Abstract
Integrating loop-mediated isothermal amplification (LAMP) with capacitively coupled contactless conductivity detection (C4D), we have developed an electrical sensor for the simultaneous amplification and detection of specific sequence DNA. Using the O26-wzy gene as a model, the amount of initial target gene could be determined via the threshold time obtained by monitoring the progression of the LAMP reaction in real time. Using the optimal conditions, a detection limit of 12.5 copy/μL can be obtained within 30 min. Monitoring the LAMP reaction by C4D has not only all the advantages that existing electrochemical methods have, but also additional attractive features including being completely free of carryover contamination risk, high simplicity and extremely low cost. These benefits all arise from the fact that the electrodes are separated from the reaction solution, that is C4D is a contactless method. Hence in proof of principle, the new strategy promises a robust, simple, cost-effective and sensitive method for quantitative determination of a target gene, that is applicable either to specialized labs or at point-of-care.
The determination of target nucleic acids is of great importance for fundamental research and applied technology1,2. In order to amplify trace amounts of DNA to a detectable level, amplification methods based on various principles have been developed very fast in recent decades3. Loop-mediated isothermal amplification (LAMP), which eliminates the rapid thermocycling demand, is highly specific because four primers recognize six separate regions of the target sequence in order for the amplification to proceed4,5. Moreover, its efficiency is outstanding, as upward of ~109 copies accumulate from less than 10 copies of input template within an hour or two4. Hence this gene analysis method is becoming particularly popular and represents a revolution in molecular biology by reducing cost, turnaround time and complexity4,6.
The efficient determination of a target gene with LAMP relies on the monitoring of amplification reaction. Many methods, such as those based on fluorescence, turbidity, gel electrophoresis, electrochemistry, enzyme-linked immunosorbent assay and lateral flow dipstick, have been developed for monitoring the biochemical reaction7. Amongst them, optical and electrochemical methods have been in the forefront because they allow the real time monitoring of the LAMP progression, and provide quantitative reports automatically. However, the optical readout methods require not only highly precise and expensive instruments but also sophisticated numerical algorithms to interpret the data7,8,9. The alternative, electrochemical methods, are faster, lower cost, simpler and can be more readily be miniaturized by eliminating the requirement of photoelectric converter10. Unfortunately, for continuously monitoring the progression of the LAMP reaction in real time with contact electrodes, either with voltammetry11,12,13,14 or potentiometry15,16, there is still the issue of low reproducibility due to the fouling and passivation of working electrode, by biological species in the amplification reaction vessel. Furthermore, accompanied with this issue of electrode fouling is the increased risk of carryover contamination. Thus, safer, simpler and more robust methods for monitoring the performance of LAMP reactions are required.
The LAMP reaction can be represented by the following equations:
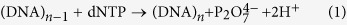 |
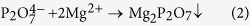 |
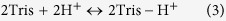 |
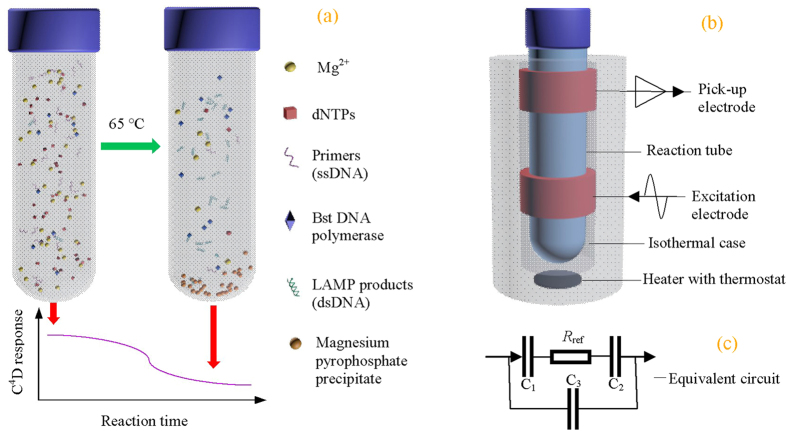
Where dsDNA is synthesized at the expense of consuming primers and dNTPs8. An insoluble salt, magnesium pyrophosphate precipitate17,18,19 and protons17,20 are also produced. The consumption of primers and dNTPs, plus the yield of precipitate, leads to a decrease of the overall ionic strength (as illustrated in Fig 1A), which is what we exploit for monitoring the LAMP reaction via the change in conductivity21. Based on this principle, herein an electrical sensor for monitoring the LAMP amplification reaction in real time using a capacitively coupled contactless conductivity detection (C4D) was developed (as illustrated in Fig. 1B,C). The new strategy not only has the same advantages that existing electrochemical methods have, but also solves all the challenges existing electrochemical methods face. We anticipate it will enable the creation of a high throughput, portable device for simple, cost-effective and rapid nucleic acid analysis that is suitable both for working in specialized labs and at points of care.
