Preface
Soft connective tissues at steady state are yet dynamic; resident cells continually read environmental cues and respond to promote homeostasis, including maintenance of the mechanical properties of the extracellular matrix that are fundamental to cellular and tissue health. The mechanosensing process involves assessment of the mechanics of the matrix by the cells through integrins and the actomyosin cytoskeleton, and is followed by a mechano-regulation process that includes the deposition, rearrangement, or removal of matrix to maintain overall form and function. Progress toward understanding the molecular, cellular, and tissue scale effects that promote mechanical homeostasis has helped identify key questions for future research.
Keywords: mechanosensing, mechanoregulation, integrins, actomyosin, extracellular matrix
Introduction
The extracellular matrix (ECM) is fundamental to the form and function of soft connective tissues. Cells within these tissues establish the ECM during development, maintain it in health, remodel it during adaptations, and repair it in response to disease and injury 1. Conversely, the ECM influences many cellular functions, including migration, growth, differentiation, and even survival 2. This reciprocal relationship was recognized over 30 years ago and has remained a central concept in cell biology 3. Importantly, cell-matrix interactions not only involve the chemical composition and structural organization of the ECM, but also its mechanical properties. Thus, cells must sense and regulate ECM mechanics to promote mechanical homeostasis, that is, to maintain tissue-level structural integrity and functionality.
Mechanical loads acting on a tissue are perceived by resident cells as stimuli that are transmitted through, or exerted on, constituents of the extracellular matrix, matrix receptors, and intracellular structures. As detailed below, mechanical homeostasis thus involves ECM constituents such as the collagens and elastin that support and transmit loads; transmembrane receptors for these constituents, primarily the integrins that connect extracellular and intracellular structures plus their associated linker proteins (such as talin and vinculin) that connect the receptors to the cytoskeleton; and actin filaments, non-muscle myosin, and associated proteins that constitute the cytoskeleton and transmit mechanical loads or signals within the cell (Fig. 1). Much has been learned since the mid-1970s about how cells sense and regulate the mechanical properties of the ECM 4, 5, but the motivation for study has generally been to understand developmental processes, disease progression, or wound healing 6, 7. In contrast, we consider how mechanical loads on transmembrane complexes and cytoskeletal structures are fundamental to the cell–matrix interactions that govern mechanical homeostasis in health. The central idea is that health requires that cells first sense the mechanics of the matrix and then regulate it to maintain the desired properties; loss of these complementary homeostatic processes leads to fibrosis, mechanical failure, or other pathologies. Toward this end, we focus on integrative mechanosensing and mechanoregulation of the ECM across different length and time scales to understand mechanical homeostasis of the ECM.
Fig. 1. Key components in soft connective tissue mechanical homeostasis.
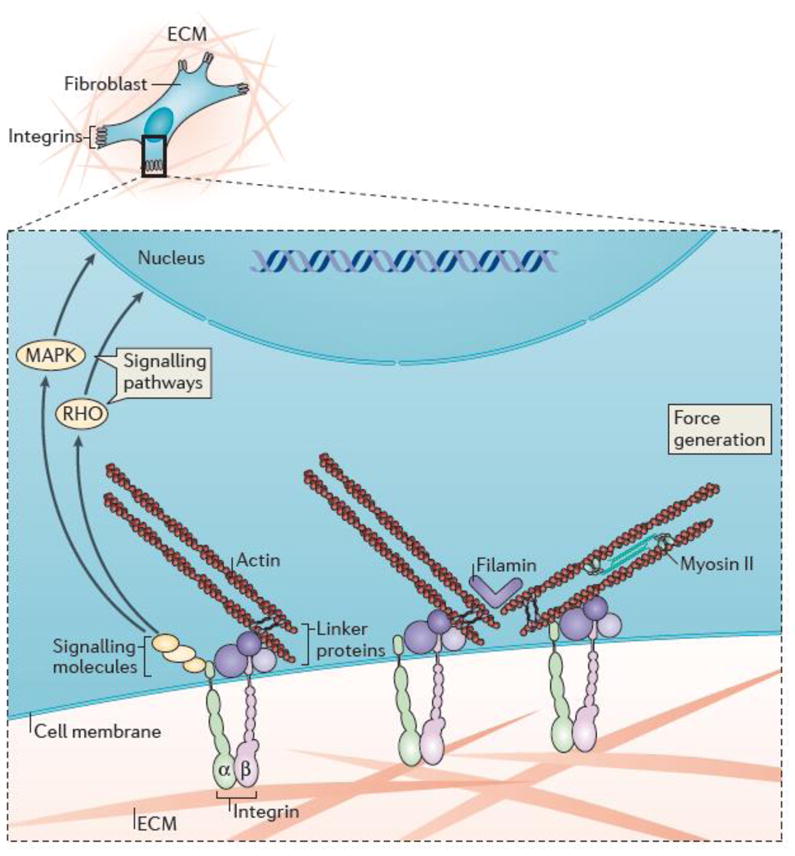
Schematic drawing depicting a fibroblast embedded in extracellular matrix (ECM) consisting primarily of collagen, fibronectin, and glycosaminoglycans, with an expanded view showing cell-matrix interactions and associated intracellular structures. In particular, cells interact mechanically with the ECM via heterodimeric transmembrane receptors called integrins, which in turn interact with intracellular signaling molecules (including focal adhesion kinase (FAK) and Src) and physically connect to cytoskeletal actin via a host of linker proteins (including talin, vinculin, filamin, the ILK-PINCH-parvin complex, and α-actinin). Key signaling pathways associated with integrin activation include the Rho-Rho kinase and mitogen-activated protein kinase (MAPK) pathways. The mechano-stimulation of cells is complemented in most situations by chemo-stimulation via soluble ligands.
Key players in mechanical homeostasis
In order to understand mechanical homeostasis, it is important to first summarize the key players — the ECM, effectors and sensors.
The substrate
Although the ECM comprises over 300 proteins, 200 glycoproteins, and 30 proteoglycans 8, its mechanical properties often depend largely on three constituents: elastic fibers, fibrillar collagens, glycosaminoglycans (GAGs) and the related proteoglycans (PGs). Elastic fibers consist of a core of elastin and a surrounding sheath of microfibrils, including the glycoproteins fibrillin and fibulin. These fibers endow tissues with extensibility (elastic fibers can extend up to 150% without failure) and resilience (the ability to recoil upon unloading); they are also the most biologically, chemically, and thermally stable constituents of the ECM 9, 10. Elastic fibers are deposited and organized prior to adulthood and have long half-lives (for example, 50 to 70 years in human arteries 9). They thereby provide a “mechanical memory” in that they are prestressed due to somatic growth and recoil when unloaded from their homeostatic state. Because functional elastic fibers cannot be organized in adulthood, any mechanical damage or proteolytic degradation that they undergo results in irreversible changes in tissue form and function. Two prime examples are aging-induced stiffening of elastic arteries and the wrinkling of skin, both of which arise in part from loss of elastic fiber integrity via normal degradation kinetics or mechanical fatigue. Mutations in the genes for elastin or elastin-associated glycoproteins are responsible for Williams syndrome and Marfan syndrome, amongst others 10.
