Abstract
Non-visual arrestins (β-arrestin-1 and β-arrestin-2) are adaptor proteins that function to regulate G protein-coupled receptor (GPCR) signaling and trafficking. β-arrestins are ubiquitously expressed and function to inhibit GPCR/G protein coupling, a process called desensitization, and promote GPCR trafficking and arrestin-mediated signaling. β-arrestin-mediated endocytosis of GPCRs requires the coordinated interaction of β-arrestins with clathrin, adaptor protein 2 (AP2) and phosphoinositides. These interactions are facilitated by a conformational change in β-arrestin that is thought to occur upon binding to a phosphorylated activated GPCR. In this review, we provide an overview of the key interactions involved in β-arrestin-mediated trafficking of GPCRs.
Keywords: arrestin, receptor, phosphorylation, endocytosis, clathrin, adaptin, phosphoinositides
β-arrestins and GPCR trafficking
Many transmembrane signaling systems consist of specific G protein-coupled receptors (GPCRs) that transduce the binding of extracellular stimuli into intracellular signaling. GPCRs modulate the activity of numerous intracellular effectors and ultimately regulate a myriad of biological processes. To ensure that extracellular stimuli are translated into intracellular signals of appropriate magnitude and duration, most signaling cascades are tightly regulated. GPCRs are subject to three principle modes of regulation: (i) desensitization, where a receptor becomes refractory to continued stimuli; (ii) internalization, where receptors are physically removed from the cell surface by endocytosis; and (iii) down-regulation, where total cellular receptor levels are decreased (Fig. 1). GPCR desensitization is primarily mediated by second messenger dependent kinases and by GPCR kinases (GRKs). GRKs specifically phosphorylate activated GPCRs and initiate the recruitment of arrestins, which mediate receptor desensitization, endocytosis and signaling (Krupnick and Benovic 1998).
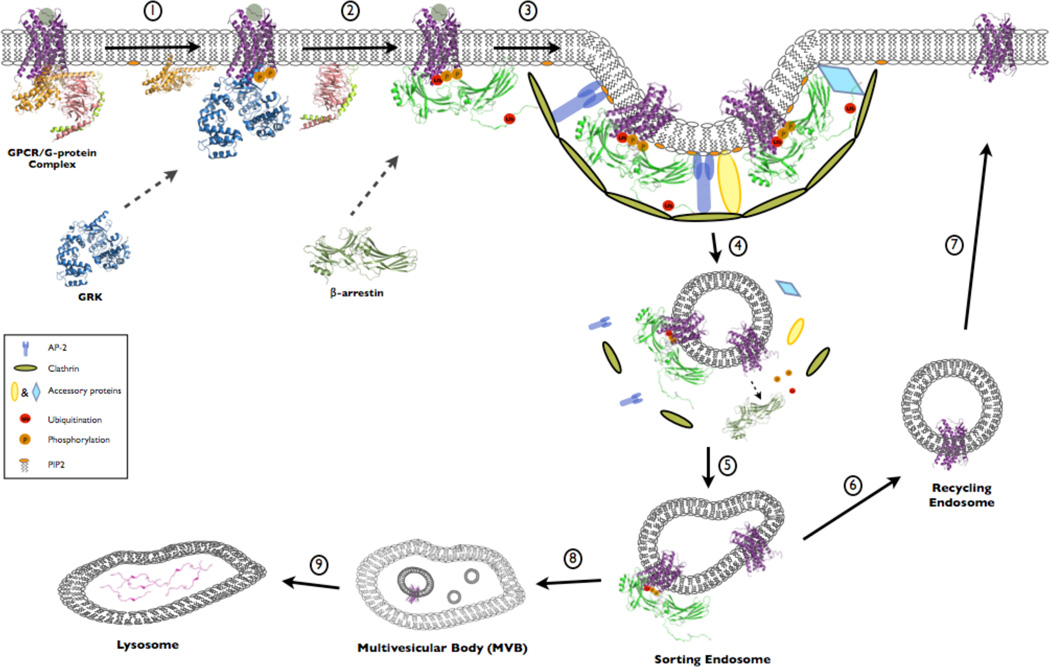
Figure 1. Role of β-arrestins in GPCR trafficking.
(1) Agonist binding to a GPCR results in heterotrimeric G protein activation leading to dissociation of Gα from Gβγ subunits. This promotes GRK association with the agonist-bound GPCR which mediates receptor phosphorylation and (2) promotes β-arrestin recruitment to the receptor. (3) β-arrestin association with the phosphorylated GPCR mediates conformational changes in arrestin that promote association of the GPCR-β-arrestin complex with the endocytic machinery and subsequent endocytosis (4). GPCRs then traffic to sorting endosomes (5) and ultimately either recycle back to the plasma membrane through recycling endosomes (6 and 7) or are sorted to lysosomes where they are degraded (8 and 9).