Figure 1. Principle of the electrical sensor for the determination of target DNA by combining LAMP and C4D.
(a) Schematic representation of the LAMP reaction and the consequent conductivity response obtained with C4D. (b) Structure of the electrical sensor, which includes two electrodes of C4D and an electronic heater with programmable thermostat. (c) The practical equivalent circuit of the C4D detector.
Results
Characterization of the electrical sensor
Using 0.1, 0.2, 0.3, 0.4 and 0.5 M KCl as probes, the custom built electrical sensor was characterized, focusing on the sensitivity and stability of the C4D (Supplementary Note 1 and Supplementary Fig. 1–3). At room temperature, a sensitivity of 871 mV/M was obtained with the optimal conditions of an excitation amplitude of 16 V and an excitation frequency of 2.0 MHz. Moreover, the temperature inside the sensor can be kept stable at 65 °C. At this temperature the C4D also shows high sensitivity and stability.
Using a NMR sample tube as a reaction vessel, a series of LAMP reactions (200 μL per sample) were implemented in the electrical sensor when the temperature was programmed to be 65 °C. We observed that high efficiency and specificity of LAMP reactions could be obtained with this set of primers22 using the optimized conditions (Supplementary Note 2 and Supplementary Fig. 4).
LAMP reaction leading decrease of conductivity response
With 1.25 × 104 copy/μL template DNA or herring sperm DNA, we collected the conductivity responses of the LAMP reaction solutions at room temperature before and after the incubation, respectively. As shown in Fig. 2, the mean output potential value of the negative post-reaction solutions is 1.478 ± 0.001 V. It is not significantly different from the mean value obtained from the same solution prior to performing the amplification reaction. By contrast, the mean value of the positive post-reaction solutions is 1.467 ± 0.002 V, which represents a decrease of ~10 mV compared to the pre-reaction solution, suggesting a significant decrease of conductivity. This result is attributed mainly to the consumption of reactants and the formation of the magnesium pyrophosphate precipitate21 (Supplementary Note 3 and Supplementary Fig. 5). In addition, the phosphate backbone of the produced dsDNA in the reaction solution can adsorb positive ions (e.g., [K+], [Na+], [Mg2+]), leading to a decrease of overall ionic mobility23. In conclusion, successful amplification leads to a detectable decrease of conductivity response of the LAMP reaction solution; at the endpoint the change of conductivity can be monitored rapidly (<2 s) with the electrical sensor, being free of tube-open operation.
Figure 2. Conductivity responses of pre- and post- LAMP reaction solutions.
