Abstract
Prior work demonstrated dependence of the change in blood pressure during the Valsalva maneuver (VM) on the extent of thoracic hypovolemia and splanchnic hypervolemia. Thoracic hypovolemia and splanchnic hypervolemia characterize certain patients with postural tachycardia syndrome (POTS) during orthostatic stress. These patients also experience abnormal phase II hypotension and phase IV hypertension during VM. We hypothesize that reduced splanchnic arterial resistance explains aberrant VM results in these patients. We studied 17 POTS patients aged 15–23 yr with normal resting peripheral blood flow by strain gauge plethysmography and 10 comparably aged healthy volunteers. All had normal blood volumes by dye dilution. We assessed changes in estimated thoracic, splanchnic, pelvic-thigh, and lower leg blood volume and blood flow by impedance plethysmography throughout VM performed in the supine position. Baseline splanchnic blood flow was increased and calculated arterial resistance was decreased in POTS compared with control subjects. Splanchnic resistance decreased and flow increased in POTS subjects, whereas splanchnic resistance increased and flow decreased in control subjects during stage II of VM. This was associated with increased splanchnic blood volume, decreased thoracic blood volume, increased heart rate, and decreased blood pressure in POTS. Pelvic and leg resistances were increased above control and remained so during stage IV of VM, accounting for the increased blood pressure overshoot in POTS. Thus splanchnic hyperemia and hypervolemia are related to excessive phase II blood pressure reduction in POTS despite intense peripheral vasoconstriction. Factors other than autonomic dysfunction may play a role in POTS.
Keywords: vasoconstriction, splanchnic arterial resistance, mesenteric artery, autonomic dysfunction, orthostatic intolerance
In previous work we demonstrated (31) the dependence of decrease in blood pressure on degree of thoracic hypovolemia during phase II of the Valsalva maneuver in healthy volunteers. We later showed that splanchnic hypervolemia during the Valsalva maneuver determines the magnitude of decrease in thoracic blood volume during early phase II of the maneuver (31). This, in turn, governs pressure recovery during late phase II (31).
Prior work has shown that postural tachycardia syndrome (POTS), characterized by excessive tachycardia when upright in association with symptoms of orthostatic intolerance, is linked to thoracic hypovolemia (31). In POTS, thoracic hypovolemia may be produced by absolute hypovolemia, as observed in a variant of POTS with low peripheral blood flow (15), or may be produced by an excessive redistribution of blood volume away from the thoracic compartment, as observed in other variants (33). One such subset of POTS patients with redistributive thoracic hypovolemia, we designated “normal-flow POTS.” This variant is characterized by normovolemia, normal cardiac output, and normal peripheral blood flow while resting supine. During orthostasis, however, there is thoracic hypovolemia, splanchnic hypervolemia, and intense peripheral vasoconstriction (34).
The literature indicates that abnormalities of the Valsalva maneuver occur in POTS (17, 24). Sandroni et al. (24), in particular, found an exaggerated early phase II decrease in blood pressure, with proportionate reduction in late phase II blood pressure. They also found a significantly larger phase IV systolic blood pressure.
The findings of Sandroni et al. are intriguing because in prior studies we found (31) that increased splanchnic and thoracic blood volume changes are closely related to decreased early and late phase II blood pressure. Also, enhanced splanchnic hypervolemia and thoracic hypovolemia during orthostatic challenge are key features of normal-flow POTS (33).
Therefore, we investigated the hypothesis that decreased early phase II blood pressure and reduced late phase II pressure recovery in POTS are related to increased thoracic hypovolemia generated by splanchnic hypervolemia and associated splanchnic hyperemia. We further proposed that fluid redistribution during the Valsalva maneuver in POTS augments reflex- mediated peripheral vasoconstriction. Although inadequate to correct blood pressure during phase II, peripheral vasoconstriction results in the excessive phase IV hypertension observed in these patients. Our results indeed demonstrate that during the Valsalva maneuver splanchnic resistance is greatly decreased in POTS patients relative to control subjects, with associated increase in splanchnic blood flow, whereas peripheral resistance is increased and blood flow is decreased compared with control subjects. Persistently elevated peripheral resistance during phase IV accounts for the exaggerated hypertensive response in POTS.
MATERIALS AND METHODS
Subjects and Experimental Outline
To test these hypotheses we studied 17 normal-flow POTS patients and 10 healthy volunteer control subjects. POTS patients were referred for symptoms of orthostatic intolerance lasting for longer than 6 mo. Orthostatic intolerance was defined by the presence of light-headedness, headache, fatigue, neurocognitive deficits, palpitations, nausea, blurred vision, and shortness of breath or heat while upright with no other medical explanation for the symptoms. In all patients, POTS was confirmed with a screening upright tilt table test at 70°. POTS was diagnosed by symptoms of orthostatic intolerance during the screening tilt test associated with an increase in sinus heart rate of >30 beats/min or to a rate of >120 beats/min during the first 10 min of tilt, as defined in the adult literature (18, 25). We used occlusion cuffs placed around the lower limb 10 cm above a mercury in Silastic strain gauge (Hokanson) to measure supine calf blood flow by strain gauge plethysmography (SGP). Measurements were made in the supine position at the beginning of experiments after a 30-min resting period. Blood flow was estimated in the supine position by standard venous occlusion methods (8), using rapid cuff inflation to a pressure below diastolic pressure to prevent venous egress. Arterial inflow in units of milliliters per 100 milliliters of tissue per minute was estimated as the rate of change of the rapid increase in limb cross-sectional area. We subdivided the POTS patients after the tilt test on the basis of calf blood flow. For normative purposes we had previously collected calf blood flow data from more than 50 healthy volunteer subjects spanning prior research protocols. For purposes of this study, “normal” calf blood flow was defined as >1.2 ml·min−1 ·100 ml tissue−1, which is the smallest calf blood flow that we have measured in control subjects, and <3.6 ml·min−1 ·100 ml tissue−1, which is the largest calf blood flow we have measured in control subjects. We defined normal-flow POTS patients as POTS patients falling between these limits. Seventeen normal-flow POTS patients aged 15–23 yr were identified in this manner (median = 17.8 yr; 4 male, 13 female). No POTS patients were taking any medication at the time of testing.
There were 10 healthy volunteers aged 15–23 yr (median = 18.5 yr; 3 male, 7 female). All control subjects were free from systemic illnesses. They were taking no medications. All subjects had normal ECGs and echocardiograms and had no other evidence for cardiovascular illness. We excluded subjects with a history of syncope or orthostatic intolerance. There were no trained competitive athletes or bedridden subjects. Informed consent was obtained from subjects or from parents and subjects in those less than 18 yr old. All protocols were approved by the Committee for the Protection of Human Subjects (Institutional Review Board) of New York Medical College.
We assessed changes in blood pressure and heart rate and estimated changes in thoracic, splanchnic, pelvic, and calf segmental blood volumes, segmental blood flows, and segmental arterial resistances (defined below) by impedance plethysmography throughout the Valsalva maneuver, which was performed in the supine position. The Valsalva response has been shown to be strongly posture dependent (5, 29) as well as blood volume dependent (5). We therefore chose to perform the maneuver in the supine position in normovolemic subjects only to separate autonomic stimuli arising from orthostasis or absolute hypovolemia from stimuli due to the Valsalva maneuver. All subjects had blood volume assessed by indocyanine green (ICG) dye dilution methods (see below).
