Abstract
The ability to adaptively inhibit responses to tempting/distracting stimuli in the pursuit of goals is an essential set of skills necessary for adult competence and wellbeing. These inhibitory capacities develop throughout childhood, with growing evidence of important maturational changes occurring in adolescence. There also has been intense interest in the role of social adversity on the development of executive function, including inhibitory control. We hypothesized that the onset of adolescence could be a time of particular opportunity/vulnerability in the development of inhibition due to the large degree of maturational changes in neural systems involved in regulatory control. We investigated this hypothesis in a longitudinal study of adolescents by examining the impact of socioeconomic status (SES) on the maturation of inhibition and concurrent brain function. Furthermore, we examined gender as a potential moderator of this relationship, given evidence of gender‐specificity in the developmental pathways of inhibition as well as sex differences in adolescent development. Results reveal that lower SES is associated with worse behavioral inhibition over time and a concurrent increase in anterior cingulate (ACC) activation, but only in girls. We also found that lower SES girls exhibited decreased ACC ↔ dorsolateral prefrontal cortex (dlPFC) coupling over time. Our findings suggest that female adolescents with lower SES appear to develop less efficient inhibitory processing in dlPFC, requiring greater and relatively unsuccessful compensatory recruitment of ACC. In summary, the present study provides a novel window into the neural mechanisms by which the influence of SES on inhibition may be transmitted during adolescence. Hum Brain Mapp 36:3194–3203, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: socioeconomic status, development, inhibition, adolescence, anterior cingulate cortex, longitudinal, dorsolateral prefrontal cortex, connectivity
INTRODUCTION
The ability to prevent tempting or distracting stimuli from interfering with goals is essential for success in most spheres of life, including academic and professional performance, health, and overall wellbeing [Moffitt et al., 2011]. However, reduced inhibitory capacity has been observed in youth with lower socioeconomic status (SES) [Farah et al., 2006], which may impair long‐term success. Given that inhibition development has been linked to socioeconomic processes [Bradley and Corwyn, 2002; Feinstein, 2003], such contextual factors may be particularly influential during phases of accelerated development, such as adolescence [Forbes and Dahl, 2010]. Thus, elucidating the manner in which reduced inhibitory capacity develops during adolescence may inform efforts to alleviate the impact of social inequality.
Inhibition is often conceptualized as the capacity to stop responses that are no longer adaptive or prevent distracting stimuli from derailing thought or action. Research in adults indicates that successful inhibition relies on top‐down control instantiated in anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (dlPFC), among other regions [Aron et al., 2004; Kelly et al., 2004]. Recent models of inhibition have emphasized the interaction between ACC and dlPFC. For example, Banich [2009] proposed that dlPFC biases task representations to be congruent with goals, and ACC resolves any remaining competition. Between the 3rd and 5th year of life, children show significant increases in inhibition [Zelazo et al., 2003]. The next period of accelerated inhibition development is adolescence [Bunge and Wright, 2007; Davidson et al., 2006], where performance improves until it plateaus after the second decade of life [Eigsti et al., 2006; Luna et al., 2004].
The neuromaturational trajectory of inhibition systems is protracted across adolescence [Crone and Dahl, 2012], which may permit flexibility in the development of these systems, allowing for adaptive adjustment to contextual demands. Although this flexibility may often confer advantages, it may also create an extended period of vulnerability to the potentially detrimental impact of lower SES. Indeed, research suggests that inhibition is impaired in low SES adolescents [Farah et al., 2006], but the cross‐sectional nature of such evidence does not allow us to determine whether these effects emerge during adolescence or are due to the impact of SES earlier in life. For example, early life stress is linked to impaired adolescent inhibition and associated brain activation differences [Mueller et al., 2010]. However, stress related to socioeconomic risk factors may play a different role during adolescence than in early life. Understanding these effects is critical for pinpointing protective factors during development. Therefore, a critical gap remains in our understanding of the influence of SES on adolescent development of inhibition.
Research has delineated several important gender differences in types of stressors and their specific effects during early adolescence, including dramatic increases in female depression after pubertal onset [Kessler et al., 2001]. Research in adults has found gender specificity in the neural mechanisms instantiating inhibition, suggesting gender‐specific pathways in inhibition development. For example, men activate ACC more than women during inhibition (controlling for performance differences) and the relationship between ACC activation and impulsivity was positive in men but negative in women [Liu et al., 2012]. Adolescence may be key period for gender differences to emerge.
