Abstract
Low-flow postural tachycardia syndrome (POTS) is associated with increased plasma angiotensin II (ANG II) and reduced neuronal nitric oxide (NO), which decreases NO-dependent vasodilation. We tested whether the ANG II type 1 receptor (AT1R) antagonist losartan would improve NO-dependent vasodilation in POTS patients. Furthermore, if the action of ANG II is dependent on NO, then the NO synthase inhibitor nitro-l-arginine (NLA) would reverse this improvement. We used local heating of the skin of the left calf to 42°C and laser-Doppler flowmetry to assess NO-dependent conductance [percent maximum cutaneous vascular conductance (%CVCmax)] in 12 low-flow POTS patients aged 22.5 ± 0.8 yr and in 15 control subjects aged 22.0 ± 1.3 yr. After measuring the baseline local heating response at three separate sites, we perfused individual intradermal microdialysis catheters at those sites with 2 μg/l losartan, 10 mM NLA, or losartan + NLA. The predrug heat response was reduced in POTS, particularly the plateau phase reflecting NO-dependent vasodilation (50 ± 5 vs. 91 ± 7 %CVCmax; P < 0.001 vs. control). Losartan increased baseline flow in both POTS and control subjects (from 6 ± 1 to 21 ± 3 vs. from 10 ± 1 to 21 ± 2 %CVCmax; P < 0.05 compared with predrug). The baseline increase was blunted by NLA. Losartan increased the POTS heat response to equal the control subject response (79 ± 7 vs. 88 ± 6 %CVCmax; P = 0.48). NLA decreased both POTS and control subject heat responses to similar conductances (38 ± 4 vs. 38 ± 3 %CVCmax; P < 0.05 compared with predrug). The addition of NLA to losartan reduced POTS and control subject conductances compared with losartan alone (48 ± 3 vs. 53 ± 2 %CVCmax). The data suggest that the reduction in cutaneous NO-dependent vasodilation in low-flow POTS is corrected by AT1R blockade.
Keywords: lasers, autonomic nervous system
chronic orthostatic intolerance is associated with the postural tachycardia syndrome (POTS) in a majority of cases (13, 17, 23, 31, 32). POTS is defined by an excessive increase in heart rate during orthostatic challenge associated with symptoms of orthostatic intolerance (23). Our laboratory has described a subset of POTS patients designated “low-flow POTS” (29) in which there is marked upright tachycardia associated with supine pallor, acrocyanosis, and hypovolemia. There are often resting tachycardia, subnormal cardiac output, and subnormal regional blood flows with marked peripheral vasoconstriction (29). Increased sympathetic outflow measured by microneurography has been found in similar patients by other investigators (8).
We recently observed increased plasma angiotensin II (ANG II) in low-flow POTS. Increased ANG II was related to hypovolemia and reduced body mass (26, 30) and associated with widespread reductions of blood flows in regional circulations (29) including the skeletal muscle and the cutaneous circulation (18, 28). Although increased plasma ANG II was not universally found, we reasoned that there could also be changes of tissue-level ANG II that might not be reflected in increased concentrations in the blood. Abnormalities of the renin-angiotensin-aldosterone system have been previously reported in POTS by other investigators (13, 21).
We also recently found decreased cutaneous microvascular nitric oxide (NO)-dependent vasodilation in low-flow POTS (18). Decreased NO-dependent increases in cutaneous vascular conductance (CVC) was confirmed, and our observations were extended using microdialysis techniques; we demonstrated that the NO-dependent plateau of the local heating response (15, 19), used as a NO bioassay, was highly attenuated in low-flow POTS patients compared with control subjects. This indicated reduced NO bioavailability in these patients. We also showed that the plateau response was primarily neuronal NO synthase (nNOS) dependent. However, the NO synthase (NOS)-dependent response to acetylcholine, used to measure endothelium-dependent NO-mediated vasodilation, was preserved in POTS patients. Taken together, the data suggested that the NO of nNOS origin (nNO), but not the NO of endothelial NOS origin (eNO), is reduced in low-flow POTS (27).
Although ANG II can directly produce vasoconstriction (34), one of its most potent vasoconstrictive actions occurs through ANG II type 1 receptor (AT1R)-dependent interactions with the reduced NADP (NADPH) oxidase. This results in the enhanced production of reactive oxygen species (ROS) (10 – 12), specifically superoxide, which scavenges NO and produces peroxynitrite. Thus a potent oxidative/nitrative agent is produced, whereas available NO is reduced (35).
We hypothesized that AT1R activation decreases NO bioavailability through an increase in oxidant stress in POTS patients. Therefore, we hypothesized that the antagonism of the AT1R with losartan would increase the local heating response through NO-dependent mechanisms, which are sensitive to the NOS inhibitor nitro-l-arginine (NLA).
METHODS
Subjects
We studied the effects of an AT1R antagonist and NOS inhibition in POTS patients by comparing them with healthy volunteer subjects.
