Abstract
Background and purpose
Medical treatment of rheumatoid arthritis (RA) has changed dramatically over the last 15 years, including immune modulation. We investigated the risk of revision for infection after primary total hip replacement (THR) in patients with rheumatoid arthritis over a 16-year period, and compared it with that in THR patients with osteoarthritis (OA).
Patients and methods
We identified 13,384 THRs in RA patients and 377,287 THRs in OA patients from 1995 through 2010 in a dataset from the Nordic Arthroplasty Register Association (NARA). Kaplan-Meier survival curves, with revision for infection as the endpoint, were constructed. Cox regression analyses were performed to calculate the relative risk (RR) of revision for infection adjusted for age, sex, fixation technique, and year of primary surgery.
Results
RA patients had a 1.3 times (95% CI 1.0–1.6) higher risk of revision for infection. After 2001, this risk increased more for RA patients than for OA patients. During the first 3 months and from 8 years postoperatively, the risk of revision for infection was higher in RA patients with THRs fixated with antibiotic-loaded cement than in corresponding OA patients.
Interpretation
We found a slightly higher overall risk of revision for infection in RA patients than in OA patients, but this difference was only present after 2001. In THRs with antibiotic-loaded cement, the risk of very early and late infections leading to revision was higher in RA patients than in OA patients.
Rheumatoid arthritis (RA) patients are particularly vulnerable to infections due to the nature of the disease (immunopathy and ongoing inflammation), general disability, comorbidity, and medication (Mutru et al. 1985, Doran et al. 2002). The increasing use of immune-modulating agents, particularly biologics, in the treatment of RA during the last decade may increase this risk of infection (Bongartz et al. 2006, Winthrop et al. 2008, Komano et al. 2011). RA often leads to joint destruction, so patients with RA are at risk of requiring joint replacement surgery. Before biologics were used, around 25% of all RA patients with 16–20 years of observation needed at least 1 large joint replacement (Wolfe and Zwillich 1998, Kapetanovic et al. 2008). Around 2–3% of all total hip replacements (THRs) in the Nordic Arthroplasty Register Association (NARA) dataset have been performed on RA patients (Havelin et al. 2009, Makela et al. 2014b).
The frequency of prosthetic joint infection is reported to be as low as 1–2% after hip or knee replacement (Zimmerli et al. 2004), and the frequency of surgical revision due to infection is even lower (Pedersen et al. 2010, Schrama et al. 2010, Dale et al. 2012). In a previous study of RA patients with THRs from the Norwegian Arthroplasty Register, the risk of revision for infection was similar to that in osteoarthritis (OA) patients within 6 years of primary THR, whereas there was a higher risk of revision for infection in RA patients than in patients with OA from 6 years postoperatively. The overall risk of revision for infection was not significantly different in the 2 diagnostic groups (Schrama et al. 2010).
Knowing that treatments for RA patients have improved dramatically in the last 10–15 years, we found it important to assess whether there is increased infection risk, which would require a large patient population to be followed over a long period. The collaboration between the Nordic arthroplasty registers—in the form of the NARA—has resulted in a large dataset on THR (Havelin et al. 2009, Makela et al. 2014a). This dataset gives the opportunity to study rare events in selected patient groups, such as revision due to infection after THR in patients with RA.
Based on what we know about increased infection risk associated with immunosuppressive treatments in general, we hypothesize that the new aggressive treatment strategies for RA that have evolved over the last decades including higher doses (e.g. of methotrexate), frequent use of combination regimes (e.g. methotrexate, hydroxochloroquine, and sulfasalazine), and the use of biologics, may make the patients more susceptible to infections—in this case, prosthesis infections.
The main objective of our study was therefore to estimate the risk of revision for infection after primary THR in RA patients relative to that in patients with OA, to evaluate whether today’s RA patients are at greater risk of prosthesis infection. We also wanted to evaluate risk factors for revision because of infection and to study the effect of the length of time from primary THR to revision.
Patients and methods
The Danish Hip Arthroplasty Register was established in 1995 (Lucht 2000), the Finnish Hip Arthroplasty Register in 1980 (Paavolainen et al. 1991), the Norwegian Hip Arthroplasty Register in 1987, and the Swedish Hip Arthroplasty Register in 1979 (Havelin et al. 2000, Malchau et al. 2002). Denmark, Finland, Norway, and Sweden (with 25 million inhabitants) have similar healthcare systems, personal identity numbers, and census registries. This enables combination and comparison of the arthroplasty registries. The collaboration between the registries, the NARA, was established in 2007 (Havelin et al. 2009, Makela et al. 2014a). For the present study, we defined a common set of parameters containing only data that all the registries could provide, and we reached a consensus on the definition of several variables. In all the registries, reporting of infection as the cause of revision reflects the surgeon’s opinion based on clinical information and findings at surgery. The data are not edited according to postoperative culture results. From 1995 through 2010, a total of 390,671 primary THRs with the diagnoses RA or OA were identified in the NARA and included in the study (Table 1). Bilateral THRs were treated as 2 independent observations, since bilaterality has been shown to have a negligible influence on the risk of revision for infection (Lie et al. 2004, Ranstam and Robertsson 2010, Dale et al. 2012). The primary outcome measure was revision for infection following primary THR and only infections leading to revision of the prosthesis (removal or exchange of prosthetic parts) were included, since minor soft-tissue procedures were not reported to all national registries.
