Abstract
Background and purpose
Ceramic-on-ceramic (CoC) bearings were introduced in total hip arthroplasty (THA) to reduce problems related to polyethylene wear. We compared the 9-year revision risk for cementless CoC THA and for cementless metal-on-polyethylene (MoP) THA.
Patients and methods
In this prospective, population-based study from the Danish Hip Arthroplasty Registry, we identified all the primary cementless THAs that had been performed from 2002 through 2009 (n = 25,656). Of these, 1,773 THAs with CoC bearings and 9,323 THAs with MoP bearings were included in the study. To estimate the relative risk (RR) of revision, we used regression with the pseudo-value approach and treated death as a competing risk.
Results
444 revisions were identified: 4.0% for CoC THA (71 of 1,773) and 4.0% for MoP THA (373 of 9,323). No statistically significant difference in the risk of revision for any reason was found for CoC and MoP bearings after 9 years of follow-up (adjusted RR = 1.3, 95% CI: 0.72–2.4). Revision rates due to component failure were 0.5% (n = 8) for CoC bearings and 0.1% (n = 6) for MoP bearings (p < 0.001). 6 patients with CoC bearings (0.34%) underwent revision due to ceramic fracture.
Interpretation
When compared to the “standard” MoP bearings, CoC THA had a 33% higher (though not statistically significantly higher) risk of revision for any reason at 9 years.
Aseptic loosening is the most frequent cause of revision after total hip arthroplasty (THA) (Australian Orthopaedic Association 2013, Danish Hip Arthroplasty Registry 2013). This is associated with polyethylene wear debris, which can stimulate an adverse local host response that results in bone resorption and aseptic loosening of the prosthesis (Jacobs et al. 1994). Ceramic-on-ceramic (CoC) bearings were introduced for THAs in 1970 (Boutin 1972) to reduce the problem of wear due to friction and that of loosening, which was a result of osteolysis caused by wear particles (Hannouche et al. 2005).
Although CoC bearings have shown low wear rates and are used in young and active patients (Hannouche et al. 2005), there are some concerns of fracture of the ceramic acetabular liner (Min et al. 2007) or fracture of the ceramic head (Habermann et al. 2006). Furthermore, dislodgement of the acetabular ceramic insert has been reported for the sandwich design (Akagi et al. 2004). Finally, squeaking and other noises can occur in THAs with CoC bearings (Jarrett et al. 2009). All these concerns may lead to revision surgery.
Survivorship of CoC THAs after a mean follow-up time of 5–12 years has been described in some previous studies (Garcia-Rey et al. 2009, Johansson et al. 2011, D’Antonio et al. 2012), but these studies had small sample sizes and involved very few hospitals and clinics, thus reducing the generalizability of the findings. Based on data from the Danish Hip Arthroplasty Registry (DHR), we therefore conducted a population-based cohort study to determine the revision risk and to investigate the causes of revision of cementless CoC THAs, comparing them to those of “standard” MoP THAs.
Patients and methods
There are approximately 5.6 million inhabitants in Denmark. All Danish citizens are guaranteed tax-funded, “free” medical care on admission to hospitals or outpatient clinics. Every Danish citizen is given a personal 10-digit identification number that allows unambiguous linkage between all the medical databases in Denmark.
Data sources
The DHR is a nationwide, population-based clinical database that was founded on January 1, 1995 and validated in 2004 (Pedersen et al. 2004). The DHR holds prospectively collected data on primary THAs, revisions, and—to some extent—postoperative complications. In 2012, 50 orthopedic departments and private clinics reported to the registry (Danish Hip Arthroplasty Registry 2013). In annual reports, the completeness of the data is calculated at the individual level as the proportion of THAs reported to the DHR out of the total number of THAs reported to the National Patient Registry (NPR) and/or the DHR. The NPR is considered to be the gold standard—due to the fact that the hospital is reimbursed only after registration of a surgical procedure. In 2012, the degree of completeness was 97% for primary THAs and 90% for THA revisions (Danish Hip Arthroplasty Registry 2013).
The Civil Registration System (CRS) was established in 1968. It contains data on vital status and residence for the entire Danish population (Pedersen et al. 2006). Thus, the CRS provides complete follow-up information on the entire study population.
The NPR was established in 1977. It contains data on all admissions and discharges from hospitals in Denmark, including the dates of admission and discharge, the surgical procedures performed, and up to 20 diagnoses for every discharge. Since 1994, diagnoses have been classified according to the Danish version of the International Classification of Diseases, tenth edition (Andersen et al. 1999). Since 1995, data on outpatients and emergency visits have been included in the registry. Data from the NPR were used to determine the complete hospitalization history of patients included in the study population. As a measure of comorbidity, we computed the Charlson comorbidity index (CCI) score for each patient at the time of surgery (Charlson et al. 1987, Thygesen et al. 2011). This index is based on 19 major disease categories, including cardiovascular, cerebrovascular, chronic pulmonary, liver, renal, and gastrointestinal diseases, diabetes, and solid and hematological tumors. Admissions from each category are weighted with 1, 2, 3, or 6 points. These weights are summed to provide the index score. We defined 3 comorbidity levels: a score of 0 (low), given to patients with no previous record of diseases included in the CCI; a score of 1–2 (medium); and a score of 3 or more (high) (de Groot et al. 2003).