Collagen is the most abundant protein in the human body; it exists in over 25 types, the most common being fibrillar types I and III. In contrast to elastic fibers, collagen fibers endow connective tissues with its material stiffness (how much stress changes when strained) and strength (the maximum stress at failure). They also have relatively short half-lives (see Box 1) and thus are not subject to mechanical fatigue. Rather, their remodeling (which involves their reorientation or cross-linking) or turnover (their rate of synthesis and degradation) under stress is critical to connective tissue homeostasis. Collagen fibers are built hierarchically, from molecules (~300 nm long and 1.5 nm in diameter) to fibrils (20–100 nm in diameter) to fibers (0.5 to 20 μm in diameter); cells must therefore sense and regulate collagen across these different length scales. The cell-mediated fibrillogenesis of type I collagen is aided by associations of this fibrillar collagen with other constituents of the ECM, including fibronectin, type V collagen, and the proteoglycan biglycan. Mutations in the genes for collagen I and III are responsible for osteogenesis imperfecta and Ehlers-Danlos syndrome, among other conditions, while mutations in constituents associated with collagen I and III lead to similar structural defects 11. Like elastic fibers, the contributions of collagen fibers to the overall structural integrity of tissues depends on fiber density, orientation, undulation, cross-linking, prestress, and interactions with other matrix components. Interestingly, given their very different times of deposition and prestresses, elastic fibers influence the stiffness of collagen fibers by affecting their undulation in vivo 12. Loss of elastic fiber integrity thus affects overall tissue mechanics in at least two important ways. GAGs are high molecular weight molecules consisting of repeating disaccharide units; they are highly negatively charged due to attached sulfate and carboxyl groups. PGs consist of GAGs attached to a protein core. GAGs and PGs perform multiple functions, including sequestrating growth factors. Additionally, they are extensively hydrated and thereby contribute to the compressive stiffness of connective tissues. Maintenance of ECM mechanical properties depends on the continual synthesis, incorporation, and degradation of these structural and other structural constituents 1, 13, 14, which interact in complex and not yet fully understood ways.
Box 1. Terms useful in mechanics and mechanobiology.
Understanding mechanical homeostasis requires an appreciation of cell and tissue level mechanics. Toward this end, it is important to note some basic terminology and definitions.
Stress is a measure of “force intensity”, given as force per (oriented) area and typically reported in units of one newton per square meter (N/m2), which is called a pascal (Pa). Note that 1 nN/μm2 = 1 kPa (where nN indicates a nano-newton and μm indicates a micron, namely 1 nN = 10−9 N and 1 μm = 10−6 m), which shows equivalence between units used in molecular and cell/tissue level studies.
Strain is a normalized measure of deformation that indicates changes in lengths or angles within a material, typically induced by applied stresses. Strain is dimensionless and sometimes represented as a percent change.
Material stiffness is a measure of resistance to deformation, literally how stress changes in response to strain; the inverse of stiffness is compliance, a measure of how strain changes in response to stress. An ideal material having an infinite material stiffness is said to be rigid. In contrast, structural stiffness combines the effects of material stiffness and geometry. For example, a thin-walled tube composed of a stiff material can have the same structural stiffness as a thick-walled tube composed of a compliant material. As noted in the text, it is the material stiffness that appears to be conserved in arteries while structural stiffness changes with changes in pressure.
Strength is a measure of resistance to material damage or failure; it is the maximum value of stresses that can be tolerated prior to failure. The terms hard and soft reflect a resistance to penetration or being scratched and thus particular aspects of strength.
Elastic describes a mechanical behavior that does not dissipate energy, thus the material returns to its original geometry when unloaded. Inelastic behaviors include viscous (fluids), plastic (an irreversible shear-induced deformation common in ductile metals), and damage, which includes fatigue (that is, loss of strength due to repeated mechanical loading). Viscoelastic refers to combined fluid-like and solid-like behavior. Viscoelastic responses are often elastic (that is, energy preserving) on short time scales but viscous (that is, energy dissipative) when force is maintained over longer times. Silly putty is an excellent example of a material that exhibits viscoelasticity, as are cytoskeletal networks. These mechanical behaviors [are often quantified via relationships between stress and strain, or their rates, which necessitates the determination of values of specific material parameters. Young’s modulus (also known as the tensile modulus or elastic modulus) is such a parameter for materials exhibiting a linear stress–strain behavior under small deformations; the material stiffness is the same in these materials independent of the stress or strain. Nonlinear behaviors characteristic of soft connective tissues and the cytoskeleton require different material parameters for their description. Finally, note that an exponential stress-strain behavior results in a linear relationship between stiffness and stress. Hence, an increased prestress supports an increased initial stiffness, which in turn often affects cell phenotype.
The effectors
Fibroblasts are the primary cells that build and maintain the ECM in most soft connective tissues. They can secrete the elastin, different types of collagens, glycoproteins, and GAGs that constitute a specific tissue, and they coordinate their synthetic and mechanical machinery to organize the constituents that give rise to the overall structural organization, and thus mechanical properties, of the tissues. They can also secrete proteases, most notably members of the matrix metalloproteinase family, that degrade the various structural constituents; 15. Fibroblasts can differentiate into myofibroblasts when stimulated by transforming growth factor-beta (TGF-β) under conditions of high tensile stress, which increases both their ability to synthesize ECM components and their contractile capacity 14, 16. The latter is due, in part, to the incorporation of smooth muscle α-actin within the cytoskeletal stress fibers and an increase in the clustering of integrins at focal adhesions. This phenotype is often associated with fibrotic pathologies or aberrant wound healing and will not be discussed further here. Although many other cell types, including macrophages, contribute either directly or indirectly to mechanical homeostasis in connective tissues, we focus on mechanotransduction in fibroblasts.
The sensors
The main cellular components that mediate the sensing and regulation of ECM mechanics are the integrins that bind matrix proteins, the associated cytoskeletal and signaling proteins of the focal adhesions, and the actomyosin cytoskeleton (Fig. 1). A second set of important players are the signaling components that regulate the assembly of these structures; these are primarily the Rho family small GTPases and their downstream effectors such as Rho-associated protein kinase (ROCK), myosin light chain kinases, and so on. In principal, every component in the mechanical linkage between the ECM and the actin that bears force is a potential mechanotransducer 17, though some components likely transmit force without mechanotransduction, that is, without converting force into meaningful biochemical signals. Talin and vinculin provide one linkage between integrins and actin; the ILK–PINCH–parvin pathway provides another, as do filamin and α-actinin 17 (Fig 1). Inhibiting or altering these components leads to the altered sensing of stiffness or stress and strain through the ECM. As discussed in detail below, mechano-sensing is thought to be mediated by force-induced changes in protein conformations or the kinetics of assembly and disassembly of protein complexes. A critical concept relevant to all mechanosensing through integrin-mediated adhesions is that baseline stress or prestress from endogenous contractility tunes the cells’ responses to external forces 18. Thus, tension from endogenous actomyosin on these linkages modulates their subsequent responses to externally applied forces. This aspect greatly complicates efforts to unravel mechanosensory pathways since inhibitors can have indirect effects by altering cytoskeletal organization and/or decreasing prestress. This facet needs to be taken into account when interpreting many experimental results.
Mechanobiological phenomena in tissue
Mechanobiology refers both to how biological systems sense and respond to mechanical signals and how they exert force and control the mechanical properties of their surroundings. Mechanobiological effects span the full range of biological organization from molecules to cells to organisms, but here we focus on the tissue level where the ECM plays a central role. Whereas ECM was once thought to serve only a structural role (maintaining tissue form under mechanical loads and providing a physical support system for cell adhesion and migration), we now know that it also serves an important instructional role (providing biochemical and biomechanical cues that influence a range of cell activities, including migration, adhesion, phenotypic modulation, and survival). An understanding of the mechanobiology of tissues thus requires a direct link between molecular mechanisms and tissue-level phenomena. It is challenging, however, to reconcile detailed descriptions of molecular mechanisms with coarse-grained mechanical quantities, including stress, strain, and stiffness. These quantities (Box 2), which cannot be sensed directly at a molecular scale 19, are nevertheless regulated to maintain homeostatic values13, 20 and are fundamental descriptors of tissue-level form and function. For example, interstitial arterial cells (that is, smooth muscle cells and fibroblasts) establish a preferred matrix stiffness during development and then tend to maintain this value over a lifetime, at least in the absence of disease or injury 21. Thus, arterial wall stiffness is similar within a single species despite many genetic variations or alterations in blood pressure 22, and across multiple species (indeed, from lobsters to whales) despite large variations in ECM composition, blood pressure, and body size 23. Similar observations hold for diverse connective tissues including tendons, skin, the heart, and so forth 24, 25.
Box 2. Loading rates affect matrix composition.