POTS patients were referred to the Hypotension Center for the evaluation of signs and symptoms of chronic orthostatic intolerance lasting at least 3 mo. Symptoms of chronic orthostatic intolerance included dizziness, exercise intolerance, headache, fatigue, memory problems, palpitations, nausea, blurred vision, pallor, and abnormal sweating while upright, which improve with recumbence and which have no other medical explanation. Orthostatic intolerance was defined by the presence of symptoms while upright relieved by recumbence, which had no other medical explanation. The diagnosis of POTS was made in these patients during a screening tilt-table test to 70° upright for a maximum of 10 min. POTS was diagnosed when there were symptoms of orthostatic intolerance during tilt associated with an increase in sinus heart rate of >30 beats/min or to a rate exceeding 120 beats/min during 10 min of tilt (17, 22). During the same visit, POTS patients were partitioned on the basis of supine calf blood flow into those patients with decreased blood flow designated “low-flow POTS” (<1.2 ml · 100 ml−1 tissue · min−1) and into those patients without it. We have previously shown that POTS patients can be classified into normal-, low-, and high-flow groups based on venous occlusion plethysmography measurements of calf blood flow (28, 29). Prior work indicated that 1.2 ml/100 ml tissue/min was the lowest calf blood flow that we found in healthy volunteers during experiments in >100 subjects. We measured calf blood flow by venous occlusion strain-gauge plethysmography (9) while supine. For the current study, patients were retained if they belonged to the low-flow POTS group.
Using these methods, we recruited 12 low-flow POTS patients (all women, all Caucasian, aged 17–26 yr, median age 22 yr). Fifteen healthy volunteer subjects were also recruited (all women, all Caucasian, aged 16.5–25.5 yr, median age 22.5 yr) and studied after a screening upright tilt at 70° demonstrated normal orthostatic response. The volunteer subjects served as a control group and were recruited from among adolescents and young adults referred for an innocent heart murmur. This precluded the participation of subjects with mitral valve prolapse. Subjects with a history of syncope or orthostatic intolerance were specifically excluded.
For all experiments, subjects fulfilled certain criteria. Only subjects free from cutaneous, systemic, and cardiovascular diseases were eligible. Subjects were not taking any medication and refrained from alcohol and caffeinated beverages for at least 24 h before the study. There were no smokers or trained competitive athletes. Informed consent was obtained, and the Committee for the Protection of Human Subjects (Institutional Review Board) of New York Medical College approved all protocols. Women were enrolled without regard to the phase of their menstrual cycle, except that none were menstruating during the testing procedures.
Protocol
General protocol
Microdialysis catheters were used to infuse drugs locally (Fig. 1). Before microdialysis catheter insertion, laser-Doppler flow (LDF) was measured over each of three insertion sites to estimate baseline flows for later use to determine when the area had recovered from the trauma of catheter insertion. Laser probes were removed, and three microdialysis catheters were inserted. After recovery, LDF was measured while perfusing the catheters with lactated Ringer solution, and values were recorded for 10 min. After this, LDF was recorded during local heating. A recovery period followed, requiring 30 – 60 min. To evaluate the influence of AT1R blockers on the local heating response, we perfused one catheter with the AT1R antagonist losartan and repeated local heating. To separate the contribution of the local heat response during AT1R blockade that is due to NO from other ANG II-mediated vasoconstrictive effects, we perfused a second catheter with the nonisoform-specific NOS inhibitor NLA combined with losartan and repeated local heating. To evaluate the component of the local heating response that is unrelated to NO, we perfused a third catheter with NLA alone and repeated local heating. Before the effects of the drugs were tested, lactated Ringer was perfused through all of the catheters, and subjects were allowed to recover before the introduction of drugs, which were dissolved in the same lactated Ringer vehicle.
Fig. 1.
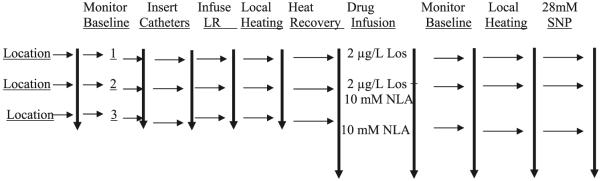
The scheme for the described studies. Locations for microdialysis catheters were randomly designated 1–3, and baseline laser-Doppler flow was measured. Microdialysis catheters were then inserted and perfused with vehicle [sterile lactated Ringer solution (LR)], and laser Doppler flow was monitored until levels returned to preinsertion values. Initial local heating responses were performed, and after return of laser Doppler to preheating values, drugs were infused for at least 40 min while laser Doppler flow was monitored. Local heating was repeated once again while the indicated drug continued to be perfused. Los, losartan; NLA, nitro-l-arginine; SNP, sodium nitroprusside.