Table 1.
Patient characteristics
| RA | OA | p-value | |
|---|---|---|---|
| No. of THRs | 13,384 | 377,287 | |
| Mean age (SD), years | 62 (14) | 69 (9.6) | < 0.001 |
| % females | 76% | 60% | < 0.001 |
| No. of primary THRs | < 0.001 | ||
| 1995–2001 | 7,047 | 130,613 | |
| 2002–2010 | 6,337 | 246,674 | |
| Type of fixation, n (%) | 0.3 | ||
| Cemented | 8,633 (65) | 245,464 (65) | |
| Uncemented | 3,034 (23) | 83,547 (22) | |
| Hybrida | 1,143 (8.5) | 31,619 (8.4) | |
| Inverse hybrid | 574 (4.3) | 16,657 (4.4) | |
| Mean follow-up (SD), years | 7.0 (4.3) | 6.1 (4.1) | < 0.001 |
Hybrid: cemented stem and uncemented cup.
Firstly, we compared the overall risk of revision for infection after primary THR in patients with RA to that in patients with OA during 1995–2010 (Figure 1). We then evaluated the potential influence of biologics on the infection risk in patients with RA. Since we had no data on drug use in these patients, the risk of revision for infection was analyzed in 2 different time periods: from 1995 to 2001, and from 2002 to 2010. TNF-α inhibitors were introduced as treatment for RA in a few patients in Denmark, Finland, Norway, and Sweden in 1999–2000 (Hjardem et al. 2005, Nordstrom et al. 2006, Soderlin and Geborek 2008). From 2000, the use of TNF-α inhibitors increased steadily (Hjardem et al. 2005, Soderlin and Geborek 2008). Using 2001 as the cutoff, we attempted to divide the patients into 1 group (n = 6,337) (2002–2010) in which a considerable proportion of RA patients (20–30%) (Kvien et al. 2005) received treatment with biologics and 1 group (n = 7,047) (1995–2001) in which few or no RA patients had such treatment. RA and OA patients were compared in the 2 time periods, and the risk of revision for infection in the 2 diagnostic groups was compared in order to evaluate the possible influence of important changes in medical treatment in the second time period. Finally, separate analyses were performed for evaluation of the prosthetic fixation method on revision rate.
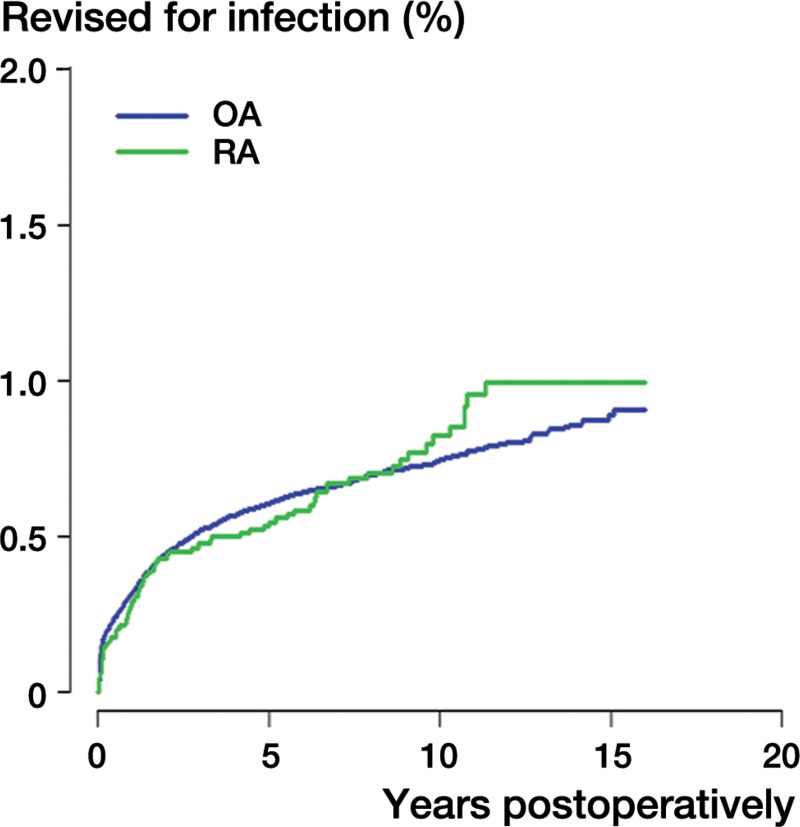
Figure 1.