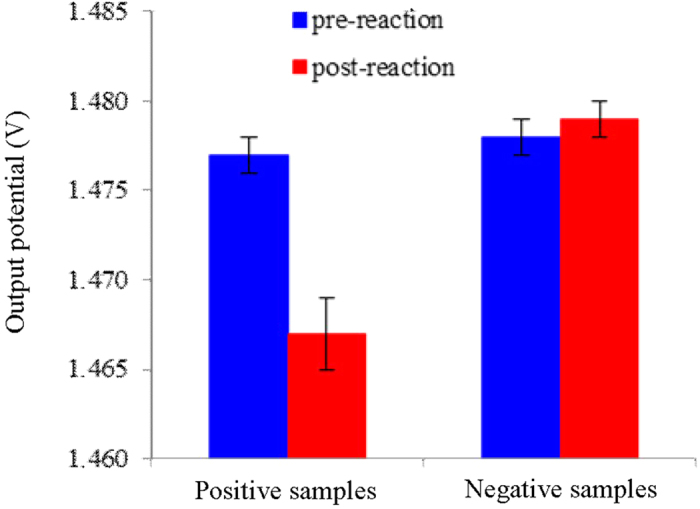
Five positive samples contained 1.25 × 104 copy/μL template DNA. While the control five negative samples contained the same amount of herring sperm DNA. All the samples were loaded in the same NMR sample tube in turn, and incubated at 65 °C for 12 min, respectively, followed by cooling to room temperature. Conductivity measurements were all carried out with the electrical sensor at 22 °C. Excitation amplitude: 16 V; Excitation frequency: 2.0 MHz.
Real time monitoring the progression of the LAMP reaction
We prepared the positive (containing 1.25 × 107 copy/μL target DNA) and the negative (containing 1.25 × 107 copy/μL herring sperm DNA) LAMP reaction samples at room temperature, followed by loading them into two NMR sample tubes, respectively. Real time collection of the conductivity responses of the reaction solutions in turn was conducted once the reaction tubes were inserted into the electrical sensor, in which the temperature was kept 65 °C. As shown in Fig. 3, for both cases during the first 3 minutes the output potentials increase rapidly because the rise in temperature leads to an increase in ion mobility24,25. With regards to the negative sample, the output potential comes to a plateau in the following 2 min, and remains stable for all the rest period, indicating no reaction proceeded. In contrast, for the positive sample the output potential begins to drop sharply at the approximately 294th second, indicating the beginning of the detectable decrease of conductivity, which is contributed to the performance of DNA amplification. This time point was defined as threshold time (Tt). It is significantly shorter than that observed with either electrochemical methods11,14,15 or optical methods19,26,27, suggesting a faster response. The sharp decline in the conductivity continues for ~110 s, suggesting a rather high efficiency of the biochemical reaction. Thereafter the decrease of output potential slows down to a gentle decline, indicating that the amplification reaction slows considerably, which may be due to the inhibition of polymerase activity by the fall of pH16, or the decrease of the concentration of Mg2+ 28. Note, the usage of more ThermoPol® reaction buffer than the commended dosage, e.g., ≥1.2×, benefits to obtain stable curves of conductivity responses (see Supplementary Note 4 and Supplementary Fig. 6). In conclusion, the outcomes show that the electrical sensor can not only provide the temperature condition required for the LAMP reaction, but also monitor the progression of the biochemical reaction in real time under the selected conditions.
Figure 3. Real time monitoring the conductivity response of the LAMP reactions with the electrical sensor.
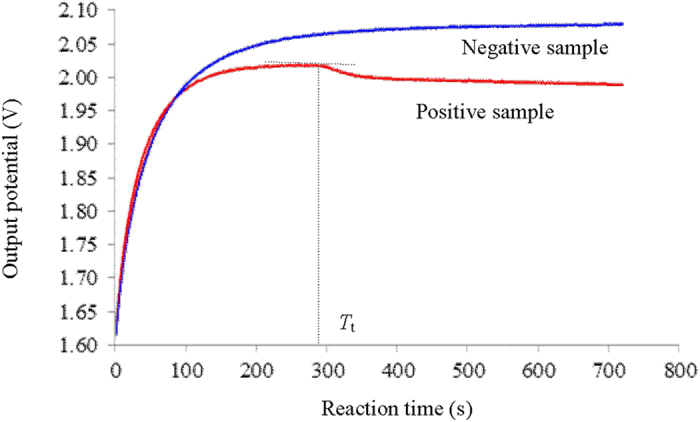
The positive and the negative LAMP samples contained 1.25 × 107 copy/μL template DNA and 1.25 × 107 copy/μL herring sperm DNA, respectively. The output potential value was collected at 1-second interval. Excitation amplitude: 16 V; Excitation frequency: 2.0 MHz.