Present work focused on the impact of SES on the development of inhibition during adolescence. Adolescents completed an inhibition task (Go/NoGo) while functional magnetic resonance imaging (fMRI) data were collected. Data were collected at two time points (2 years apart), and the relationships between SES and maturation in task performance and brain activation were examined, along with the potential moderating effect of gender.
We hypothesized that lower SES would be linked to impairment in the development of inhibition over time. Specifically, we predicted that low SES adolescents would evidence less improvement in accuracy over time or potentially even degradation in inhibitory control over time. This impairment in behavioral inhibition should be associated with greater compensatory recruitment of the neural circuitry supporting inhibition (e.g., dlPFC and dACC). With regard to gender differences, we predicted that SES‐related deficiency in inhibition would be reflected to a greater degree in dACC in boys, given evidence that men recruit ACC to a greater degree than women [Liu et al., 2012].
MATERIALS AND METHODS
Participants
Participants were recruited from the community through advertisements, flyers, and demographically targeted phone lists. Exclusion criteria were: current/lifetime psychiatric disorders, braces, history of head injury, serious medical illness, psychotropic medication, alcohol, or illicit drug use. Data were collected ∼2 years apart (mean = 2.0, sd = 0.22).
fMRI data were collected from 78 participants at both time points. Thirteen participants were removed due to motion ≥5 mm at one or both times, and two participants were removed because of motion artifacts. In the final sample (N = 63, 44% female), mean age for females at time 1 = 11.3 years (sd = 0.72), time 2 = 13.5 (0.88), mean age for males at time 1 = 12.3 (0.63), time 2 = 14.4 (0.60). The younger age interval in girls was selected intentionally because of our focus on the onset of adolescence, because pubertal maturation typically begins 1–2 years earlier in girls than boys. Thus, the genders were matched on level of pubertal development (mean Tanner stage for females at time 1 = 2.7 [1.0], time 2 = 4.5 [0.72], for males at time 1 = 2.9 [0.90], time 2, 4.5 [0.86]). Given that Tanner staging includes a gender‐specific component (breast/gonad development), it is also important to examine the component shared across genders (pubic hair development: mean for females at time 1 = 2.7 [1.1], time 2 = 4.5 [0.71], for males at time 1 = 2.8 [1.0], time 2, 4.3 [1.0]). Importantly, t‐tests showed no significant gender differences at either time point (P's > 0.39).
Socioeconomic Status
SES was measured with the Hollingshead Four Factor Index [Hollingshead, 1975] via parent report obtained during the second time point. Scores covered the range of social strata (mean = 42, sd = 11, range = 17–61), were representative of the Pittsburgh area, and did not differ by gender (t 61 = 0.78, P = 0.44).
Go/NoGo Paradigm
Participants completed a commonly used block‐design variant of the Go/NoGo paradigm [Horn et al., 2003]. Participants viewed a sequence of 120 letters, presented for 0.5 s each, and divided into six blocks: three Go, three NoGo, presented ABBABA. Participants were instructed to respond to targets (any letter except V), and 75% of trials were targets. Stimuli were presented in a pseudo‐randomized order within each block: Go blocks had 20 targets, and NoGo had 10 targets and 10 nontargets.
Behavioral Analyses
Accuracy and mean reaction time (RT) were calculated for NoGo and Go trials (from Go block) separately. For NoGo trials, errors of commission were used and RT calculated from incorrect responses. For Go trials, errors of omission were used and RT calculated from correct hits. Accuracy/RT for Go was subtracted from NoGo to isolate inhibition‐related variance. NoGo versus Go accuracy/RT for time 1 was subtracted from time 2 to isolate change in inhibition‐related variance over time. Behavioral data were not available for seven participants at time 1 and one participant at time 2. For these participants, the time 2 (time 1) value was used for time 1 (time 2). Univariate analysis of covariance (ANCOVA)s were calculated with SES, gender, and the SES × gender interaction as predictors.
fMRI Data Acquisition and Processing
Participants were scanned in a Siemens 3T Allegra scanner. Functional data were acquired with a gradient echo planar imaging sequence (34 axial slices, 3 mm thick, interleaved collection, TR/TE = 2000/25 ms, FOV= 20 cm, matrix = 64 × 64). Analyses were implemented in FMRIB's software library (FSL) [Jenkinson et al., 2012]. Data were motion‐corrected, high‐pass filtered (1/136 Hz cutoff), spatially smoothed (FWHM = 5 mm), slice‐timing corrected, and intensity‐normalized.
