Abstract
Contemporaneous associations between circulating maternal organochlorines and measures of fetal heart rate and motor activity were evaluated. A panel of 47 organochlorines (OCs), including pesticides and polychlorinated biphenyls (PCBs), was analyzed from serum of 50 pregnant women at 36 weeks gestation. Data were empirically reduced into four factors and six individual compounds. All participants had detectable concentrations of at least one-quarter of the assayed OCs and, in general, higher socioeconomic level was associated with higher OC concentrations. Fetal heart rate measures were not consistently associated with maternal OCs. In contrast, one or more indicators of greater fetal motor activity were significantly associated with higher levels of the DDT and low chlorinated OC factors and five of the six individual compounds (heptachlor epoxide, trans nonachlor, oxychlordane, and PCBs 18 and 52). This preliminary demonstration of associations between fetal motor activity and maternal concentrations of persistent and pervasive environmental contaminants suggests that fetal assessment may be useful in ascertaining the potential early effects of these compounds on development.
Keywords: fetus, pesticides, PCBs, organochlorine, neurodevelopment, prenatal, motor activity
A substantial body of evidence has accumulated suggesting that maternal exposure during pregnancy and/or mobilization of bioaccumuled lipophilic compounds in maternal tissue to pesticides1–3 and PCBs4–6 has deleterious effects on offspring development. Efforts to identify adverse outcomes of prenatal exposures have focused on measurement of postnatal neurodevelopmental indicators as persistent expressions of underlying neural disruption to the developing fetal brain at the time of the exposure7. However, interpretation of associations generated by observational studies in human populations can be clouded by the degree to which prenatal and postnatal exposures covary and characteristics of the child-rearing environment that may be linked to both the prenatal exposure and the child development outcome8. In general, the more distal child performance is measured from the prenatal exposure, the greater the need for reliance on statistical controls to mitigate the influence of potential confounders introduced in the postnatal environment.
This issue can be circumvented, to some extent, by beginning the process of measuring neurodevelopment in vivo during the time of exposure. Although measuring the fetus provides different challenges than those inherent to measuring the child, quantification of the most conspicuous expressions of fetal neurodevelopment is possible. The developmental progressions of patterns of fetal heart rate and motor activity, in particular, have been well-documented. Measurement of phasic or non-phasic features of heart rate patterns in infancy and childhood has had a distinguished history in developmental science as marker of physiological regulation of the autonomic nervous system and its correspondence to performance and behavior9–13. Fetal heart rate, along with variation in rate, is the most accessible and studied aspect of fetal functional development. As gestation advances, fetal heart rate declines as metrics of variability increase14–17. These trajectories have been attributed, in part, to increased parasympathetic innervation of the heart18, 19. Measurement of a specific component of variability expressed as episodic and self-limiting surges or accelerations in heart rate is central to clinical antepartum assessment20. After 28 weeks gestation, characteristics of fetal heart rate, including baseline variability and accelerations, are significantly predictive of temperament characteristics in infancy11, 21 and developmental outcomes through the third year of life22, 23.
Similarly, motor activity is a key developmental parameter and marker of individual differences in both the fetus and child. Greater inhibitory control of motor activity is considered to be a hallmark of early child development24. Fetal motor activity is not as readily measured or consistently defined as fetal heart parameters, but also develops in predictable ways15, 25, 26. Spontaneously generated movements can be observed in the late embryonic/early fetal period27 and over gestation motor activity patterns coalesce into bouts of rest and activity that correspond to heart rate changes28. Describing developmental trends in motor activity can be challenging due to variation in movement criteria across studies29. In general, the number of movements declines slightly during the second half of gestation while duration and amplitude or vigor increases25, 26, 30, 31. There is wide inter-individual variability in fetal motor activity32 and although evidence is limited, more active fetuses tend to become more active infants21, 33, 34 and young children35. Lack of sufficient motor activity or a decline in its expression is a source of clinical obstetric concern36.
There is general consensus that these indicators provide information on development of the fetal nervous system37–39. Two types of studies support this position. One compares fetal heart rate and/or motor activity in normally developing fetuses to those with conditions or exposures that affect postnatal function. This includes congenital anomalies related to the nervous system40, 41, intrauterine growth restriction42, 43, maternal diabetes44 and pharmaceutical chemical exposures, including SSRIs45 and opioids46. The second type of study evaluates acute effects of experimentally manipulated exposures, for example, alcohol47, cigarette smoking48 or methadone49, 50. Together, this research has been referred to as “fetal behavioral teratology”51.
The current study applies fetal neurodevelopment methods to the long-standing interest in documenting potential effects on postnatal child development as a result of prenatal exposures to environmental contaminants52. The human fetus is well-exposed to these compounds. Maternal circulating levels of organochlorines are highly correlated with those measured in umbilical cord blood53, 54. Organochlorine pesticides and PCBs are present in placental tissue55, 56 and, importantly, are detectable in amniotic fluid57 which is continually swallowed and excreted by the fetus. This pilot study evaluates whether there are detectable associations between contemporaneous maternal OC levels and fetal heart rate patterns and motor activity near the end of pregnancy. Data were collected during the 36th week of pregnancy to allow maximal opportunity for maturation of both fetal heart rate and motor development.
Methods
Participants
Participants were 50 normotensive, non-smoking women with normally progressing pregnancies carrying singleton fetuses. Recruitment was conducted principally through self-referral based on flyers posted in the local academic and hospital communities. However, to ensure distribution across a range of socioeconomic strata, targeted recruitment was also directed at Medicaid-eligible pregnant women recruited through solicitation at a prenatal clinic serving an urban community. Sociodemographic characteristics are presented in Table 1. As expected, women in the lower socioeconomic status (SES) group were significantly younger, less educated, had lower income, and were less likely to be married than women in the upper SES group. Most women in the upper SES group lived in suburban (53.8%) or rural (15.4%) locales, while almost all (95%) women in the lower SES women lived in the urban center (X2 = 19.8, p < .001) in the vicinity of the recruitment hospital. With one exception, all offspring were delivered at term of normal birth weight, with 5 minute Apgar scores ≥ 8, and without postnatal complications (Table 1). One neonate had undetected prenatal growth restriction (i.e., term delivery but less than 2500 g; low SES group) and was excluded from the analysis. The project was approved by the University Institutional Review Board and all women provided written consent.
Table 1.