Thus all determinations are compared with baseline determinations obtained during the perfusion of the vehicle alone. In this experimental design (shown in Fig. 1), the baseline values represent a placebo, and because each catheter was perfused with only a drug (or drug combination), washout periods were not needed to obtain the described measurements. After baseline heat response was complete and the patient had fully recovered, each of these drugs or drug combinations was perfused for 40 min during a run-in period. After drug administration, a second postdrug baseline was recorded for each microdialysis catheter. Local heating was then repeated while drug infusions continued, and the skin response to local heating was recorded. After the recovery from this second local heating, and as a final infusion, 28 mM sodium nitroprusside (SNP) were delivered through each microdialysis catheter to obtain maximum blood flow.
Use of heat/reheat assessment
We have performed pilot testing using the heat and repeat-heat model. In brief, we performed heat/ reheat testing in six healthy control subjects aged 18 –22 yr and in 5 low-flow POTS patients aged 17–22 yr. Two catheters placed at least 8 cm apart in the leg of the same subject were perfused with Ringer solution at 2 μl/min. The area of each catheter was heated, recovered from heat to baseline, and then reheated. We compared averaged plateau responses within catheters (comparing heat and reheat plateaus of the same catheter) and between catheters both during the heat and during the reheat. For each catheter, we obtained the difference in time-average plateau values during heat and reheat (within catheter difference). We also obtained the difference in the time-average plateau between catheters during heat and reheat. We used the F-statistic to test differences between variances within and between catheters for control and POTS subjects. We used the paired t-test to compare heat and reheat within a catheter with the heat response between two catheters placed in subjects.
Instrumentation
All testing was conducted in a temperature-controlled room (~25°C) at least 2 h after subjects ate a light breakfast. Skin temperature was continuously monitored by the LDF probes used to make the skin blood flow measurements. The measurements were made in the left calf. Since all of the experiments were performed with the subject supine, the leg was at the level of the heart throughout all procedures. The subjects were instrumented with three microdialysis catheters placed at least 5 cm apart and inserted in the dermal space of the lateral aspect of the left calf after hair was gently removed from the insertion site. Each site was cooled with an ice pack before the catheter insertion to reduce discomfort. Each probe (MD-2000 linear microdialysis probes; Bioanalytical Systems, West Lafayette, IN) had a 10-mm microdialysis membrane section that was placed in the intradermal space using a 25-gauge needle as an introducer. The catheters were randomly designated 1, 2, and 3. The molecular mass cutoff was nominally 30,000 Da. However, under constant perfusion, a practical cutoff was closer to 5,000 Da.
After placement, all catheters were initially perfused with Ringer solution at 2 μl/min. An integrating LDF probe (Probe 413; Perimed) containing seven individual probe tips (each contained a separate transmitting and receiving fiber) was then placed directly over each microdialysis catheter to measure skin blood flow, designated as LDF. LDF was thereafter recorded until the values were similar to those measured over the same area before the catheter insertion. The return of LDF to approximately preinsertion values indicated recovery from the trauma of catheter emplacement and usually occurred by 60 –90 min (1), designated as the recovery period. When necessary, longer times were allowed until preinsertion LDF was reached.
Drug infusions
Once baseline LDF values were obtained and local heating response was measured under untreated conditions, subjects received perfusate containing 2 μg/l losartan, 2 μg/l losartan + 10 mM NLA, and 10 mM NLA in catheters 1-3, respectively, at a rate of 2 μl/min. We used NLA instead of NG-nitro-l-arginine methyl ester (l-NAME) because the latter can act as a muscarinic receptor antagonist (3, 5). Losartan infusion resulted in a significant increase in baseline LDF with an increase in %CVC to a plateau by ~20 min. Losartan in 2 μg/l concentration was chosen based on prior human use during microdialysis experiments (7). This dose was also the minimum concentration that produced a significant and unequivocal increase in baseline blood flow and maximum %CVC (%CVCmax) in control subjects, indicating vasodilation that was related to AT1R blockade. The 10-mM dose of NLA was chosen on the basis of pilot studies, which showed this to be the minimum concentration yielding a maximum attenuation of the NO-dependent (15, 19) local heating plateau. Furthermore, in pilot studies we did find a dose-dependent increase in baseline vasodilation with an increasing dose of losartan, although these increasing doses did not affect the local heating plateau.
Local heating
Once baseline LDF values were obtained, the areas under each laser were gradually heated at 1°C/10 s to 42°C for at least 30 min until a plateau was reached. The area underneath the heating unit was ~3 cm2. The heat was turned off to allow for the recovery to baseline LDF. Work by Kellogg et al. (15) and Minson et al. (19) indicates that the local heating response is determined by NO. These investigators and others have demonstrated that local heating produces an initial peak vasodilation suppressed by EMLA cream that may be mediated by neurogenic reflexes and neuropeptides (14, 20). This first peak is followed by a nadir and then a NO-dependent plateau, which is blunted by NOS inhibition.
Monitoring
Heart rate was monitored by electrocardiography, and right upper-extremity blood pressures (BPs) were measured by finger plethysmography (Finometer; TNO) intermittently recalibrated against oscillometry in the right arm. Mean arterial pressure (MAP) was obtained by averaging the signal over 5 min. The Finometer MAP was always compared with oscillometry [using the formula of MAP = (systolic arterial pressure + 2 × diastolic arterial pressure)/3] since the Finometer BP can wander during active procedures. However, since these were no active procedures, the Finometer and oscillometric BPs were in agreement.