Regression analyses were performed on the processed functional time series. Two predictors, one for each stimulus type block (Go blocks, NoGo blocks), were included (fixation unmodeled). Due to the block design of the paradigm, all trials were included irrespective of accuracy. To create the comparison of interest, β values for NoGo blocks were contrasted against the β values for Go. β maps were nonlinearly warped via FMRIB's non‐linear image registration tool (FNIRT) into a common stereotaxic space (MNI152 standard with FSL). To examine activation change over time, a fixed effects analysis was conducted for each participant modeling both the mean and difference (time 2 – 1) over time. Positive values on these comparisons indicated that NoGo activation (relative to Go) was greater at time 2 (relative to time 1).
Three group‐level hierarchical linear models were calculated. The dependent variable for all models was the within‐participant change in NoGo versus Go activation. The first modeled the mean change in NoGo versus Go activation over time, the second modeled the main effects of gender and SES, and the third modeled the gender × SES interaction. Two‐tailed t‐tests were conducted. Based on a priori hypotheses, a mask was used to constrain the number of voxels under consideration to prefrontal cortex (created using FSL's Harvard‐Oxford atlas: superior/middle/inferior frontal gyri, frontal pole, anterior/subcallosal cingulate, paracingulate, frontal‐medial, and orbitofrontal cortex). Gaussian random‐field theory was used to correct for multiple comparisons (via Cluster) with a voxel‐level threshold of z ≥ 2.81 and an overall error rate of P ≤ 0.05. Change in activation over time was correlated with change in task performance over time to assess the relationship between brain activation and behavior (Spearman correlations used with accuracy).
Connectivity Analyses
Given research supporting the importance of ACC‐dlPFC coupling in successful inhibition, we examined connectivity (psychophysiological interactions) between ACC and dlPFC. Specifically, the mean (across voxels) timeseries was extracted for the dACC/rACC cluster and two dlPFC (right middle frontal gyrus (MFG)) clusters activated by the NoGo > Go main effect. Hierarchical linear models were computed via statistical package for the social sciences (SPSS)'s MIXED, with participant as nesting variable, and Time (the two data collection periods) and TR as repeated factors. The level 1 covariance matrix was modeled with a lag 1 autoregressive function. Level 1 fixed effects were the hemodynamic response convolved task predictors, the dACC/rACC time series, and the interaction of ACC and NoGo versus Go. Task predictors were NoGo versus Go (weighted +1 for NoGo, −1 for Go, 0 elsewhere), NoGo and Go sum (weighted +1 for NoGo and Go, 0 elsewhere), and the temporal derivatives. A predictor modeling the mean time series across intracerebral voxels was included as a level 1 nuisance covariate to model brain‐wide signal fluctuations that could confound estimates of connectivity [Fox et al., 2009]. Time was the level 2 fixed effect, and SES the level 3 fixed effect. Initial models tested the SES × Time × ACC × Condition (NoGo vs. Go) interaction, and interactions were decomposed by (median) splitting by SES and then by Time.
Exploratory Analyses of Puberty
Given research linking the development of executive control processes, such as inhibition, more closely to age than puberty‐specific processes, we did not expect puberty to be the driving force in SES‐related differences in the development of inhibition. However, because of growing evidence of the impact pubertal processes during this developmental period, particularly on social and affective maturation, it is possible that such processes may impact SES‐specific inhibition development. Therefore, we conducted exploratory analyses to examine the impact of pubertal development, using Tanner staging (based on physical exam by a trained nurse) as the measure of puberty.
First, we tested whether puberty accounted for observed activation findings. Mean activation in observed clusters was extracted for each participant, for each time point then entered into repeated‐measures ANCOVAs in SPSS (time was the repeated measure). Predictors were gender, SES, and change in puberty over time, along with the two‐ and three‐way interactions. No significant interactions with puberty emerged, indicating that the observed activation effects were not driven specifically by pubertal change. Second, we recomputed the connectivity analyses, again adding pubertal change (and related interactions). Similar to the activation findings, no significant interactions with puberty emerged, indicating that the observed connectivity effects were not driven specifically by pubertal change.
RESULTS
Behavioral Analyses
To determine the impact of gender and SES on the development of behavioral inhibition, change over time in accuracy and RT was examined. The Go condition served as a baseline to isolate inhibition‐specific variance (vs. processing speed). SES and gender did not predict accuracy individually (P's < 0.05). SES and gender interacted to predict change over time in NoGo (commission) versus Go (omission) errors (F (1,59) = 7.2, P < 0.01, R 2 = 0.11). SES was associated with better accuracy over time in girls (r = 0.39, P = 0.04), whereas a marginal negative association was found in boys (r = −0.33, P = 0.054; Fig. 1). This pattern was present when only NoGo accuracy was examined, indicating that this effect is not dependent on using Go as a baseline. Additionally, this effect remained when mean (over time) age was included as a nuisance covariate. Gender predicted change over time in NoGo (incorrect trials) versus Go (correct trials) RT (F (1,60) = 6.8, P = .01, R 2 = 0.10), with girls showing faster, and boys showing slower, relative RT over time. This pattern remained when only NoGo RT was examined, but was no longer significant when mean (over time) age was included as a covariate of no interest. Neither SES nor the SES × gender interaction predicted change in RT.