Characteristics of study participants and offspring by socioeconomic status
| All N=50 |
Upper SES N=30 |
Lower SES N=20 |
T/χ2 a | |
|---|---|---|---|---|
| Maternal measures | ||||
| Age (years) | ||||
| Mean (SD) | 27.6 (5.7) | 30.9 (3.7) | 22.7 (4.4) | 7.14*** |
| Race [N (%)] | ||||
| African-American | 19 (38.0%) | 2 (6.7%) | 17 (85.0%) | |
| Non-Hispanic white | 31 (62.0%) | 28 (93.3%) | 3 (15.0%) | 31.25*** |
| Education [N (%)] | ||||
| < HS diploma | 10 (20.0%) | 0 (0%) | 10 (50.0%) | |
| HS diploma | 8 (16.0%) | 2 (6.7%) | 6 (30.0%) | |
| > HS diploma | 32 (64.0%) | 28 (93.3%) | 4 (20.0%) | 29.17*** |
| Household income level [N (%)] | ||||
| ≤ $40,000 | 23 (60.0%) | 4 (13.3%) | 19 (95.0%) | |
| > $40,000 | 26 (40.0%) | 26 (86.7%) | 1 (5.0%) | 31.34*** |
| Marital status [N (%)] | ||||
| Married | 30 (60.0%) | 28 (93.3%) | 2 (10.0%) | |
| Single | 20 (40.0%) | 2 (6.7%) | 18 (90.0%) | 34.72*** |
| Parity [N (%)] | ||||
| 0 | 27 (54.0%) | 18 (60.0%) | 9 (45.0%) | |
| 1 | 11 (22.0%) | 7 (23.3%) | 4 (20.0%) | |
| ≥2 | 12 (24.0%) | 5 (16.7%) | 7 (35.0%) | 2.24 |
| Body Mass Index | ||||
| Mean (SD) | 25.3 (5.6) | 24.5 (3.9) | 26.5 (7.0) | −1.32 |
| Serum total lipids (g/dL) | ||||
| Mean (SD) | 897.04 (168.9) | 946.49 (172.3) | 822.87 (136.3) | 2.69** |
| Infant measuresb | ||||
| Gestational age | ||||
| Mean (SD) | 39.4 (1.1) | 39.1 (1.1) | 39.9 (1.1) | −2.39* |
| Birth weight | ||||
| Mean (SD) | 3422.9 (390.8) | 3492.5 (381.5) | 3322.1 (391.5) | 1.52 |
| 5 minute Apgar score | ||||
| Mean (SD) | 8.9 (.3) | 8.9 (.4) | 8.9 (.3) | 0.28 |
| Female sex | ||||
| [N (%)] | 21 (42%) | 12 (40%) | 9 (45%) | .12 |
T-tests used for continuous measures; Pearson’s Chi-square test for categorical measures.
Some birth outcomes not available for one infant.
p < 0.05;
p <.01; p < .001.
Procedure
Data were collected during the 36th week of gestation. Prenatal visits were scheduled at 13:30 and women were instructed to eat 1.5 hours prior to the visit but not thereafter. Women were positioned in a modified left lateral recumbent position. A brief ultrasound scan was administered to determine optimal transducer location, followed by 50 minutes of undisturbed monitoring and recording. Upon completion, blood samples were collected by venipuncture (20 ml), allowed to clot at room temperature for at least 1 hour, and then centrifuged. At the time of specimen collection, women had been fasting for approximately 2.5 hours. Serum was transferred to two glass vials and shipped to the Centers for Disease Control and Prevention (CDC) for analysis. Data were collected between September 1999 and December 2001 with the following distributions: 1999 (n = 11); 2000 (n = 27); 2001 (n = 12).
Fetal data collection and analysis
Fetal data were generated by a Toitu (MT320) fetal actocardiograph. This monitor detects fetal movement and fetal heart rate with a single wide array transabdominal Doppler transducer and processes this signal through a series of autocorrelation techniques. The actograph detects fetal movements by preserving the remaining signal after band-passing frequency components of the Doppler signal that are associated with FHR and maternal somatic activity. Reliability studies comparing actograph based versus ultrasound visualized fetal movements have found the performance of this monitor to be highly accurate in detecting both fetal motor activity and quiescence58–60.
Fetal data were collected from the output port of the monitor and digitized at 1000 Hz through an internal A/D board using streaming software. Off-line processing of fetal data, including application of algorithms to interpolate fetal heart rate for segments with artifact, was accomplished through customized software (GESTATE; James Long Company). Extracted cardiac measures include fetal heart rate, fetal heart rate variability, calculated as the standard deviation of fetal heart rate values per period, and the magnitude (i.e., area under the curve) of episodic accelerations in fetal heart rate identified using standard clinical parameters as each time fetal heart rate values attained 10 bpm above baseline for greater than or equal to 15 s. Fetal movement data represent raw voltage values generated from the actograph, calibrated by multiplying by a conversion factor with adjustment for offset, and scaled from 0 to 100 in arbitrary units (a.u.s). Fetal motor activity was quantified in three primary ways. The overall fetal movement signal during the recording was measured by averaging the signal values per minute and weighting each by squaring to amplify the signal to noise ratio (total movement). Individual movement bouts were identified each time the actograph signal attained amplitude of 15 units and ended when there was a cessation of 15 unit signals for at least 10 sec. The area under the curve for each movement was taken and averaged (movement size). Finally, the total time the fetus spent moving during the observation period was computed by multiplying the number of movements by the mean duration of each in seconds (time moving). Sample digitized fetal heart rate and motor data are presented in Figure 1.
Figure 1.
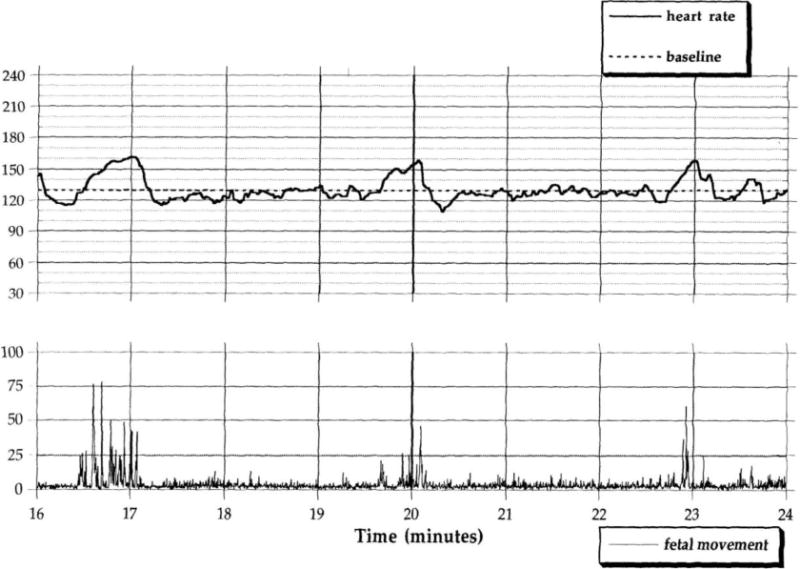
Examples of 8 minute segments of digitized fetal heart rate and fetal movement data in a 36 week fetus. The upper figure reflects a period of fairly brief movements (i.e., at minutes 16.5, 20, and 23) against a background of quiescence associated with self-limited accelerations in fetal heart rate. The lower figure shows periods of more persistent, unregulated movement and highly variable fetal heart rate.