Protocol
Tests began in a temperature-controlled room after an overnight fast. An intravenous catheter was placed in the right antecubital fossa. After a 30-min acclimatization period, tests were performed in the following order, allowing at least 15 min for recovery in between: supine cardiac output and blood volume by the ICG dye dilution technique, calf blood flow and arterial resistance measurement by SPG, and supine quantitative Valsalva maneuver with impedance plethysmography (IPG) measurements. We validated impedance measurements of thoracic blood flow against dye dilution cardiac outputs; we validated impedance measurements of calf blood flow against SPG; and we validated impedance measurements of splanchnic blood flow against the exponential decay coefficient of the concentration of ICG, which approximates portal blood flow divided by blood volume within a constant representing hepatic dye extraction (see below).
Details of Method
Peripheral blood flow and peripheral arterial resistance
We used venous occlusion SGP in all subjects to measure calf blood flow. Supine measurements in units of milliliters per 100 milliliters of tissue per minute were made at the beginning of experiments, and measurements were compared with impedance estimates of blood flow. Measurements were made in a standard manner with excluded ankle circulation. We have used these techniques previously (30, 34).
Heart rate, respiration, and blood pressure monitoring
Electrocardiogram strips were monitored continuously. Relative respiratory volume was measured with a respiratory inductance plethysmograph placed around the maximum thoracic circumference and attached to a Respitrace monitor (NIMS Scientific). Respitrace signals were only used as an aid to help to delimit the Valsalva maneuver. Upperextremity blood pressure was continuously monitored with a finger arterial plethysmograph (Finometer, FMS, Amsterdam, The Netherlands) placed on the right index or right middle finger calibrated against an oscillometric blood pressure cuff and recalibrated automatically. ECG, respiratory, and Finometer pressure data were interfaced to a personal computer through an analog-to-digital (A/D) converter (DATAQ, Milwaukee, WI). All data were multiplexed with strain gauge and impedance data and were effectively synchronized.
Dye dilution measurement of blood volume
We used the ICG dye dilution technique to measure blood volume and cardiac output (1) and to estimate splanchnic blood flow in terms of portal uptake of the dye (26). We used a spectrophotometric finger photosensor (DDG, Nihon-Kohden) validated by prior clinical studies (11, 13). The dye decay curve is a monoexponential V0exp − (Kt), where V0 is extrapolated dye concentration at time 0, K represents clearance by the liver divided by blood volume and t is time: clearance = (1 − hematocrit) · Q·E (26), where Q is portal blood flow and E is the hepatic dye extraction ratio.
We measured the hematocrit and extrapolated the dye decay curve to the time of dye injection (t = 0), yielding estimated blood volume. A log-linear curve fit to the exponential decay yields the parameter K, which was used to estimate portal blood flow and thus splanchnic blood flow within a constant.
IPG to measure changes in segmental blood volumes and blood flows
IPG has been used to detect internal volume shifts (6), including those produced during orthostatic stress (2, 3). We have used this technique to provide estimates of blood volume shifts during the Valsalva maneuver (31, 32). We used a Tetrapolar High-Resolution Impedance Monitor four-channel digital impedance plethysmograph (UFI) to measure volume shifts in four anatomic segments designated the thoracic segment, the splanchnic segment, the pelvic segment incorporating lower pelvis to the knee, and the leg segment (2, 21, 32, 39). Ag/AgCl ECG electrodes were attached to the left foot and left hand, which served as current injectors. Other electrodes were placed in pairs representing anatomic segments as follows: ankle to upper calf just below the knee (leg segment), knee to iliac crest (pelvic segment), iliac crest to midline xyphoid process (splanchnic segment), and midline xyphoid process to supraclavicular area (thoracic segment). The IPG introduces a high-frequency (50 kHz), low-amperage (0.1 mA root mean squared) constant-current signal between the foot and hand electrodes that is insensible to the subjects. Electrical resistance values were measured by using the segmental pairs as sampling electrodes. Anatomic features were selected as the most appropriate locations for comparing changes within and across patients. This combination of electrodes gives highly reproducible changes in computed segmental blood flows and volume shifts and has been tested in a wide range of experiments by our group (21, 39). The distance between the sampling electrodes (L) was measured carefully with a tape measure. We also measured the relevant circumferences of calf, thigh, hips, waist, and chest to obtain approximate volume contents of each anatomic segment. We estimated the change in blood volume in each segment during the Valsalva maneuver from the formula Δsegmental blood volume (ml) = ρ · (L2/R0R1) · ΔR (6), where ρ is electrical conductivity of blood estimated as 53.2*exp(Hct*0.022) (where Hct is hematocrit, or packed cell volume, which we measured) given by Geddes and Kidder (7), R0 is the baseline resistance of a specific segment, R1 is the resistance during the Valsalva maneuver, and ΔR is the change in resistance (R1 − R0) in a specific segment during the maneuver; ρ was regarded as constant during the maneuver.
IPG was also used to measure segmental blood flows (21). Changes in fluid compartment volumes and transient blood flows have been quantitated during orthostasis (2, 21). Impedance cardiography has been used to assess changes in cardiac output during the Valsalva maneuver (22). Pulsatile resistance changes were used to compute the time derivative ∂R/∂t, which we used to obtain the total (ml/min) and relative (ml·100 ml body tissue−1 ·min−1) blood flow responses of each body segment during test conditions.
Blood flow was estimated for an entire anatomic segment from the formula (Fig. 1) (6), where HR is heart rate, ρ is the density of blood, L is the distance between the centers of the electrodes, T is the ejection period, R is the pulsatile resistance, and R0 is the baseline resistance. Respiratory artifact was removed from the signal by a custom Fourier-based frequency selection technique followed by a six-pole digital Butterworth filter.
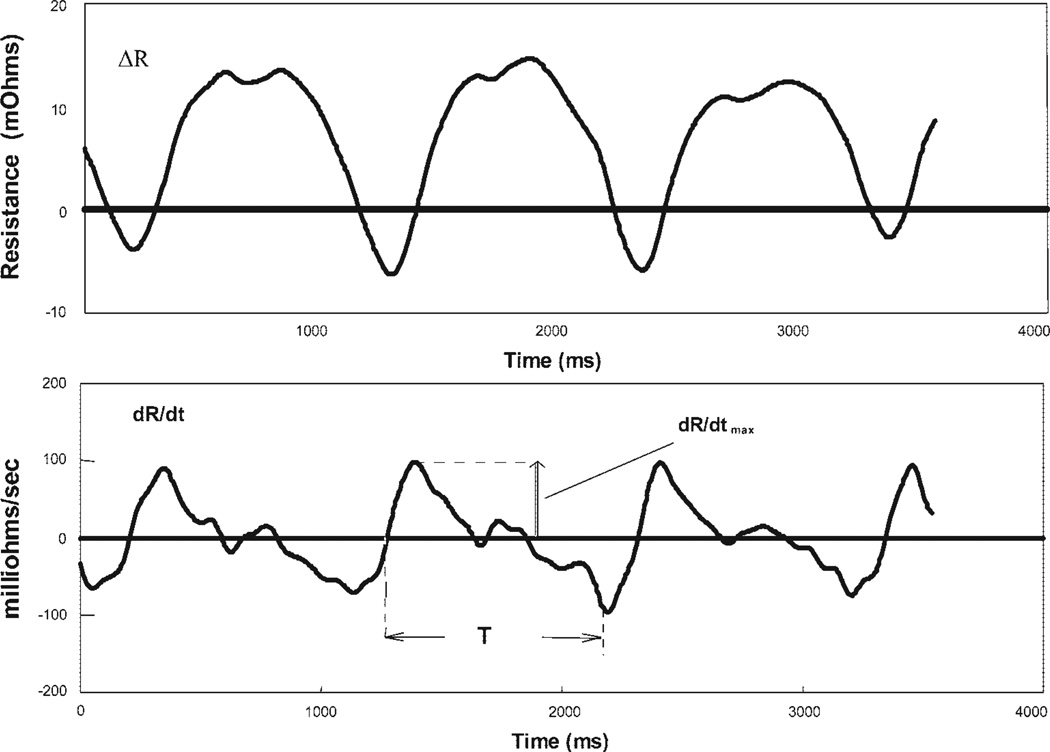
Fig. 1.