Figure 1.
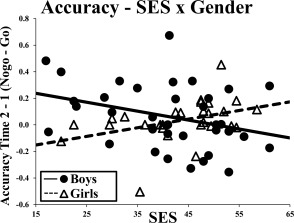
Socioeconomic status and change over time in NoGo versus go accuracy in girls and boys. SES, Socioeconomic Status. The scatterplot illustrates change in NoGo versus Go accuracy over time seperately in girls and boys.
Mean NoGo Versus Go Brain Activation Across Time
To determine which regions were activated by NoGo (vs. Go) across both time points, mean (across participants and time points) NoGo versus Go brain activation was calculated. Seven clusters emerged in which NoGo was greater than Go (Table 1) in regions consistent with past research (46). No regions emerged in which Go was greater than NoGo.
Table 1.
Mean NoGo versus go brain activation across time
| Region | Direction of effect | Cluster size (mm3) | Max z‐value | Cluster P‐value | Location | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| dACC/paracingulate (BA 6/24/32) | ↑ | 9,280 | 5.22 | <0.001 | 6 | 24 | 34 |
| L IFG (BA 47) | ↑ | 2,304 | 6.42 | 0.031 | −34 | 20 | −12 |
| R dlPFC (BA 9/44/45/47) | ↑ | 11,504 | 5.88 | <0.001 | 46 | 18 | −6 |
| R MFG (BA 9/10) | ↑ | 4,448 | 4.49 | 0.003 | 34 | 54 | 30 |
| L SFG (BA 6/8) | ↓ | 2,872 | −4.51 | 0.016 | −26 | 16 | 48 |
| M OFC/rACC (BA 10/11/32) | ↓ | 10,504 | −5.16 | <0.001 | 0 | 30 | −20 |
| L OFC/IFG/MFG (BA 10/11/46/47) | ↓ | 11,480 | −5.02 | <0.001 | −38 | 38 | −12 |
Note: L, left; R, right; M, medial; SFG/MFG/IFG, superior/middle/inferior frontal gyrus; dlPFC, dorsolateral prefrontal cortex; dACC, dorsal anterior cingulate cortex; rACC, rostral anterior cingulate cortex; OFC, orbitofrontal cortex; BA, Brodmann's area; ↑, increased activation; ↓, decreased activation; Location, location of max z‐value.
Change Over Time in NoGo Versus Go Brain Activation
To determine which brain regions exhibited change over time in activation to NoGo (vs. Go), mean (across participants) change over time in NoGo versus Go brain activation was calculated. Clusters emerged in dorsal anterior cingulate (dACC) and left dlPFC (Fig. 2 and Table 2).
Figure 2.
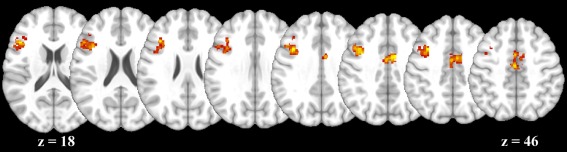
Change over time in NoGo versus Go brain activation. The figure illustrates the two clusters in cingulate and left dorsolateral prefrontal cortex that exhibited increased NoGo versus Go activation over time. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Change in NoGo versus go brain activation over time
| Region | Direction of effect | Cluster size (mm3) | Max z‐value | Cluster P‐value | Location | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| M dACC (BA 24) | ↑ | 2,768 | 4.20 | 0.012 | 10 | 0 | 38 |
| L dlPFC (BA 9/45/46) | ↑ | 6,736 | 4.19 | <0.001 | −38 | 22 | 28 |
Note: L, left; M, medial; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; BA, Brodmann's area; ↑, increased activation over time; Location, location of max z‐value.