Study population
For patients registered with CoC bearings, the fixation method was cementless in 97.1%, hybrid in 2.7%, and “other” in 0.1%. The study population included patients undergoing cementless THA with either CoC or MoP bearings who were being operated for 1 of the following diagnoses: primary osteoarthritis (OA), inflammatory arthritis, femoral head osteonecrosis, and sequelae from childhood hip disorder.
In the DHR, the registration of THA bearings started in 2002. This study population consisted of all primary cementless THAs registered in the DHR with surgery between January 1, 2002 and September 15, 2009 (n = 25,656). When a patient received bilateral THA operations, only the first was included in the study due to the statistical assumption of independent observations. Thus, 3,572 THAs were excluded due to bilaterality. Patients diagnosed with hip fracture (n = 2,097) and “other” diagnoses (n = 201) and patients with ceramic-on-polyethylene (n = 5,171), metal-on-metal (n = 2,100), or “other” types of bearings (n = 565) were excluded. Furthermore, patients with an acetabular component with a dual-mobility liner (n = 306) were excluded. We also excluded 520 patients who were registered with missing information regarding bearings. Of these, 18 patients had a metal liner and could therefore not have CoC or MoP bearings. Hence, 502 patients with missing information on articulation could possibly have had either CoC or MoP bearings. Patients who were registered without information on diagnosis (n = 16), femoral head size (n = 4), and duration of surgery (n = 8) were also excluded.
11,096 cementless THAs (1,773 CoC and 9,323 MoP) with complete patient information on sex, age group, diagnosis, comorbidity, year of surgery, femoral head size, and duration of surgery were included in the final analysis.
Types of ceramic bearings
According to the manufacturer (CeramTec, Plochingen, Germany), BIOLOX forte was introduced in 1995 and BIOLOX delta in 2004. Distributors of the prosthetic components were contacted to obtain information on the types of ceramic bearings that were used with the specific acetabular and femoral components from 2002 to September 15, 2009. Distributors were supplied with information on the specific component brand and its period in use. At the patient level, the femoral head size was also taken into account to determine the ceramic bearing type implanted.
Medical records
In order to identify patients with fracture of a ceramic component, medical records were reviewed for 14 patients who had revision surgery due to component failure.
Statistics
Patients were followed from the date of primary surgery until revision, death, emigration, or the end of the study period (September 15, 2010), whichever came first. Revision was defined as a new surgical procedure including complete or partial exchange or removal of the prosthetic components. When death is treated as censored information in survival analysis, it will result in overestimation of the revision rates (Gillam et al. 2010). We therefore performed multivariable regression with the pseudo-value approach (Klein et al. 2007), treating death as a competing risk to estimate the relative risk (RR) for any revision with 95% confidence intervals (CIs), and a cumulative incidence curve was constructed. Adjustments were made for the patient- and surgery-related factors presented in Table 1. Subanalyses were performed at 2, 4, 6, and 8.7 years of follow-up in order to evaluate early and medium-term revision risk. We performed stratified analyses on potentially influencing factors, including sex; age under or over 60 years; comorbidity; osteoarthritis (OA) as diagnosis; femoral heads 28 mm or smaller; and femoral heads larger than 28 mm. All stratified analyses were performed at 8.7 years of follow-up—except for femoral head sizes larger than 28 mm, which had a maximum follow-up of 7.5 years. The primary outcome was revision for any reason. Revisions for aseptic loosening, dislocation, and other causes at 8.7-years follow-up were analyzed and these were secondary outcomes.
Table 1.
Characteristics of patients with ceramic-on-ceramic (CoC) and metal-on-polyethylene (MoP) total hip arthroplasty. Values are numbers of patients and percentages (%) for each group
| CoC n = 1,773 | MoP n = 9,323 | p-value | |
|---|---|---|---|
| Sex | 0.001 | ||
| Female | 835 (47) | 4,792 (51) | |
| Male | 938 (53) | 4,531 (49) | |
| Age groups, years | < 0.001 | ||
| ≤ 49 | 356 (20) | 539 (6) | |
| 50–59 | 576 (33) | 2,068 (22) | |
| 60–69 | 744 (42) | 4,238 (46) | |
| 70–79 | 91 (5) | 2,069 (22) | |
| ≥ 80 | 6 (0) | 409 (4) | |
| Diagnosis | < 0.001 | ||
| Primary OA | 1,471 (83) | 8,373 (90) | |
| Femoral head osteonecrosis | 67 (4) | 258 (3) | |
| Arthritis | 53 (3) | 193 (2) | |
| Childhood hip disorders | 182 (10) | 499 (5) | |
| Charlson comorbidity index at surgery | < 0.001 | ||
| Low | 1,350 (76) | 6,324 (68) | |
| Medium | 363 (21) | 2,447 (26) | |
| High | 60 (3) | 552 (6) | |
| Year of surgery | < 0.001 | ||
| 2002 | 167 (10) | 867 (9) | |
| 2003 | 210 (12) | 922 (10) | |
| 2004 | 339 (19) | 1,088 (12) | |
| 2005 | 345 (19) | 1,187 (13) | |
| 2006 | 238 (13) | 1,235 (13) | |
| 2007 | 190 (11) | 1,277 (14) | |
| 2008 | 153 (9) | 1,426 (15) | |
| 2009, until September 15 | 131 (7) | 1,321 (14) | |
| Femoral head size, mm | < 0.001 | ||
| ≤ 27 | 1 (0) | 139 (2) | |
| 28 | 652 (37) | 6,066 (65) | |
| 32 | 922 (52) | 1,926 (21) | |
| 36 | 193 (11) | 1,066 (11) | |
| ≥ 40 | 5 (0) | 126 (1) | |
| Duration of surgery, min | < 0.001 | ||
| ≤ 59 | 505 (29) | 3,925 (42) | |
| 60–89 | 899 (51) | 4,202 (45) | |
| 90–119 | 286 (16) | 917 (10) | |
| ≥ 120 | 83 (5) | 279 (3) |
Revision rates per 100 person-years (with CI) were calculated as the number of revisions within each group divided by the total risk time for the same group. Chi-square test was performed to compare proportions between the 2 bearing groups, and the 2-sample Wilcoxon rank-sum test was used to compare ages and follow-up times because of skewness. For ages and follow-times, medians and interquartile ranges (IQR) are given. Any p-value < 0.05 was considered significant. Statistical analyses were carried out with Stata software, release 13.1.