Laboratory Methods for Chemical Analyte Analysis
Serum samples were tested for 11 pesticides and 36 polychlorinated biphenyl (PCB) congeners chosen based on a previously described method61. Briefly, 1 mL of serum was spiked with isotopically-labeled analogues of each OC pesticide and PCB of interest. The serum samples were dispersed over hydromatrix, lyophilized to remove residual water, and statically extracted under pressure at high temperature (i.e. pressurized fluid extraction) using dichloromethane and hexane. The concentrated residues were further cleaned using gel permeation chromatography to eliminate residual biogenic material. The final concentrated extracts were analyzed using gas chromatography-high resolution mass spectrometry with isotope dilution quantification. Quality control materials and blank samples were included in each run to ensure valid sample analysis. Limits of detection (LODs) were in the low pg/g range with relative standard deviations < 15%. Total cholesterol and triglyceride concentrations were determined using commercially available enzymatic methods. Measurements with concentrations below the LOD were imputed based upon the average LOD of each analyte, replacing the concentration with a value equal to the LOD divided by the square root of 262. Due to the lipophilic nature of these organochlorine compounds, analyte concentrations were adjusted by the maternal total serum lipid concentration63.
Statistical analysis
Given the large number of OCs relative to the small sample size, we adopted an empirical approach to data reduction. Principal component analysis (PCA) and factor analysis, using the PCA and FACTOR commands in Stata 11 (College Station, TX) were used to generate factors of analytes that displayed collinearity, consistent with data reduction methods used in other studies of maternal PCB and pesticide burden64, 65. The compositions of the final factors were based on iterative evaluation. Scores then were created for each factor based on the PCA components that had loading scores ≥0.50. Quartiles for each component analyte were added, and the sum divided by the number of components in that factor, thereby assigning equal weight to each component. Analytes that did not load on any factor, had detectable concentrations ≥0.1pg/g, and were present at detectable levels in greater than 85% of the sample were analyzed individually. Kruskal-Wallis equality of populations rank tests were used to compare exposure levels between sociodemographic groups. Due to the exploratory nature of this project, we elected to use a simple bivariate approach to the primary analyses of associations between exposures and fetal heart rate and motor measures focused on Pearson correlation coefficients with standard two-sided significance testing. We adjusted for multiple comparisons using a false discovery rate (FDR) approach, and we allowed for a FDR of 20% due to the exploratory nature of the study. Preliminary analyses revealed that data from one fetus included significant artifact from persistent hiccups which affect fetal heart rate66 and introduce artifact into the fetal movement signal; this case was removed from analyses between maternal OCs and fetal measures.
Results
Principal components analysis
One PCB congener (PCB149) was eliminated due to perfect collinearity with PCB151. Twenty-three of the remaining 46 lipid-adjusted compounds loaded on four unique factors. These four factors predominantly reflected constellations of low (FACTlow), moderate (FACTmid), and highly-chlorinated compounds (FACThigh) plus a DDT factor (FACTDDT), and accounted for 80% of the variance. Fifteen of the 23 compounds that did not load on any factor and were detected in fewer than 85% of the samples and two others with very low concentrations (PCB 151, PCB 189; < 0.1pg/g) were not examined further. The remaining three pesticides, oxychlordane (OXY), heptachlor epoxide (HEP), and trans nonachlor (TNA) and three PCB congeners (18, 52, 209) were examined individually in remaining analyses. Table 2 presents the geometric mean concentrations for the factors, their individual components, and the remaining compounds that were analyzed separately. Descriptive data for the entire suite of individual analytes is available in Supplementary Table 1.
Table 2.
Overall geometric mean (GM) values for factors (score) and individual analytes (pg/g) stratified by socioeconomic status
| Concentrations | N (%) > LOD | All N=49 |
High SES N=30 |
Low SES N=19 |
Ka |
|---|---|---|---|---|---|
| Factors | |||||
| DDT Factor (FACTDDT) | 49 (100%) | 2.51 | 2.67 | 2.25 | 2.80+ |
| p,p’-DDE (PDE) | 49 (100%) | 205.09 | 211.03 | 196.02 | 1.05 |
| o,p’-DDT (ODT) | 38 (78%) | 0.48 | 0.42 | 0.59 | 2.70+ |
| p,p’-DDT (PDT) | 49 (100%) | 3.99 | 3.99 | 3.98 | 0.61 |
| Beta hexachlorocyclohexane (BHC) | 49 (100%) | 5.05 | 6.31 | 3.54 | 24.86*** |
| Low Chlorinated factor (FACTlow) | 49 (100%) | 2.50 | 2.46 | 2.58 | 0.23 |
| Hexachlorobenzene (HCB) | 49 (100%) | 36.44 | 35.37 | 38.20 | 0.04 |
| PCB 28 | 49 (100%) | 7.17 | 6.40 | 8.56 | 1.18 |
| PCB 66 | 47 (94%) | 1.60 | 1.68 | 1.49 | 0.01 |
| Mid Chlorinated factor (FACTmid) | 49 (100%) | 2.51 | 2.82 | 2.03 | 7.28** |
| PCB 74 | 49 (100%) | 6.67 | 7.93 | 5.06 | 12.46*** |
| PCB 99 | 49 (100%) | 5.52 | 6.27 | 4.32 | 3.26+ |
| PCB 105 | 46 (94%) | 1.52 | 1.64 | 1.37 | 1.18 |