Data and Statistical Analysis
Laser-Doppler skin blood flows were measured in arbitrary perfusion units. Continuous LDF data were collected at a sampling rate of 200 Hz during experiments. Data from the lasers were multiplexed and interfaced to a personal computer through an analog-to-digital converter (DI-720; DATAQ Industries, Milwaukee, WI) using custom data acquisition software. LDF data were converted to units of CVC by dividing by the MAP. CVC measurements were then converted to a %CVCmax by dividing the CVC by the maximum CVC achieved after the administration of 28 mM SNP at the end of the experiments. This fraction was converted to a percentage by the multiplication by 100. Conductance data are therefore displayed as %CVCmax.
Changes in baseline LDF before drugs and after drugs were compared by two-way ANOVA. The results are shown and reported as means ± SE. All other comparisons were made by repeated-measures ANOVA to look at differences in the local heating response between pre- and postdrug infusion using the particular microdialysis catheter as the within factor. We also compared postdrug responses to losartan alone with responses to losartan + NLA and compared postdrug responses to NLA alone with losartan + NLA using catheters as the between factor and subjects as the within factor. Group responses were obtained by averaging the heat response data over all subjects in the POTS group and comparing the resulting curve with heat response data over all subjects in the control group. The data were obtained from individual heating curves before and after drugs. Table 2 shows the results from two-way ANOVA. We compared the initial peak %CVCmax response, the nadir in %CVCmax following the first peak, and the plateau phase in %CVCmax before and after drug treatment.
Table 2.
Magnitudes of heat responses
| Control Subjects | POTS Patients | |
|---|---|---|
| n | 15 | 12 |
| Baseline, %CVCmax | ||
| Predrugs | 10±1 | 6±1* |
| Losartan | 21±3† | 21±2† |
| NLA | 9±1 | 8±1 |
| Losartan + NLA | 13±3 | 15±7 |
| First thermal peak, %CVCmax | ||
| Predrugs | 71±6 | 49±4* |
| Losartan | 67±6 | 56±6 |
| NLA | 49±5† | 46±3 |
| Losartan + NLA | 49±4† | 49±3 |
| Nadir, %CVCmax | ||
| Predrugs | 48±5 | 29±5* |
| Losartan | 47±4 | 51±4† |
| NLA | 27±2† | 24±3 |
| Losartan + NLA | 29±2† | 33±4 |
| Plateau, %CVCmax | ||
| Predrugs | 91±7 | 50±5* |
| Losartan | 88±6 | 79±7† |
| NLA | 38±4† | 38±3† |
| Losartan + NLA | 53±2† | 48±3 |
Values are means ± SE; n, number of patients in each group. NLA, nitro-L-arginine.
P < 0.05 smaller than control subjects;
P < 0.05 smaller than predrugs %CVCmax.
Results were calculated using the Statistical Package for the Social Sciences software version 11.0. The value for P was <0.05.
RESULTS
The mean values of heat/reheat plateaus and two catheter comparisons were not different (P = 0.87). Thus either heat/reheat or separate catheter measurements gave similar heating plateaus on average. The variance between the catheters was significantly greater between than within catheters (F = 11.1; P < 0.01 for control subjects and F = 9.7; P < 0.025 for POTS subjects). Thus site difference affects the variability of heating results more than the variability of reheat results. Of all the manipulations we have used, heat/reheat appears most repeatable. Figure 2 shows the results from a representative pilot experiment in which a microdialysis catheter was inserted, and after recovery, the LDF response to local heating was measured. The figure shows that under predrug conditions, there were no significant changes in the heat response over time and that the elicited response of the skin to local heating is repeatable over time.
Fig. 2.
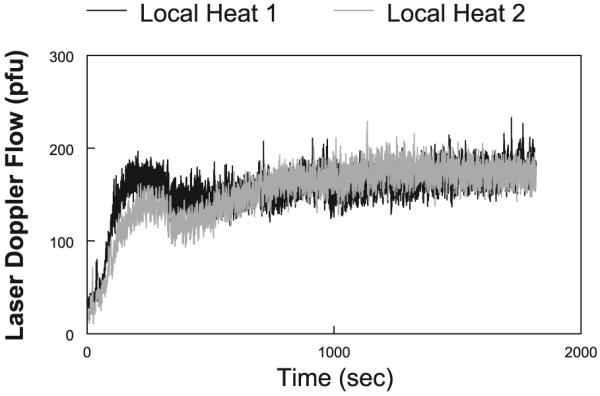
Local heating response performed in a representative control subject. Heating was performed (local heating 1), and the site was allowed to cool to its original preheated temperature. Heating was then repeated (local heating 2). The curves are nearly superimposable. The data provide a time control as well as a repeatability control for our experiments. pfu, arbitrary perfusion units.