Ethics
The study was approved by the Danish Data Protection Agency (journal no. 2010-41-4926).
Results
Description of the study population (Tables 1 and 2)
Table 2.
Specific designs of acetabular and femoral components and type of ceramic used in total hip arthroplasty with ceramic-on-ceramic bearings. Values are numbers of patients and percentage (%) of total number
| No. n = 1,773 | Company | Period in use | No. of BIOLOX forte | No. of BIOLOX delta | No. of either BIOLOX forte or delta | No. of unknown ceramic components | |
|---|---|---|---|---|---|---|---|
| Acetabular component | |||||||
| Plasmacup SC | 792 (45) | Aesculap | 2002–2009 | 764 | 28 | - | - |
| Lineage | 312 (18) | Wright | 2004–2009 | 312 | 0 | - | - |
| Trident PSL a | 125 (7) | Stryker | 2004–2008 | 125 | 0 | - | - |
| Exceed ABT | 93 (5) | Biomet | 2006–2009 | - | 93 | - | - |
| Trident hemispherical b | 73 (4) | Stryker | 2005–2007 | 73 | 0 | - | - |
| Duraloc Option | 63 (4) | DePuy | 2002–2005 | 63 | 0 | - | - |
| Mallory-Head | 51 (3) | Biomet | 2002–2009 | - | 28 | - | 23 |
| Trilogy | 51 (3) | Zimmer | 2002–2009 | 0 | 51 | - | - |
| Anca-Fit | 47 (3) | Wright | 2002–2005 | 47 | 0 | - | - |
| C2a Taper b | 44 (2) | Biomet | 2007–2009 | 44 | - | - | - |
| Pinnacle | 41 (2) | DePuy | 2004–2009 | 0 | 41 | - | - |
| Prototyl-E | 19 (1) | Wright | 2009 | - | - | 19 | - |
| 12 other cups | 62 (3) | 2002–2009 | - | - | - | 62 | |
| In total | 1,428 | 241 | 19 | 85 | |||
| Femoral component | |||||||
| Bicontact | 769 (43) | Aesculap | 2002–2009 | 720 | 29 | 20 | - |
| Anca-Fit | 409 (23) | Wright | 2002–2009 | 391 | 18 | - | - |
| Symax | 195 (11) | Stryker | 2004–2008 | 195 | 0 | - | - |
| Bi-Metric | 178 (10) | Biomet | 2002–2009 | - | - | - | 178 |
| Corail | 102 (6) | DePuy | 2002–2009 | 31 | 15 | 56 | - |
| Profemur R | 18 (1) | Wright | 2006–2007 | 18 | 0 | - | - |
| S-ROM | 18 (1) | DePuy | 2002–2004 | 18 | 0 | - | - |
| 18 other stems | 84 (5) | 2002–2009 | - | - | - | 84 | |
| Total | 1,373 | 62 | 76 | 262 |
Ceramic liner titanium-enchased.
Sandwich design of the ceramic liner.
16% of the patients had CoC bearings and 84% had MoP bearings. The median follow-up time was 5.0 (3.1–6.5) years for CoC bearings and 3.9 (2.0–5.9) years for MoP bearings (p < 0.001). More males received CoC THAs than MoP THAs. The median patient age was 59 (52–65) years for CoC and 65 (59–70) years for MoP (p < 0.001). A greater proportion of patients with CoC THAs had been diagnosed with sequelae from a childhood hip disorder (p < 0.001) and more CoC patients than MoP patients had a CCI score equal to zero (p < 0.001). 60% of patients with CoC THA had their surgery during the period 2002–2005, whereas only 44% of patients with MoP THA had surgery then (p < 0.001). Patients with CoC THA had a higher proportion of 32-mm or larger femoral head sizes than patients with MoP THA (63% vs. 33%; p < 0.001). The most frequent cup/stem combinations were Plasmacup SC/Bicontact (42%), Lineage/Anca-Fit (16%), and Trident PLS/Symax (7%) for CoC THA and Trilogy/collarless Bi-Metric (34%), Mallory-Head/collarless Bi-Metric (19%), and Pinnacle/Corail (5%) for MoP THA. For patients with CoC THA, 81% (1,428 of 1,773) had a liner and 77% (1,373 of 1,773) had a femoral head made of BIOLOX forte.