| PCB 118 | 49 (100%) | 8.72 | 10.30 | 6.69 | 5.57* |
| PCB 138–158 | 48 (98%) | 8.11 | 9.50 | 6.34 | 9.10** |
| PCB 157 | 31 (63%) | 0.23 | 0.34 | 0.13 | 4.82* |
| High Chlorinated factor (FACThigh) | 49 (100%) | 2.51 | 2.94 | 1.82 | 15.85*** |
| Mirex (MIR) | 47 (96%) | 1.19 | 1.59 | 0.97 | 15.20*** |
| PCB 153 | 49 (100%) | 19.02 | 23.66 | 13.47 | 12.17*** |
| PCB 156 | 43 (88%) | 1.64 | 2.52 | 0.83 | 15.85*** |
| PCB 170 | 44 (90%) | 2.92 | 4.32 | 1.58 | 17.87*** |
| PCB 172 | 29 (59%) | 0.19 | 0.27 | 0.11 | 4.47* |
| PCB 180 | 49 (100%) | 10.32 | 13.73 | 6.58 | 17.70*** |
| PCB 187 | 45 (92%) | 3.00 | 4.56 | 1.55 | 6.79** |
| PCB 194 | 44 (90%) | 1.61 | 2.46 | 0.82 | 16.34*** |
| PCB 196–203 | 41 (84%) | 0.81 | 1.33 | 0.37 | 15.85*** |
| PCB 206 | 49 (100%) | 1.68 | 1.94 | 1.35 | 4.04* |
| Unloaded organochlorine pesticides and polychlorinated biphenyls (PCBs) | |||||
| Heptachlor epoxide (HEP) | 47 (96%) | 3.98 | 4.87 | 2.90 | 11.60*** |
| Oxychlordane (OXY) | 47 (96%) | 7.25 | 9.47 | 4.76 | 16.01*** |
| Trans nonachlor (TNA) | 49 (100%) | 11.20 | 13.97 | 7.90 | 16.84*** |
| PCB 18 | 45 (90%) | 1.35 | 1.57 | 1.05 | 0.89 |
| PCB 52 | 45 (90%) | 1.02 | 0.99 | 1.07 | 0.01 |
| PCB 209 | 47 (96%) | 0.80 | 0.82 | 0.77 | 0.43 |
Kruskal-Wallis equality-of-proportions rank test
Note.
p <.10;
p < .05;
p < .01;
p < .001.
Associations between fetal measures and OCs
Fetal heart rate
Descriptive information for the three fetal cardiac measures is as follows: fetal heart rate, M = 141.0, sd = 7.7; fetal heart rate variability, M = 9.0, sd = 2.3; acceleratory area, M = 494.7, sd = 132.6. Maternal OCs were unrelated to fetal rate and variability. The third cardiac measure, acceleration size, displayed significant or trend level associations with two OC factors and two individual pesticides. Higher maternal FACThigh and OXY levels were associated with less robust accelerations in heart rate (rs = −0.41 and −0.31, respectively, ps < .05); this relationship neared significance for both FACTDDT and TNA (rs for both = −0.27, ps < .07).
Fetal motor activity
Descriptive information for the three fetal movement variables is as follows: total movement, M = 52.6, sd = 69.6; movement area, M = 84.0, sd = 140.5; time spent moving, M = 825.0, sd = 648.7. Correlation coefficients and statistical significance for associations between the final organochlorine variables and fetal motor measures are presented in Table 3. Significant, positive associations were detected between at least one of the fetal movement measures and FACTDDT, FACTlow, each of the three individual pesticides (HEP, TNA, and OXY), and two of the three individually analyzed PCBs (18 and 52). While associations with FACTmid did not attain significance, the associations were also consistently positive. No associations with either FACThigh or PCB 209 neared or attained significance with fetal movement measures; these are not included in Table 3.
Table 3.
Unadjusted associations between fetal movement measures and maternal OC concentrations for all participants (N = 48)
| Factors | Unloaded compounds | |||||||
|---|---|---|---|---|---|---|---|---|
| DDT Factor (FACTDDT) | Low Chlorinated Factor (FACTlow) | Mid Chlorinated factor (FACTmid) | Heptachlor epoxide (HEP) | Trans nonachlor (TNA) | Oxychlordane (OXY) | PCB 18 | PCB 52 | |
| Total movement | .29* | .33* | .20 | .29* | .31* | .26+ | .21 | .52** |
| Movement size | .34* | .37** | .21 | .30* | .30* | .30* | .35* | .48** |
| Time moving | .22 | .24+ | .15 | .20 | .14 | .21 | .27+ | .43** |
p < .10;
p < .05;
p < .01. Figure legend
Multiple comparisons
We tested 27 associations among fetal movement indicators and organochlorine variables. Allowing for a false discovery rate of 20%, all associations observed at an alpha of 0.1 (p ≤ 0.10) remained significant using this approach.
Socioeconomic status and OCs
The final column in Table 2 provides results for comparisons between SES groups for the factors, their components, and the remaining compounds. Concentrations for the upper SES group were significantly higher for FACTmid and FACThigh and for each of the unloaded pesticides; FACTDDT displayed a trend level difference. Because maternal age differed significantly by SES and is a plausible source of the differences in OC exposure, post hoc analyses were conducted with SES groups combined. Older women had significantly higher levels of the mid and high chlorinated factors (FACTmid, r = 0.43, p < .01, FACThigh r = 0.74, p < .0001), each individual pesticide (HEP r = 0.31, p < .05; TNA r = 0.37, p < .01; and OXY r = 0.46, p < .001), and a trend level for higher DDT concentrations (FACTDDT, r = .27, p = .06.). Age was unrelated to individual PCB levels.
Evaluation of sociodemographic factors as potential confounders in the observed relations between maternal OCs and fetal measures indicated that neither SES nor maternal age was related to fetal heart rate, variability or motor activity measures. However acceleration area was significantly reduced in fetuses of lower SES women (t (46) = −3.05, p < .01) and negatively related to maternal age (r = −0.38, p < .01). Given the association with both dependent and independent measures, controlling for SES and maternal age eliminated the significant and near significant associations between acceleratory area and the four OCs noted above.