All soft connective tissues are subject to mechanical loading, including the ever present effects of gravity on earth, yet the rate of loading differs considerably across tissues and species. In the human, for example, heart rates of 60 to 70 beats per minute (bpm) subject heart tissue and arteries to high loading rates, respiratory rates of 12 to 20 breaths per minute subject lung tissue to intermediate rates, and most skin is subject to nearly static loading. Skeletal muscle and tendons can experience high loading rates during vigorous exercise, but low loading rates during rest. The half-life of fibrillar collagen has been reported to differ by ~5 fold between arteries (~22 days) and skin (~95 days) in middle-aged to older humans; related values are ~20 days for the heart, ~25 days for skeletal muscle, ~27 days for lungs, and ~52 days for tendons/ligaments 149. Interestingly, these findings suggest that the half-life of collagen may be less in tissues subjected to higher loading rates, consistent with the general expectation that replacement should be more frequent in tissues subjected to more demanding mechanical environments. Somewhat related, the ratio of elastic to collagen fibers also tends to correlate with loading rate in arteries. For example, this ratio in carotid arteries (in the neck) decreases from mice (heart rate of ~600 bpm) to rats (~300 bpm), rabbits (~230 bpm), dogs (~90 bpm), and humans (~60 bpm), with in vivo axial prestretch similarly decreasing from mouse to human 150. Interestingly, arterial elastic fibers emerged on an evolutionary timescale with the appearance of closed circulatory systems that are subjected to pulsatile loading and thus are found exclusively in vertebrates. However, a detailed understanding of the molecular mechanisms by which cells sense and regulate matrix in quasi-static versus dynamic mechanical environments remain largely unknown.
To promote mechanical homeostasis in health, cells must use negative feedback mechanisms that sense changes within the ECM and restore values back to normal (Fig. 2). For example, under normal conditions, acute increases in stiffness should trigger mechanisms that render the ECM more compliant, whereas acute decreases in stiffness should trigger pathways that result in stiffening. By contrast, diverse pathologies appear to result from either a loss of negative feedback 26–29 or a switch to positive feedback mechanisms (Fig. 2). For example, acute increases in stress/strain can result in continued stiffening, often referred to as fibrosis. Given the fundamental role of integrins in both sensing and mechanically regulating matrix, it is not surprising that recent anti-fibrotic therapeutic strategies target integrins 30, 31. Notwithstanding the importance of understanding failed mechanisms in disease, our focus is on the normal mechanisms that ensure proper form and function of soft connective tissues (Fig. 3).
Fig. 2. Feedback loops regulate extracellular matrix structure and function.
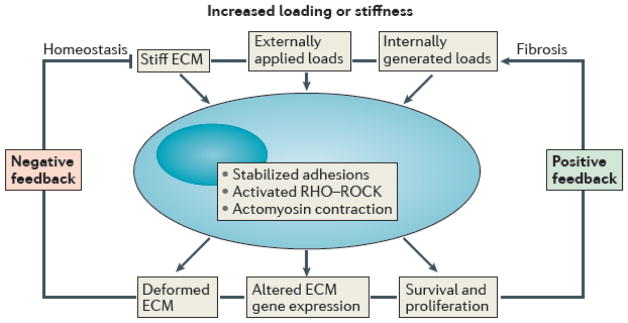
Flow chart of the effects of increased mechanical loading or matrix stiffness on the cellular responses that lead either to a homeostatic regulation of matrix properties (negative feedback loop) or fibrotic conditions (positive feedback loop). In both cases, stabilized focal adhesions of greater number or size and increased actomyosin contractility, often via the Rho–Rho kinase pathway, play central roles. The precise molecular mechanisms responsible for these feedback loops remain unknown, particularly for the negative feedback that is required, by definition, for homeostasis.
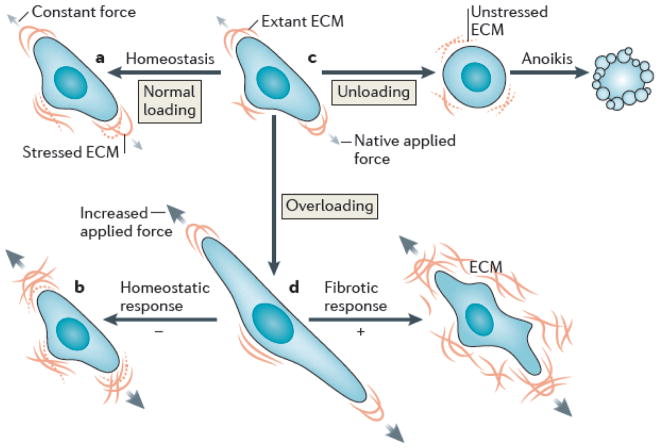
Fig. 3. Cell-matrix interactions in health and disease.
Schematic drawing of a normal cell and its mechanical interaction with extant matrix that is stressed or strained due to native applied forces (indicated by the grey arrows) (top row, center). Shown, too, is both a cell ensuring homeostatic maintenance of matrix under constant forces, despite the continual degradation of stressed matrix (top row, left) and a homeostatic remodeling in response to increased applied forces, that is, overloading (black arrows; bottom row, left). In contrast, loss of signaling via the matrix can lead to a special form of apoptosis called anoikis (top row, right) whereas pathologic signaling in response to overloading can lead to a fibrotic response (bottom row, right). Note, in particular, that homeostasis ultimately requires the balanced production and removal of constituents, with the new constituents having the same mechanical properties as the old. These properties include stiffness, orientation, and prestress.
Cellular regulation of ECM
Cells establish the ECM during development and subsequently determine its composition, structure, and mechanical properties, a process that is regulated by mechanics.
Mechanical stress within the matrix
Soft connective tissues exhibit a nonlinear relationship between stress and strain that is approximately exponential. An interesting property of this relationship is that stiffness relates linearly to stress 32, hence, the cellular control of ECM stress is equivalent to controlling ECM stiffness. Considerable understanding of the regulation of matrix stress has come from studying tissue equivalents, often collagen or fibrin gels seeded with fibroblasts. For example, when seeded within initially stress-free but mechanically constrained collagen gels, fibroblasts adhere to the matrix and contract, which develops tensile stresses that within hours tend to a steady state 33; this process has been called tensional homeostasis 7. Of particular note, if this endogenous stress is externally increased or decreased, the cells return the stress toward the original level. Because the matrix stiffens proportionally with the stress, tensional homeostasis represents one method to regulate matrix stiffness. It is important to note that a “residual matrix tension” remains when the cells’ actomyosin machinery is disrupted in these stressed gels 34, which suggests that the cells lock in the stresses (or strains), perhaps by cross-linking the remodeled matrix.
Regardless of the precise mechanisms, tensional homeostasis appears to establish a favorable mechanical environment for cell function. Interestingly, the measured levels of endogenous stress in tissue equivalents (~3 to 5 kPa) 35, 36 are comparable to the levels of stress that have been measured at focal adhesions (~3 to 5.5 kPa) 37, 38. This observed consistency in established levels of stress across spatial scales for different matrices and cells (including non-contractile smooth muscle cells and fibroblasts) suggests that there is a “homeostatic target” value of interstitial stress not unlike the well-known target value of wall shear stress for endothelial cells in large arteries, which is ~1.5 Pa in humans 39. Mechanical homeostasis is thus achieved in the short-term though negative feedback characterized by matrix reorganization and cross-linking, and in the long-term by balanced matrix degradation and the deposition of constituents under the appropriate pre-stress.
ECM turnover
All constituents of the ECM have finite half-lives (Box 1) and most are renewed via proteolysis and synthesis, the notable exception being elastin. Such turnover of ECM is difficult to study in vivo, however, and is poorly recapitulated in vitro. Fortunately, computational models have provided some insight into the roles of ECM turnover in mechanical homeostasis in native tissues under physiologic conditions. These models suggest that mechanical homeostasis in soft connective tissue depends primarily on four key factors 40: rates of ECM production, rates of ECM removal, the mechanical properties of the ECM constituents, and the degree of prestress that is built into these constituents when deposited. It is well known that rates of matrix synthesis correlate positively with altered mechanical loading 13, 41, as do rates of protease synthesis. That is, increasing mechanical loading tends to increase both the cellular production and removal of structural constituents 42, 43, as would be required for a process governed by negative feedback. Indeed, the mechanical state of the matrix can also influence the rate of degradation by proteases, with increased stress tending to be protective 44, which would also contribute to reducing stress via the retention of matrix. It is intuitive that the structural integrity of a tissue depends upon the mechanical properties of the constituents it comprises 45. Recall, for example, that competent elastic fibers endow a tissue with resilience while collagen fibers contribute primarily to the stiffness and strength.