Risk of any revision (Table 3)
Table 3.
Crude and adjusted relative risk (RR) of revision for any cause, with 95% confidence intervals (CIs), in total hip arthroplasty (THA) with ceramic-on-ceramic (CoC) and metal-on-polyethylene (MoP) bearingsa
| Patients at the start of the period (n) | Revisions performed within the period (%) | Crude RR (95% CI) | Adjusted RR (95% CI) | |
|---|---|---|---|---|
| At 2-year follow-up (0 to 2 years postoperatively) | ||||
| CoC | 1,773 | 48 (2.7) | 0.91 (0.67–1.24) | 1.18 (0.65–2.13) |
| MoP | 9,323 | 274 (2.9) | 1 (ref.) | 1 (ref.) |
| At 4-year follow-up (2 to 4 years postoperatively) | ||||
| CoC | 1,519 | 15 (1.0) | 0.95 (0.72–1.26) | 1.12 (0.70–1.81) |
| MoP | 7,065 | 62 (0.9) | 1 (ref.) | 1 (ref.) |
| At 6-year follow-up (4 to 6 years postoperatively) | ||||
| CoC | 1,135 | 4 (0.4) | 0.91 (0.68–1.21) | 1.03 (0.60–1.77) |
| MoP | 4,501 | 26 (0.6) | 1 (ref.) | 1 (ref.) |
| At 8.7-year follow-up (6 to 8.7 years postoperatively) | ||||
| CoC | 543 | 4 (0.8) | 1.02 (0.74–1.39) | 1.33 (0.72–2.43) |
| MoP | 2,230 | 11 (0.5) | 1 (ref.) | 1 (ref.) |
Adjustments were made for sex, age, diagnosis of primary THA, comorbidity, year of surgery, femoral head size, and duration of surgery.
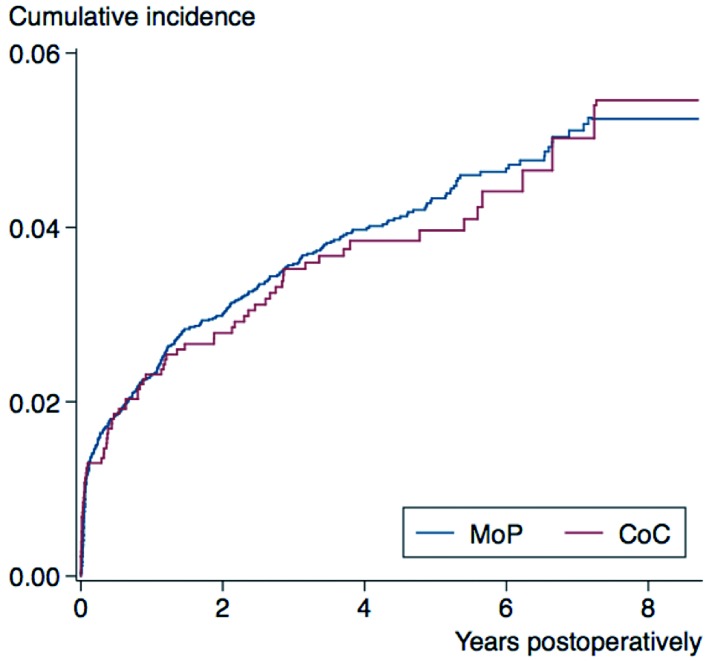
The entire study population had 444 revisions (4.0%): 4.0% (71 of 1,773) for CoC THA and 4.0% (373 of 9,323) for MoP THA. Revision rates were 0.84 (0.66–1.06) per 100 person-years for CoC bearings and 0.97 (0.88–1.08) per 100 person-years for MoP bearings. At 8.7 years of follow-up, the cumulative incidence for any revision was 5.4% (4.0–7.1) for CoC THA and 5.3% (4.7–5.9) for MoP THA (Figure). At 2, 4, 6, and 8.7 years of follow-up, there was no significant difference in the risk of revision of CoC THA and MoP THA for any reason.
Cumulative incidence for any revision of cementless total hip arthroplasty with ceramic-on-ceramic (CoC) and metal-on-polyethylene (MoP) bearings. See Table 3 for the relative risk of any revision at 2, 4, 6, and 8.7 years of follow-up.
Stratified analyses: risk of revision for any reason
For women, men, patients who were younger than 60 years, patients aged 60 years or older, patients diagnosed with OA, or patients who had no comorbidity (CCI score = 0), any comorbidity (CCI score > 0), or 28-mm or smaller femoral head, no significant differences in revision risk were found for CoC bearings and MoP bearings at 8.7 years of follow-up. At 7.5 years of follow-up, the revision risk was similar in the 2 bearing groups for femoral head sizes greater than 28 mm.
Causes of revision (Tables 4 and 5)
Table 4.