SES, Gender, and SES × Gender Moderation
SES, gender, and the two‐way interaction were examined as predictors of NoGo versus Go activation change over time to determine whether the development of inhibition depended on gender and SES. A cluster emerged in dACC/rostral anterior cingulate (rACC) in which lower SES was associated with increased NoGo (vs. Go) activation over time, but only in girls (Fig. 3, cluster size = 3,712, maz z‐value = 4.29, cluster P‐value < 0.001, xyz = −2, 40, 28). This pattern remained when only NoGo (vs. fixation) was examined and when accuracy change over time was included as a nuisance covariate. Effects of SES and gender were not significant. Given that our 5 mm motion cutoff may be considered liberal, we reran this analysis after excluding participants with motion ≥3 mm (n = 20). Importantly, the cluster remained significant (and largely identical in shape/extent) in this reduced sample, suggesting that motion‐related variance does not account for this finding.
Figure 3.
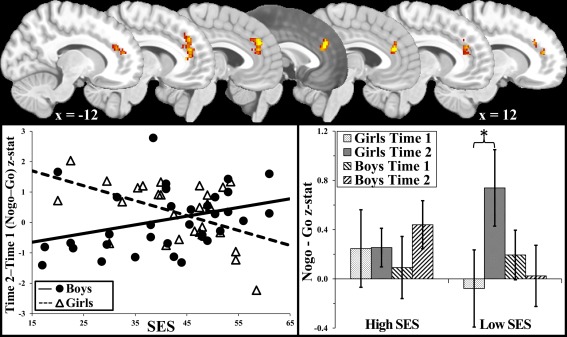
Socioeconomic status and gender moderate change over time in NoGo versus Go brain activation. ACC, anterior cingulate cortex; SES, socioeconomic status. Top figure illustrates the cluster in anterior cingulate cortex in which socioeconomic status (SES) was negatively related to increased NoGo versus Go activation over time only in girls (visualized at sequential sagital slices every 4 mm, going from x = −12 to 12). The scatterplot visualizes the relationship between SES and change in activation seperately for girls and boys. The bar graph further breaks down the interaction, illustrating the level of activation for higher and lower SES (median split) girls and boys seperately for times 1 and 2. The error bars represent ± standard deviation. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Given that the boys and girls were matched in developmental stage (as assessed by Tanner stage) rather than age, it is possible that the observed interactions with gender were actually driven by differences in age between the groups. Two sets of analyses were carried out to rule out this possibility. First, analyses were repeated with mean age (across time) as a nuisance covariate. Second we age‐matched the genders and recomputed analyses. Specifically, we divided participants based on within‐gender median splits and retained only the older girls and the younger boys. With this grouping, mean age was actually slightly higher in the female group (time 1 = 11.9, time 2 = 14.2) than in the male group (time 1 = 11.8, time 2 = 13.9). We then recomputed the SES × Time × Gender interaction analyses. Importantly, effects remained significant, both when including mean age as a covariate and when using the reduced samples. Thus, age differences between the genders do not appear to account for the observed interactions with gender.
Analyses were repeated within each gender to determine whether the relationship was independently significant in each group. A cluster emerged for girls in a similar dACC/rACC location and with a similar activation pattern as the SES × gender cluster (cluster size = 1,416, maz z‐value = 3.69, cluster P‐value = 0.034, xyz = ‐8, 34, 36). No clusters emerged for boys.
To assess the impact of ACC activation on behavior, NoGo versus Go d/rACC change over time activation was correlated with accuracy change over time in girls. Increased ACC activation over time predicted worse accuracy over time (r = −0.41, P = 0.03).
ACC Connectivity with dlPFC
It is possible that the increases in ACC activation over time in lower SES girls were due to weakened top‐down biasing of ACC by dlPFC. Thus, we examined connectivity between ACC and the two regions of dlPFC activated by the NoGo versus Go main effect. Specifically, we tested whether SES moderated change in NoGo (vs. Go) connectivity over time in girls. A significant SES × Time × ACC time course × Condition (NoGo vs. Go) interaction emerged for the cluster located in right inferior and middle frontal gyri (λ = −0.01, P = 0.01). This effect remained when mean (across time) age was included as a nuisance covariate. The interaction was decomposed by dividing into high and low SES (median split) and recomputing the model in each sample. The Time × ACC × Condition interaction was significant in both groups (low SES: λ = 0.12, P < 0.01; high SES: λ = 0.05, P = 0.05). Interactions were further decomposed by recomputing the model for each time point. The ACC × Condition interaction was significant only for lower SES girls at time 1 (Fig. 4; low SES at time 1: λ = 0.11, P < 0.01; low SES at time 2: λ = −0.01, P = 0.68; high SES at time 1: λ = 0.03, P = 0.12; high SES at time 2: λ = −0.02, P = 0.22). This pattern remained when only NoGo is examined. The interaction with the second dlPFC cluster was not significant.
Figure 4.