Performance of quantifying target gene
Figure 4 shows the typical conductivity responses recorded in real time of LAMP reactions with different concentration of initial template DNA. We observed that the larger the amount of initial template DNA is, the shorter the Tt is, similar to that appears in real time electrochemical monitoring11,14,29, turbidity monitoring18 and fluorescence monitoring26,27. The insert of Fig. 4 shows the plot of Tt versus log10 initial concentration of template DNA. Over the four orders of magnitude concentration range of template DNA investigated, from 1.25 × 107 copy/μL to 1.25 × 104 copy/μL, there is a linear correlation between Tt versus log concentration. These results show the quantity of the template DNA of an unknown concentration can be determined by comparing the Tt value with the Tt values of the template DNA of known concentrations. The velocity of LAMP reaction may depend on the nature of template DNA such as G/C or A/T ratio in amplified region. However, the velocity of LAMP reaction may not affect the quantitative determination by this method since the linearity between Tt and the initial amount of template DNA is independent of the velocity of LAMP reaction30. Using serial dilutions of the O26-wzy gene sample, the limit of detection was determined to be 12.5 copy/μL with an incubation time of 30 min, which is lower than that by existing real-time electrochemical method14. The sensitivity is 10 times higher than that obtained by conventional PCR method (35 cycles), which is in agreement with previous report20.
Figure 4. Typical conductivity responses of LAMP reactions with different concentration of initial template DNA.
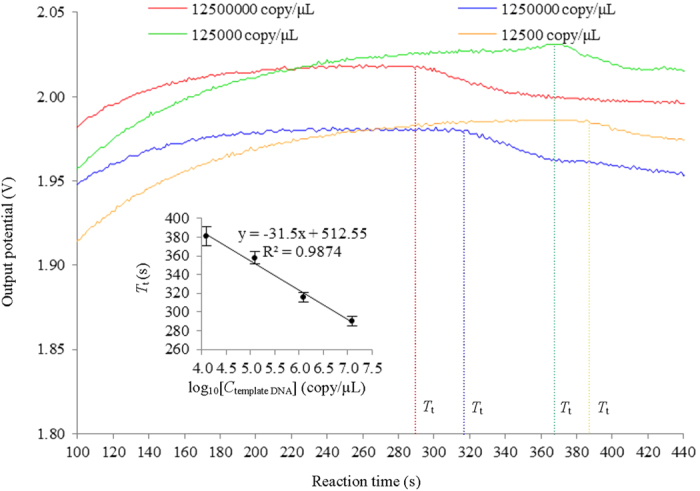
The insert shows a plot of Tt versus log10 initial concentration of template DNA (n = 5). The DNA amplification and monitoring in real time were performed with the same parameters in Fig. 3.
Discussion
The portable electrical sensor is composed of two key components. As been characterized, the electrical heater with a programmable thermostat allows us to keep the temperature inside at a desired stable isothermal level; the sensitive C4D system allows us to monitor the conductivity response of the solution in the reaction tubes in real time. Hence, simultaneous amplification and detection sequence-specific DNA can be implemented by LAMP. Moreover, it also opens the door to investigate the thermodynamic and kinetic mechanisms of many chemical and biochemical reactions, in which change of ionic activity are involved. Note, the associated electronics could be easily miniaturised to a thumb nail size or less31, promising the development of portable and high throughput instrumentations.
In LAMP reaction four or six primers are used to recognize six or eight distinct regions of the target gene sequence, so that the specificity is extremely high4,7,8. Thus, even indirect methods for monitoring the reaction can be employed to perform the determination of target gene32. Among the real-time monitoring methods, turbidity shows relatively low sensitivity and slow response19. With the help of fluorescence, both the sensitivity and the response speed can be improved significantly at the expense of higher running cost, complex handling procedures7,27 and non-negligible inhibitory potential from the probes employed32. Furthermore, the necessity of optical-electrical signal transferring components increases the complexity in miniaturizing instrumentations and hence manufacturing costs7,33. These optical-based methods have an outstanding advantage, however, they are free of carryover contamination risk due to the absence of any tube-open operation. By contrast, electrochemical methods, including voltammetry11,12,13,14, conductivity21 and potentiometry15,16, have the advantages of not only the inherent miniaturization and portability, but also the independence from sample turbidity, low-cost/low-power requirements and compatibility with microfabrication technology. However, even under optimal conditions there are still several challenges for monitoring the LAMP reaction with electrochemical methods, e.g., inhibition from the indicators and high risk of carryover contamination7.