Dimensions and Supine Hemodynamic Data
Data are shown in Table 1. POTS patients weighed less than control subjects (P < 0.05) and had a proportionately smaller body mass index (P < 0.025). The supine heart rate was increased in POTS patients compared with control subjects (P < 0.001). Calf blood flow in low-flow POTS was decreased by the study design. Calf arterial resistance was proportionately increased in POTS patients (P < 0.001). Resting LDF measured in perfusion units was decreased in POTS patients compared with control subjects (P < 0.05), as was resting %CVCmax. There was no significant difference in the maximum LDF following SNP perfusion comparing control with POTS subjects.
Table 1.
Dimensions and supine hemodynamics
| Control Subjects | POTS Patients | |
|---|---|---|
| n | 15 | 12 |
| Age, yr | 22±1.3 | 22.5±0.8 |
| Weight, kg | 62±2 | 55±2* |
| Height, cm | 168±2 | 167±3 |
| BMI, kg/m2 | 22.8±0.8 | 19.3±.6 |
| Supine HR, beats/min | 62±2 | 86±3* |
| Supine systolic BP, mmHg | 120±3 | 120±4 |
| Diastolic systolic BP, mmHg | 67±2 | 71±3 |
| Pulse pressure, mmHg | 53±2 | 48±3 |
| Venous occlusion calf blood flow, ml·100 ml−1 · min−1 |
2.7±0.2 | 0.83±0.12* |
| Calf arterial resistance, ml·100 ml −1 · min −1 /mmHg |
34±4 | 77±8* |
| Maximum laser-Doppler flow with sodium nitroprusside, pfu |
188±10 | 177±12 |
| Resting laser-Doppler flow, pfu | 18.4±1.0 | 12.7±2.3* |
| Resting %CVCmax | 9.9±1.7 | 7.2±1.5* |
Values are means ± SE; n, number of patients in each group. POTS, postural tachycardia syndrome; BMI, body mass index; HR, heart rate; BP, blood pressure; %CVCmax, percent maximum cutaneous vascular conductance.
P < 0.05 smaller than control.
pfu, Arbitrary perfusion units.
The Effects of Losartan, NLA, and Losartan + NLA on Baseline LDF
Baseline laser-Doppler %CVCmax data are shown for POTS and control subjects in Fig. 3. %CVCmax is shown before and after losartan, before and after NLA, and before and after losartan + NLA. Before the administration of drugs, baseline %CVCmax was significantly decreased in POTS patients compared with control subjects (P < 0.05 for all groups in Fig. 3). After losartan was given, baseline %CVCmax was significantly and comparably increased for both POTS and control subjects (P < 0.001). NLA alone did not affect baseline %CVCmax. However, the addition of NLA to losartan significantly blunted the increase in %CVCmax for losartan alone in both POTS patients (P < 0.05) and control subjects (P < 0.01).
Fig. 3.
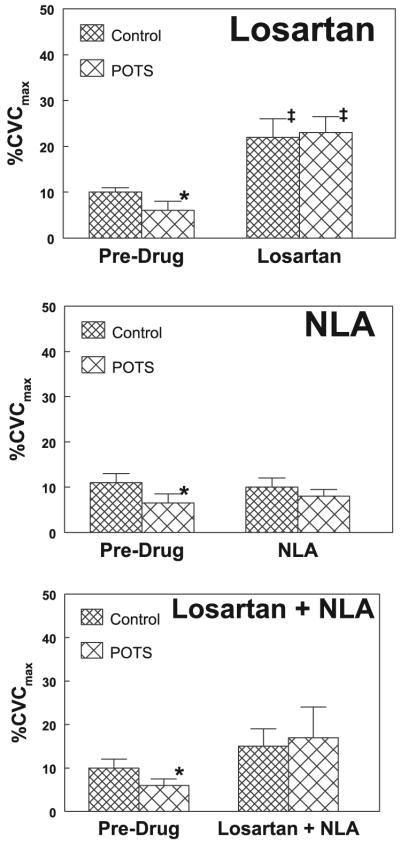
Baseline (preheating) percent maximum cutaneous vascular conductance (%CVCmax) before and after administration of Los, NLA, and Los + NLA. Postural tachycardia syndrome (POTS) baseline conductance was reduced predrug compared with control subjects. Los caused an increase in conductance in both POTS and control subjects, which reached similar values (top). NLA did not significantly change baseline conductance, although POTS and control subject conductances became similar (middle). The addition of NLA to Los resulted in a marked blunting of Los-induced vasodilation (bottom), which was not significantly different from predrug conductances. *P < 0.05 compared with control; ‡P < 0.05 compared with predrug.
The Effects of Losartan, NLA, and Losartan + NLA on the Local Heating Response
Table 2 shows %CVCmax measured at key points along the heating curves averaged over all subjects. A description of the local heat response in control and POTS subjects and the effects of drugs on key points in time are given in Baseline, First Thermal Peak, Nadir, and Plateau sections. The key points included baseline, the first thermal peak, the nadir, and the plateau. There was no effect of the drug treatments on the maximum CVC (P = 0.4).