Discussion
This pilot study provides evidence for modest, but significant, positive associations between prenatal maternal organochlorine levels and fetal motor activity. Findings are based on a sample of women with normally progressing pregnancies drawn from populations without specific occupational or agricultural exposures. Consistent with a recent report of the persistent nature of these chemical analytes in the blood of pregnant women in the United States based on NHANES 2003–2004 data67, all fifty participants had detectable concentrations of at least one-quarter of the analyzed organochlorines despite the fact that they have been banned for over three decades. Our data were generated by the same laboratory that analyzed the NHANES data, and of the analytes reported both here and in that report (i.e., hexachlorobenzene, p,p′-DDE, PCBs 118, 153, 138–158 and 180), geometric concentrations in the current study were somewhat higher for all analytes except PCB138–158. All participants resided in the Baltimore metropolitan area at the time of assessment. Urban and suburban populations may be exposed to persistent OC compounds from environmental sources68, and a number of banned OC compounds were detected in the airshed69 and watershed of the local Chesapeake Bay region during the late 1990s and early 2000s.
Higher concentrations of seven of the ten individual or aggregated organochlorine compounds evaluated in this study were positively associated with one or more measures indicative of greater or more vigorous fetal motor activity. This includes the low chlorinated factor, comprised of hexachlorobenzene and two PCB congeners, the DDT factor, all three individual pesticides and two of the three individually analyzed PCBs. What does this portend for postnatal development? Few existing studies have measured both prenatal and postnatal activity level, but those that do have reported modest but significant associations between fetal motor activity and motor activity shortly after birth34, 70, during infancy21, 33, 35 and through age 235. Unlike measures of fetal heart rate, fetal activity levels do not necessarily correspond to a continuum of optimality. Although prolonged periods of absent fetal motor activity are clinically ominous, neither high nor low levels within the normal range are considered indicative of “better” development, with the exception of two reports that higher fetal motor activity is predictive of more mature infant motor function30, 71. Thus, as in the postnatal period, fetal activity level may be best regarded as a dimension of temperament72, 73. Nonetheless, worth noting are recent reports of links between prenatal organochlorine exposure and both teacher-rated74 and laboratory-based performance measures75 of child behaviors that reflect the continuous temperament dimensions underlying attention deficit hyperactivity disorder (ADHD). However, we caution against making the leap that increased fetal motor activity portends this childhood outcome as only one report examines this to date. Mothers of children with ADHD reported higher levels of felt fetal motor activity as compared to mothers of control children; however reliance on retrospective maternal recollection of prenatal activity after childhood diagnosis threatens interpretability of this study76.
Contrary to expectations based on the use of heart rate variability as an index of the development of autonomic regulation in infancy and childhood, significant associations between maternal organochlorines and fetal heart rate and variability were not detected. This is consistent with the lack of associations reported between prenatal and contemporaneous organophosphate levels and similar heart rate measures during early childhood77. Although initial bivariate analyses suggested negative associations between acceleratory magnitude, these associations did not persist when SES and maternal age, which were significantly associated with both the exposures and the outcome variable, were controlled. A larger sample would provide opportunity to better parse the contributing role of each. In addition, our ability to detect associations with fetal heart rate variability per se may be a result of a measurement limitation. Fetal heart rate variable quantification was based on the standard Doppler method available at the time of the study which detects motion of the fetal heart. More recent technology affords detection and timing of the fetal electrocardiogram (fECG) thereby providing a more precise metric of beat to beat variability and may unmask additional associations.
This is not the first report of higher OC concentrations in more socioeconomically advantaged women than in less advantaged women. Consistent results have been reported in population-based cohorts of pregnant women in Spain in the mid 2000’s78, 79. Analysis of stored serum from a New York City sub-cohort of the National Collaborative Perinatal Project (1959–1962) revealed that pregnant African-American women of higher income levels had higher PCB concentrations than pregnant African-American women with lower incomes80. Results based on another New York cohort of primiparous women reported higher DDE and PCB concentrations in Caucasian women than other race/ethnic groups, but also found the Caucasian women were older81. Those authors suggested that maternal age might explain the association, since maternal age remained significant in multivariate models, while race did not81. Maternal age has also been shown to be associated with higher OC levels in at least two other studies of pregnant women in the U.S.82, 83. Older women have longer and presumably more diverse opportunities to encounter OC sources, and there was an eight year differential in age between women in the upper and lower SES groups in the current sample. However, since more educated women tend to delay child-bearing, variation in maternal OC concentrations between groups reflects a persistent confounder in fetal exposure.
The prenatal period is not monolithic; neuromaturation commences early in gestation and is expressed through more complex differentiation of function over time. The cross-sectional design of this study cannot provide information on whether these associations, if real, are the result of acute or more persistent alterations to the developing fetal nervous system. Moreover, because data were collected near the end of pregnancy, it is not possible to identify when maternal OCs exert biological influence, the degree to which the trajectory of development has been altered, and whether associations with motor activity earlier in development would have been more or less pronounced. In late pregnancy, fetal motor activity is subject to increasing mechanical constraints of the uterus in tandem with increasing fetal size; it is possible that measurement of fetal motor activity earlier in gestation when there is less external constraint may yield larger associations.
These data provide preliminary support for detectable effects of maternal organochlorine exposures on fetal motor activity. Data were collected using digitized actigraphy methodology that eliminates the need for continuous ultrasound visualization. Interpretation is limited by a small sample, which restricted our ability to examine interactions among contaminants or to employ more sophisticated mixture models. Moreover, the lack of specific hypothesis testing makes it difficult to interpret the pattern of significant and non-specific findings between different factors and compounds. Yet, even in animal models that can provide controlled administration of compounds, reports of associations between behaviors – including activity level – with some congeners, but not others, is not uncommon84.
Given the susceptibility of the developing fetus to environmental exposures, and the amplification of effects over time generated from minor deviations early in development, there is clear need for better understanding of the effects of organochlorines and other substances on the developing fetus and child85. Data collection on the fetus can provide a window into the early potential effects of these compounds on neurodevelopment and may be useful in efforts to advance regulatory efforts to protect vulnerable populations.
Supplementary Material
Acknowledgments
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) 2R01 HD27592, awarded to J. A. DiPietro and a pilot project funded by the National Institute of Environmental Health Sciences (NIEHS) Center grant ES03819.
Contributor Information
Janet A. DiPietro, Email: jdipietr@jhsph.edu.
Meghan F. Davis, Email: mdavis@jhsph.edu.
Kathleen A Costigan, Email: kcostig@jhmi.edu.
Dana Boyd Barr, Email: dbbarr@emory.edu.
