor M. J. et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. British journal of cancer 103, 870–876, 10.1038/sj.bjc.6605855 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology 57, 2224–2234, 10.1002/hep.26057 (2013). [DOI] [PubMed] [Google Scholar]
- Gomez D., Morris-Stiff G., Toogood G. J., Lodge J. P. & Prasad K. R. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 97, 513–518, 10.1002/jso.21001 (2008). [DOI] [PubMed] [Google Scholar]
- Coussens L. M. & Werb Z. Inflammation and cancer. Nature 420, 860–867, 10.1038/nature01322 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan D. C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer treatment reviews 39, 534–540, 10.1016/j.ctrv.2012.08.003 (2013). [DOI] [PubMed] [Google Scholar]
- Sugiura T., Uesaka K., Kanemoto H., Mizuno T. & Okamura Y. Elevated Preoperative Neutrophil-to-lymphocyte Ratio as a Predictor of Survival After Gastroenterostomy in Patients with Advanced Pancreatic Adenocarcinoma. Annals of surgical oncology 20, 4330–4337, 10.1245/s10434-013-3227-8 (2013). [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Wuertz B. R. & Furcht L. T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clinical cancer research : an official journal of the American Association for Cancer Research 10, 4895–4900, 10.1158/1078-0432.ccr-03-0760 (2004). [DOI] [PubMed] [Google Scholar]
- Kuang D. M. et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. Journal of hepatology 54, 948–955, 10.1016/j.jhep.2010.08.041 (2011). [DOI] [PubMed] [Google Scholar]
- Sun Z. & Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. The Lancet. Oncology 5, 182–190, 10.1016/s1470-2045(04)01414-7 (2004). [DOI] [PubMed] [Google Scholar]
- Horne Z. D. et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. The Journal of surgical research 171, 1–5, 10.1016/j.jss.2011.03.068 (2011). [DOI] [PubMed] [Google Scholar]
- Krenn-Pilko S. et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. British journal of cancer 110, 2524–2530, 10.1038/bjc.2014.163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz M. et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. British journal of cancer 110, 435–440, 10.1038/bjc.2013.785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A. R. et al. Neutrophil-lymphocyte ratio predicts overall and recurrence-free survival after liver transplantation for hepatocellular carcinoma. Hepatology research : the official journal of the Japan Society of Hepatology 43, 757–764, 10.1111/hepr.12019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Lou L., Ye J. & Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ open 5, e006404, 10.1136/bmjopen-2014-006404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang W. & Feng L. J. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PloS one 9, e111906, 10.1371/journal.pone.0111906 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal D., Eroglu C., Kurtul N., Oguz A. & Tasdemir A. Are Neutrophil/Lymphocyte and Platelet/Lymphocyte Rates in Patients with Non-Small Cell Lung Cancer Associated with Treatment Response and Prognosis? Asian Pac. J. Cancer Prev. 14, 5237–5242, 10.7314/apjcp.2013.14.9.5237 (2013). [DOI] [PubMed] [Google Scholar]
- Yao Y. W., Yuan D. M., Liu H. B., Gu X. L. & Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunology Immunotherapy 62, 471–479, 10.1007/s00262-012-1347-9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon N. A. et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors following stereotactic radiation therapy for early-stage non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 10.1097/jto.0000000000000399 (2014). [DOI] [PubMed] [Google Scholar]
- Kacan T. et al. Could the Neutrophil to Lymphocyte Ratio be a Poor Prognostic Factor for Non Small Cell Lung Cancers? Asian Pac. J. Cancer Prev. 15, 2089–2094, 10.7314/apjcp.2014.15.5.2089 (2014). [DOI] [PubMed] [Google Scholar]
- Pinato D. J. et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. British journal of cancer, 10.1038/bjc.2014.145. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. et al. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. Journal of cancer research and clinical oncology 138, 2009–2016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramukai S. et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan Multinational Trial Organisation LC00-03. European journal of cancer 45, 1950–1958, 10.1016/j.ejca.2009.01.023 (2009). [DOI] [PubMed] [Google Scholar]
- Tomita M., Shimizu T., Ayabe T., Yonei A. & Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer research 31, 2995–2998 (2011). [PubMed] [Google Scholar]
- Cedres S. et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 14, 864–869, 10.1007/s12094-012-0872-5 (2012). [DOI] [PubMed] [Google Scholar]
- Botta C. et al. Systemic inlammatory status at baseline predicts bevacizumab beneit in advanced non-small cell lung cancer patients. Cancer Biology and Therapy 14, 469–475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget P. et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Annals of surgical oncology 20 Suppl 3, S650–660, 10.1245/s10434-013-3136-x (2013). [DOI] [PubMed] [Google Scholar]
- Jafri S. H., Shi R. & Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC cancer 13, 10.1186/1471-2407-13-158. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G. N. et al. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol, 10.1111/ajco.12273 (2014). [DOI] [PubMed] [Google Scholar]
- Choi J. E. et al. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer medicine, 10.1002/cam4.428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- E Y., He N., Wang Y. & Fan H. Percutaneous transluminal angioplasty (PTA) alone versus PTA with balloon-expandable stent placement for short-segment femoropopliteal artery disease: a metaanalysis of randomized trials. Journal of vascular and interventional radiology : JVIR 19, 499–503, 10.1016/j.jvir.2007.12.446 (2008). [DOI] [PubMed] [Google Scholar]
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674, 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A. & Balkwill F. Cancer-related inflammation. Nature 454, 436–444, 10.1038/nature07205 (2008). [DOI] [PubMed] [Google Scholar]
- Pillay J. et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. Journal of Clinical Investigation 122, 327–336, 10.1172/jci57990 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura T. et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. Journal of hepatology 58, 58–64, 10.1016/j.jhep.2012.08.017 (2013). [DOI] [PubMed] [Google Scholar]
- Yang X., Huang Y., Feng J. F. & Liu J. S. Prognostic significance of neutrophil-to- lymphocyte ratio in esophageal cancer: a meta-analysis. OncoTargets and therapy 8, 789–794, 10.2147/ott.s77099 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. J. et al. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World journal of gastroenterology : WJG 21, 2807–2815, 10.3748/wjg.v21.i9.2807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W.-K. et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. Bmc Cancer 14, 10.1186/1471-2407-14-117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-X. et al. Prognostic role of neutrophil- to- lymphocyte ratio in colorectal cancer: A systematic review and meta- analysis. International Journal of Cancer 134, 2403–2413, 10.1002/ijc.28536 (2014). [DOI] [PubMed] [Google Scholar]
- Tierney J. F., Stewart L. A., Ghersi D., Burdett S. & Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16, 10.1186/1745-6215-8-16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]