Here, therefore, we emphasize an often ignored aspect of mechanical homeostasis in soft connective tissues – that newly deposited matrix must be incorporated within extant matrix under stress to ensure tissue maintenance over long periods of nearly constant loading (Fig. 3). That is, computational models suggest that tissue form and function can be maintained only if the structural constituents that are degraded are replaced with new constituents that have the same properties, including the same level of prestress-induced stiffness 46, 47.
Growing experimental evidence supports this concept of the mechanoregulation of matrix stress and hence stiffness. Although collagen fibrils can self-assemble in vitro via purely thermodynamic mechanisms, their assembly is regulated in vivo by many additional binding partners 48, including fibronectin and biglycan. Proper organization in vivo also requires direct cellular control. Indeed, it now appears that the fibrillogenesis of collagen I and III by both fibroblasts and smooth muscle cells requires fibronectin and integrins 49, 50; the former may serve as a scaffold on which the collagen molecules are deposited or on which the cells can act, while the requirement for integrins implies that cells must actively organize the secreted molecules. Indeed, fibroblasts appear to organize collagen fibers via active repetitive cycles of cellular protrusion into the matrix, binding to the matrix, contracting to draw in the matrix, and then releasing the matrix, with Rho kinase and myosin II playing important roles 50–52. Rho kinase is important in sustaining myosin II activation, which, with complementary actin polymerization, allows cells to forcefully act on the matrix. In addition, cell-mediated organization of matrix likely mimics the aforementioned residual matrix tension that cells establish in vitro in collagen gels 16, 34, which would enable the cells to coordinate the organization of both new and pre-existing ECM such that they do not have to actively maintain the tension that they build into to the matrix. Rather, the incorporation of this tension is likely accomplished via the crosslinking of prestressed matrix constituents, a process which may be mechanically regulated as well 53. In summary, cells often actively organize the matrix through their integrins, with the actomyosin machinery allowing them to pull or push on fibers that can subsequently be entrenched to establish a new mechanical state 53.
Perhaps the most direct evidence that cells prestress matrix is that actomyosin activity is required for fibronectin to be assembled into fibrils 54. In particular, it appears that soluble, folded fibronectin secreted into the extracellular space binds to α5β1 integrins and then is unfolded via actin-mediated contractility to expose otherwise cryptic binding sites that promote the assembly of multiple fibronectin molecules into fibrils 55. As noted above, appropriately unfolded, that is prestressed, fibronectin aids in collagen fibrillogenesis, which is a major contributor to the material stiffness of most soft tissues. During embryonic development, fibroblasts use special extensions of the cell membrane termed fibropositors, which are powered by actomyosin activity, to guide the deposition of prestressed collagen fibers 56. These membrane structures allow the cell to orient the collagen (within these directed channels) as it is incorporated within the extant matrix. Whether it occurs during development or in a mature organism, cell-mediated collagen fibrillogenesis involves a remarkable, multistep sequence that results in the assembly of an organized matrix. Perhaps guided by the prestressed fibronectin, fibroblasts use targeted48, integrins (for example, α2β1) to pull on and orient collagen I fibrils as they assemble into fibers 56. This process also involves accessory proteins such as collagen V as well as those that modulate overall fiber diameter, including the proteoglycan decorin.
Similar processes of prestressing seem to be involved in the formation of elastic fibers from the secreted, soluble elastin that first aggregates on the cell surface 57. Associated proteins and glycoproteins, such as the fibulins and fibrillins, similarly participate in the coordinated assembly of elastic fibers that confer structural stiffness as well as resilience 58. Again, cell mediation via appropriate integrins (for example, α5β1 and αvβ3) appear to play an important role 59 by allowing the cells to hold onto and control the fibers mechanically.
ECM influences on cells
Once established by the cells, the ECM then provides the cells with important biomechanical and biochemical cues that guide their behavior.
How matrix stiffness influences cells
Fibroblasts are highly sensitive to mechanical stimuli and the mechanical properties of their matrix 60, a characteristic that they share with many other cell types, including smooth muscle and epithelial cells. Cells spread more and develop larger focal adhesions and actin stress fibers on stiff than compliant matrices61. They also exert higher tractions on stiff surfaces, whereas they downregulate myosin-dependent contractility on more compliant ones 62. Cell migration speed shows a biphasic dependence on stiffness, being maximal at intermediate levels63, 64; yet, when cells encounter an interface between materials of different stiffness, they migrate preferentially to the stiff surface 65. Matrix stiffness also regulates cell cycle progression. In endothelial cells, this occurs through activation of the small GTPase Rac1 which leads to induction of cyclin D 66. Matrix stiffness also controls gene expression and cell fate, as, for example, in directing the differentiation of mesenchymal stem cells 67. In this context, matrices direct differentiation toward lineages, the normal mechanical environment of which approximates that level of stiffness; for example, compliant substrates favor differentiation toward neural and adipocyte fates where in vivo stiffness is low. The Yap and Taz proteins of the Hippo pathway have recently been implicated in transcriptional effects of matrix stiffness and cytoskeletal organization 68, and they appear to contribute to the stiffness-dependent regulation of matrix gene expression and cell cycle progression among other effects.
Finally, matrix stiffness can influence the ultimate fate decision: compliant matrices induce the apoptosis of anchorage dependent cells 62. This phenomenon may relate to the more general requirement for matrix attachment in survival 69, 70 (Fig 3), most likely because integrin signaling molecules such as focal adhesion kinase are suppressed on compliant ECM. There are additional implications, however. Similar effects may be important in wound healing by promoting myofibroblast apoptosis once tissue repair is complete and cell-induced tension decreases; failed apoptosis is linked both to scarring and scleroderma, the latter being a lethal disease characterized by connective tissue stiffening 71.
Interestingly, the stiffness at which the ECM can influence cell phenotype depends on the cell type. Fibroblasts and endothelial cells increase their spreading and the assembly of their cytoskeleton into actin stress fibers and focal adhesions at ~3 kPa, whereas neutrophil spreading is insensitive to substrate stiffness down to 2 Pa 72 and preosteocytes increase spreading and cytoskeletal organization at ~60 kPa 73. Other factors also influence cell spreading in response to stiffness: cell-cell adhesions permit spreading on more compliant substrates 72, as does the incorporation of the glycosaminoglycan hyaluronan within the matrix 74. Inhibiting myosin reverses the effects of stiffness on fibroblasts, such that decreasing matrix stiffness increases rather than decreases cell spreading and proliferation 75. A siRNA screen identified a number of genes within protein kinase pathways that altered the sensing of stiffness by fibroblasts, including components, the depletion of which allowed spreading and elongation on compliant matrix 76. Stiffness sensing thus represents a tunable cellular response, and not a simple mechanical effect.
How force on the ECM influences cells
Cells also respond to mechanical loads imposed on their matrix or adhesive substrate. These loads induce matrix strains, and associated stresses, which promote assembly of the cytoskeleton into actin stress fibers and focal adhesions 77 and drive a variety of signaling cascades 78. One key pathway involves the translocation of Mal-or myocardin family actin-associated transcription factors to the nucleus, which bind to elements in many cytoskeletal and adhesion proteins to induce their expression 79.