Thoracic impedance plethysmography traces obtained after filtering with a 6-pole Butterworth filter. Top: change in resistance (ΔR) as a function of time (t). Bottom: ∂R/∂t vs. time. Ejection time T and maximum change in resistance with time (∂R/∂tmax) are indicated. Blood flow is calculated as , where HR is heart rate, ρ is the electrical conductivity of blood, L is the segment length, T is ejection time, and R0 is baseline electrical resistance.
IPG flows are expressed in units of milliliters per minute for each defined anatomic segment. Normalization to tissue volume was performed by dividing by estimated segmental volume. Data were interfaced to a personal computer through the A/D converter and were multiplexed with strain gauge and impedance data.
Quantitative Valsalva maneuver
The quantitative Valsalva maneuver was performed with the subject supine by exhaling with an open glottis into a mouthpiece connected to the mercury column of a sphygmomanometer with a small air leak. The air leak enabled the glottis to remain open during the slow exhalation. Thus a pressure of 40 mmHg (at the mouthpiece and presumably at the pleural level) was maintained for at least 15 s (or until 1 patient fainted). When pressure was released, care was taken to prevent deep breathing. Two attempts with 10 min of intervening quiet breathing were made to obtain an adequate Valsalva maneuver with sustained intraoral pressure. The first adequate exhalation was used for data acquisition. Blood pressure, electrocardiogram, heart rate, and thoracic impedance were recorded continuously throughout the maneuver. The classic Valsalva maneuver blood pressure response has four phases (10). Phase 0 refers to the period preceding exhalation. A brief increase in blood pressure immediately follows the onset of exhalation and is denoted phase I. This is followed by a decrease in blood pressure and an increase in heart rate during early phase II (II-E). Late phase II (II-L) starts at the lowest systolic blood pressure and is usually marked by recovery of blood pressure with persistent or enhanced tachycardia. Once exhalation is complete, the release of strain restores normal negative intrathoracic pressure, leading to a blood pressure decrease in phase III. This is followed by the pressure overshoot of phase IV with associated reflex bradycardia.
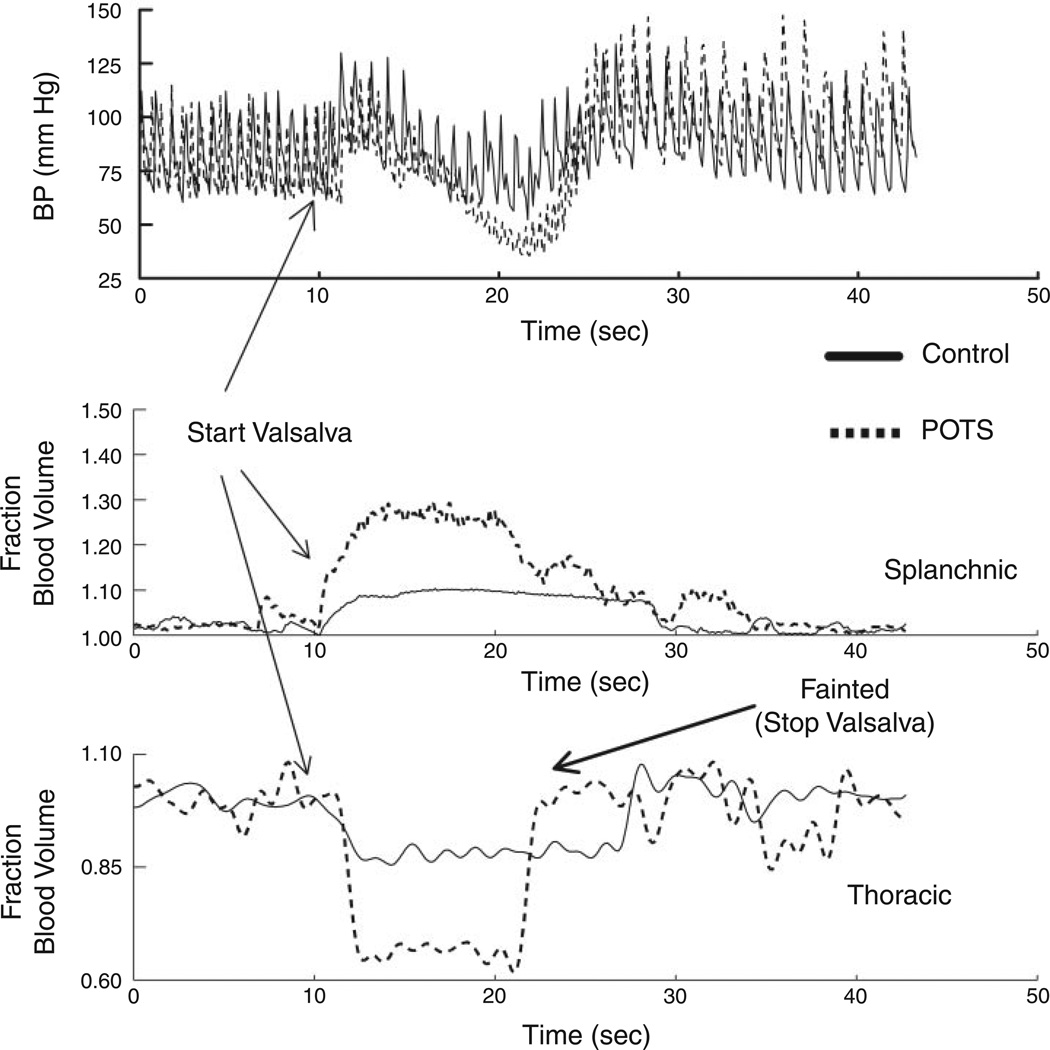
Two representative Valsalva maneuvers, one from a POTS patient and the other from a healthy volunteer, appear in Fig. 2. These depict blood pressure and blood flow changes.
Fig. 2.