Kaplan-Meier failure curves with revision for infection as the endpoint for rheumatoid arthritis (RA) and osteoarthritis (OA) patients.
Statistics
Survival analyses with revision for infection as the end point were performed for the total study population and for the THRs in RA patients and OA patients separately. Revision of the implant was defined as surgical removal or exchange of the whole or any part of the implant. Follow-up time was calculated from primary THR until first revision for infection, until the patient was censored at death or emigration, until date of revision if the THR was revised for other causes than infection, or until the end of the period studied on December 31, 2010. Survival curves were generated using the Kaplan-Meier method. Cox regression analyses were performed to estimate the relative risk (RR with 95% CI) of revision for infection adjusted for age (continuous), sex, year of primary surgery, and method of fixation (Table 2). The RR is an estimate of the relative difference in revision risk between the groups at any given time throughout the observation period, and all RRs given are adjusted estimates. We chose to adjust for age as a continuous variable as opposed to performing stratified analyses for age categories, due to the low number of events in the RA population. Additional Schoenfeld residual analyses were performed to detect any changes in revision risk with increasing time since the primary surgery, in uncemented THRs, and where antibiotic-loaded cement was used—comparing RA and OA patients (Figure 3). The hybrids and reverse hybrids were included in these analyses together with the fully cemented prostheses in the antibiotic-loaded cement group. Adjusted RR estimates were further established for predefined follow-up intervals, i.e. the first 3 months, 3 months to 2 years, 2 to 8 years, and longer than 8 years, using an extended Cox model including time-dependent covariates. This largely follows the widely used Coventry prosthetic joint infection classification into early infections (< 3 months), delayed infections (3 months to 2 years), and late infections (> 2 years) (Coventry 1975). The cutoff at 8 years was based on examination of the course of the curve in Figure 3B. Any p-values of 0.05 or less were considered to be statistically significant. The statistical analysis was performed using SPSS version 20.0 and the R statistical software package, version 3.0.2.
Table 2.
Relative risk of revision for infection according to sex, age, diagnosis, year of surgery, and type of fixation, calculated using Cox regression analysis
| No. of revisions | RR | 95% CI | p-value | |
|---|---|---|---|---|
| Age | 2,315 | 1.0 | 0.99–1.01 | 0.1 |
| Sex | ||||
| Female | 1,044 | 1 (ref.) | ||
| Male | 1,271 | 1.9 | 1.8–2.1 | < 0.001 |
| Diagnosis | ||||
| OA | 2,228 | 1 (ref.) | ||
| RA | 87 | 1.3 | 1.0–1.6 | 0.04 |
| Year of primary surgery | ||||
| 1995–2001 | 858 | 1 (ref.) | ||
| 2002–2010 | 1,457 | 1.4 | 1.3–1.5 | < 0.001 |
| Fixation | ||||
| AB+ cement a | 1,632 | 1 (ref.) | ||
| Uncemented | 490 | 0.9 | 0.8–1.0 | 0.1 |
| AB– cement a | 193 | 1.4 | 1.2–1.6 | < 0.001 |
AB+ cement: antibiotic-loaded cement. AB– cement: cement without antibiotics.
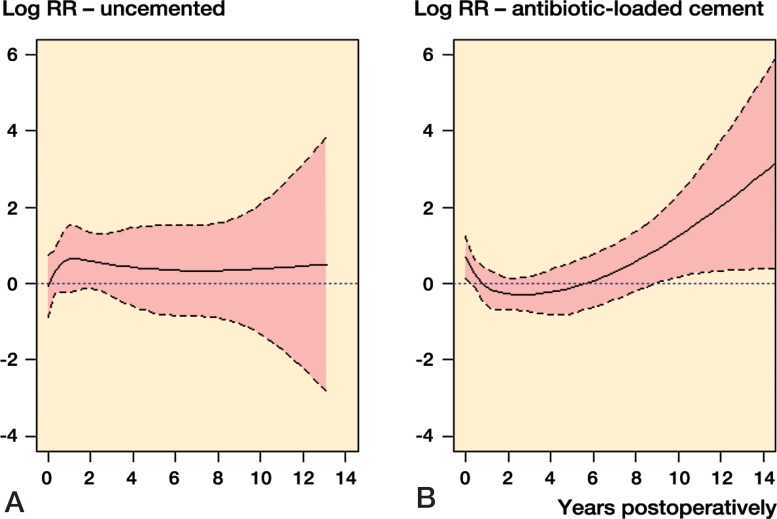
Figure 3.
Log relative risk (RR) estimates of revision for infection in patients with rheumatoid arthritis (solid line) vs. osteoarthritis (0-line, reference) are shown by year after the primary surgery. Broken lines show the 95% confidence intervals.