Conversely, matrix metalloproteinase genes are induced in dermal fibroblasts when ECM stress is decreased using either matrix unloading or actomyosin inhibitors to reduce tension 80. These in vitro studies also identified tenascin-C as a key tension-dependent gene 60, 81; its transcription increased in response to tension, consistent with its expression in vivo at sites of high tension 82. Tenascin-C is a matrix protein that, in vitro, reduces cellular interactions with other matrix proteins, such as fibronectin, decreases Rho activity, and reduces the contraction of collagen gels by the cells 83. These results might suggest that tenascin-C is a component of the negative feedback loop that promotes mechanical homeostasis under conditions of high stress. Yet, its deletion in mice reduces fibrosis 84–86, though this might be due to tenascin-C modulating the inflammatory responses. Clearly, the role of tenascin-C in stress responses is not fully understood.
Responses of the actin cytoskeleton to loads
The actin cytoskeleton underlies many cellular responses to matrix loading, and its response is highly sensitive to the associated magnitude, direction, and timescale of loading. Gels of F-actin with crosslinking proteins exhibit viscoelasticity (Box 2), initially showing strain stiffening and then passive stress relaxation after longer time periods 87, 88. Consequently, over short times, cells can resist deformation as a strain-stiffening material but also relax via viscoelastic mechanisms 89. Cells also respond to cyclic loading by actively remodeling and reorienting their cytoskeleton, thus, over longer times they may adapt to accommodate matrix deformations and actively relax the stress further toward the original (pre-loading) values 90. For a given cell-type, the extent of cytoskeletal realignment can depend on the frequency and magnitude of the applied load 91, although no realignment occurs on very compliant substrates 92. Responses to stretch also involve Rho GTPases, which are activated by stretch and affect subsequent cytoskeletal responses 93, 94. The predominant model, then, is that the actin cytoskeleton undergoes initial passive rearrangements, which activate signaling pathways that mediate subsequent active responses. These phenomena, however, are at best partially understood. A complete understanding will integrate physical models of cytoskeletal mechanics 95–97 with the signaling pathways and active responses that govern cytoskeletal remodeling.
Additional cellular cues from the ECM
Although our focus is on mechanotransduction and matrix homeostasis, the ECM also provides myriad signals to resident cells that complement mechanical cues. A prime example of this is the effect of functional elastic fibers, consisting of elastin and elastin-associated glycoproteins, on smooth muscle cells within arteries. Experiments with mice that are null or haploinsufficient for elastin showed that competent elastic fibers promote vascular smooth muscle cells to transition from a migratory, synthetic phenotype that exists in development to a mature, quiescent, contractile phenotype 98, 99. Conversely, damage to or degradation of elastic fibers promotes a shift toward the synthetic phenotype, which likely contributes to different arterial pathologies 28, 98.
Integrin signaling is also determined by the organization and composition of the matrix, not just its physical properties. For example, the proliferation of smooth muscle cells is inhibited by collagen that is assembled into fibrils but promoted by non-fibrillar or degraded collagen under conditions where mechanical properties are unchanged 100. These effects occur in part because different integrins, which transduce distinct signals 101, bind preferentially to different forms of collagen 102. The organization of the matrix, which could govern the spatial arrangement of the integrins and hence their downstream signals, could also be important. In vitro studies have shown that the spatial organization of integrin ligands can critically regulate cellular responses 103, 104. Evidence for such effects with matrix in vivo is lacking, but it is an attractive hypothesis.
Limitations of these studies
Though important advances have been made using simplified model systems, it is important to recognize their limitations. Much has been learned about cell mechanics and mechanobiology from plating cells onto coverslips coated with a thin layer of a gel such as acrylamide, for which material stiffness can be systematically varied by changing its cross-linking density or structural stiffness can be varied by changing the gel thickness. However, their material stiffness is not a fully independent variable, as differences in crosslinking density can also alter matrix protein anchoring and substrate porosity 105. Furthermore, the materials utilized for these substrates typically show a linear mechanical response over a wide range of strains, rather than the strongly nonlinear (strain-stiffening, that is stiffness increases with extension) behavior typical of native matrix 45, 106.
The geometry of these substrates can also influence their physical properties. In vivo, matrix proteins are organized into linear fibers with structures spanning many length scales, which is poorly modeled by matrix proteins uniformly deposited onto experimental substrates. Lastly, when a cell pulls on a compliant substrate attached to a rigid surface, the resulting deformations are strongly localized, decaying exponentially with distance from the point of application of the force; the range of the deformations is approximately the thickness of the substrate 68. On the other hand, when a cell contracts on or within a 3D matrix, the induced deformations are relatively long-ranged, and roughly according to the inverse-square of the distance from the cell 107. Furthermore, the organization of the matrix into stiff fibers allows mechanical information to be conveyed farther. Simply stated, tension applied at one end of a fiber can propagate over its entire length, provided that it is not cross-linked to other filaments. Alternatively, long-range propagation can be viewed as a consequence of the nonlinear rheological properties of the matrix 108, 109
Molecular aspects of mechanotransduction
While major questions remain, a good deal has been learned about the molecular mechanisms by which integrin mediated adhesions sense the properties of and the forces transmitted through the ECM.
How tension regulates focal adhesions
Integrin-mediated adhesions often strengthen or stabilize under force 110, 111. Forces acting across matrix-integrin-cytoskeletal linkages are thought to initiate signals by unfolding protein domains and changing binding affinities. The matrix component fibronectin was the first protein for which this was shown; forces expose binding sites in fibronectin that promote its self-assembly into fibrils upon stretching 112. FRET reporters reveal that fibronectin unfolds within fibrils in response to actomyosin-dependent forces 54. Single molecule experiments also showed that the integrin-cytoskeletal linkers talin and filamin undergo domain unfolding upon stretching. Stretching talin enables it to bind vinculin 113, 114, which in turn binds actin and reinforces the link between integrins and actin 115, 116. Applying force to filamin within actin gels enhances its ability to bind to integrin peptides, but reduces its binding to the Rac inhibitor FilGAP 117. This switch may mediate the suppression of Rac activity when cells are stretched 118. Single molecule studies show further that applying 2 pN to 5 pN forces to an isolated filamin A construct consisting of domains 20–21 increases its binding to integrin, glycoprotein Ib, and migfilin peptides 119. Studies in live cells using fluorescence-based molecular force sensors also reveal tension across filamin 120 and the talin-vinculin assembly (115; Kumar and Schwartz, unpublished data); calibrated sensors similarly place the force within the 2 pN to 5 pN range for vinculin and talin. Thus, there is good evidence that the unfolding of protein domains under physiological forces can alter protein interactions or activities and thus chemical signaling that is important in mechanosensing.
The integrin-ligand bond exhibits “catch bond” behavior, converting to a long-lived state in response to applied force 121, 122. Interestingly, this effect is increased at high loading rates and is enhanced by cyclic force application, which is perhaps indicative of a “molecular memory.” The conformational landscape for integrins is highly complex and could directly mediate these effects, but active signaling through downstream components may contribute. For example, myosin-dependent tension can recruit vinculin to focal adhesions via focal adhesion kinase (FAK) and Src-mediated phosphorylation of paxillin, a known binding partner for vinculin 123. Vinculin recruited through this pathway could further stabilize adhesions. Finally, actin filaments stabilize under tension, decreasing both spontaneous depolymerization rates 124 and reducing their sensitivity to severing by cofilin 125. As actin scaffolds are essential for focal adhesion stability, this effect can also influence the lifetime of focal adhesions 126.
Mechanotransduction and the actin cytoskeleton
Total force transmission increases with matrix stiffness. In one view, this is regulated locally by the interaction of the steadily flowing F-actin with dynamic focal adhesions 127. The essential ideas are captured by the concept of a “focal adhesion clutch” (Fig. 4). In this model, actin filaments, driven by some combination of pushing from polymerization at the leading edge of the cell and pulling from the central myosin filaments, are thought to flow backward over the immobile, matrix-bound integrins. Linker proteins are driven backward at intermediate speeds, slowing the actin flow and transmitting force through a sort of “friction” 128–130. Matrix stiffness is believed to affect this system primarily by changing the loading rate of the matrix-integrin-cytoskeleton linkage 131. On compliant substrates, rearward movement of the actin cytoskeleton is buffered by the deformation of the matrix, which slows the loading rate on adhesions; on stiff substrates, the force on a focal adhesion increases faster. Alternatively, recent studies have suggested that the cellular response to stiffness resides within the regulatory mechanisms of the cytoskeleton that control the overall level of contractility 132–134 or the orientation of actin stress fibers 135, 136.