Representative Valsalva maneuvers, one from a postural tachycardia syndrome (POTS) patient (dashed lines) and the other from a healthy volunteer control subject (solid lines). Top: arterial blood pressure (BP). Middle: fractional change in splanchnic blood volume. Bottom: fractional change in thoracic blood volume. There is a reciprocal relation between thoracic and splanchnic blood volume changes. A greater fall in arterial pressure is associated with greater changes in segmental volumes in POTS. The decrease in blood pressure in the POTS patient was sufficient to produce loss of consciousness, which terminated the maneuver.
The blood pressure response was quantified during straining and during the pressure overshoot. We recorded the maximum decline in blood pressure to indicate the end of phase II-E. Typically this occurred at ~7 s into exhalation. Blood pressure thereafter increased while exhalation was maintained. We recorded the point of maximum systolic blood pressure preceding release to indicate phase II-L. Early blood pressure changes are generally regarded as independent of the sympathetic nervous system, which requires at least some seconds to exert any effect (23, 38).
Calculation of arterial resistance
Total baseline peripheral resistance was calculated as the mean arterial pressure (MAP) divided by the cardiac index obtained from ICG dye dilution measurements. Impedance estimates of blood flow were used to compute segmental arterial resistance during phase 0, phase II-E, phase II-L, and phase IV of the Valsalva maneuver.
Expiratory pressure was assumed to be equal to atmospheric pressure during phases 0 and IV. Therefore, we calculated resistance = MAP/segmental blood flow.
Expiratory pressure was assumed to equal 40 mmHg during phases II-E and II-L, with an assumed similar increase in right atrial pressure. Therefore, we used the formula resistance = (MAP − 40)/segmental blood flow.
Statistics
All tabular and graphic results are reported as means ± SE. Cardiac index, blood volume, calf blood flow by SPG, resting heart rate, and resting mean and systolic blood pressures were compared by multiple corrected unpaired t-test between control and POTS subjects. Segmental blood flows during phase II and segmental volume changes during the Valsalva maneuver were compared by ANOVA, and then individual comparisons were made with unpaired t-tests if significance was found. Changes in fractional segmental blood flows and arterial resistances were performed for each segment by two-way ANOVA.
RESULTS
All subjects, with one exception, were able to perform the supine quantitative Valsalva maneuver, maintaining the expiratory pressure at 40 mmHg for at least 15 s. The single exception was a young man with POTS whose phase II hypotension was so severe that he lost consciousness. His Valsalva maneuver is shown in Fig. 2. General results are shown in Table 1 and in Figs. 2–6.
Table 1.
Patient dimensions and hemodynamic data
| Control | POTS | |
|---|---|---|
| Weight, kg | 72±5 | 69±4 |
| Height, cm | 167±4 | 167±3 |
| Body surface area, m2 | 1.76±0.09 | 1.70±0.05 |
| Normalized blood volume, ml/kg | 65±5 | 68±6 |
| Cardiac index (by ICG), l· min−1·m−2 | 3.5±0.5 | 4.3±0.6 |
| Total peripheral resistance, mmHg·l−1·min·m2 | 31±4 | 23±5 |
| Venous occlusion calf blood flow, ml·100 ml−1·min−1 | 2.3±0.4 | 2.7±0.3 |
| HR phase 0, beats/min | 63±5 | 81±3* |
| Systolic BP phase 0, mmHg | 118±5 | 120±3 |
| MAP phase 0, mmHg | 82±4 | 89±4 |
| Impedance blood flows phase 0, ml/min | ||
| Thoracic | 5,805±824 | 4,975±720 |
| Splanchnic | 1,245±220 | 2,247±366* |
| Pelvic | 662±171 | 618±127 |
| Leg | 112±18 | 93±14 |
| % Change in blood volume during Valsalva maneuver | ||
| Thoracic | −9±1 | −29±8* |
| Splanchnic | 6±1 | 20±8* |
| Pelvic | 2±1 | 1±1 |
| Leg | 1±1 | 1±1 |
Values are means ± SE. POTS, postural tachycardia syndrome; ICG, indocyanine green; HR, heart rate; BP, blood pressure; MAP, mean arterial pressure.
P < 0.05 difference compared with control subjects.
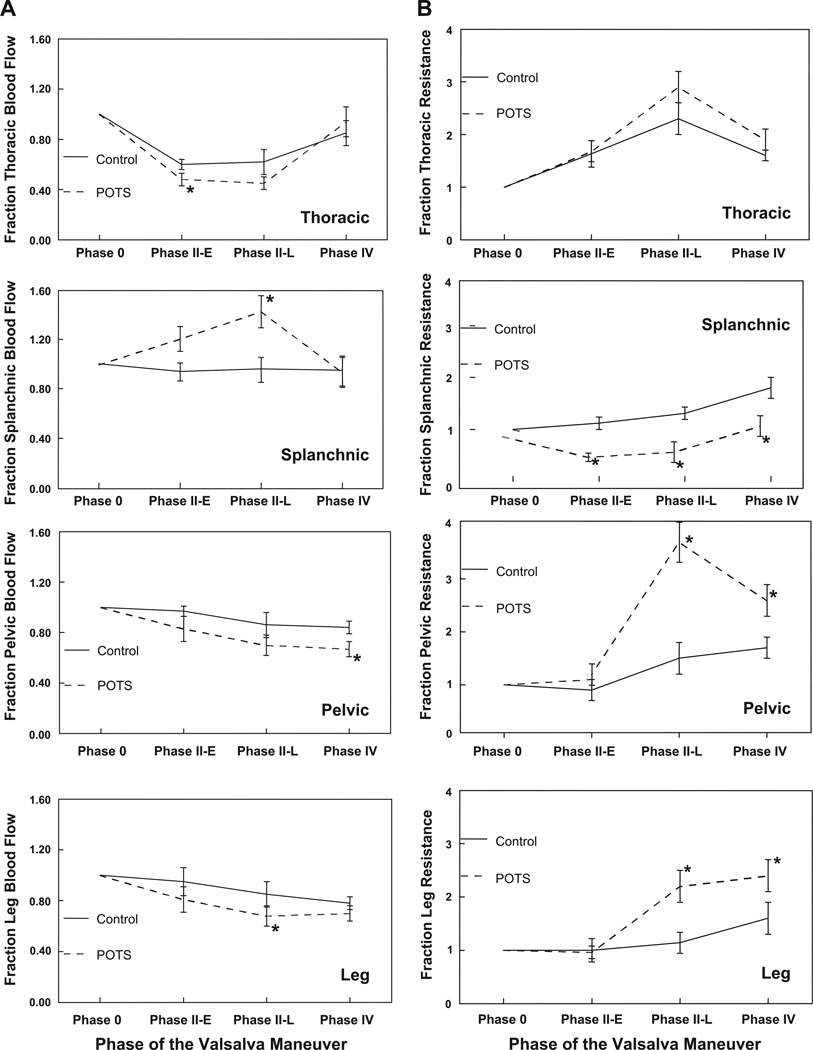
Fig. 6.
A: fractional changes in segmental blood flow. Fractional splanchnic blood flow was increased during phase II of the Valsalva maneuver for POTS patients compared with control subjects, whereas pelvic blood flow was decreased in phase IV and leg blood flow decreased in phase II-E. Thoracic blood flow was reduced in POTS during stage II of the maneuver. B: fractional changes in segmental arterial resistance. Splanchnic resistance was decreased and pelvic and leg resistances were increased in POTS compared with control subjects. *P < 0.05 compared with control.