References
- 1.Bouchard M, Chevrier J, Harley K, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel S, Wetmur J, Chen J, Barr D, Canfield R, Wolff M. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr D, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forns J, Lertxundi N, Aranbarri A, Murcia M, Gascon M, Martinez D, et al. Prenatal exposure to organochlorine compounds and neuropsychological development up to two years of life. Environ Int. 2012;45:72–77. doi: 10.1016/j.envint.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson J, Jacobson S. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 6.Schantz S, Widholm J, Rice D. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winneke G. Appraisal of neurobehavioral methods in environmental health research: the developing brain as a target for neurotoxic chemicals. Int J Hyg Environ Health. 2007;210:601–609. doi: 10.1016/j.ijheh.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson J, Jacobson S. Methodological considerations in behavioral toxicology in infants and children. Dev Psychol. 1996;32:390–403. [Google Scholar]
- 9.Bornstein MH, Suess PE. Physiological self-regulation and information processing in infancy: cardiac vagal tone and habituation. Child Dev. 2000;71:273–287. doi: 10.1111/1467-8624.00143. [DOI] [PubMed] [Google Scholar]
- 10.Dunster KR. Physiologic variability in the perinatal period. Clin Perinatol. 1999;26:801–809. [PubMed] [Google Scholar]
- 11.Snidman N, Kagan J, Riordan L, Shannon D. Cardiac function and behavioral reactivity during infancy. Psychophysiology. 1995;32:199–207. doi: 10.1111/j.1469-8986.1995.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 12.Calkins S. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Dev Psychobiol. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Fox NA, Porges S. The relation between neonatal heart period patterns and developmental outcome. Child Dev. 1985;56:28–37. [PubMed] [Google Scholar]
- 14.Dawes GS, Moulden M, Sheil O, Redman CWG. Approximate entropy, a statistic of regularity, applied to fetal heart rate data before and during labor. Obstet Gynecol. 1992;80:763–768. [PubMed] [Google Scholar]
- 15.DiPietro JA, Caulfield LE, Costigan KA, Merialdi M, Nguyen RHN, Zavaleta N, et al. Fetal neurobehavioral development: a tale of two cities. Dev Psychol. 2004;40:445–456. doi: 10.1037/0012-1649.40.3.445. [DOI] [PubMed] [Google Scholar]
- 16.van Leeuwen P, Lange S, Bettermann H, Gronemeyer D, Hatzmann W. Fetal heart rate variability and complexity in the course of pregnancy. Early Hum Dev. 1999;54:259–269. doi: 10.1016/s0378-3782(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 17.Nijhuis I, ten Hof J, Mulder E, Nijhuis J, Narayan H, Taylor D, et al. Numerical fetal heart rate analysis: nomograms, minimal duration of recording, and intrafetal consistency. Prenat Neonatal Med. 1998;3:314–322. [Google Scholar]
- 18.Dalton K, Dawes GS, Patrick JE. The autonomic nervous system and fetal heart rate variability. Am J Obstet Gynecol. 1983;146:456–462. doi: 10.1016/0002-9378(83)90828-1. [DOI] [PubMed] [Google Scholar]
- 19.Freeman RK, Garite TJ, Nageotte MP. Physiologic basis of fetal monitoring. In: Freeman RK, Garite TJ, Nageotte MP, editors. Fetal Heart Rate Monitoring. 2nd. Baltimore, MD: Williams & Wilkins; 1991. pp. 7–20. [Google Scholar]
- 20.Ware DJ, Devoe LD. The nonstress test: reassessment of the “gold standard”. Clin Perinatol. 1994;21:779–796. [PubMed] [Google Scholar]
- 21.DiPietro J, Hodgson DM, Costigan KA, Johnson TRB. Fetal antecedents of infant temperament. Child Dev. 1996;67:2568–2583. [PubMed] [Google Scholar]
- 22.Bornstein MH, DiPietro JA, Hahn CS, Painter K, Haynes OM, Costigan KA. Prenatal cardiac function and postnatal cognitive development: an exploratory study. Infancy. 2002;3:475–494. [Google Scholar]
- 23.DiPietro JA, Bornstein MH, Hahn CS, Costigan KA, Achy-Brou A. Fetal heart rate and variability: Stability and prediction to developmental outcomes in early childhood. Child Dev. 2007;78:1788–1798. doi: 10.1111/j.1467-8624.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaton W, McKeen N, Campbell D. The waxing and waning of movement: implications for psychological development. Dev Rev. 2001;21:205–223. [Google Scholar]
- 25.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of gross fetal body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am J Obstet Gynecol. 1982;142:363–371. doi: 10.1016/s0002-9378(16)32375-4. [DOI] [PubMed] [Google Scholar]
- 26.ten Hof J, Nijhuis IJM, Mulder EJH, Nijhuis JG, Narayan H, Taylor DJ, et al. Longitudinal study of fetal body movements: nomograms, intrafetal consistency and relationship with episodes of heart rate patterns A and B. Pediatr Res. 2002;52:568–575. doi: 10.1203/00006450-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 27.deVries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behaviour. I. Qualitative aspects. Early Hum Dev. 1982;7:301–322. doi: 10.1016/0378-3782(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 28.Groome LJ, Watson JE. Assessment of in utero neurobehavioral development: I. Fetal behavioral states. J Matern Fetal Investig. 1992;2:183–194. [Google Scholar]
- 29.ten Hof J, Nijhuis IJM, Mulder EJH, Nijhuis JG, Narayan H, Taylor DJ, et al. Quantitative analysis of fetal generalized movements: methodological considerations. Early Hum Dev. 1999;56:57–73. doi: 10.1016/s0378-3782(99)00035-3. [DOI] [PubMed] [Google Scholar]
- 30.DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson J, et al. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010;81:115–130. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roodenburg PJ, Wladimiroff JW, van Es A, Prechtl HFR. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Hum Dev. 1991;25:19–35. doi: 10.1016/0378-3782(91)90203-f. [DOI] [PubMed] [Google Scholar]
- 32.deVries J, Fong B. Normal fetal motility: an overview. Ultrasound Obstet Gynecol. 2006;27:701–711. doi: 10.1002/uog.2740. [DOI] [PubMed] [Google Scholar]
- 33.Degani S, Leibovitz Z, Shapiro I, Ohel G. Twins’ temperament: early prenatal sonographic assessment and postnatal correlation. J Perinatol. 2009:1–6. doi: 10.1038/jp.2008.238. [DOI] [PubMed] [Google Scholar]