A role for β-arrestins in agonist-promoted internalization of GPCRs was first discovered in 1996 (Ferguson et al. 1996; Goodman et al. 1996). These initial studies focused on the β2-adrenergic receptor (β2AR) while more recent studies have demonstrated that β-arrestins promote the trafficking of many GPCRs as well as additional classes of receptors (Moore et al. 2007; Shenoy and Lefkowitz 2011). Mechanistic insight into this process has revealed an essential role for the coordinated interaction of β-arrestins with the GPCR (Vishnivetskiy et al. 1999, 2011), clathrin (Krupnick et al. 1997; Kang et al. 2009), adaptor protein 2 (AP2) (Laporte et al. 1999, 2000; Kim and Benovic 2002; Schmid et al. 2006; Burtey et al. 2007) and phosphoinositides (Gaidarov et al. 1999; Milano et al. 2006). Moreover, β-arrestin binding to the GPCR appears to induce a conformational change that promotes interaction with the endocytic machinery, thereby linking the binding and trafficking events (Kim and Benovic 2002; Xiao et al. 2004; Nobles et al. 2007).
General structure of β-arrestins
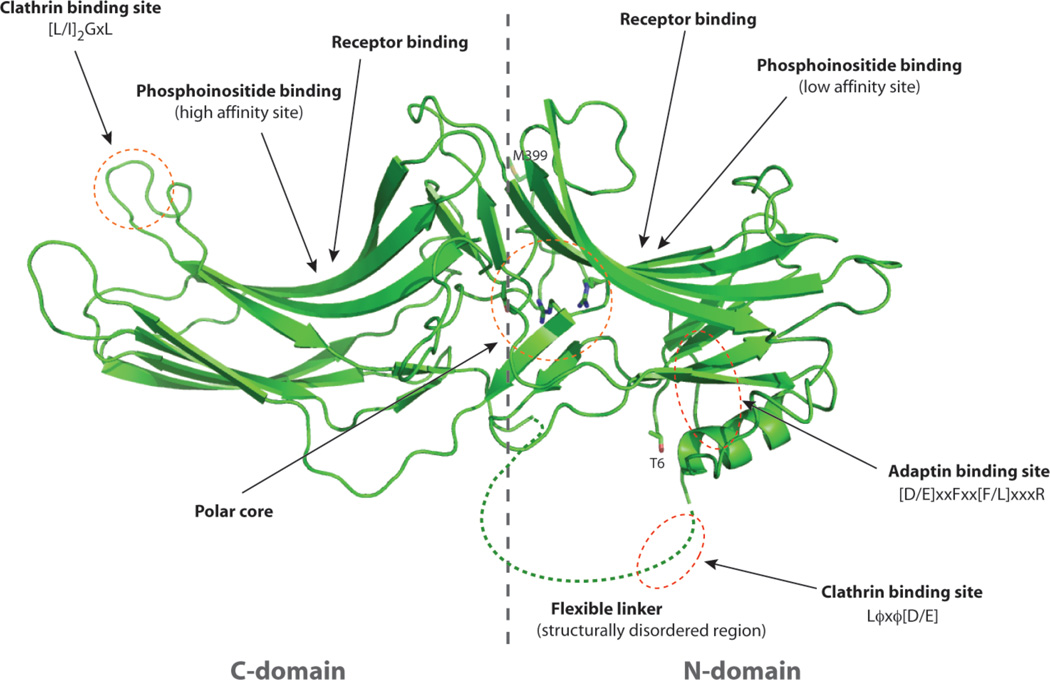
The four mammalian arrestins fall into two classes, visual and non-visual, and X-ray structures for all four family members have been solved. Arrestins can be divided into two major domains, the N-domain and C-domain, with each domain primarily consisting of anti-parallel β-sheets connected by short flexible loops (Fig. 2). The N- and C-domains are connected by a short “hinge region” while the C-tail is connected by a flexible linker to the C-domain and contains a short β strand that interacts with a lateral β strand of the N-domain. The overall structure is stabilized by a polar core of buried salt bridges and by a three-element interaction involving the first β strand, an α-helix in the N-domain and the C-terminal tail (Han et al. 2001; Milano et al. 2002, 2006; Kang et al. 2009; Zhan et al. 2011). The polar core is comprised of charged residues from the amino terminus (Asp-29 in β-arrestin-1), N-domain (Arg-169), C-domain (Asp-290 and Asp-297), and C-terminal tail (Arg-393) thus bringing different parts of the molecule together to maintain a basal conformation. The residues involved in formation of the polar core are highly conserved, suggesting that this structural element is critical for the function of all arrestins. Because the buried side chains of the polar core achieve neutrality by an elaborate network of electrostatic interactions, it has been suggested that disturbance of the polar core by introduction of a phosphate group from the receptor promotes structural changes that result in an active conformation of arrestin (Hirsch et al. 1999). Indeed, two of the five polar core residues, namely Arg169 and Asp290 in β-arrestin-1 (Han et al. 2001) and Arg170 and Asp291 in β-arrestin-2 (Zhan et al. 2011) are particularly important for arrestin selectivity for binding to activated phosphorylated receptors (Vishnivetshy et al. 1999).