Baseline
Baseline data are shown in Table 2. There is a lower baseline %CVCmax in POTS patients before drugs compared with the control subjects. Baseline %CVCmax increased to a similar level in POTS and control subjects after losartan was given. The increase in baseline with losartan was blunted by the addition of NLA. There was no difference between groups for the NLA + losartan site. NLA alone did not change baseline in either group.
First thermal peak
Before drugs, the %CVCmax of the first thermal peak was reduced in POTS patients compared with control subjects (P < 0.01). After losartan, NLA, or losartan + NLA perfusion, the %CVCmax of the peak was similar to control subjects. However, the %CVCmax of the first peak was reduced (P < 0.001) after the perfusion with NLA alone or after the perfusion with losartan + NLA compared with predrug in control subjects only.
Nadir
Before drugs, the %CVCmax of the nadir was reduced in POTS patients compared with control subjects (P < 0.025). After perfusion with losartan, the nadir increased in POTS patients to %CVCmax, which was similar to control subjects. The control subject nadir was unchanged. After NLA, the %CVCmax of the nadir was significantly reduced compared with baseline for control subjects such that control subject nadir and POTS patient nadir were now similar. The nadir was unchanged in POTS patients during NLA administration.
Plateau
Before drugs, the plateau %CVCmax was markedly reduced in POTS patients compared with control subjects (P < 0.001). After losartan, the plateau was unchanged in control subjects but increased to equal the %CVCmax of the control subject plateau in POTS patients. The plateau decreased to similar low %CVCmax in both groups after NLA (P < 0.001). The addition of losartan to NLA (losartan + NLA) caused a small increase in plateau %CVCmax compared with NLA alone (P = 0.5) and a large decrease in %CVCmax compared with losartan alone (P < 0.001).
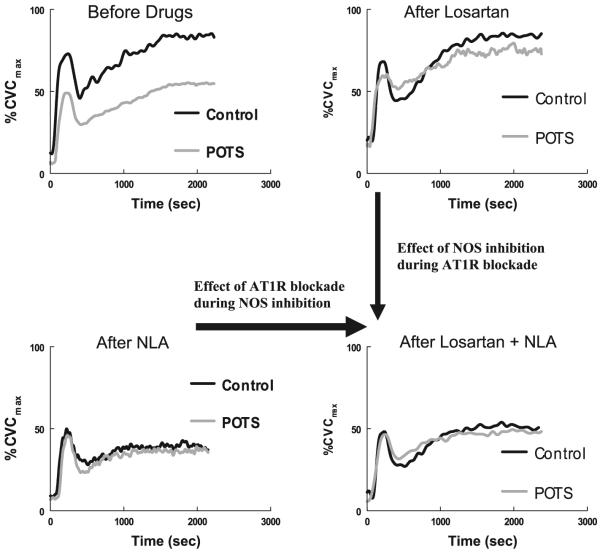
These results may be more readily appreciated in Fig. 4 in which the averaged local heat response curves are shown for POTS and control subjects before and after drugs. From these curves it can be seen that losartan alone improves the overall local heat response for POTS patients from the predrug result such that the heat response becomes similar to the control subject response. NLA alone reduces the overall local heat response in both control and POTS subjects to a similar low level. Thus the response to nonisoform-specific NOS inhibition produces similar heat responses in both groups. Losartan + NLA given in combination also resulted in similar local heating responses in control and POTS subjects. Comparing losartan + NLA with NLA alone showed an increased heat response, suggesting that there is a component of AT1R blockade that is independent of NOS inhibition. Therefore, there is a component of ANG II-mediated vasoconstriction that is independent of NO. Comparing losartan + NLA with losartan alone also shows that, during losartan administration, NLA produces similar reductions in the heat response in both POTS and control subjects. This indicates that once the effects of ANG II are antagonized by losartan, NOS inhibition produces equivalent reductions in available NO.
Fig. 4.
Heating response expressed as %CVCmax before and after administration of Los, NLA, and Los + NLA. POTS heating response was reduced predrug compared with control subjects (top, left). Los caused an increase in conductance in POTS so that the response approximated control subjects (top, right). NLA greatly reduced the heating response in POTS and control subjects (bottom, left) such that POTS and control subject responses became similar. The addition of NLA to Los resulted in a marked blunting in Los-induced vasodilation (bottom, right). POTS and control subject responses remained similar. The response to Los + NLA (bottom, right) was increased compared with the response to NLA alone (bottom, left), indicating that ANG II exerts some constrictive effects that are independent of nitric oxide.
DISCUSSION
Our main findings are as follows.