The main indications for total hip arthroplasty (THA) revision registered in the Danish Hip Arthroplasty Registry. For each type of THA bearing, numbers and percentages (%) are given regarding the causes of revision listed. The bearings included were ceramic-on-ceramic (CoC) and metal-on-polyethylene (MoP)
| CoC n = 71 | MoP n = 373 | p-value | |
|---|---|---|---|
| Aseptic loosening | 10 (0.6) | 43 (0.5) | 0.6 |
| Osteolysis without loosening | 0 (0.0) | 3 (0.0) | 0.5 |
| Deep infection | 6 (0.3) | 61 (0.7) | 0.1 |
| Femoral bone fracture | 9 (0.5) | 56 (0.6) | 0.6 |
| Dislocation | 22 (1.2) | 156 (1.7) | 0.2 |
| Component failure | 8 (0.5) | 6 (0.1) | < 0.001 |
| Pain | 9 (0.5) | 26 (0.3) | 0.1 |
| Other | 7 (0.4) | 22 (0.2) | 0.2 |
Table 5.
Characteristics of patients who were revised for fracture of the ceramic component
| Acetabular liner |
Femoral head |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Sex | Age at primary surgery | Years from primary surgery to revision | Component brand | Component fractured | Type of ceramic | Component brand | Component fractured | Type of ceramic | Size, mm |
| 1 | F | 53 | 4.8 | Duraloc Option | No | BIOLOX forte | S-ROM | Yes | BIOLOX forte | 28 |
| 2 | F | 60 | 7.2 | Plasmacup SC | Yes | BIOLOX forte | Bicontact | No | BIOLOX forte | 28 |
| 3 | M | 46 | 1.4 | Plasmacup SC | No | BIOLOX forte | Bicontact | Yes | BIOLOX forte | 28 |
| 4 | F | 65 | 5.7 | Plasmacup SC | No | BIOLOX forte | Bicontact | Yes | BIOLOX forte | 28 |
| 5 | F | 52 | 3.2 | Anca-Fit | Yes | BIOLOX forte | Anca-Fit | Yes | BIOLOX forte | 28 |
| 6 | M | 42 | 2.4 | Mallory-Head | Yes | Unknown | Bi-Metric collarless | Yes | BIOLOX delta | 28 |
8 CoC THAs were revised due to component failure. The proportion of revisions due to component failure was higher for CoC bearings than for MoP bearings (p < 0.001). Of the 8 patients who were registered as having component failure as the cause of revision, 6 patients had ceramic fracture and 2 patients had impingement between the stem-neck and the rim of the liner. In the 6 patients with ceramic fracture, 3 patients had an isolated ceramic head fracture, 1 patient had an isolated ceramic liner fracture, and 2 patients had fracture of both the ceramic head and liner. Thus, 5 patients had ceramic head fracture and 3 had ceramic liner fracture. Ceramic component fracture occurred at a median of 4.0 (1.4–7.2) years after primary surgery, and all the patients who had ceramic fracture had a 28-mm femoral head implanted. The causes of revision of MoP THA in patients registered with component failure as the cause of revision were: subluxation/instability (n = 2), subsidence of the cementless stem (n = 1), deep infection (n = 1), wear of the polyethylene liner (n = 1), and malposition of the acetabular component (n = 1).
Compared to MoP THA patients, patients with CoC THA had half the proportion of revision due to deep infection. The proportion due to dislocation was also lower, but these findings were not statistically significant.
Only 2 patients in the CoC group had revision registered as being due to “mechanical noises” and “squeaking”. For MoP, no revisions were performed because of noise from the THA.
There were no statistically significant differences in the risk of revision due to aseptic loosening (adjusted RR = 0.84, CI: 0.21–3.4), dislocation (adjusted RR = 1.2, CI: 0.29–5.3), and all other causes (adjusted RR = 1.1, CI: 0.14–8.8) between CoC bearings and MoP bearings at 8.7 years.
Discussion
In this nationwide, population-based study from the DHR involving 11,096 patients, CoC bearings did not have a statistically significantly higher overall risk of revision than MoP bearings after the maximum follow-up period of 8.7 years.
The main findings compared to other studies
In the Australian Orthopaedic Association National Joint Replacement Registry (AOA NJRR), the cumulative incidence of revision of CoC THA, with OA as diagnosis, at 10 years was 5.3%, which is similar to our findings after 8.7 years of follow-up. In studies with smaller series, a 5-year survival of 98% (Johansson et al. 2011) and a 6.7-year survival of 94–98% (Garcia-Rey et al. 2009) were found, corresponding to our findings. The main cause of revision in both studies was aseptic loosening. In contrast to this study, Khatod et al. (2014) found an 87% higher risk of aseptic revision in 510 CoC THAs than in 20,631 THAs with metal-on-highly cross-linked polyethylene. The median follow-up period in their study of 2.9 (IQR: 1.2–5.1) years and inclusion of all types of fixation method makes comparison between these findings and our findings difficult. Aseptic loosening can be caused by wear debris (Jacobs et al. 1994), and hip simulator studies have shown reduced wear rates for CoC bearings compared to MoP bearings.