A.Uncemented total hip replacements with 22 revisions for infection in RA.
B.Total hip replacements with antibiotic-loaded cement as fixation and 55 revisions for infection in RA.
Results
Patients with OA were generally older than the RA patients. We found no important difference in mean age in the 2 diagnostic groups when comparing the 2 time periods (mean age for RA patients was 61 years in the first period and 62 years in the second, and in OA patients it was unchanged at 69 years). We found no influence of increasing age on the risk of revision for infection (Table 2).
Overall results for RA and OA patients
Of the 390,671 THRs included (377,287 in the OA group and 13,384 in the RA group), 2,315 were revised for infection. Of these revisions, 2,228 were in OA patients and 87 were in RA patients. The incidence of revision for infection was 0.6% in OA patients and 0.7% in RA patients. The overall risk of revision for infection was higher in RA patients than in OA patients (RR = 1.3, 95% CI: 1.0–1.6) (Table 2 and Figure 1). Men had a statistically significantly higher risk of revision for infection than women (RR = 1.9, CI: 1.8–2.1).
Infection risk associated with RA according to time of primary surgery
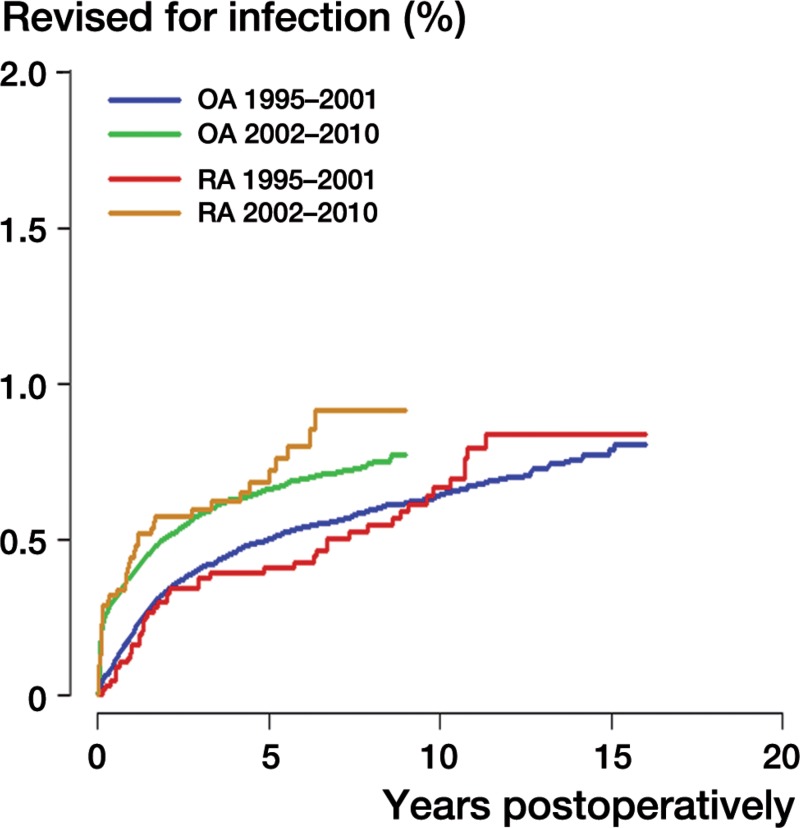
For all patients and in both groups (RA and OA), the risk of revision for infection in the second period (2002–2010) was statistically significantly higher than in the first period (1995–2001) (RR = 1.4, CI: 1.3–1.6) (Table 2 and Figure 2). For both diagnoses, this increase in infection risk was significant. In the first period, the risk of revision for infection was no higher for RA patients than for OA patients (RR = 1.1, CI: 0.8–1.5) (Figure 2 and Table 3) whereas in the second period, RA patients had a higher risk of revision for infection than OA patients (RR = 1.4, CI: 1.0–1.8) (Figure 2 and Table 3).
Figure 2.
Kaplan-Meier failure curves for rheumatoid arthritis (RA) and osteoarthritis (OA) patients in the periods 1995–2001 and 2002–2010 with revision for infection as the endpoint.
Table 3.
Relative risk of revision for infection according to age, sex, and type of fixation for both diagnoses and time periods, calculated using Cox regression analysis
| No. of revisions | RR | 95% CI | p-value | |
|---|---|---|---|---|
| RA 1995–2001 | 44 | 1 (ref.) | ||
| RA 2002–2010 | 43 | 1.9 | 1.2–3.1 | 0.006 |
| OA 1995–2001 | 814 | 1 (ref.) | ||
| OA 2002–2010 | 1,414 | 1.4 | 1.3–1.5 | < 0.001 |
| OA 1995–2001 | 814 | 1 (ref.) | ||
| RA 1995–2001 | 44 | 1.1 | 0.8–1.5 | 0.5 |
| OA 2002–2010 | 1,414 | 1 (ref.) | ||
| RA 2002–2010 | 43 | 1.4 | 1.0–1.8 | 0.05 |
Effect of mode of fixation
There was no difference in frequency between the RA group and the OA group in terms of method of implant fixation (cemented, hybrid, or cementless) (Table 1). A higher risk of revision for infection was seen in THRs where cement without antibiotics was used than in THRs with antibiotic-loaded cement (RR = 1.4, CI: 1.2–1.6) (Table 2). Additional analysis also revealed a higher risk of revision for infection in THRs with cement without antibiotics than in uncemented THRs (RR = 1.5, CI: 1.2–1.8).