AT1R Activation Contributes to the Regulation of Baseline Blood Flow, and a Large Part of this Contribution Relates to its Effects on NO
This observation is depicted in Fig. 3, which shows that losartan increases baseline flow in POTS and control subjects and that this increase is blunted by the addition of NLA. The data from this figure also imply that resting blood flow and conductance are reduced in low-flow POTS, which is consistent with the physical findings of cool skin and acrocyanosis in this population. It is interesting to note that once losartan is administered, the differences in the local cutaneous conductance between POTS and control subjects disappear. This may indicate that all of the resting skin blood flow differences between patients and control subjects can be eliminated by AT1R blockade. AT1R and ANG II are found within the skin. Angiotensin appears to be produced by local angiotensinogen, renin, and ANG I-converting enzyme systems (24). This suggests but does not prove that ANG II can affect the heat response. ANG II is a protean-signaling molecule with diverse effects on vascular and metabolic regulation. It may, therefore, prove difficult to specify the exact mechanism by which ANG II receptor blockade eliminates differences in CVC. For example, it is known that ANG II exerts its vasoconstrictive effects in different ways including increasing in the nerve activity and facilitation of norepinephrine release (6). However, our prior results do not indicate differences in norepinephrine-mediated vasoconstriction between POTS patients and control subjects (18).
In addition, although ANG II can directly produce vasoconstriction (34), one of its most potent vasoconstrictive actions occurs through its AT1R-dependent interactions with the NADPH oxidase, resulting in the increased production of ROS (10 –12). Superoxide is specifically increased and scavenges NO-producing peroxynitrite. In vitro data also suggest that ROS can induce vasoconstriction through Rho-kinase-dependent mechanisms (2). Rho-kinase has been shown to have an increasingly important role in cold-induced vasoconstriction (33) and connects well with a hypothesis including increased ROS. The production of excessive ROS along with reduced available cutaneous NO is the most likely mechanisms responsible for our observations (35).
Cutaneous NO is Deficient in Low-Flow POTS and is Related to (Caused by) ANG II
From Table 2 and Fig. 4, it is clear that the local heat response is highly attenuated in low-flow POTS patients compared with control subjects. This is not a new finding and has been demonstrated using similar microdialysis and iontophoretic techniques in prior publications (18, 27). The remainder of results depicted in Fig. 4 are novel. The most striking feature is that once NLA, losartan, or their combination is given, the local heating response for control subjects and low-flow POTS patients become equivalent. However, the effects of NLA and losartan are polar opposites in which NLA decreases heat responses to a common low level, which is slightly less than the predrug response of the POTS patients, whereas decreased AT1R activity and, presumably, ANG II increase the POTS response so that POTS and control subjects respond similarly. This suggests that the elimination of local NO production equalizes the heat response between POTS and control subjects by attenuating NO-mediated effects especially in control subjects, whereas the elimination of AT1R effects equalizes the heat response by intensifying NO-mediated effects in POTS patients.
Figure 4 also shows that the NO-mediated plateau phase is not completely blocked in POTS patients because the addition of NLA causes a further decrease in the plateau from the predrug state. However, POTS is a highly heterogeneous illness. In grouping patients on the basis of leg blood flow, we have reduced heterogeneity and obtained a useful classification scheme for generating verifiable hypotheses. However, there is almost certainly room for the further refinement of the low-flow POTS group into more specific sub-subgroups. Thus it is likely that the attenuation of the heat response is present in varying degrees among low-flow POTS patients.
Of interest is also the effect of NOS inhibition during AT1R blockade, i.e., the comparison between the response to losartan alone versus losartan + NLA. The addition of NLA to losartan reduces the local heat response but incompletely compared with the %CVCmax observed with NLA alone. This implies that AT1R blockade exerts effects that are NO independent or, alternatively, that the dilating effects of ANG II type 2 receptors become important once AT1Rs are effectively antagonized (16).
An Interpretation for the Mechanistic Pathophysiology of Low-Flow POTS Patients
Our current data imply that NO is locally reduced in the skin of low-flow POTS patients compared with control subjects because of the effects of AT1R activation, suggesting the involvement of ANG II. Data from these and other experiments can be tentatively assembled into a unified picture that may help our understanding of the pathophysiology of low-flow POTS. Our previous data has shown a reduction in nNO coupled with peripheral vasoconstriction, reduced cardiac output, and increased total peripheral resistance (29) sometimes associated with an increase in plasma ANG II (26). The current data show that AT1R activation can account for observed abnormalities in the local heating response in low-flow POTS and, thus, for local NO deficiency. We expect that these results can be generalized to other microvascular circulations. Increased ANG II and decreased NO act reciprocally to increase central and peripheral sympathoexcitation (36). This is particularly important in states of excessive sympathetic activation such as heart failure (37) in which it appears to be related to oxidative stress (4). Whether a similar sympathoexcitation occurs in low-flow POTS and is the cause of the increase in ANG II in low-flow POTS remains to be determined.
Limitations
We studied the cutaneous circulation, which has unique autonomic control. Our recent work indicates that flow-regulation abnormalities in low-flow POTS occur throughout the circulatory system and that the local flow abnormalities that occur in the skin may be generalized. There is a paucity of quantitative comparisons of local regulatory mechanisms in humans. However, it is clear that the myogenic response, venoarteriolar reflex, and reactive hyperemia occur in the skin and are abnormal in low-flow POTS patients (28).