The C4D method employed here in the electrical sensor shares all the merits that these existing electrochemical methods have. Moreover, the unique nature, i.e. the separation between the electrode and reaction solution34, highlights dramatic advances by solving completely all the problems the electrochemical methods faced, leading to 1) capacity of realizing successive monitoring non-invasively; 2) free of any probes, indicators or labels; 3) complete elimination of carryover contamination risk; 4) extremely simplicity of operation; 5) extremely low cost. Among these advantages listed above, it is worth a special emphasis on the elimination of carryover contamination because the LAMP reaction may lead to incorrect results upon contamination of even a small quantity of amplification product4,8. In conclusion, the electrical sensor has the advantages that optical and electrochemical methods have, meanwhile eliminates their disadvantages, though at present the sensitivity is a little lower than some other schemes14,32,33 (≤1 copy/μL). The fluctuation of base-line in the present conductivity outputs is another challenge. It is probably be overcome by selecting more suitable reaction vessel. Hence, as a proof of principle, the new strategy promises a superior quantitative determination of target gene, applying either in specialized labs or at the point of care.
Methods
The electrical sensor
As illustrated in Fig. 1B, the electrical sensor was composed of a C4D and an electronic heater with thermostat. The C4D included two metal electrodes (an excitation electrode and a pick-up electrode). The practical equivalent circuit is shown in Fig. 1C. The two electrodes, the insulating tube and the electrolyte solution form two coupling capacitances C1 and C2. And there was also a stray capacitance arising from direct capacitive coupling between the two electrodes through air (C3)34,35. The solution in the reaction tube was equivalent to a resistor R. An ER225 C4D System (eDAQ Pty Ltd., Australia) was used to provide an AC source (maximum peak to peak amplitude of 40 V) and an AC current pick-up unit. Thus, an alternating current path was formed. The application of an AC voltage on the excitation electrode led to an AC current flowing through the AC path. From the AC current obtained by the AC current pick-up unit, the conductivity detection of the solution in the tube could be implemented. The property of the C4D was characterized referring to the method previous reported24,35,36, because the geometry and placement of the sensing electrodes play very important roles in the signal coupling and sensitivity37. The electronic heater with a programmable thermostat could provide an isothermal temperature condition (precision: ±0.3 °C), over the range of room temperature −120 °C. Commonly, the temperature could be stable in about 20 min after the appointment.
LAMP
We retrieved sequences of O-antigen gene clusters of Escherichia coli serogroups O26 from GenBank using accession numbers AF529080 (http://www.ncbi.nlm.nih.gov/nuccore/AF529080). Within the cluster, serogroup-specific O26-wzy gene was selected as target to design LAMP primers. A dsDNA fragment related to O26-wzy gene (190 bp in length), which was inserted in pUC57-Amp (2710 bp in length), was synthesized by GENEWIZ Inc. (USA), and was used as template DNA. Primers were synthesized in Genework Pty Ltd. (Sydney, Australia) with the sequences referred to Wang et al.22. We listed the detailed data in Supplementary Note 5 and Supplementary Table 1.