The steady-state wear rate for alumina liners in an alumina head-alumina cup combination was 0.004 mm3 per million cycles over 14 million cycles in a hip simulator study—in contrast to 13 mm3 per million cycles for polyethylene liners in MoP bearings in the same study (Clarke et al. 2000). In other hip simulator studies, under severe microseparation conditions, BIOLOX forte showed steady-state wear rates of 1.3 mm3 per million cycles (Stewart et al. 2001); in contrast, the steady-state wear rates for BIOLOX delta components was 0.12 mm3 per million cycles (Stewart et al. 2003). Mean wear rates for BIOLOX forte CoC bearings retrieved after a minimum of 6 months in situ were reported to be 0.6 mm3/year for femoral heads and 0.5 mm3/year for acetabular liners (Lusty et al. 2007). Thus, simulator studies have shown less wear with CoC bearings, which—together with more bio-inert debris than polyethylene wear debris (Christel 1992)—may reduce the risk of aseptic loosening in CoC THAs, although this has not yet been shown in any study and should only become apparent with longer follow-up time. In the present study and in the above-mentioned studies with short- to medium-term follow-up (Garcia-Rey et al. 2009, Johansson et al. 2011, Khatod et al. 2014), revision due to aseptic loosening could certainly be related to fixation of the components rather than to wear.
A serious complication with ceramic implants is fracture, which may lead to reoperation and to a poor prognosis. One study showed that at a mean follow-up of 5 years after revision due to ceramic head fracture in 24 patients with BIOLOX forte ceramic bearings, 5 patients needed a second revision and 2 of them underwent a third revision (Koo et al. 2014). The reported incidence of ceramic component fracture varies from 0.01% to 3.5% (Ha et al. 2007, D’Antonio and Sutton 2009, Traina et al. 2011). In reports with the highest incidence of ceramic liner fracture, a sandwich design with a layer of polyethylene interposed between the ceramic liner and the acetabular shell was implanted (Ha et al. 2007, Lopes et al. 2012). The risk of fracture using BIOLOX forte (which is made of alumina) has been reduced with the introduction of BIOLOX delta, which is made of zirconia platelet-toughened alumina—making the material more resistant to fracture in ex vivo studies (Piconi et al. 2003). In the present study, 5 of the 6 patients who were revised due to ceramic fracture had BIOLOX forte-on-BIOLOX forte bearings. Other studies have found a prevalence of BIOLOX forte component fracture of 0–2% in smaller series (Yeung et al. 2012, Epinette and Manley 2014). All 6 patients with ceramic fracture had 28-mm femoral heads implanted, which are more prone to fracture than larger head sizes (D’Antonio and Sutton 2009, Traina et al. 2011). Furthermore, Traina et al. (2011) reported that 28-mm femoral heads designed to accept a short neck taper have a higher prevalence of fracture than heads that result in a longer taper. The DHR does not contain information on neck length, and such data are not included in this study. Apart from the material itself, there may be additional causes of ceramic fractures: both head and liner fractures could be due to trauma; debris (e.g. blood or fat) could be interposed between the neck taper or metal shell and the ceramic component; or there could have been course handling of the ceramic component during surgery. Moreover, head failure is associated with dislocation or a mismatch in design between the metal taper of the femoral neck and the ceramic head; and liner failure may be due to malpositioning of the implant or malseating of the ceramic liner into the metal shell (Traina et al. 2011).
None of the patients registered with component failure as the cause of revision had revision due to stem breakage. 1 patient with MoP bearings had revision due to component failure: wear of the polyethylene liner. 2 patients with MoP THA were revised due to problems related to the primary surgery: cup malpositioning and subsidence of the cementless stem, which may have been too small. 1 patient with MoP THA who was registered with component failure as the cause of revision actually had revision due to deep infection―which is a clear misclassification of the cause of revision. The incidence of revision due to deep infection of MoP THA was more than twice the incidence of revision due to deep infection of CoC THA. This finding was not statistically significant, but the trend has been seen in the National Joint Registry for England, Wales and Northern Ireland (2014).
CoC THAs have been described to make “squeaking”, “clicking”, “grinding”, “popping”, and “snapping” noises (Jarrett et al. 2009, Schroder et al. 2011). This complication might lead to revision surgery. We found only 2 patients (0.1%) who underwent revision due to noises from the CoC bearings. This is in accordance with a newly published meta-analysis, which has found an incidence of revision for squeaking of 0.2% (Owen et al. 2014). Our data are not conclusive in terms of the types of noises that lead to revision, as this information is not reported to the DHR.
Methodological considerations
The strengths of our study include the population-based design with prospectively collected data, the large sample size, and the complete follow-up, which limit possible selection and information bias. The medical databases that provide data to our study have independently registered data and they have documented moderate-to-high overall validity (Andersen et al. 1999, Pedersen et al. 2004, Pedersen et al. 2006). In addition, the DHR has a coverage (hospitals/clinics reporting to the registry) of over 95% and a completeness of 97% for primary THA (Danish Hip Arthroplasty Registry 2013); thus, the results are widely generalizable. In addition, all the CoC THAs that were revised for ceramic fracture were validated by searching in the medical files.