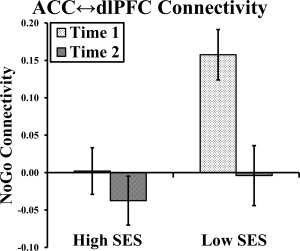
Socioeconomic status moderates connectivity between anterior cingulate and dorsolateral prefrontal cortex during NoGo in girls. Note: ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; SES, socioeconomic status. The graph illustrates the level of connectivity in girls between the ACC cluster that showed increased activation over time in lower SES girls and one of the dlPFC clusters that evidenced the main effect of NoGo versus Go. For visualization purposes, the graph shows connectivity for the NoGo period on the y axis, but this pattern holds when compared to the Go baseline. The error bars represent ± standard error of the λ's.
Given that our 5‐mm motion cutoff may be considered liberal, we reran the interaction analysis after excluding participants with motion ≥3 mm (n = 20). Importantly, the interaction remained significant in this reduced sample, suggesting that motion‐related variance does not account for this finding.
DISCUSSION
Lower SES appears to have a significant detrimental impact on the development of inhibition [Farah et al., 2006], and the present study provides a novel window into the neural mechanisms by which this impact may be transmitted to behavior during early adolescence. We found that lower SES was associated with both decreased behavioral inhibition (as indexed by NoGo vs. Go accuracy) and increased dACC/rACC activation over a 2‐year period, but only in girls. Notably, increased ACC activation over time also predicted worse accuracy over time in girls, suggesting that this change in ACC activation may be involved in the decrease in behavioral inhibition. Together with the large body of research supporting a role for this ACC region in compensatory inhibition [Banich, 2009], our findings suggest that female adolescents with lower SES develop less efficient inhibition, requiring greater (relatively unsuccessful) compensatory recruitment of ACC.
Recent models of inhibition have emphasized the interaction between ACC and dlPFC, which exhibited a mean increase over time in the present study but was not significantly moderated by SES. For example, Banich [2009] proposed that dlPFC biases task representations to be congruent with goals, and ACC resolves any remaining competition. In other words, individuals with inefficient dlPFC biasing of task representations must initiate compensatory processing in ACC to maintain performance. This proposal is supported by research indicating that the observed region of ACC shows robust structural connectivity with dlPFC [Beckmann et al., 2009].
Given that SES did not moderate dlPFC, one interpretation of present findings is that female adolescents with lower SES recruit dlPFC to the same degree as their high SES counterparts. However, this processing may be inefficient, requiring increased ACC engagement to resolve the remaining competition. We performed a preliminary test of this hypothesis by examining ACC ↔ dlPFC connectivity. Consistent with hypotheses, lower SES girls exhibited high levels of NoGo connectivity at time 1, which decreased to near zero at time 2. This suggests that dlPFC performed appropriate top‐down biasing at time 1, but this biasing failed to occur at time 2, leaving ACC to resolve the remaining competition. Thus, present findings provide preliminary support for the proposal that inhibitory difficulties in lower SES girls are due to a disruption in a brain network that includes ACC and right dlPFC.
In light of evidence that women generally exhibit better inhibition than men [Bjorklund and Kipp, 1996], the present finding that SES was related to worse inhibition over time only in girls may appear a bit puzzling. Although inhibition difficulties are in general more common in boys, our results suggest that the onset of adolescence may represent a window of vulnerability for girls who go through this maturational period under conditions of social adversity (as indexed by low SES). If replicated by other studies, this may also imply an opportunity for early intervention/prevention targeting low SES girls during this period of development given that these inhibitory capacities may be more malleable to external influences and thus more impacted by social factors, both negative and positive during adolescence. These findings also raise provocative questions as to why early adolescent females in low SES environments may be more vulnerable to negative developmental trajectories in inhibition—including the possibility that the specific types of social stressors experienced by girls going through this maturational window in low SES environments may be a contributing factor.
Although not significant, it is interesting that SES exhibited the opposite relationship with both behavior and ACC activation in boys (behavioral effects in boys were marginally significant). In contrast to girls, these differences appear to be driven by behavior/brain activation changes in the higher SES group for boys. For example, higher SES boys evidenced increased ACC activation over time (although this effect was nonsignificant). Thus, it is possible that SES also impacts the development of inhibition in boys during this developmental period, with higher SES boys evidencing relatively worse accuracy and greater ACC recruitment over time. Of course, no inferences should be made about these effects given that they did not reach conventional levels of significance. However, the presence of a marginally significant behavioral effect in boys hints that SES also plays an important role in inhibitory development in boys.
How Does SES Impact Brain Function?