Adjusted RR estimates for predefined follow-up intervals revealed a trend of a higher risk of revision in the RA group than in the OA group for uncemented THRs, throughout the study period (Figure 3A). For the antibiotic-loaded cement group, a higher relative risk of revision for infection was found for RA patients than for OA patients during the first 3 postoperative months (RR = 1.8, CI: 1.1–3.0; p = 0.01), and after 8 years (RR = 2.7, CI: 1.2–6.3; p = 0.02) (Figure 3B).
Discussion
We found an increased risk of revision for infection in RA patients than in OA patients. This was not found in a previous publication from the Norwegian Arthroplasty Register (Schrama et al. 2010). In that study, the RA population was considerably smaller (n = 4,167, with only 25 patients revised for infection). However, the current finding has been confirmed in other publications (Fitzgerald et al. 1977, Poss et al. 1984, Bongartz et al. 2008). Furthermore, in a previous study, total knee replacements (TKRs) were included in addition to THRs and a higher risk of revision of TKRs for infection was seen in RA patients than in OA patients (Schrama et al. 2010).
Another finding was the increased risk of revision for infection from the first to the second time period in both patient groups. This has also been shown and discussed in a recent paper on infection in THRs from the NARA dataset by Dale et al. (2012).
More revisions for infection in RA patients in 2002–2010
We found that the difference in risk of revision for infection between RA and OA patients emerged after 2002; no difference was seen in the period 1995–2001. A move towards accepting patients with more comorbidity for THR surgery may have taken place during the study period, but we have little reason to believe that this would have occurred to a greater extent in the RA population than in the OA population. However, joint replacement surgery in RA patients has declined during recent years (Fevang et al. 2007, Jamsen et al. 2013), and those still needing surgery would be patients with a long disease duration or non-responders to treatment, who may have particularly high disease activity. The latter group would have an increased risk of infection in general (Au et al. 2011), due to ongoing inflammation. Furthermore, the use of steroids would probably be greater in this group, possibly contributing to the increased infection risk (Akkara Veetil and Bongartz 2012). Another possible reason for the increased risk of revision for infection in RA patients in the latter period is the use of immune-modulating biologic drugs, although studies on the impact of these drugs in the context of joint replacement surgery have so far been conflicting (Talwalkar et al. 2005, Wendling et al. 2005, Giles et al. 2006, den Broeder et al. 2007, Ruyssen-Witrand et al. 2007, Gilson et al. 2010, Kawakami et al. 2010, Momohara et al. 2011, Suzuki et al. 2011, Berthold et al. 2013).
A change in strategy in treatment of RA with early intensive treatment aimed at remission was adopted during the last study period (Smolen et al. 2010a, Vermeer et al. 2011). Superior results have been shown with this strategy, but patients generally use higher doses of methotrexate and are often on combination regimes (with or without biologics and/or steroids). This might have led to greater impairment of the immune system, making patients more susceptible to prosthetic joint infection.
A 40% increase in risk of revision for infection in RA patients relative to OA patients (RR = 1.4, Table 3) in 2002–2010 of an already uncommon outcome (0.6% revision for infection in our study) still makes the absolute risk low.
Many factors that influenced the risk of infection leading to revision (treatment policy, operating technique, diagnostics, awareness, etc.) may have changed with time. The possible changes in these factors are unlikely to have differed between RA and OA patients undergoing THR. By comparing RA patients with the large OA group, we tried to control for these factors when evaluating the risk of revision for infection in the 2 time periods. We studied the possible influence of the changed medical treatment of RA over time on the risk of revision for infection.
Effect of mode of fixation
Another finding of this study was that in RA patients with antibiotic-loaded cement, no difference in revision risk was seen compared to OA patients (except for the first 3 months), until an increased risk was evident in RA patients from 8 years postoperatively (Figure 3B). It appears that the antibiotics protected the THRs in RA patients against infection during the period from 3 months to 8 years postoperatively, although we cannot explain why this would not also be the case during the first 3 months. Our results concur with the findings of Josefsson and Kolmert (1993) that gentamicin-loaded cement is effective in infection prophylaxis for longer than 5 years but shorter than 10 years postoperatively.