Our study also had several limitations. Although the DHR has been validated regarding a number of parameters, no validation regarding the registration of type of bearings has been made. We excluded 502 THAs that had no information registered concerning the bearings. We performed additional regression analyses for the 2 worst-case scenarios, presuming that these 502 THAs had either CoC or MoP bearings. Including the 502 THAs in the CoC group, the adjusted RR for revision for any reason was 1.2 (CI: 0.48–2.8) at 8.7 years for CoC THA compared to MoP THA. Including the 502 THAs in the MoP group provided an adjusted RR for revision for any reason of 1.3 (CI: 0.69–2.5) at 8.7 years for CoC THA compared to MoP THA. In both scenarios—before exclusion of the 502 patients as well as after their exclusion—no statistically significant difference in the RR of revision for any reason at 8.7 years was found. It is assumed that exclusion of the 28 patients with unregistered information on diagnosis, femoral head size, and/or duration of surgery would have no influence on the results in this large study population. We only included patients with cementless THAs in order to reduce the confounding effect of fixation. Adjustments for many confounders have been made, but there is still the possibility of unmeasured confounding, because the registries do not contain data on height, weight, BMI, and level of physical activity before and after surgery. Registration of causes of revision of THAs has never been validated in the DHR. Misclassification of causes of revision and also the lack of registration of revision in the DHR was unlikely to be related to the registration of the type of bearings for primary THAs; this produced a bias towards null. Furthermore, the median follow-up was longer for the CoC group than for the MoP group. This should be taken into account when interpreting the results, as the number of revisions—and especially revision due to aseptic loosening—would most likely increase with longer follow-up.
Conclusion
At 8.7 years of follow-up, CoC THA had a 33% higher risk of revision for any reason than MoP THA, but this was not statistically significant. CoC THA had a significantly higher incidence of revision due to component failure. The incidences of ceramic head and liner fracture were 0.28% and 0.17%, respectively.
Acknowledgments
CV, ABP, PKA, and SO designed the study protocol. CV and ABP collected the data. Analyses were planned by CV, ABP, PKA, and SO and were carried out by CV. CV wrote the initial draft of the manuscript, which was critically revised by CV, ABP, PKA, and SO.
We thank the Danish Rheumatism Association for financial support for this study.
No competing interests declared.
References
- Akagi M, Nonaka T, Nishisaka F, Mori S, Fukuda K, Hamanishi C. Late dissociation of an alumina-on-alumina bearing modular acetabular component . J Arthroplasty. 2004;19(5):647–51. doi: 10.1016/j.arth.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences . Dan Med Bull. 1999;46(3):263–8. [PubMed] [Google Scholar]
- Australian Orthopaedic Association. National Joint Replacement Registry, Annual Report 2013. 2013.
- Boutin P. [Total arthroplasty of the hip by fritted aluminum prosthesis. Experimental study and 1st clinical applications] . Rev Chir Orthop Reparatrice Appar Mot. 1972;58(3):229–46. [PubMed] [Google Scholar]
- CeramTec BIOLOX® delta - Nanocomposite for Arthroplasty. The Fourth Generation of Ceramics
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation . J Chronic Dis. 1987;40(5): 373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Christel PS. Biocompatibility of surgical-grade dense polycrystalline alumina . Clin Orthop Relat Res. 1992;(282):10–8. [PubMed] [Google Scholar]
- Clarke IC, Good V, Williams P, Schroeder D, Anissian L, Stark A, Oonishi H, Schuldies J, Gustafson G. Ultra-low wear rates for rigid-on-rigid bearings in total hip replacements . Proc Inst Mech Eng H. 2000;214(4):331–47. doi: 10.1243/0954411001535381. [DOI] [PubMed] [Google Scholar]
- D’Antonio JA, Sutton K. Ceramic materials as bearing surfaces for total hip arthroplasty . J Am Acad Orthop Surg. 2009;17(2):63–8. [PubMed] [Google Scholar]
- D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years . Clin Orthop Relat Res. 2012;470(2):373–81. doi: 10.1007/s11999-011-2076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Hip Arthroplasty Registry Annual Report 2013. 2013.
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods . J Clin Epidemiol. 2003;56(3):221–9. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- Epinette JA, Manley MT. No differences found in bearing related hip survivorship at 10-12 years follow-up between patients with ceramic on highly cross-linked polyethylene bearings compared to patients with ceramic on ceramic bearings . J Arthroplasty. 2014;29(7):1369–72. doi: 10.1016/j.arth.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Garcia-Rey E, Cruz-Pardos A, Garcia-Cimbrelo E. Alumina-on-alumina total hip arthroplasty in young patients: diagnosis is more important than age . Clin Orthop Relat Res. 2009;467(9):2281–9. doi: 10.1007/s11999-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam MH, Ryan P, Graves SE, Miller LN, de Steiger RN, Salter A. Competing risks survival analysis applied to data from the Australian Orthopaedic Association National Joint Replacement Registry . Acta Orthop. 2010;81(5):548–55. doi: 10.3109/17453674.2010.524594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha YC, Kim SY, Kim HJ, Yoo JJ, Koo KH. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty . Clin Orthop Relat Res. 2007;458:106–10. doi: 10.1097/BLO.0b013e3180303e87. [DOI] [PubMed] [Google Scholar]