The present study cannot determine the specific mechanism by which SES impacts neurodevelopment of inhibition, it can only support the existence of such an impact. One potential conduit is increased level of stress derived from living in lower SES environments [Baum et al., 1999]. Living in a higher stress environment may require a greater basal cognitive load (e.g., increased monitoring of the environment), which may deplete resources [Hagger et al., 2010]. Furthermore, as individuals move from childhood to adolescence, those living in a lower SES environment may experience an increasing set of stressors. Specifically, parents can mitigate the impact of stress by providing a buffer [McLoyd, 1998], and parents in lower SES circumstances may be less able to provide monitoring/support to buffer these stresses. One can also speculate about the ways in which girls in these circumstances may experience different types of social stressors after the onset of puberty [Ge et al., 1996]. Thus, one interpretation of present findings is that the stress conferred by living in a lower SES environment becomes increasingly salient and/or more impactful during early adolescence for girls. In many ways, this could parallel findings that stressors and biological maturational factors at puberty interact to contribute to increased rates of depression in adolescent girls.
Implications for Development
Research indicates that the combination of low SES and difficulty with inhibition during adolescence is associated with an increased risk of developing several maladaptive patterns of behavior, including gambling, drug, and alcohol use [Auger et al., 2010; Clark et al., 2005]. Present findings indicate that, at least in girls, this behavior may result in part from inefficient ACC processing and disrupted ACC‐dlPFC coupling, leading to deficient behavioral inhibition. Thus, adolescence may be a particularly salient target for prevention/intervention efforts aimed at such maladaptive behavior. For example, cognitive training of inhibition increases both behavioral inhibition and activation in ACC and dlPFC [Houde et al., 2000]. Accordingly, engagement in such training may provide a means to prevent/remediate the impact of lower SES on both the behavioral and neural mechanisms involved in inefficient inhibition.
Interestingly, research has identified female‐specific effects of SES in adults, and present findings may provide insight into the neuromaturational mechanisms by which these effects develop. For example, low SES women are more likely to be obese than men of similar SES [Wang and Beydoun, 2007]. Research suggests a link between body weight and inefficient inhibition. For example, a recent study examined the relationship between body mass index (BMI) in female adolescents and inhibition using a food‐specific version of the Go/NoGo task [Batterink et al., 2010]. Higher BMI predicted both reduced behavioral inhibition and concurrent reduction in dlPFC activation. Thus, one factor leading to differences in obesity may be a female‐specific impact of SES on behavioral inhibition and concurrent ACC activation/coupling with dlPFC.
Given growing evidence indicating that pubertal processes play a critical role in social and affective maturation during adolescence [Crone and Dahl, 2012], we conducted exploratory analyses to determine whether pubertal development over the 2‐year interval was driving SES‐specific development in inhibition. Although no significant interactions with puberty were observed in the present work, it remains crucial to examine puberty when investigating the influence of SES on developmental trajectories, because lower SES has been linked to an earlier onset of puberty in girls [James‐Todd et al., 2010] and there is evidence that pubertal hormones may influence motivational as well as social and emotional processes in ways that could impact developmental trajectories [Crone and Dahl, 2012].
Strengths and Limitations
The study benefits from a number of strengths, including the longitudinal design, which allows for more powerful and accurate tests of development and remains uncommon in the developmental neuroscience literature. Also, the study focused on a specific window of development, specifically examining a 2‐year interval near the onset of adolescence. Additionally, we examined the impact of both behavior and neural function, which allows for convergent support.
Several limitations must also be considered. First, we cannot determine the precise mechanism by which SES impacts neural and behavioral development. Future research could investigate whether stressful life events—or particular types of social adversities—might mediate the relationship between SES and neural/behavioral development. An additional limitation is that the present sample encompassed the full range of SES, which does not allow for in‐depth examination of gradations within lower SES youth. Future research using a lower‐SES targeted sample could provide more clarity in the neural processes involved in inhibition in these individuals. Although interesting directions for future research, we do not believe that these complexities undermine the inferences of the present study.
In summary, the present study provides novel insight into the neuromaturational pathways by which socioeconomic factors may impact inhibition. Specifically, we demonstrated that lower SES female adolescents show decreased behavioral inhibition/ACC‐dlPFC connectivity and increased compensatory ACC activation over a 2‐year period. To our knowledge, this is the only study to show that lower SES is associated with a decline in inhibition over development, rather than a smaller increase or delay. Present findings refine our knowledge of the timing and specificity of the impact of SES on inhibition, which may help improve prevention/intervention efforts. For example, SES research and intervention/prevention efforts often focus solely on early childhood, and our findings suggest that this focus should be expanded (not shifted) to include adolescence. In addition, present findings support the importance of examining the moderating role of gender in neurodevelopmental research on SES.