After 8 years, the risk of revision for infection increased in the RA patients, probably because of the higher susceptibility to blood-borne infections connected to the diagnosis and possibly due to the immune-modulating medical treatment (Stinchfield et al. 1980, Ainscow and Denham 1984). Not only the extra volume but probably also the surface properties regarding bacterial adherence and colonization of the now inactive bone cement might reinforce this susceptibility (Oga et al. 1988).
Strengths and limitations
One strength of our study was that it was based on a population of about 25 million inhabitants in 4 countries, with high completeness of data and coverage in the registries (Soderman et al. 2000, Pedersen et al. 2004, Espehaug et al. 2006). The positive predictive value of the registered RA diagnosis is also high (Pedersen et al. 2004). Consequently, the population of THR patients was large, giving a large cohort of RA patients with THRs for the long-term evaluation of the rare prosthetic joint infections.
Some limitations of the study must also be considered. We lack information on what medical treatment was used in the individual patient. We may only assume that patients in this study were treated in accordance with the recommendations for patients with RA at the time (Smolen et al. 2010b). In addition, the number of revisions for infection performed in RA patients was low (n = 87 in 13,384 RA patients), but even so, our material on hip replacements in RA patients is among the largest published. Another limitation is that in a recent study from the Swedish Hip Arthroplasty Register, the completeness of reporting of early infection after THR was found to be 67% (Lindgren et al. 2014). However, we believe that the completeness of reporting is most likely the same for patients with different diagnoses. With the use of OA patients as controls, a difference detected between the 2 patient groups can be considered reliable. We lacked information on comorbidity before THR, which is well known to differ between RA and OA patients, and may affect the risk of revision due to infection following THR (Rud-Sorensen et al. 2010). However, we have no reason to believe that this difference has changed over time. If there is any difference, we might expect a reduction in comorbidity with time in RA patients due to improved medical treatment. Finally, no information on antibiotic prophylaxis was included in the study. Such data have been shown to vary widely—within countries as well as between them. We cannot rule out the possibility of RA patients having different prophylactic antibiotic regimens from OA patients, although this is not the case in our department.
In conclusion, we found a higher risk of revision caused by prosthetic joint infection in patients with RA than in those with OA. The difference was only present from 2002 onward. The increased risk of revision for infection in RA patients coincided with a change in treatment strategy for these patients, with more aggressive immunmodulating therapy. For THRs with antibiotic-loaded cement, a higher risk of very early and late infections leading to revision was seen in RA patients than in OA patients.
Acknowledgments
JCS, BTF, and AMF performed the analyses. JCS and BTF wrote the manuscript. All the authors contributed to interpretation of the analyses and to critical revision of the manuscript.
We thank the orthopedic surgeons in Denmark, Finland, Norway, and Sweden for conscientious reporting of THAs, and the staff of the 4 national registries for their thorough quality assurance of registrations.
No competing interests declared.
References
- Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements . J Bone Joint Surg Br. 1984;66(4):580–2. doi: 10.1302/0301-620X.66B4.6430907. [DOI] [PubMed] [Google Scholar]
- Akkara Veetil BM, Bongartz T. Perioperative care for patients with rheumatic diseases . Nat Rev Rheumatol. 2012;8(1):32–41. doi: 10.1038/nrrheum.2011.171. [DOI] [PubMed] [Google Scholar]
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis . Ann Rheum Dis. 2011;70(5):785–91. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- Berthold E, Geborek P, Gulfe A. Continuation of TNF blockade in patients with inflammatory rheumatic disease. An observational study on surgical site infections in 1,596 elective orthopedic and hand surgery procedures . Acta Orthop. 2013;84(5):495–501. doi: 10.3109/17453674.2013.842431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials . Jama. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis . Arthritis Rheum. 2008;59(12):1713–20. doi: 10.1002/art.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coventry MB. Treatment of infections occurring in total hip surgery . Orthop Clin North Am. 1975;6(4):991–1003. [PubMed] [Google Scholar]
- Dale H, Fenstad AM, Hallan G, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty . Acta Orthop. 2012;83(5):449–58. doi: 10.3109/17453674.2012.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study . J Rheumatol. 2007;34(4):689–95. [PubMed] [Google Scholar]
- Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study . Arthritis Rheum. 2002;46(9):2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- Espehaug B, Furnes O, Havelin LI, Engesaeter LB, Vollset SE, Kindseth O. Registration completeness in the Norwegian Arthroplasty Register . Acta Orthop. 2006;77(1):49–56. doi: 10.1080/17453670610045696. [DOI] [PubMed] [Google Scholar]
- Fevang BT, Lie SA, Havelin LI, Engesaeter LB, Furnes O. Reduction in orthopedic surgery among patients with chronic inflammatory joint disease in Norway 1994 . Arthritis Rheum. 2007;57(3):529–32. doi: 10.1002/art.22628. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RH, Jr., Nolan DR, Ilstrup DM, Van Scoy RE, Washington JA, 2nd, Coventry MB . Deep wound sepsis following total hip arthroplasty . J Bone Joint Surg Am. 1977;59(7):847–55. [PubMed] [Google Scholar]
- Giles JT, Bartlett SJ, Gelber AC, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis . Arthritis Rheum. 2006;55(2):333–7. doi: 10.1002/art.21841. [DOI] [PubMed] [Google Scholar]
- Gilson M, Gossec L, Mariette X, et al. Risk factors for total joint arthroplasty infection in patients receiving tumor necrosis factor alpha-blockers: a case-control study . Arthritis Res Ther. 2010;12(4):R145. doi: 10.1186/ar3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties . Acta Orthop Scand. 2000;71(4):337–53. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs . Acta Orthop. 2009;80(4):393–401. doi: 10.3109/17453670903039544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjardem E, Hetland ML, Ostergaard M, Krogh NS, Kvien TK. Danish Database for Biological Therapies in Rheumatology Study G. Prescription practice of biological drugs in rheumatoid arthritis during the first 3 years of post-marketing use in Denmark and Norway: criteria are becoming less stringent . Ann Rheum Dis. 2005;64(8):1220–3. doi: 10.1136/ard.2004.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 . Acta Orthop. 2013;84(4):331–7. doi: 10.3109/17453674.2013.810519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson G, Kolmert L. Prophylaxis with systematic antibiotics versus gentamicin bone cement in total hip arthroplasty. A ten-year survey of 1,688 hips . Clin Orthop Relat Res. 1993;292:210–4. [PubMed] [Google Scholar]
- Kapetanovic MC, Lindqvist E, Saxne T, Eberhardt K. Orthopaedic surgery in patients with rheumatoid arthritis over 20 years: prevalence and predictive factors of large joint replacement . Ann Rheum Dis. 2008;67(10):1412–6. doi: 10.1136/ard.2007.086710. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Ikari K, Kawamura K, et al. Complications and features after joint surgery in rheumatoid arthritis patients treated with tumour necrosis factor-alpha blockers: perioperative interruption of tumour necrosis factor-alpha blockers decreases complications? . Rheumatology (Oxford) 2010;49(2):341–7. doi: 10.1093/rheumatology/kep376. [DOI] [PubMed] [Google Scholar]
- Komano Y, Tanaka M. Nanki T, et al. and Group RS. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for Longterm Safety . J Rheumatol. 2011;38(7):1258–64. doi: 10.3899/jrheum.101009. [DOI] [PubMed] [Google Scholar]
- Kvien TK, Heiberg, Lie E, et al. A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases . Clin Exp Rheumatol. 2005;23(Suppl 39):S188–94. [PubMed] [Google Scholar]
- Lie SA, Engesaeter LB, Havelin LI, Gjessing HK, Vollset SE. Dependency issues in survival analyses of 55,782 primary hip replacements from 47,355 patients . Stat Med. 2004;23(20):3227–40. doi: 10.1002/sim.1905. [DOI] [PubMed] [Google Scholar]
- Lindgren JV, Gordon M, Wretenberg P, Karrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register . BMC Musculoskelet Disord. 2014;15(1):384. doi: 10.1186/1471-2474-15-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht U. The Danish Hip Arthroplasty Register . Acta Orthop Scand. 2000;71(5):433–9. doi: 10.1080/000164700317381081. [DOI] [PubMed] [Google Scholar]
- Makela KT, Matilainen M, Pulkkinen P, et al. Failure rate of cemented and uncemented total hip replacements: register study of combined Nordic database of four nations . BMJ. 2014a;348:f7592. doi: 10.1136/bmj.f7592. [DOI] [PubMed] [Google Scholar]
- Makela KT, Matilainen M, Pulkkinen P, et al. Countrywise results of total hip replacement . Acta Orthop. 2014b;85(2):107–16. doi: 10.3109/17453674.2014.893498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register . J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- Momohara S, Kawakami K, Iwamoto T, et al. Prosthetic joint infection after total hip or knee arthroplasty in rheumatoid arthritis patients treated with nonbiologic and biologic disease-modifying antirheumatic drugs . Mod Rheumatol. 2011;21(5):469–75. doi: 10.1007/s10165-011-0423-x. [DOI] [PubMed] [Google Scholar]
- Mutru O, Laakso M, Isomaki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis . Br Med J (Clin Res Ed) 1985;290(6484):1797–9. doi: 10.1136/bmj.290.6484.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom DC, Konttinen L, Korpela M, et al. Classic disease modifying anti-rheumatic drugs (DMARDs) in combination with infliximab. The Finnish experience . Rheumatol Int. 2006;26(8):741–8. doi: 10.1007/s00296-005-0054-7. [DOI] [PubMed] [Google Scholar]