Comparative Central and Peripheral Blood Flows
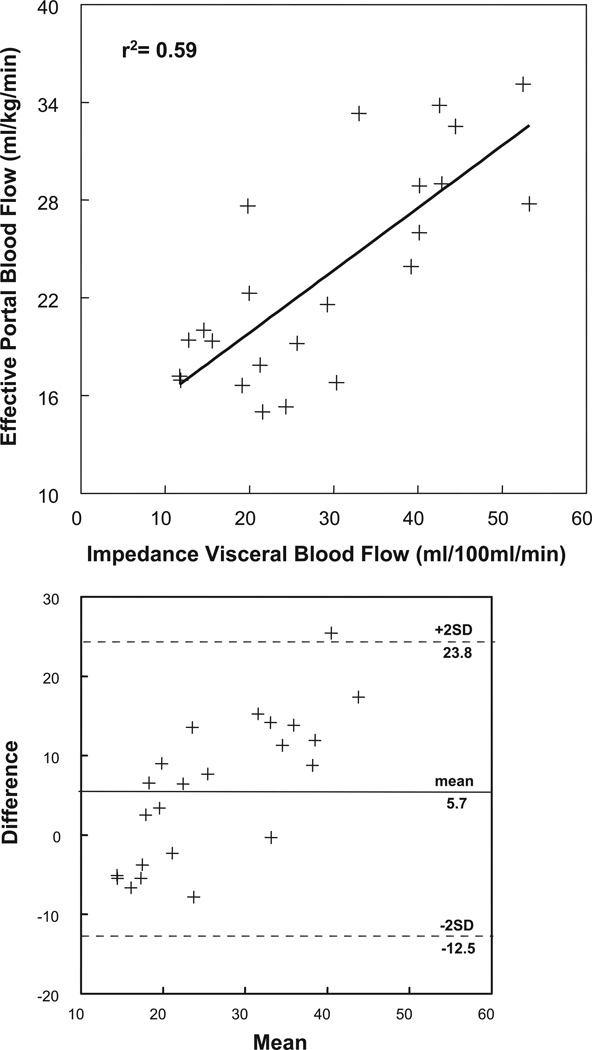
Supine resting calf blood flow measured by SPG was 2.3 ± 0.4 ml·100 ml tissue−1 ·min−1 for control subjects and was not different at 2.7 ± 0.3 ml±100 ml tissue−1 ±min−1 for POTS patients. This compared with impedance estimates for resting calf blood flow of 2.8 ± 0.8 ml±100 ml tissue−1 ±min−1 (r = 0.78) for control subjects and 3.1 ± 0.8 ml±100 ml tissue−1 ±min−1 (r = 0.68) for POTS patients. Similarly, cardiac index measured by ICG technique was 3.5 ± 0.5 l ±min−1 ±m−2 for control subjects and was not different at 4.3 ± 0.6 l ±min−1 ±m−2 for POTS patients. This compares favorably to impedance estimates for cardiac index of 3.2 ± 0.6 l ±min−1 ±m−2 (r = 0.72) for control subjects and 3.9 ± 0.7 l ±min−1 ±m−2 (r = 0.69) for POTS patients. Splanchnic blood flow estimated by impedance plethysmography compared with splanchnic blood estimated by ICG dye dilution exponential decay is shown in Fig. 3. Data show a correlation coefficient of 0.7 and were obtained from all subjects while supine and in a resting state. Figure 3, bottom, compares the two methods with a Bland-Altman plot. There are both fixed and proportional biases but no nonuniformities of error.
Fig. 3.
Top: splanchnic blood flow estimated by impedance plethysmography (x-axis) compared with splanchnic blood flow estimated by indocyanine green (ICG) dye dilution technique (y-axis). The graph was prepared with data from all subjects. The flow methods correlated with Pearson coefficient = 0.70. Bottom: comparison of the two methods with a Bland-Altman plot. There are both fixed and proportional biases but no nonuniformities of error.
Resting Hemodynamics and Size Measurements
As shown in Table 1, weight, height, and body surface area were similar for both groups. Blood pressure, blood volume, cardiac index, calf blood flow, and total peripheral resistance were not different between control and POTS subjects. Resting heart rate was significantly increased above control in the POTS group (P < 0.025).
Also as shown in Table 1, IPG measurements showed that resting supine splanchnic blood flow was significantly elevated in POTS patients compared with control subjects (P < 0.05). There were no significant differences in resting, phase 0 thoracic, pelvic, or calf IPG blood flows.
Blood Pressure, Heart Rate, and Segmental Blood Volume Changes During Valsalva Maneuver
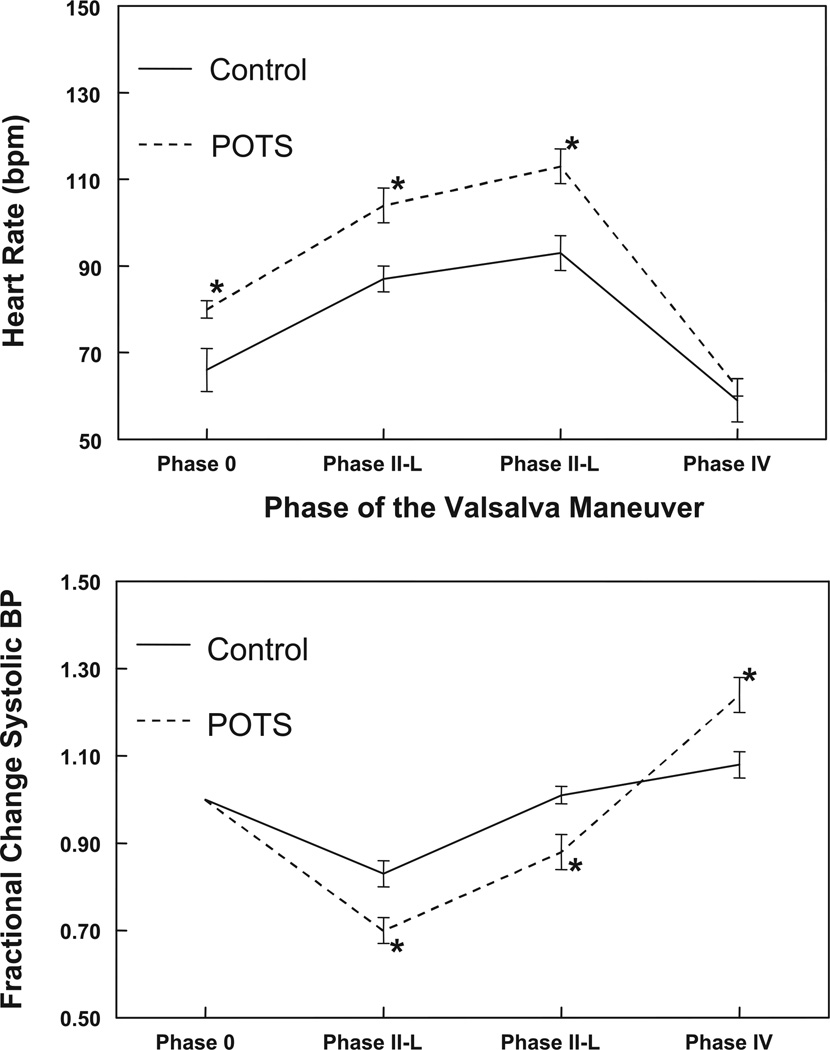
Figure 4 shows the changes in blood pressure and heart rate during the Valsalva maneuver. Heart rate was elevated compared with control in phases 0, II-E, and II-L (P < 0.05). Systolic blood pressure, expressed in fractions of the resting systolic blood pressure, was decreased in POTS patients compared with control subjects in phases II-E and II-L (P < 0.03) and increased compared with control subjects in phase IV (P < 0.01).