The LAMP reaction solution in the NMR sample tube (D = 3.0 mm, Norell, Inc., USA) contained 0.2 μM outer primers, 0.8 μM loop primers, 1.6 μM inner primers, 1.2 mM of each dNTPs, 1.2 × ThermoPol® reaction buffer, 8 μL Bst DNA polymerase, 6 mM MgSO4 and 8 μL of template DNA. The concentrations of some reactants and supporting species in the LAMP reaction solution, i.e. primers, dNTPs, Bst DNA polymerase, ThermoPol® reaction buffer, Mg2+ and betaine, which affect the performance of the reaction significantly4,28, were optimized, referring to the methods reported previously21,29. We characterized the specificity and efficiency of the reaction with gel electrophoresis8,21, visual assessment via white precipitate7,20 and colorimetric detection8,18,38,39. A Power PAC300 electrophoresis apparatus and a Gel Doc XR + System (Biorad, USA) were used for agarose gel electrophoresis and detection by employing SYBR GOLD dye (Life Technologies Australia Pty Ltd.). Note, we performed the electrophoresis analysis with 5 times diluted concentration of the post LAMP reaction solution.
End point detection of the results of LAMP reactions
We prepared a 200 μL LAMP reaction solution at room temperature (22 ± 0.5 °C), followed by loading it into a NMR sample tube. Then the tube was inserted into the electrical sensor, in which room temperature was kept. With an excitation frequency of 2.0 MHz and an excitation amplitude of 16 V, We collected the output potential of its conductivity response. After the reaction solution was incubated at 65 °C for 12 min, it cooled down to room temperature. Then we collected the output potential again with the same parameters.
Real time monitoring the progression of the LAMP reaction
The preparation and load of the reaction solution was at room temperature. Then we inserted the NMR sample tube loaded with reaction solution into the electrical sensor, in which the temperature was programmed to keep 65 °C. We began to collect the output potential with the ER225 C4D System in real time at a speed of 1 point per second from the beginning of the incubation. Unless otherwise stated, an excitation frequency of 2.0 MHz and an excitation amplitude of 16 V were selected; and the record lasted for 12 min. Another sample could be implemented as soon as the former was finished, without the step for renewing working electrodes.
Performance of quantifying target gene
Serial dilution method was used to study the performance of quantifying O26-wzy gene. We prepared a series of LAMP reaction samples, in which contained 1.25 × 100, 1.25 × 101, 1.25 × 102, 1.25 × 103, 1.25 × 104, 1.25 × 105, 1.25 × 106, 1.25 × 107 and 1.25 × 108 copy/μL, respectively. Then we performed the amplification in the electrical sensor by incubating at 65 °C. Meanwhile, we recorded the conductivity responses in real time, respectively. The reaction time, at which the output potential started to drop sharply, was defined as threshold time (Tt). Average Tt from 5 samples for each concentration of template DNA was plotted against log10 concentration of template DNA. Error bars represent the variation (RSD) between different samples. Generally, we continued to record the output potential for another 5 min after the appearance of Tt. The PCR control experiments were performed by referencing to Wang et al.22 with minor modification. In brief, the F3 and B3 were used as the upper- and down-stream primers, respectively. The PCR reaction mixture (25 μL) contained 1 × PCR buffer, 4 mM MgCl2, 0.2 mM each dNTP, 0.25 μM each primer, 1.5 U of GoTaq Hot Start Polymerase (Promega, Madison, WI) and 1 μL template DNA. The conditions for the PCR were as follows: denaturation at 94 °C for 20 s, annealing at 60 °C for 30 s, and extension at 72 °C for 50 s in a Mastercycler gradient (Eppendorf, Germany), totally for 35 cycles. The PCR results were evaluated by the electrophoresis with 2% agarose gel.
Additional Information
How to cite this article: Zhang, X. et al. Quantitative determination of target gene with electrical sensor. Sci. Rep. 5, 12539; doi: 10.1038/srep12539 (2015).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge financial support from the Australian Research Council Centre of Excellence Scheme (CE140100036) and the 973 Program (2015CB453303) of China. The author Xuzhi Zhang engages in scientific research in UNSW sponsored by China Scholarship Council (CSC).
Footnotes
Author Contributions Conceived and designed the experiments: X.Z., Q.L., J.J.G. and X.J. Performed the experiments: X.Z., C.J. and R.T. Prepared figures: X.Z. and Y.L. Wrote the paper: X.Z. and J.J.G.