Fig. 4. Force-mediated regulation of integrin adhesions.
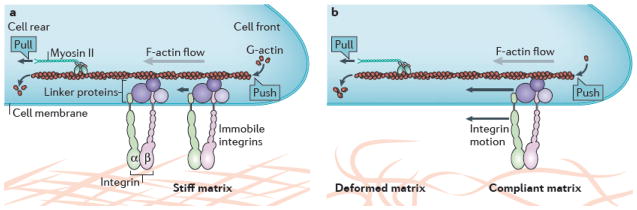
a| Schematic drawing of the “focal adhesion clutch”. The immobile integrins are coupled to the filamentous actin (F-actin) via linker proteins (for example, talin and vinculin) that can move (as indicated by the small arrows) as the F-actin moves rearward under pushing forces from actin polymerization or pulling forces from myosin II activity. A stiff matrix resists this force. b| A compliant matrix deforms under the force of F-actin flow (as indicated by the compressed actin fibers), which reduces the net loading rate on intracellular components and results in an altered cellular response.
Integrin specificity in mechanical homeostasis
Different matrix protein–integrin pairs also show distinct mechanotransduction properties and pathways. Integrin αvβ3 requires protein tyrosine phosphatase alpha (RPTPα) to respond to force, as measured by both bead trapping and differential spreading on compliant versus stiff substrates, whereas β1 integrins are RPTPα-independent 137, 138. RPTPα also co-localizes and co-precipitates with αvβ3, but not β1. Using a slightly different assay, α5β1 was found to mediate most of the total adhesion of cells to fibronectin coated beads, as measured by the resistance of these integrins to detachment under force; yet, the cyclic application of force caused a stiffening of the associated cytoskeleton that required αvβ3 binding and was not seen with beads bound through α5β1 139. Measurements of traction force on substrates of increasing stiffness showed that adhesion strengthening on fibronectin required αvβ3, that is, it was absent when cells bound through α5β1 alone 140. A requirement for αvβ3 in these assays is far from universal, however, as numerous studies report stiffness-dependent cell spreading and traction force on collagen-coated substrates, which only binds β1 integrins 63, 141.
These diverse results can only be explained by a model in which events within focal adhesions trigger signaling pathways that govern cell responses to force. The exact features remain unknown, but we postulate that both total force levels and loading rates alter the dynamics within focal adhesions to influence signaling outputs, which then feedback to control functions such as actin polymerization and force generation, both of which are fundamental to ECM sensing and regulation.
Conclusions/Perspectives
Because of the finite life-spans of individual cells and the finite half-lives of matrix constituents, connective tissues undergo continual turnover while exposed to mechanical loads, whether quasi-static or dynamic. Hence, to maintain overall form and function, resident cells must continually assess the structural integrity of the matrix and maintain, remodel, or repair ECM constituents as appropriate. Importantly, the comparative stability of healthy matrix properties over much of a lifetime implies that organization, and thus stiffness, must be under homeostatic control. That is, mechanisms must exist to detect changes and promote homeostasis. This notion is supported by studies showing substantial reversal of fibrosis in fatty liver disease after weight loss or pharmacological treatment 142, 143. Regression of breast cancer after chemotherapy similarly leads to decreased stiffness of the stromal tissue in some patients (V. Weaver, personal communication). Stiffening is not always readily reversible, however. Arteries steadily stiffen with age; they are currently the target of “de-stiffening” therapies, though results to date have been modest 144. Hypertension exacerbates this process, and pharmacologically reducing blood pressure has not been found to reverse stiffness 145. This tissue-specific effect may be driven by the extreme, repetitive mechanical stresses experienced by arteries and the irreversible loss of elastin, which is essential for vessel wall elasticity and homeostasis.
Surprisingly, the nature of the negative feedback loop(s) required for homeostatic control of stiffness is largely unknown (Fig 2). COX2-dependent arachidonic acid metabolites have been implicated in maintaining compliance in arteries 146 and lung 147. In the arterial study, however, high density lipoprotein controlled COX2 expression, whereas in the lung study, stiffer substrates decreased COX2 expression and prostaglandin release, which facilitated the increase in contractility. Thus, COX2-dependent pathways do not appear to participate in the regulatory circuits that mediate homeostasis.
What has been identified and extensively studied in vitro is a positive feedback loop that would be predicted to lead to fibrosis. Plating cells on or in stiff matrix leads to increased contractility and the formation of actin stress fibers, suppression of collagen-degrading proteases, and increased collagen gene expression 60, 71, 148. Cells also show increased responsiveness to TGF-β, which leads to further collagen synthesis and the suppression of protease activity 13, 14. A major question, then, is why has this fibrotic pathway been so easy to study while so little is known about the negative feedback loop for homeostasis? One likely possibility is that, in vitro culture, which usually uses serum in the medium, mimics wound healing rather than quiescent, native conditions. The selection in tissue culture of cells that grow well on stiff tissue culture plastic in serum-containing medium may further bias cell phenotype.
Several major outstanding questions remain: How do tissues maintain normal matrix organization and tissue stiffness? What pathways mediate the negative feedback loop that prevents progression toward stiffer matrix and higher cell contractility? What governs the switch between these two states, the homeostatic one that maintains health versus the fibrotic one that compromises the function of many tissues? Lastly, can we devise treatments to break the fibrotic cycle and restore the homeostatic state? Understanding the regulatory pathways in detail and the factors that govern switching between these states is likely to be the best route forward.
Acknowledgments
This work was supported, in part, by grants from the US National Institutes of Health (R01 HL105297 to JDH and PO1 GM98412 to MAS), US National Science Foundation (CMMI-116142 to JDH), Sackler Program at Yale University (to ERD and JDH), and the Connecticut Stem Cell Fund grant 12SCA09 (to ERD).
Glossary
- Homeostasis
An active promotion of equilibrium by biological systems. Homeostasis is a process, not a state. It requires both a sensor and an effector mechanism.
- Integrins
Heterodimeric transmembrane protein complexes that are fundamental to mechanically linking the extracellular matrix and cytoskeleton, particularly actin filaments.
- Integrin Linker Proteins
Intracellular proteins such as talin, filamin, α-actinin, PINCH, parvin, vinculin, and paxillin provide vital links between the cytosolic domain of the integrins and the cytoskeleton.
- Actomyosin Machinery
Combination of thin (actin) and thick (myosin) cytoskeletal filaments that enable forceful contractions powered by ATP. Inclusion of smooth muscle α-actin into actomyosin structures based on non-muscle myosin results in “stress fibers” that contract more forcefully.
- Fibropositors
Membrane-associated structures in embryonic cells that aid in the organized deposition of collagen within the extracellular space. They depend on actomyosin activity.
- Catch bond
Most molecular bonds, covalent or non-covalent, increase their off-rates under tension, exhibiting so-called slip bond behavior where the bond weakens under force. There is a small fraction of bonds, however, where off rates decrease under tension (within a certain range), thus, strengthening under force and behaving instead as “catch bonds”.
- Quasi-static
A dynamical process that nevertheless occurs slowly enough that it can be considered as a series of equilibria.
Biographies
Jay Humphrey earned a Ph.D. in engineering science and mechanics from Georgia Tech and completed postdoctoral research in cardiology at Johns Hopkins. His lab currently studies vascular homeostasis and disease progression, with particular interest in the effects of aging, hypertension, and aneurysmal development on wall mechanobiology.
Eric Dufresne earned a Ph.D. in physics from the University of Chicago and did postdoctoral work in soft matter at Harvard. His lab studies a range of synthetic and biological systems. Current biological interests include the mechanics of epithelial cells, active transport in bacteria, and the development of photonic nanostructures.