- Habermann B, Ewald W, Rauschmann M, Zichner L, Kurth AA. Fracture of ceramic heads in total hip replacement . Arch Orthop Trauma Surg. 2006;126(7):464–70. doi: 10.1007/s00402-006-0173-y. [DOI] [PubMed] [Google Scholar]
- Hannouche D, Hamadouche M, Nizard R, Bizot P, Meunier A, Sedel L. Ceramics in total hip replacement . Clin Orthop Relat Res. 2005;(430):62–71. doi: 10.1097/01.blo.0000149996.91974.83. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Shanbhag A, Glant TT, Black J, Galante JO. Wear debris in total joint replacements . J Am Acad Orthop Surg. 1994;2(4):212–20. doi: 10.5435/00124635-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Jarrett CA, Ranawat AS, Bruzzone M, Blum YC, Rodriguez JA, Ranawat CS. The squeaking hip: a phenomenon of ceramic-on-ceramic total hip arthroplasty . J Bone Joint Surg (Am) 2009;91(6):1344–9. doi: 10.2106/JBJS.F.00970. [DOI] [PubMed] [Google Scholar]
- Johansson HR, Johnson AJ, Zywiel MG, Naughton M, Mont MA, Bonutti PM. Does acetabular inclination angle affect survivorship of alumina-ceramic articulations? . Clin Orthop Relat Res. 2011;469(6):1560–6. doi: 10.1007/s11999-010-1623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatod M, Cafri G, Namba RS, Inacio MC, Paxton EW. Risk factors for total hip arthroplasty aseptic revision . J Arthroplasty. 2014;29(7):1412–7. doi: 10.1016/j.arth.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time . Stat Med. 2007;26(24):4505–19. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- Koo KH, Ha YC, Kim SY, Yoon KS, Min BW, Kim SR. Revision of ceramic head fracture after third generation ceramic-on-ceramic total hip arthroplasty . J Arthroplasty. 2014;29(1):214–8. doi: 10.1016/j.arth.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Lopes R, Philippeau JM, Passuti N, Gouin F. High rate of ceramic sandwich liner fracture . Clin Orthop Relat Res. 2012;470(6):1705–10. doi: 10.1007/s11999-012-2279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty PJ, Watson A, Tuke MA, Walter WL, Walter WK, Zicat B. Orientation and wear of the acetabular component in third generation alumina-on-alumina ceramic bearings. An analysis of 33 retrievals . J Bone Joint Surg (Br) 2007;89(9):1158–64. doi: 10.1302/0301-620X.89B9.19282. [DOI] [PubMed] [Google Scholar]
- Min BW, Song KS, Kang CH, Bae KC, Won YY, Lee KY. Delayed fracture of a ceramic insert with modern ceramic total hip replacement . J Arthroplasty. 2007;22(1):136–9. doi: 10.1016/j.arth.2005.12.012. [DOI] [PubMed] [Google Scholar]
- National Joint Registry for England, Wales and Northern Ireland 11th Annual Report. 2014.
- Owen DH, Russell NC, Smith PN, Walter WL. An estimation of the incidence of squeaking and revision surgery for squeaking in ceramic-on-ceramic total hip replacement: a meta-analysis and report from the Australian Orthopaedic Association National Joint Registry. Bone Joint J. 2014;96-B(2):181–7. doi: 10.1302/0301-620X.96B2.32784. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Johnsen S, Overgaard S, Soballe K, Sorensen HT, Lucht U. Registration in the danish hip arthroplasty registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications . Acta Orthop Scand. 2004;75(4):434–41. doi: 10.1080/00016470410001213-1. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons . Dan Med Bull. 2006;53(4):441–9. [PubMed] [Google Scholar]
- Piconi C, Maccauro G, Muratori F, Brach Del Prever E. Alumina and zirconia ceramics in joint replacements . J Appl Biomater Biomech. 2003;1(1):19–32. [PubMed] [Google Scholar]
- Schroder D, Bornstein L, Bostrom MP, Nestor BJ, Padgett DE, Westrich GH. Ceramic-on-ceramic total hip arthroplasty: incidence of instability and noise . Clin Orthop Relat Res. 2011;469(2):437–42. doi: 10.1007/s11999-010-1574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T, Tipper J, Streicher R, Ingham E, Fisher J. Long-term wear of HIPed alumina on alumina bearings for THR under microseparation conditions . J Mater Sci Mater Med. 2001;12(10)(12):1053–6. doi: 10.1023/a:1012802308636. [DOI] [PubMed] [Google Scholar]
- Stewart TD, Tipper JL, Insley G, Streicher RM, Ingham E, Fisher J. Long-term wear of ceramic matrix composite materials for hip prostheses under severe swing phase microseparation . J Biomed Mater Res B Appl Biomater. 2003;66(2):567–73. doi: 10.1002/jbm.b.10035. [DOI] [PubMed] [Google Scholar]
- Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients . BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina F, Tassinari E, De FM, Bordini B, Toni A. Revision of ceramic hip replacements for fracture of a ceramic component: AAOS exhibit selection . J Bone Joint Surg (Am) 2011;93(24):e147. doi: 10.2106/JBJS.K.00589. [DOI] [PubMed] [Google Scholar]
- Yeung E, Bott PT, Chana R, Jackson MP, Holloway I, Walter WL, Zicat BA, Walter WK. Mid-term results of third-generation alumina-on-alumina ceramic bearings in cementless total hip arthroplasty: a ten-year minimum follow-up . J Bone Joint Surg (Am) 2012;94(2):138–44. doi: 10.2106/JBJS.J.00331. [DOI] [PubMed] [Google Scholar]