- 34.Groome L, Swiber M, Holland S, Bentz L, Atterbury J, Trimm R. Spontaneous motor activity in the perinatal infant before and after birth: stability in individual differences. Dev Psychobiol. 1999;35:15–24. doi: 10.1002/(sici)1098-2302(199907)35:1<15::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 35.DiPietro JA, Bornstein MH, Costigan KA, Pressman EK, Hahn CS, Painter K, et al. What does fetal movement predict about behavior during the first two years of life? Dev Psychobiol. 2002;40:358–371. doi: 10.1002/dev.10025. [DOI] [PubMed] [Google Scholar]
- 36.Velazquez M, Rayburn W. Antenatal evaluation of the fetus using fetal movement monitoring. Clin Obstet Gynecol. 2002;45:993–1004. doi: 10.1097/00003081-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Krasnegor N, Fifer W, Maulik D, McNellis D, Romero R, Smotherman W. Fetal behavioral development: a transdisciplinary perspective for assessing fetal well-being and predicting outcome. Prenat Neonatal Med. 1998;3:185–190. [Google Scholar]
- 38.Amiel-Tison C, Gosselin J, Kurjak A. Neurosonography in the second half of fetal life: a neonatologist’s point of view. J Perinat Med. 2006;34:437–446. doi: 10.1515/JPM.2006.088. [DOI] [PubMed] [Google Scholar]
- 39.Nijhuis IJM, ten Hof J. Development of fetal heart rate and behavior: indirect measures to assess the fetal nervous system. Eur J Obstet Gynecol Reprod Biol. 1999;87:1–2. doi: 10.1016/s0301-2115(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 40.Maeda K, Morokuma S, Yoshida S, Ito T, Pooh R, Serizawa M. Fetal behavior analyzed by ultrasonic actocardiogram in cases with central nervous system lesions. J Perinat Med. 2006;34:398–403. doi: 10.1515/JPM.2006.079. [DOI] [PubMed] [Google Scholar]
- 41.Morokuma S, Fukushima K, Otera Y, Yumoto Y, Tsukimori K, Ochiai M, et al. Ultrasound evaluation of fetal brain dysfunction based on behavioral patterns. Brain Dev. 2013;35:61–67. doi: 10.1016/j.braindev.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Graatsma E, Mulder E, Vasak B, Lobmaier S, Pildner von Steinburg S, Schneider K, et al. Average acceleration and deceleration capacity of fetal heart rate in normal pregnancy and pregancies complicated by fetal growth restriction. J Matern Fetal Neonatal Med. 2012;25:2517–2522. doi: 10.3109/14767058.2012.704446. [DOI] [PubMed] [Google Scholar]
- 43.Nijhuis IJM, ten Hof J, Mulder EJ, Nijhuis JG, Narayan H, Taylor D, et al. Fetal heart rate in relation to its variation in normal and growth retarded fetuses. Eur J Obstet Gynecol Reprod Biol. 2000;89:27–33. doi: 10.1016/s0301-2115(99)00162-1. [DOI] [PubMed] [Google Scholar]
- 44.Kainer F, Prechtl H, Engele H, Einspieler C. Assessment of the quality of general movements in fetuses and infants of women with type-I diabetes mellitus. Early Hum Dev. 1997;50:13–25. doi: 10.1016/s0378-3782(97)00089-3. [DOI] [PubMed] [Google Scholar]
- 45.Mulder E, Ververs F, de Haus R, Visser G. Selective serotonin reuptake inhibitors affect neurobehavioral development in the human fetus. Neuropsychopharmacology. 2011;36:1961–1971. doi: 10.1038/npp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansson L, DiPietro JA, Elko A, EL Williams Milio L, Velez M. Pregnancies exposed to methadone, methadone and other substances, and poly-drugs without methadone: a comparison of fetal neurobehaviors and infant outcomes. Drug Alcohol Depend. 2012;122:213–219. doi: 10.1016/j.drugalcdep.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulder EJ, Morssink LP, van der Schee T, Visser GHA. Acute maternal alcohol consumption disrupts behavioral state organization in the near term fetus. Pediatr Res. 1998;44:774–779. doi: 10.1203/00006450-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 48.Coppens M, Vindla S, James D, Sahota D. Computerized analysis of acute and chronic changes in fetal heart rate variation in association with maternal smoking. Am J Obstet Gynecol. 2001;185:421–426. doi: 10.1067/mob.2001.115992. [DOI] [PubMed] [Google Scholar]
- 49.Jansson LM, DiPietro JA, Elko A. Fetal response to maternal methadone administration. Am J Obstet Gynecol. 2005;193:611–617. doi: 10.1016/j.ajog.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 50.Wouldes T, Roberts A, Pryor J, Bagnall C, Gunn T. The effect of methadone treatment on the quantity and quality of human fetal movement. Neurotoxicol Teratol. 2004;26:23–34. doi: 10.1016/j.ntt.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Visser G, Mulder E, Ververs FT. Fetal behavioral teratology. J Matern Fetal Neonatal Med. 2010;23:14–16. doi: 10.3109/14767058.2010.517717. [DOI] [PubMed] [Google Scholar]
- 52.Fein G, Schwartz P, Jacobson S, Jacobson J. Environmental toxins and behavioral development: a new role for psychological research. Am Psychol. 1983:1188–1197. doi: 10.1037//0003-066x.38.11.1188. [DOI] [PubMed] [Google Scholar]
- 53.Bergonzi R, Specchia C, Dinolfo M, Tomasi C, DePalma G, Frusca T, et al. Distribution of persistent organochlorine pollutants in maternal and feotal tissues: data from an Italian polluted urban area. Chemosphere. 2009;76:747–754. doi: 10.1016/j.chemosphere.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 54.Covaci A, Jorens P, Jacquemyn Y, Schepens P. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298:45–53. doi: 10.1016/s0048-9697(02)00167-5. [DOI] [PubMed] [Google Scholar]
- 55.Ando M, Saito H, Eakisaka L. Gas chromatographic and mass spectometric analysis of polychlorinated biphenyls in human placenta and cord blood. Environ Res. 1986;41:14–22. doi: 10.1016/s0013-9351(86)80163-3. [DOI] [PubMed] [Google Scholar]
- 56.Bergonzi R, DePalma G, Specchia C, Dinolfo M, Tomasi C, Frusca T, et al. Persistent organochlorine compounds in fetal and maternal tissues: evaluation of their potential influence on several indicators or fetal growth and health. Sci Total Environ. 2011;409:2888–2893. doi: 10.1016/j.scitotenv.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Luzardo O, Mahtani V, Troyano J, Alvarez de la Rosa M, Padilla-Perez A, Zumbado M, et al. Determinants of organochlorine levels detectable in the amniotic fluid of women from Tenerife Island (Canary Islands, Spain) Environ Res. 2009;109:607–613. doi: 10.1016/j.envres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Besinger RE, Johnson TRB. Doppler recordings of fetal movement: clinical correlation with real-time ultrasound. Obstet Gynecol. 1989;74:277–280. [PubMed] [Google Scholar]