Martin Schwartz earned a Ph.D. in physical chemistry at Stanford and did postdoctoral research in biology at MIT. His lab currently works on signaling by integrins and mechanotransduction in the vascular system, especially fluid shear stress sensing, vascular remodeling, and atherosclerosis.
Footnotes
Conflicts of Interest: none
References
- 1.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–99. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 3.Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–62. [PubMed] [Google Scholar]
- 4.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–23. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnans C, Chou J, Werb Z. Remodeling the extracellular matrix in development and diseases. Nat Rev Cell Mol Biol. 2014;15 doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.JKM, Ou G, Weaver VM. Deconstructing extracellular matrix assembly: a multi-scale road map. Nat Rev Cell Mol Biol. 2014;15 doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arribas SM, Hinek A, Gonzalez MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006;111:771–91. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- 11.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferruzzi J, Collins MJ, Yeh AT, Humphrey JD. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for Marfan syndrome. Cardiovasc Res. 2011;92:287–95. doi: 10.1093/cvr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191:475–7. doi: 10.1126/science.128820. This work provided the first demonstration that cyclic mechanical loading induced smooth muscle cells to increase their production of extracellular matrix. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 17.Ciobanasu C, Faivre B, Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol. 2013;92:339–48. doi: 10.1016/j.ejcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163–79. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphrey JD. Stress, strain, and mechanotransduction in cells. J Biomech Eng. 2001;123:638–41. doi: 10.1115/1.1406131. [DOI] [PubMed] [Google Scholar]
- 20.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem Biophys. 2008;50:53–78. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]
- 22.Bersi MR, Ferruzzi J, Eberth JF, Gleason RL, Humphrey JD. Consistent Biomechanical Phenotyping of Common Carotid Arteries from Seven Genetic, Pharmacological, and Surgical Mouse Models. Annals of Biomedical Engineering. 2014;42:1207–1223. doi: 10.1007/s10439-014-0988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–13. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 24.Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- 25.Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- 26.Cook JR, et al. Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome. J Clin Invest. 2014;124:1329–39. doi: 10.1172/JCI71059. This paper provided evidence that an organ-level pathology resulted from abnormal mechanosensing related to a genetic mutation in an extracellular matrix protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganesh SK, et al. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-beta expression and connective tissue features. FASEB J. 2014 doi: 10.1096/fj.14-251207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell biology. Dysfunctional mechanosensing in aneurysms. Science. 2014;344:477–9. doi: 10.1126/science.1253026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Gerber EE, et al. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013;503:126–30. doi: 10.1038/nature12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal SK. Integrins and cadherins as therapeutic targets in fibrosis. Front Pharmacol. 2014;5:131. doi: 10.3389/fphar.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. Springer; 1993. Springer. [Google Scholar]
- 33.Delvoye P, Wiliquet P, Leveque JL, Nusgens BV, Lapiere CM. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol. 1991;97:898–902. doi: 10.1111/1523-1747.ep12491651. These results provided an early demonstration in vitro that fibroblasts establish a steady-state endogenous tension when introduced into an initially stress-free collagen gel. [DOI] [PubMed] [Google Scholar]
- 34.Marenzana M, Wilson-Jones N, Mudera V, Brown RA. The origins and regulation of tissue tension: identification of collagen tension-fixation process in vitro. Exp Cell Res. 2006;312:423–33. doi: 10.1016/j.yexcr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legant WR, et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods. 2010;7:969–71. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 38.Tan JL, et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–9. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphrey JD, Rajagopal KR. A constrained mixture model for growth and remodeling of soft tissues. Mathematical Models & Methods in Applied Sciences. 2002;12:407–430. [Google Scholar]
- 41.Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J Vasc Res. 1998;35:93–103. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 42.O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1) Hypertension. 2000;36:319–24. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 43.Prajapati RT, Chavally-Mis B, Herbage D, Eastwood M, Brown RA. Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen. 2000;8:226–37. doi: 10.1046/j.1524-475x.2000.00226.x. This paper showed that fibroblasts embedded in a 3-D collagen matrix apply contractile forces to maintain a constant level of macroscopic tension. [DOI] [PubMed] [Google Scholar]
- 44.Ruberti JW, Hallab NJ. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun. 2005;336:483–9. doi: 10.1016/j.bbrc.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 45.Fung YC. Biomechanics: mechanical properties of living tissues. Springer-Verlag; New York: 1993. [Google Scholar]
- 46.Valentin A, Humphrey JD. Evaluation of fundamental hypotheses underlying constrained mixture models of arterial growth and remodelling. Philos Trans A Math Phys Eng Sci. 2009;367:3585–606. doi: 10.1098/rsta.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cyron C, Wilson JS, Humphrey JD. Mechanobiological stability: a new paradigm to understand the enlargement of aneurysms. J Roy Soc. 2014 doi: 10.1098/rsif.2014.0680. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–81. doi: 10.1074/jbc.M206286200. The results in this paper demonstrate that cells actively contribute, in vivo, to collagen fibrillogenesis via an integrin mediated process. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Van Den Diepstraten C, D’Souza SJ, Chan BM, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol. 2003;163:1045–56. doi: 10.1016/s0002-9440(10)63464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7:157–64. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 52.Dahlmann-Noor AH, Martin-Martin B, Eastwood M, Khaw PT, Bailly M. Dynamic protrusive cell behaviour generates force and drives early matrix contraction by fibroblasts. Exp Cell Res. 2007;313:4158–69. doi: 10.1016/j.yexcr.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huelsz-Prince G, Belkin AM, VanBavel E, Bakker EN. Activation of extracellular transglutaminase 2 by mechanical force in the arterial wall. J Vasc Res. 2013;50:383–95. doi: 10.1159/000354222. [DOI] [PubMed] [Google Scholar]
- 54.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–99. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Canty EG, et al. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–63. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czirok A, et al. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez F, Dietz HC. Fibrillin-rich microfibrils: Structural determinants of morphogenetic and homeostatic events. J Cell Physiol. 2007;213:326–30. doi: 10.1002/jcp.21189. [DOI] [PubMed] [Google Scholar]
- 59.Bax DV, et al. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem. 2003;278:34605–16. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- 60.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–20. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 62.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 63.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–27. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein EA, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–8. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 68.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 69.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–43. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 72.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 73.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci U S A. 2005;102:4300–5. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chopra A, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35:71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mih JD, Marinkovic A, Liu F, Sharif AS, Tschumperlin DJ. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J Cell Sci. 2012;125:5974–83. doi: 10.1242/jcs.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prager-Khoutorsky M, et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol. 2011;13:1457–65. doi: 10.1038/ncb2370. This paper used an siRNA screen to identify genes linked to tyrosine kinase signaling that alter cell rigidity sensing, demonstrating that these responses involve active signaling rather than purely mechanical mechanisms. [DOI] [PubMed] [Google Scholar]
- 77.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–15. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Developmental Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 79.Iyer KV, Pulford S, Mogilner A, Shivashankar GV. Mechanical Activation of Cells Induces Chromatin Remodeling Preceding MKL Nuclear Transport. Biophysical Journal. 2012;103:1416–1428. doi: 10.1016/j.bpj.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lambert CA, Colige AC, Munaut C, Lapiere CM, Nusgens BV. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol. 2001;20:397–408. doi: 10.1016/s0945-053x(01)00156-1. [DOI] [PubMed] [Google Scholar]
- 81.Chiquet-Ehrismann R, et al. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J Cell Biol. 1994;127:2093–101. doi: 10.1083/jcb.127.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackie EJ, Thesleff I, Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol. 1987;105:2569–79. doi: 10.1083/jcb.105.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200:488–99. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- 84.Carey WA, Taylor GD, Dean WB, Bristow JD. Tenascin-C deficiency attenuates TGF-ss-mediated fibrosis following murine lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L785–93. doi: 10.1152/ajplung.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Karef A, et al. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211:86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 86.Nishioka T, et al. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2010;298:H1072–8. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 87.Mizuno D, Tardin C, Schmidt CF, Mackintosh FC. Nonequilibrium mechanics of active cytoskeletal networks. Science. 2007;315:370–3. doi: 10.1126/science.1134404. [DOI] [PubMed] [Google Scholar]