References
- Rosi N. L. et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312, 1027–1030 (2006). [DOI] [PubMed] [Google Scholar]
- Patolsky F., Weizmann Y. & Willner I. Redox-active nucleic-acid replica for the amplified bioelectrocatalytic detection of viral DNA. J. Am. Chem. Soc. 124, 770–771 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang C. & Xing D. Single-molecule DNA amplification and analysis using microfluidics. Chem. Rev. 110, 4910–4947 (2010). [DOI] [PubMed] [Google Scholar]
- Tanner N. A. & Evans T. C. Current protocols in molecular biology, 15.14.1–15.14.14 (John Wiley & Sons, Hoboken, 2014). [DOI] [PubMed] [Google Scholar]
- Notomi T. et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28, E63 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L. L. M. et al. Sensitive and inexpensive molecular test for falciparum malaria: Detecting plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 52, 303–306 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang X., Lowe S. B. & Gooding J. J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 61, 491–499 (2014). [DOI] [PubMed] [Google Scholar]
- Tomita N., Mori Y., Kanda H. & Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3, 877–882 (2008). [DOI] [PubMed] [Google Scholar]
- Drummond T. G., Hill M. G. & Barton J. K. Electrochemical DNA sensors. Nat. Biotechnol. 21, 1192–1199 (2003). [DOI] [PubMed] [Google Scholar]
- Ferguson B. S. et al. Integrated microfluidic electrochemical DNA sensor. Anal. Chem. 81, 6503–6508 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Recording the reaction process of loop-mediated isothermal amplification (LAMP) by monitoring the voltammetric response of 2’-deoxyguanosine 5’-triphosphate. Electroanalysis 23, 2438–2445 (2011). [Google Scholar]
- Hsieh K., Patterson A. S., Ferguson B. S., Plaxco K. W. & Soh H. T. Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care through microfluidic electrochemical quantitative loop-mediated isothermal amplification. Angew. Chem. 124, 4980–4984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Chai Y., Yuan Y., Bai L. & Yuan R. Development of an electrochemical method for Ochra toxin A detection based on aptamer and loop-mediated isothermal amplification. Biosens. Bioelectron. 55, 324–329 (2014). [DOI] [PubMed] [Google Scholar]
- Ahmed M. U., Nahar S., Safavieh M. & Zourob M. Real-time electrochemical detection of pathogen DNA using electrostatic interaction of a redox probe. Analyst 138, 907–915 (2013). [DOI] [PubMed] [Google Scholar]
- Toumazou C. et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. methods 10, 641–646 (2013). [DOI] [PubMed] [Google Scholar]
- Salm E. et al. Electrical detection of nucleic acid amplification using an on-chip quasi-reference electrode and a PVC REFET. Anal. Chem. 86, 6968–6975 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y., Nagamine K., Tomita N. & Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Bioph. Res. Co. 289, 150–154 (2001). [DOI] [PubMed] [Google Scholar]
- Le Roux C. A. et al. Development and evaluation of a real-time reverse transcription-loop-mediated isothermal amplification assay for rapid detection of rift valley fever virus in clinical specimens. J. Clin. Microbiol. 47, 645–651 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denschlag C., Vogel R. F. & Niessen L. Hyd5 gene based analysis of cereals and malt for gushing-inducing Fusarium spp. by real-time LAMP using fluorescence and turbidity measurements. Int. J. Food Microbiol. 162, 245–251 (2013). [DOI] [PubMed] [Google Scholar]
- Zhou D. et al. Establishment and application of a loop-mediated isothermal amplification (LAMP) system for detection of cry1Ac transgenic sugarcane. Sci. Rep-UK. 4, 4912 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. et al. Monitoring the progression of loop-mediated isothermal amplification (LAMP) using conductivity. Anal. Biochem. 466, 16–18 (2014). [DOI] [PubMed] [Google Scholar]