Fig. 4.
Changes in heart rate (top) and blood pressure (bottom) during the Valsalva maneuver. The heart rate was elevated compared with control in phase 0 and early (II-E) and late (II-L) phase II. Systolic blood pressure, expressed in fractions of the resting systolic blood pressure, was decreased in POTS patients compared with control subjects in phases II-E and II-L and increased compared with control subjects in phase IV. *P < 0.05 compared with control.
Figure 2 shows changes in blood pressure and associated changes in thoracic and splanchnic segmental blood volumes during the Valsalva maneuver in representative subjects. Volume change is expressed as a fractional change normalized to baseline. Directionally opposite changes in blood volumes occur with a decrease in thoracic segmental volume and a reciprocal increase in splanchnic segmental blood volume. Thoracic volume decreases whereas splanchnic volume increases more in POTS patients than in control subjects. In the POTS patient depicted in Fig. 2, blood pressure decreased so severely that he lost consciousness; the expiratory maneuver was terminated, and he rapidly regained consciousness as blood pressure increased. Table 1 shows that the average fractional decrease in thoracic blood volume was approximately three times greater in POTS patients compared with healthy control subjects (P < 0.025). Conversely, the average fractional increase in splanchnic blood volume was approximately three times as great as the increase in splanchnic volume in control subjects (P < 0.025).
Impedance Blood Flow and Arterial Resistance During Valsalva Maneuver
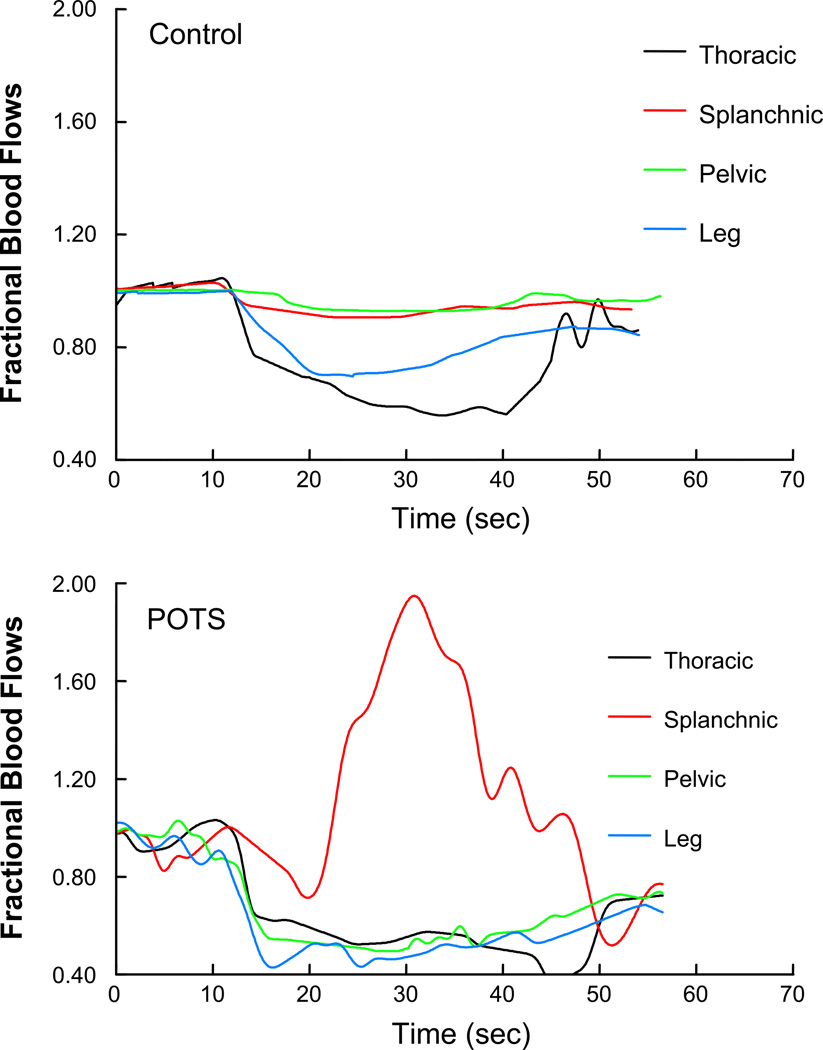
Representative segmental blood flow tracings are shown in Fig. 5. These are expressed as fractional changes in segmental blood flow to facilitate paired comparison. Splanchnic blood flow was markedly increased in the POTS subject compared with control, whereas thoracic, pelvic, and leg flows were decreased in POTS during the Valsalva maneuver.
Fig. 5.
Representative fractional changes in segmental blood flow in control (top) and POTS (bottom) subjects. Splanchnic blood flow was markedly increased in POTS compared with control, whereas thoracic, pelvic, and leg flows were decreased in POTS.
Averaged segmental blood flows and segmental arterial resistances are shown in Fig. 6. Thoracic blood flow decreased and arterial resistance increased in both POTS and control subjects during phase II. Blood flow was nearly restored to baseline in phase IV. Although thoracic blood flow tended to be lower in POTS patients and thoracic arterial resistance tended to be higher compared with control subjects, significant differences were only noted in phase II-E (P = 0.036).
Fractional splanchnic blood flow decreased and fractional splanchnic arterial resistance increased during the maneuver in control subjects. Fractional splanchnic blood flow increased significantly in POTS patients (P < 0.025) and splanchnic arterial resistance decreased (P < 0.01). Because absolute splanchnic blood flow was also significantly greater in POTS patients during phase 0, the absolute splanchnic resistance was even further decreased and the absolute splanchnic blood flow even further increased in POTS patients compared with control subjects in all phases of the Valsalva maneuver.
Pelvic and leg blood flows were further decreased and leg arterial resistances were further increased in POTS patients compared with control subjects (P < 0.05). Peripheral pelvic and leg resistances remained elevated in phase IV in both control and POTS subjects but were significantly higher in the POTS group (P < 0.025).
DISCUSSION
Our most significant findings are those of increased splanchnic blood flow, increased splanchnic hypervolemia (i.e., splanchnic pooling), and related enhanced thoracic hypovolemia compared with control subjects in our normal-flow POTS patients during the Valsalva maneuver. On the one hand, splanchnic vascular resistance appears to be markedly decreased before and during the Valsalva maneuver in POTS patients and may account in part for the increase in splanchnic venous pooling compared with control subjects. On the other hand, as illustrated in Fig. 4, pooling occurs rapidly and early in the POTS patients, which, although consistent with decreased splanchnic vascular resistance, could also indicate increased splanchnic venous tonic capacitance. Separation of these effects is difficult in vivo, requiring either splanchnic flow or pressure control during the maneuver (7), and was not feasible here. Also, splanchnic venous and arterial tone typically covary (23, 27). Thus it may be reasonable to speculate that similar reductions in splanchnic arterial and venous constriction occur in POTS.