- 88.Wachsstock DH, Schwarz WH, Pollard TD. Cross-linker dynamics determine the mechanical properties of actin gels. Biophys J. 1994;66:801–9. doi: 10.1016/s0006-3495(94)80856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trepat X, et al. Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1025–34. doi: 10.1152/ajplung.00077.2004. [DOI] [PubMed] [Google Scholar]
- 90.Webster KD, Ng WP, Fletcher DA. Tensional homeostasis in single fibroblasts. Biophys J. 2014;107:146–55. doi: 10.1016/j.bpj.2014.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jungbauer S, Gao HJ, Spatz JP, Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophysical Journal. 2008;95:3470–3478. doi: 10.1529/biophysj.107.128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faust U, et al. Cyclic Stress at mHz Frequencies Aligns Fibroblasts in Direction of Zero Strain. Plos One. 2011;6:16. doi: 10.1371/journal.pone.0028963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gawlak G, et al. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J. 2014;28:3249–60. doi: 10.1096/fj.13-245142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verma SK, et al. Rac1 and RhoA differentially regulate angiotensinogen gene expression in stretched cardiac fibroblasts. Cardiovasc Res. 2011;90:88–96. doi: 10.1093/cvr/cvq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De R, Zemel A, Safran SA. Dynamics of cell orientation. Nature Physics. 2007;3:655–659. [Google Scholar]
- 96.Schwarz US, Safran SA. Physics of adherent cells. Reviews of Modern Physics. 2013;85:1327–1381. [Google Scholar]
- 97.Livne A, Bouchbinder E, Geiger B. Cell Orientation Under Cyclic Stretching. Nature Communications. 2014;5 doi: 10.1038/ncomms4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karnik SK, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–23. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 99.Brooke BS, Bayes-Genis A, Li DY. New insights into elastin and vascular disease. Trends Cardiovasc Med. 2003;13:176–81. doi: 10.1016/s1050-1738(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 100.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–78. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 101.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 102.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–31. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 103.Altrock E, Muth CA, Klein G, Spatz JP, Lee-Thedieck C. The significance of integrin ligand nanopatterning on lipid raft clustering in hematopoietic stem cells. Biomaterials. 2012;33:3107–18. doi: 10.1016/j.biomaterials.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Cavalcanti-Adam EA, Aydin D, Hirschfeld-Warneken VC, Spatz JP. Cell adhesion and response to synthetic nanopatterned environments by steering receptor clustering and spatial location. HFSP J. 2008;2:276–85. doi: 10.2976/1.2976662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Materials. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 106.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. This paper identifies the origin and implications of strain-dependent stiffness in biological gels. [DOI] [PubMed] [Google Scholar]
- 107.Schwarz US, Safran SA. Physics of adherent cells. Reviews of Modern Physics. 2013;85:1327–1381. [Google Scholar]
- 108.Sawhney RK, Howard J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. Journal of Cell Biology. 2002;157:1083–1091. doi: 10.1083/jcb.200203069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winer JP, Oake S, Janmey PA. Non-Linear Elasticity of Extracellular Matrices Enables Contractile Cells to Communicate Local Position and Orientation. Plos One. 2009;4 doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 111.Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhong C, et al. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel B, et al. The activity of the vinculin binding sites in talin is influenced by the stability of the helical bundles that make up the talin rod. J Biol Chem. 2006;281:7458–67. doi: 10.1074/jbc.M508058200. [DOI] [PubMed] [Google Scholar]
- 115.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–6. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dumbauld DW, et al. Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions. J Cell Physiol. 2010;223:746–56. doi: 10.1002/jcp.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011 doi: 10.1038/nature10430. This paper identifies tension dependent changes in the interactions of filamin with other proteins that may mediate cytoskeletal strengthening and signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katsumi A, et al. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158:153–64. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rognoni L, Stigler J, Pelz B, Ylanne J, Rief M. Dynamic force sensing of filamin revealed in single-molecule experiments. Proc Natl Acad Sci U S A. 2012;109:19679–84. doi: 10.1073/pnas.1211274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meng F, Suchyna TM, Lazakovitch E, Gronostajski RM, Sachs F. Real Time FRET Based Detection of Mechanical Stress in Cytoskeletal and Extracellular Matrix Proteins. Cell Mol Bioeng. 2011;4:148–159. doi: 10.1007/s12195-010-0140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W, Lou J, Evans EA, Zhu C. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J Cell Biol. 2012;199:497–512. doi: 10.1083/jcb.201201091. Single molecule techniques are used to demonstrate that integrins show force and loading rate-dependent changes in conformation and stability of the integrin-ligand interaction that may underlie mechanotransduction by these receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kong F, et al. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol Cell. 2013;49:1060–8. doi: 10.1016/j.molcel.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–90. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee CY, et al. Actin depolymerization under force is governed by lysine 113:glutamic acid 195-mediated catch-slip bonds. Proc Natl Acad Sci U S A. 2013;110:5022–7. doi: 10.1073/pnas.1218407110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol. 2011;195:721–7. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marshall TW, Aloor HL, Bear JE. Coronin 2A regulates a subset of focal-adhesion-turnover events through the cofilin pathway. J Cell Sci. 2009;122:3061–9. doi: 10.1242/jcs.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gardel ML, et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. The results in this paper described the relationship between traction stress and actin retrograde flow within focal adhesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–5. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- 129.Brown CM, et al. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci. 2006;119:5204–14. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- 130.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–6. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 131.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–91. doi: 10.1126/science.1163595. Theory and experiments in this paper link matrix compliance to loading rate of ECM-integrin-cytoskeletal bonds. [DOI] [PubMed] [Google Scholar]
- 132.Edwards CM, Schwarz US. Force Localization in Contracting Cell Layers. Physical Review Letters. 2011;107:5. doi: 10.1103/PhysRevLett.107.128101. [DOI] [PubMed] [Google Scholar]
- 133.Mertz AF, et al. Scaling of Traction Forces with the Size of Cohesive Cell Colonies. Physical Review Letters. 2012;108:5. doi: 10.1103/PhysRevLett.108.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marcq P, Yoshinaga N, Prost J. Rigidity Sensing Explained by Active Matter Theory. Biophysical Journal. 2011;101:L33–L35. doi: 10.1016/j.bpj.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zemel A, Rehfeldt F, Brown AEX, Discher DE, Safran SA. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nature Physics. 2010;6:468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trichet L, et al. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6933–6938. doi: 10.1073/pnas.1117810109. In this paper, a combination of experiments and theory suggest that rigidity sensing may not be localized to focal adhesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.von Wichert G, et al. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J Cell Biol. 2003;161:143–53. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys J. 2006;90:1804–9. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci U S A. 2009;106:16245–50. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schiller HB, et al. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol. 2013;15:625–36. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 141.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suriawinata A, Fiel MI. Liver pathology in obesity. Semin Liver Dis. 2004;24:363–70. doi: 10.1055/s-2004-860865. [DOI] [PubMed] [Google Scholar]
- 143.Yu J, et al. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol. 2010;42:948–57. doi: 10.1016/j.biocel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 144.Boutouyrie P, Beaussier H, Achouba A, Laurent S, trialists E. Destiffening effect of valsartan and atenolol: influence of heart rate and blood pressure. J Hypertens. 2014;32:108–14. doi: 10.1097/HJH.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 145.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 146.Kothapalli D, et al. Apolipoprotein E-mediated cell cycle arrest linked to p27 and the Cox2-dependent repression of miR221/222. Atherosclerosis. 2013;227:65–71. doi: 10.1016/j.atherosclerosis.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu F, et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–9. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 149.Gineyts E, et al. Racemization and isomerization of type I collagen C-telopeptides in human bone and soft tissues: assessment of tissue turnover. Biochem J. 2000;345(Pt 3):481–5. [PMC free article] [PubMed] [Google Scholar]
- 150.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42:1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]