- Wang F., Jiang L., Yang Q., Prinyawiwatkul W. & Ge B. Rapid and specific detection of Escherichia coli serogroups O26, O45, O103, O111, O121, O145, and O157 in ground beef, beef trim, and produce by loop-mediated isothermal amplification. Appl. Environ. Microbiol. 78, 2727–2736 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneth B. Following ionic activity by electrochemistry during the polymerase chain reaction. Anal. Biochem. 385, 26–33 (2009). [DOI] [PubMed] [Google Scholar]
- Tanyanyiwa J., Galliker B., Schwarz M. A. & Hauser P. C. Improvedcapacitivelycoupledconductivity detector for capillary electrophoresis. Analyst 127, 214–218 (2002). [Google Scholar]
- Collins D. A., Nesterenko E. P., Brabazon D. & Paull B. In-process phase growth measurement technique in the fabrication of monolithicporous layer open tubular (monoPLOT) columns using capacitively coupled contactless conductivity. Analyst 138, 2540–2545 (2013). [DOI] [PubMed] [Google Scholar]
- Patel J. C. et al. Real-time loop-mediated isothermal amplification (RealAmp) for the species-specific identification of plasmodiumvivax. PLoS ONE 8, e54986 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. et al. Detection of periodontal pathogen Porphyromonasgingivalis by loop-mediated isothermal amplification method. FEMS Immunol. Med. Mic. 43, 233–239 (2005). [DOI] [PubMed] [Google Scholar]
- Yeh H. Y., Shoemaker C. A. & Klesius P. H. Evaluation of a loop-mediated isothermal amplification method for rapid detection of channel catfish Ictaluruspunctatus important bacterial pathogen Edwardsiellaictaluri. J. Microbiol. Methods 63, 36–44 (2005). [DOI] [PubMed] [Google Scholar]
- Veigas B. et al. Ion sensing (EIS) real-time quantitative monitorization of isothermal DNA amplification. Biosens. Bioelectron. 55, 50–55 (2014). [DOI] [PubMed] [Google Scholar]
- Mori Y., Kitao M., Tomita N. & Notomi T. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J. Biochem. Biophys. Methods 59, 145–157 (2004). [DOI] [PubMed] [Google Scholar]
- Joseph P. et al. Identification of inorganic ions in post-blast explosive residues using portable CE instrumentation and capacitively coupled contactless conductivity detection. Electrophoresis 29, 4593–4602 (2008). [DOI] [PubMed] [Google Scholar]
- Cai T., Loub G., Yang J., Xu D. & Meng Z. Development and evaluation of real-time loop-mediated isothermal amplification for hepatitis B virus DNA quantification: A new tool for HBV management. J.Clin. Virol. 41, 270–276 (2008). [DOI] [PubMed] [Google Scholar]
- Safavieh M., Ahmed M. U., Tolba M. & Zourob M. Microfluidic electrochemical assay for rapid detection and quantification of Escherichia coli. Biosens. Bioelectron. 31, 523–528 (2012). [DOI] [PubMed] [Google Scholar]
- Brito-Neto J. G. A., da Silva J. A. F., Blanes L. & do Lago C. L. Understanding capacitively coupled contactless conductivity detection in capillary and microchip electrophoresis. Part 1. Fundamentals. Electroanalysis 17, 1198–1206 (2005). [Google Scholar]
- Huang Z. et al. Design of capacitively coupled contactless conductivity detection sensor. Flow Meas. Instrum. 27, 67–70 (2012). [Google Scholar]
- Thredgold L. D. Khodakov D. A., Ellis A. V. & Lenehan C. E. On-chip capacitively coupled contactless conductivity detection using “injected” metal electrodes. Analyst 138, 4275–4279 (2013). [DOI] [PubMed] [Google Scholar]
- Mahabadi K. et al. Capacitively coupled contactless conductivity detection with dual top–bottom cell configuration for microchip electrophoresis. Electrophoresis 31, 1063–1070 (2010). [DOI] [PubMed] [Google Scholar]
- Safavieh M. et al. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst 139, 482–487 (2014). [DOI] [PubMed] [Google Scholar]
- Luo L. et al. Visual detection of high-risk human papillomavirus genotypes 16, 18, 45, 52, and 58 by loop-mediated isothermal amplification with hydroxynaphthol blue dye. J. Clin. Microbiol. 49, 3545–3550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.