Our data also indicate that there is rather intense pelvic and leg vasoconstriction, which suggests intact reflex-mediated peripheral vasoconstriction in POTS. This is apparently sufficient to produce the augmented increments in systolic blood pressure of stage IV.
Similar although less intense vasoconstriction is observed in control subjects. Vasoconstriction persists into phase IV of the Valsalva maneuver and helps to explain hypertension at this time.
Intact pelvic and leg vasoconstriction suggests intact baroreflex-mediated vasoconstriction that is appropriately evoked in response to the augmented fall in blood pressure during phase II in POTS. If peripheral sympathetically mediated vasoconstriction is intact, then there is either selective splanchnic denervation or intact autonomic splanchnic activity confounded by local vasoregulatory factors. Blunting or elimination of splanchnic sympathetic vasoconstriction without prior anatomic denervation or trauma appears unlikely. On the other hand, there is ample precedent for vasodilation produced by local factors. Thus, for example, locally mediated vasodilation occurs normally after a meal (20, 28). Similar locally mediated vasodilation may be a feature of POTS even in the absence of feeding. Biochemicals such as vasoactive intestinal polypeptide, substance P, CGRP, and NO are capable of producing vasodilation despite an intact autonomic nervous system and are known to antagonize sympathetic vasoconstrictive effects (9, 14, 16, 35). Further speculation concerning these matters remains outside of the scope of the present investigation. However, because POTS is so frequently related to prior infectious disease, it is tempting to speculate concerning involvement of persistent inflammatory activation in the vasodilatory response.
Data concerning increased splanchnic blood flow under supine resting conditions were shown previously in POTS patients by Tani et al. (37). The data of Tani et al. also showed a decrease in superior mesenteric artery blood flow and an increase in peripheral resistance on upright tilt. The difference between these results and ours may in part be related to the different perturbations used (tilt vs. Valsalva maneuver) or may be related to our stratification of POTS patients and inclusion only of normovolemic, normal-flow POTS patients. In addition our findings account for phase II pressure observations of POTS patients during the Valsalva maneuver and are consistent with the literature (24). They are consistent with our previous findings (32) of fluid redistribution from the thoracic to the splanchnic compartment. In addition, the decrease in thoracic filling, which varies from group to group, depends on blood volume, on the time-dependent changes of venous resistance and venous pressure in regional circulations, and on right atrial pressure. Intrapleural pressure is very similar to intraoral pressure (4). Right atrial pressure appears to change in direct proportion with increasing intrapleural pressure. The increase in atrial pressure may not be equal to the increase in pleural pressure. However, data indicate that the intra-atrial pressure increase is at least 70% of the intrapleural pressure increase (19). This does not qualitatively change our resistance measurements during phase II of the Valsalva maneuver and reaffirms that in normovolemic subjects thoracic filling depends strongly on venous properties. From Fig. 2 we note that changes in thoracic blood volume and changes in splanchnic blood volume are nearly mirrored in the x-axis for both control and POTS subjects. This applies to phase II-E, in which we propose splanchnic filling occurs at the expense of thoracic emptying, but also in phase II-L, in which we propose splanchnic emptying via sympathetic-mediated vasoconstriction promotes thoracic filling and blood pressure restoration in control subjects and failure of splanchnic constriction prevents such restoration in POTS patients.
Limitations
A direct measure of sympathetic activity such as muscle sympathetic nerve activity could enhance our ability to state that abnormalities in POTS occur in the presence of intact sympathetic vasoconstriction. Such instrumentation is difficult in young subjects and was therefore not pursued. In addition, measurements of intact peripheral sympathetic activity could not be used to imply intact splanchnic innervation. Directional changes in blood flow and calculated peripheral resistance suggest intact sympathetic-mediated peripheral vasoconstriction in all subjects.
The majority of the subjects were premenopausal females. Previous studies demonstrated that the hormonal fluctuations that occur during the normal menstrual cycle may alter autonomic regulation of arterial pressure during various environmental stimuli (36), although there is no apparent effect on orthostatic tolerance (12). We did not control for menstrual cycle except that female subjects were not actively menstruating during testing.
ICG estimations of portal blood flow were hampered by the lack of computation of the hepatic dye extraction ratio. As explained in materials and methods, there is an implicit assumption of equal extraction in all patients with an extraction ratio of 1.0. This may offer one reason that the Bland-Altman fixed bias is positive. We have not demonstrated whether or not the extraction ratio is different in POTS patients.
Issues remain concerning the accuracy, reliability, and validity of indirect measurement of splanchnic blood flow by impedance plethysmography. Neither the reference standard ICG method nor impedance methods as implemented in current experiments are capable of giving true absolute flow data, the green dye method (as used here) because we calculate dye clearance rather than portal or hepatic blood flow (as stated above we are missing the extraction ratio, which requires invasive catheterization) and the impedance method because it is suited for detecting relative or fractional changes in blood volume. However, invasive measures to obtain hepatic extraction ratios in each subject are beyond the scope of the present studies. Moreover, the transient measurements required during the Valsalva maneuver cannot be tested by conventional dye techniques because they rely on near steady-state measurements. This is the reason for using impedance methods in the first place. Nor has ultrasound proved useful, because the time required to retarget the ultrasound beam has prevented imaging and Doppler recording during periods of interest for the Valsalva maneuver.
Therefore, by no means do we want to equate impedance plethysmography and ICG dye dilution techniques or to imply that there is comparable accuracy between the methods. This was neither our intention nor the aim of the study. Rather, by using impedance plethysmography we are working with a technique with superior temporal resolution but imperfect accuracy that yet seems sufficient and satisfactory to compare directional changes and relative magnitudes of changes in regional blood flow and blood volume among patients and volunteer reference subjects undergoing similar testing.
Constancy of Valsalva pressure
We did not record the exhalation pressure. We did observe and “coach” all subjects during the maneuver, leading to reasonable constancy of pressure in a mouthpiece connected to a mercury manometer. However, although every effort was made to maintain nearly constant pressure of 35–40 mmHg for 15 s, it is true that occasionally pressure would fluctuate, which could potentially lead to systematic differences. We were unable to detect such differences in practice. A device to control expiratory pressure is under development.
Age
Age limitations to generalization may exist. Young adults and adolescents may not perfectly represent findings for mature adults. However, cardiovascular structure and function is essentially mature by puberty, and therefore the results can be regarded as at least qualitatively similar to those of older age groups. Moreover, younger patients generally have the advantage of the absence of confounding illness such as heart disease, renal disease, hypertension, and diabetes that may impact on autonomic or circulatory function.
ACKNOWLEDGMENTS
We thank the Chairman of Pediatrics, Dr. Leonard Newman, and members of the Division of Pediatric Cardiology, especially the Director, Dr. Michael H. Gewitz, for unflagging support and Drs. Thomas H. Hintze, David Robertson, and Phillip Low for constant inspiration and stimulation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1R01-HL-66007 and 1R01-HL-074873.