ACKNOWLEDGMENT
The authors gratefully acknowledge the conscientious work of Jill Tarr as the project director who led the recruitment and retention of the sample, and the youth and their families for their participation in the studies.
REFERENCES
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Auger N, Lo E, Cantinotti M, O'Loughlin J (2010): Impulsivity and socio‐economic status interact to increase the risk of gambling onset among youth. Addiction 105:2176–2183. [DOI] [PubMed] [Google Scholar]
- Banich MT (2009): Executive function the search for an integrated account. Curr Direc Psychol Sci 18:89–94. [Google Scholar]
- Batterink L, Yokum S, Stice E (2010): Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. Neuroimage 52:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM (1999): Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci 896:131–144. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen‐Berg H, Rushworth MF (2009): Connectivity‐based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund DF, Kipp K (1996): Parental investment theory and gender differences in the evolution of inhibition mechanisms. Psychol Bull 120:163–188. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF (2002): Socioeconomic status and child development. Annu Rev Psychol 53:371–399. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB (2007): Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol 17:243–250. [DOI] [PubMed] [Google Scholar]
- Clark DB, Cornelius JR, Kirisci L, Tarter RE (2005): Childhood risk categories for adolescent substance involvement: A general liability typology. Drug Alcohol Depend 77:13–21. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE (2012): Understanding adolescence as a period of social‐affective engagement and goal flexibility. Nat Rev Neurosci 13:636–650. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A (2006): Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 44:2037–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, Davidson MC, Lawrence Aber J, Casey BJ (2006): Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol Sci 17:478–484. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H (2006): Childhood poverty: Specific associations with neurocognitive development. Brain Res 1110:166–174. [DOI] [PubMed] [Google Scholar]
- Feinstein L (2003): Inequality in the early cognitive development of British children in the 1970 cohort. Economica 70:73–97. [Google Scholar]
- Forbes EE, Dahl RE (2010): Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain Cogn 72:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH Jr (1996): Coming of age too early: Pubertal influences on girls’ vulnerability to psychological distress. Child Dev 67:3386–3400. [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NL (2010): Ego depletion and the strength model of self‐control: A meta‐analysis. Psychol Bull 136:495–525. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975): Four factor index of social status. Unpublished working paper, Department of Sociology, Yale University.
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW (2003): Response inhibition and impulsivity: An fMRI study. Neuropsychologia 41:1959–1966. [DOI] [PubMed] [Google Scholar]
- Houde O, Zago L, Mellet E, Moutier S, Pineau A, Mazoyer B, Tzourio‐Mazoyer N (2000): Shifting from the perceptual brain to the logical brain: The neural impact of cognitive inhibition training. J Cogn Neurosci 12:721–728. [DOI] [PubMed] [Google Scholar]
- James‐Todd T, Tehranifar P, Rich‐Edwards J, Titievsky L, Terry MB (2010): The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Ann Epidemiol 20:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): FSL. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H (2004): Prefrontal‐subcortical dissociations underlying inhibitory control revealed by event‐related fMRI. Eur J Neurosci 19:3105–3112. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K (2001): Mood disorders in children and adolescents: An epidemiologic perspective. Biol Psychiatry 49:1002–1014. [DOI] [PubMed] [Google Scholar]
- Liu J, Zubieta JK, Heitzeg M (2012): Sex differences in anterior cingulate cortex activation during impulse inhibition and behavioral correlates. Psychiatry Res 201:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA (2004): Maturation of cognitive processes from late childhood to adulthood. Child Dev 75:1357–1372. [DOI] [PubMed] [Google Scholar]
- McLoyd VC (1998): Socioeconomic disadvantage and child development. Am Psychol 53:185–204. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Poulton R, Roberts BW, Ross S, Sears MR, Thomson WM, Caspi A (2011): A gradient of childhood self‐control predicts health, wealth, and public safety. Proc Natl Acad Sci USA 108:2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M (2010): Early‐life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia 48:3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA (2007): The obesity epidemic in the united states–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta‐regression analysis. Epidemiol Rev 29:6–28. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Muller U, Frye D, Marcovitch S, Argitis G, Boseovski J, Chiang JK, Hongwanishkul D, Schuster BV, Sutherland A (2003): The development of executive function in early childhood. Monogr Soc Res Child Dev 68:vii–137. [DOI] [PubMed] [Google Scholar]