We studied the calf cutaneous circulation. Our previous data indicate that flow abnormalities are widespread in low-flow POTS (25). However, whenever peripheral blood flows are studied, the most significant results occur in the lower extremities. It may be that dependence and gravitational exposure are important to observed changes.
We studied women without regard to their menstrual cycle. The phase of the menstrual cycle can exert important effects on NO-dependent mechanisms. However, there is no evidence suggesting a relationship between menstrual cycling and changes in POTS symptoms or signs.
An argument could be made to add a control experiment in which it is demonstrated that angiotensin reduces the NO-dependent cutaneous blood flow response to local heating. This indeed would be interesting to perform and would provide evidence for the direct involvement of ANG II in blunting the heat response in POTS patients. However, we believe that this is beyond the current scope, which deals with the effects of the AT1R blockade in POTS patients.
Finally, the model proposed may have some inconsistencies. For example, if the AT1R-NADPH oxidase interaction scavenges NO, then it should scavenge NO related to all isoforms including the presumed eNO synthase generation of NO from acetylcholine administration. However, this does not appear to occur. There is no definitive explanation for these observations. One possibility could be that acetylcholine stimulates vasodilation more through prostaglandins than NO. Although this has support from the literature, our own observations suggest that ~50% of the acetylcholine response is NO mediated. Another possibility is that the relevant AT1Rs blocked by losartan are in close proximity to the nNOS source. A last hypothesis is that cutaneous NO is synthesized mainly by nNOS under resting conditions and during local heating. All of these suppositions remain to be addressed.
ACKNOWLEDGMENTS
We thank members of the Department of Pediatrics, especially Dr. Leonard Newman, and the Division of Pediatric Cardiology, especially Dr. Michael H. Gewitz, for unflagging support. We also acknowledge our intellectual debt to our mentors Dr. Thomas H. Hintze and Dr. Gabor Kaley for constant inspiration and stimulation.
GRANTS
The research was supported by National Heart, Lung, and Blood Institute Grants 1-RO1-HL-66007 and 1-R01-HL-074873.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol. 1994;102:807–811. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2005;289:H243–H250. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- 3.Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. NG-nitro-l-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- 4.Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46:533–539. doi: 10.1161/01.HYP.0000179088.57586.26. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Chen CW, Hsiue TR. Comparative effects of l-NOARG and l-NAME on basal blood flow and ACh-induced vasodilatation in rat diaphragmatic microcirculation. Br J Pharmacol. 1997;120:326–332. doi: 10.1038/sj.bjp.0700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemson B, Gaul L, Gubin SS, Campsey DM, McConville J, Nussberger J, Zelis R. Prejunctional angiotensin II receptors. Facilitation of norepinephrine release in the human forearm. J Clin Invest. 1994;93:684–691. doi: 10.1172/JCI117021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frossard M, Joukhadar C, Steffen G, Schmid R, Eichler HG, Muller M. Paracrine effects of angiotensin-converting-enzyme- and angiotensin-II-receptor-inhibition on transcapillary glucose transport in humans. Life Sci. 2000;66:L147–L154. doi: 10.1016/s0024-3205(99)00679-7. [DOI] [PubMed] [Google Scholar]
- 8.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, Mosqueda-Garcia R. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–2159. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield AD, Whitney RJ, Whitney RJ. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- 10.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 11.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 13.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6:337–346. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 15.Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 16.Li XC, Widdop RE. AT2 receptor-mediated vasodilatation is unmasked by AT1 receptor blockade in conscious SHR. Br J Pharmacol. 2004;142:821–830. doi: 10.1038/sj.bjp.0705838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 18.Medow MS, Minson CT, Stewart JM. Decreased microvascular nitric oxide-dependent vasodilation in postural tachycardia syndrome. Circulation. 2005;112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 21.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 22.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74:1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 23.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 24.Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13:148–154. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 25.Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol. 2004;287:H2687–H2696. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- 26.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 2006;110:255–263. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H2161–H2167. doi: 10.1152/ajpheart.00600.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart JM, Medow MS, Montgomery LD. Local vascular responses affecting blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2003;285:H2749–H2756. doi: 10.1152/ajpheart.00429.2003. [DOI] [PubMed] [Google Scholar]
- 29.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart JM, Taneja I, Medow MS. Reduced body mass index is associated with increased angiotensin-II in young women with postural tachycardia syndrome. Clin Sci (Lond) 2007;113:449–457. doi: 10.1042/CS20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension. Evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86:1582–1588. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med. 1988;111:326–335. [PubMed] [Google Scholar]
- 33.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol. 2007;292:H1700–H1705. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 34.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 35.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 36.Zucker IH. Brain angiotensin II: new insights into its role in sympathetic regulation. Circ Res. 2002;90:503–505. doi: 10.1161/01.res.0000014287.96335.21. [DOI] [PubMed] [Google Scholar]
- 37.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]