REFERENCES
- 1.Bloomfield DA. Dye Curves: The Theory and Practice of Indicator Dye Dilution. Baltimore, MD: University Park Press; 1974. [Google Scholar]
- 2.Convertino VA, Montgomery LD, Greenleaf JE. Cardiovascular responses during orthostasis: effect of an increase in V̇O2 max. Aviat Space Environ Med. 1984;55:702–708. [PubMed] [Google Scholar]
- 3.Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- 4.Elisberg EI, Goldberg H, Snider GL. Value of intraoral pressure as a measure of intrapleural pressure. J Appl Physiol. 1951;4:171–176. doi: 10.1152/jappl.1951.4.3.171. [DOI] [PubMed] [Google Scholar]
- 5.Fritsch-Yelle JM, Convertino VA, Schlegel TT. Acute manipulations of plasma volume alter arterial pressure responses during Valsalva maneuvers. J Appl Physiol. 1999;86:1852–1857. doi: 10.1152/jappl.1999.86.6.1852. [DOI] [PubMed] [Google Scholar]
- 6.Geddes LA, Baker LE. Principles of Applied Biomedical Instrumentation. New York: Wiley; 1989. Detection of physiological events by impedance; pp. 594–600. [Google Scholar]
- 7.Geddes LA, Kidder H. Specific resistance of blood at body temperature II. Med Biol Eng. 1976;14:180–185. doi: 10.1007/BF02478745. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield AD, Whitney RJ, Whitney RJ. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- 9.Gyoda Y, Tsukada Y, Saito A, Goto K. Role of nitric oxide and neuropeptides in neurogenic vasodilatation of the guinea pig mesenteric artery. Eur J Pharmacol. 1995;279:83–92. doi: 10.1016/0014-2999(95)00142-8. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton WF, Woodbury RA, Harper HT., Jr Arterial, cerebrospinal, and venous pressures in man during cough and strain. Am J Physiol. 1944;141:42–50. [Google Scholar]
- 11.He YL, Tanigami H, Ueyama H, Mashimo T, Yoshiya I. Measurement of blood volume using indocyanine green measured with pulsespectrophotometry: its reproducibility and reliability. Crit Care Med. 1998;26:1446–1451. doi: 10.1097/00003246-199808000-00036. [DOI] [PubMed] [Google Scholar]
- 12.Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab. 2002;87:1569–1575. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- 13.Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H. Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit. 1997;13:81–89. doi: 10.1023/a:1007339924083. [DOI] [PubMed] [Google Scholar]
- 14.Itoh T, Sasaguri T, Makita Y, Kanmura Y, Kuriyama H. Mechanisms of vasodilation induced by vasoactive intestinal polypeptide in rabbit mesenteric artery. Am J Physiol Heart Circ Physiol. 1985;249:H231–H240. doi: 10.1152/ajpheart.1985.249.2.H231. [DOI] [PubMed] [Google Scholar]
- 15.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 16.Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol. 2004;286:H296–H303. doi: 10.1152/ajpheart.00668.2003. [DOI] [PubMed] [Google Scholar]
- 17.Low PA, Novak V, Spies JM, Novak P, Petty GW. Cerebrovascular regulation in the postural orthostatic tachycardia syndrome (POTS) Am J Med Sci. 1999;317:124–133. doi: 10.1097/00000441-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 19.Luster EA, Baumgartner N, Adams WC, Convertino VA. Effects of hypovolemia and posture on responses to the Valsalva maneuver. Aviat Space Environ Med. 1996;67:308–313. [PubMed] [Google Scholar]
- 20.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–196. doi: 10.1006/jsre.2000.5862. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery LD, Hanish HM, Marker RA. An impedance device for study of multisegment hemodynamic changes during orthostatic stress. Aviat Space Environ Med. 1989;60:1116–1122. [PubMed] [Google Scholar]
- 22.Patterson RP, Zhang J. Impedance cardiographic measurement of the physiological response to the Valsalva manoeuvre. Med Biol Eng Comput. 2003;41:40–43. doi: 10.1007/BF02343537. [DOI] [PubMed] [Google Scholar]
- 23.Rowell LB. Reflex control of regional circulations in humans. J Auton Nerv Syst. 1984;11:101–114. doi: 10.1016/0165-1838(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 24.Sandroni P, Novak V, Opfer-Gehrking TL, Huck CA, Low PA. Mechanisms of blood pressure alterations in response to the Valsalva maneuver in postural tachycardia syndrome. Clin Auton Res. 2000;10:1–5. doi: 10.1007/BF02291382. [DOI] [PubMed] [Google Scholar]
- 25.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74:1106–1110. doi: 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- 26.Schoemaker RC, Burggraaf J, Cohen AF. Assessment of hepatic blood flow using continuous infusion of high clearance drugs. Br J Clin Pharmacol. 1998;45:463–469. doi: 10.1046/j.1365-2125.1998.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd JT, Vanhoutte PM. Role of the venous system in circulatory control. Mayo Clin Proc. 1978;53:247–255. [PubMed] [Google Scholar]
- 28.Sieber C, Beglinger C, Jager K, Stalder GA. Intestinal phase of superior mesenteric artery blood flow in man. Gut. 1992;33:497–501. doi: 10.1136/gut.33.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Low PA. Influence of posture on the Valsalva manoeuvre. Clin Sci (Colch) 2001;100:433–440. [PubMed] [Google Scholar]
- 30.Stewart JM, Lavin J, Weldon A. Orthostasis fails to produce active limb venoconstriction in adolescents. J Appl Physiol. 2001;91:1723–1729. doi: 10.1152/jappl.2001.91.4.1723. [DOI] [PubMed] [Google Scholar]
- 31.Stewart JM, Medow MA, Bassett B, Montgomery LD. Effects of thoracic blood volume on Valsalva maneuver. Am J Physiol Heart Circ Physiol. 2004;287:H798–H804. doi: 10.1152/ajpheart.01174.2003. [DOI] [PubMed] [Google Scholar]
- 32.Stewart JM, Montgomery LD. Reciprocal thoracic-splanchnic blood volume changes during the Valsalva maneuver. Am J Physiol Heart Circ Physiol. 2005;288:H752–H758. doi: 10.1152/ajpheart.00717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart JM, Weldon A. Contrasting neurovascular findings in chronic orthostatic intolerance and neurocardiogenic syncope. Clin Sci (Lond) 2003;104:329–340. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 35.Takenaga M, Kawasaki H. Endogenous calcitonin gene-related peptide suppresses vasoconstriction mediated by adrenergic nerves in rat mesenteric resistance blood vessels. Eur J Pharmacol. 1999;367:239–245. doi: 10.1016/s0014-2999(98)00949-2. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1091–R1097. doi: 10.1152/ajpregu.00162.2003. [DOI] [PubMed] [Google Scholar]
- 37.Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, Low PA. Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS) Auton Neurosci. 2000;86:107–113. doi: 10.1016/S1566-0702(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 38.Tyden G. Aspects of cardiovascular reflex control in man. An experimental study. Acta Physiol Scand Suppl. 1977;448:1–62. [PubMed] [Google Scholar]
- 39.White DD, Montgomery LD. Pelvic blood pooling of men and women during lower body negative pressure. Aviat Space Environ Med. 1996;67:555–559. [PubMed] [Google Scholar]