- 59.DiPietro JA, Costigan KA, Pressman EK. Fetal movement detection: Comparison of the Toitu actograph with ultrasound from 20 weeks gestation. J Matern Fetal Med. 1999;8:237–242. doi: 10.1002/(SICI)1520-6661(199911/12)8:6<237::AID-MFM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 60.Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by Doppler actocardiogram and fetal B-mode imaging. Clin Perinatol. 1999;26:829–851. [PubMed] [Google Scholar]
- 61.Barr D, Weihe P, Davis M, Needham L, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere. 2006;62:1167–1182. doi: 10.1016/j.chemosphere.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 62.Gleit A. Estimation for small normal data sets with detection limits. Environ Sci Technol. 1985;19:1201–1206. doi: 10.1021/es00142a011. [DOI] [PubMed] [Google Scholar]
- 63.Akins J, Waldrep K, Bernert J. The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 64.Skrbic B, Szyrwinska K, Durisic-Mladenovic N, Nowicki P, Lulek J. Principal component analysis of indicator PCB profiles in breast milk from Poland. Environ Int. 2010;36:862–872. doi: 10.1016/j.envint.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Neta G, Goldman L, Barr D, Sjodin A, Apelberg B, Witter F, et al. Distribution and determinants of pesticide mixtures in cord serum using principal components analysis. Environ Sci Technol. 2010;44:5641–5648. doi: 10.1021/es1009778. [DOI] [PubMed] [Google Scholar]
- 66.Witter F, DiPietro J, Costigan K, Nelson P. The relationship between hiccups and heart rate in the fetus. J Matern Fetal Neonatal Med. 2007;20:289–292. doi: 10.1080/14767050601155715. [DOI] [PubMed] [Google Scholar]
- 67.Woodruff T, Zota A, Schwartz J. Environmental chemicals in pregnant women in the US: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W, Ye Y, Hu D, Ou L, Wang X. Characteristics and transport of organochlorine pesticides in urban environment. J Environ Monit. 2010;12:2153–2160. doi: 10.1039/c0em00110d. [DOI] [PubMed] [Google Scholar]
- 69.Goel A, McConnell M, Torrents A, Kuang Z, Hapeman C, Meritt D, et al. Environmental toxicology and chemistry. SETAC. 2010:1893–1906. doi: 10.1002/etc.243. [DOI] [PubMed] [Google Scholar]
- 70.Almli CR, Ball RH, Wheeler ME. Human fetal and neonatal movement patterns: Gender differences and fetal-to-neonatal continuity. Dev Psychobiol. 2001;38:252–273. doi: 10.1002/dev.1019. [DOI] [PubMed] [Google Scholar]
- 71.Richards T, Newbery H. Studies in fetal behavior: III. Can performance on test items at six months postnatally be predicted on the basis of fetal activity? Child Dev. 1938;9:79–86. [Google Scholar]
- 72.Eaton WO, Saudino KJ. Prenatal activity level as a temperament dimension? Individual differences and developmental functions in fetal movement. Inf Behav Dev. 1992;15:57–70. [Google Scholar]
- 73.Rothbart M, Ahadi S, Evans D. Temperament and personality: origins and outcomes. J Pers Soc Psychol. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- 74.Sagiv S, Thurston S, Bellinger D, Tolbert P, Altshul L, Korrick S. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school aged children. Am J Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sagiv S, Thurston S, Bellinger D, Altshul L, Korrick S. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect. 2012;120:904–909. doi: 10.1289/ehp.1104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Accardo P, Tomazic T, Fete T, Heaney M, Lindsay R, Whitman B. Maternally reported fetal activity levels and developmental diagnoses. Clin Pediatr (Phila) 1997;36:279–283. doi: 10.1177/000992289703600505. [DOI] [PubMed] [Google Scholar]
- 77.Quiros-Alcala L, Alkon A, Boyce W, Lippert S, Davis N, Bradman A, et al. Maternal prenatal and child organophosphate pesticide exposures and children’s autonomic function. Neurotoxicology. 2011;32:646–655. doi: 10.1016/j.neuro.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez M, Sunyer J, Grimalt J, Rebagliato M, Ballester F, Ibarluzea J, et al. The Spanish Environment and Childhood Research Network (INMA study) Int J Hyg Environ Health. 2007;210:491–493. doi: 10.1016/j.ijheh.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Vrijheid M, Martinez D, Aguilera I, Ballester F, Basterrechea M, Esplugues A, et al. Socioeconomic status and exposure to multiple environmental pollutants during pregnancy: evidence for environmental inequity? J Epidemiol Community Health. 2012;66:106–113. doi: 10.1136/jech.2010.117408. [DOI] [PubMed] [Google Scholar]
- 80.Borrell L, Factor-Litvak P, Volff M, Susser E, Matte T. Effect of socioeconomic status on exposures to polychlorinated biphenyls (PCBs) and dichlorodiphenlydichloreethylene (DDE) among pregnant African-American women. Arch Environ Health. 2004;59:250–255. doi: 10.3200/AEOH.59.5.250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolff M, Deych E, Ojo F, Berkowitz G. Predictors of organochlorines in New York City pregnant women, 1998–2001. Environ Res. 2005;97:170–177. doi: 10.1016/j.envres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 82.Rogan W, Gladen B, McKinney J, Carreras N, Hardy P, Thullen J, et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation Am J Public Health. 1986;76:172–177. doi: 10.2105/ajph.76.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwartz P, Jacobson S, Fein G, Jacobson J, Price H. Lake Michigan fish consumption as a source of polychlorinated biphenyls in human cord serum, maternal serum, and milk. Am J Public Health. 1983;73:293–296. doi: 10.2105/ajph.73.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johansen E, Knoff M, Fonnum F, Lausund P, Walaas S, Woien G, et al. Postnatal exposure to PCB 153 and PCB 180, but not to PCB 52, produces changes in activity level and stimulus control in outbred male Wistar Kyoto rats. Behav Brain Func. 2011;7:18. doi: 10.1186/1744-9081-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.American Academy of Pediatrics. Policy statement – Chemical management policy: prioritizing children’s health. Pediatrics. 2011;127:983–990. doi: 10.1542/peds.2011-0523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