- Oga M, Sugioka Y, Hobgood CD, Gristina AG, Myrvik QN. Surgical biomaterials and differential colonization by Staphylococcus epidermidis . Biomaterials. 1988;9(3):285–9. doi: 10.1016/0142-9612(88)90100-7. [DOI] [PubMed] [Google Scholar]
- Paavolainen P, Hamalainen M, Mustonen H, Slatis P. Registration of arthroplasties in Finland. A nationwide prospective project . Acta Orthop Scand Suppl. 1991;241:27–30. doi: 10.3109/17453679109155101. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Johnsen S, Overgaard S, Soballe K, Sorensen HT, Lucht U. Registration in the Danish Hip Arthroplasty Registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications . Acta Orthop Scand. 2004;75(4):434–41. doi: 10.1080/00016470410001213-1. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Svendsson JE, Johnsen SP, Riis A, Overgaard S. Risk factors for revision due to infection after primary total hip arthroplasty. A population-based study of 80,756 primary procedures in the Danish Hip Arthroplasty Registry . Acta Orthop. 2010;81(5):542–7. doi: 10.3109/17453674.2010.519908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty . Clin Orthop Relat Res. 1984;182:117–26. [PubMed] [Google Scholar]
- Ranstam J, Robertsson O. Statistical analysis of arthroplasty register data . Acta Orthop. 2010;81(1):10–4. doi: 10.3109/17453671003587168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rud-Sorensen C, Pedersen AB, Johnsen SP, Riis AH, Overgaard S. Survival of primary total hip arthroplasty in rheumatoid arthritis patients . Acta Orthop. 2010;81(1):60–5. doi: 10.3109/17453671003685418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyssen-Witrand A, Gossec L, Salliot C, et al. Complication rates of 127 surgical procedures performed in rheumatic patients receiving tumor necrosis factor alpha blockers . Clin Exp Rheumatol. 2007;25(3):430–6. [PubMed] [Google Scholar]
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register . Arthritis Care Res (Hoboken) 2010;62(4):473–9. doi: 10.1002/acr.20036. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force . Ann Rheum Dis. 2010a;69(4):631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs . Ann Rheum Dis. 2010b;69(6):964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlin MK, Geborek P. Changing pattern in the prescription of biological treatment in rheumatoid arthritis. A 7-year follow-up of 1839 patients in southern Sweden . Ann Rheum Dis. 2008;67(1) doi: 10.1136/ard.2007.070714. [DOI] [PubMed] [Google Scholar]
- Soderman P, Malchau H, Herberts P, Johnell O. Are the findings in the Swedish National Total Hip Arthroplasty Register valid? A comparison between the Swedish National Total Hip Arthroplasty Register, the National Discharge Register, and the National Death Register . J Arthroplasty. 2000;15(7):884–9. doi: 10.1054/arth.2000.8591. [DOI] [PubMed] [Google Scholar]
- Stinchfield FE, Bigliani LU, Neu HC, Goss TP, Foster CR. Late hematogenous infection of total joint replacement . J Bone Joint Surg Am. 1980;62(8):1345–50. [PubMed] [Google Scholar]
- Suzuki M, Nishida K, Soen S, et al. Risk of postoperative complications in rheumatoid arthritis relevant to treatment with biologic agents: a report from the Committee on Arthritis of the Japanese Orthopaedic Association . J Orthop Sci. 2011;16(6):778–84. doi: 10.1007/s00776-011-0142-3. [DOI] [PubMed] [Google Scholar]
- Talwalkar SC, Grennan DM, Gray J, Johnson P, Hayton MJ. Tumour necrosis factor alpha antagonists and early postoperative complications in patients with inflammatory joint disease undergoing elective orthopaedic surgery . Ann Rheum Dis. 2005;64(4):650–1. doi: 10.1136/ard.2004.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer M, Kuper HH, Hoekstra M, et al. Implementation of a treat-to-target strategy in very early rheumatoid arthritis: results of the Dutch Rheumatoid Arthritis Monitoring remission induction cohort study . Arthritis Rheum. 2011;63(10):2865–72. doi: 10.1002/art.30494. [DOI] [PubMed] [Google Scholar]
- Wendling D, Balblanc JC, Brousse A, et al. Surgery in patients receiving anti-tumour necrosis factor alpha treatment in rheumatoid arthritis: an observational study on 50 surgical procedures . Ann Rheum Dis. 2005;64(9):1378–9. doi: 10.1136/ard.2005.037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM. Infectious Diseases Society of America Emerging Infections N. Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: case finding through the Emerging Infections Network . Clin Infect Dis. 2008;46(11):1738–40. doi: 10.1086/587989. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Zwillich SH. The long-term outcomes of rheumatoid arthritis: a 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis . Arthritis Rheum. 1998;41(6):1072–82. doi: 10.1002/1529-0131(199806)41:6<1072::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections . N Engl J Med. 2004;351(16):1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]