Figure 2. Secondary structure of β-arrestin-1L.
Ribbon diagram of β-arrestin-1L (residues 6 to 399) indicating the N- and C-domains, the polar core and binding sites for the GPCR, phosphoinositides (high affinity site in C-domain and low affinity site in N-domain), clathrin (Lϕxϕ[D/E] and [L/I]2GxL motifs) and β2-adaptin ([D/E]xxFxx[F/L]xxxR motif).
It is believed that arrestins make an initial contact with phosphorylated receptors via adjacent lysines in the amino terminus (Vishnivetskiy et al. 2000). Biochemical data suggests that this interaction perturbs the three-element interaction, guides phosphorylated receptors to the polar core, allows the negatively charged phosphate from the receptor to interact with positively charge Arg169 (in β-arrestin-1), and ultimately causes release of the C-terminal tail from the polar core (Palczewski et al. 1991; Gurevich et al. 1998; Vishnivetskiy et al. 2000; Gurevich and Gurevich 2004). This leads to the disruption of the basal state and subsequent conformational rearrangement of arrestin. Studies monitoring arrestin conformational changes in live cells, along with other biochemical data, suggest that the arrestin amino terminus and C-terminal tail move closer upon binding to an activated receptor (Xiao et al. 2004; Charest et al. 2005). This conformational rearrangement enhances arrestin interaction with receptors and is also thought to expose binding motifs that interact with other proteins such as clathrin and AP2 (Moore et al. 2007).
β-arrestin interaction with clathrin
Clathrin is a well-studied endocytic protein that is essential for the formation of clathrin-coated pits (CCPs), which play a central role in receptor endocytosis. Clathrin is composed of a heavy and a light chain and three clathrin molecules associate to form a propeller-shaped triskelion, which is the basic structural unit of CCPs (Kirchhausen 2000). Although most GPCRs internalize via CCPs, GPCRs do not directly bind to clathrin and thus require an adaptor protein to provide a molecular link between the receptor and CCP. While the adaptor protein AP2 plays this role for some GPCRs, β-arrestins also function as adaptors to mediate endocytosis of GPCRs (Ferguson et al. 1996; Goodman et al. 1996). Upon agonist stimulation, β-arrestin-1 was found to colocalize with clathrin and the β2AR. Mechanistic studies reveal that β-arrestin-1 and -2 specifically bind to clathrin with a Kd of 10–60 nM (Goodman et al. 1996). The primary clathrin-binding site in β-arrestin, called a clathrin binding box or Lϕxϕ[D/E] motif (where ϕ is a bulky hydrophobic residue and x represents any polar amino acid), is localized in the carboxyl terminal region (residues 376–380 in β-arrestin-1) (Fig. 2). This motif is also found in many other clathrin-binding proteins such as AP2, AP180, amphiphysin, and epsin (Owen et al. 2004). Importantly, mutation or deletion of this motif in β-arrestin-1 effectively disrupts clathrin binding and receptor internalization (Krupnick et al. 1997; Kim and Benovic 2002; Burtey et al. 2007). Mutagenesis studies localized the β-arrestin binding site to the N-terminal domain of the clathrin heavy chain, specifically residues 89–100, with an invariant Glu89 and conserved Lys96 and Lys98 as critical resides that mediate β-arrestin interaction (Goodman et al. 1997). Hydrophobic and basic residues in this region of clathrin complement the hydrophobic and acidic amino acids within the Lϕxϕ[D/E] motif in β-arrestin.
Crystallographic structures of the terminal domain of the clathrin heavy chain (residues 1–363) in complex with a β-arrestin-2 peptide (ter Haar et al. 2000) as well as with full length β-arrestin-1 (Kang et al. 2009) support the predicted location of the arrestin-clathrin interface determined by mutagenesis. These structures clearly demonstrate that the Lϕxϕ[D/E] motif in β-arrestin interacts with a hydrophobic patch formed by the 1st and 2nd blades of the clathrin terminal domain. In addition, charged residues outside of the Lϕxϕ[D/E] motif form hydrogen bonds with Glu89 and Lys96 in clathrin and help to stabilize the interaction. β-arrestin-1 actually exists in two isoforms (long and short) that differ by an 8 amino acid insert between the 18th and 19th β-strands (Sterne-Marr et al. 1993; Kang et al. 2009). Interestingly, the structure of a complex between the long isoform of β-arrestin-1 (β-arrestin-1L) and clathrin revealed a second region of interaction between these proteins. This interaction was mediated by the 8 amino acid insert unique to β-arrestin-1L and a hydrophobic patch formed by 4th and 5th blades of clathrin (Kang et al. 2009) (Fig. 2). Site directed mutagenesis of the 8 amino acid insert in β-arrestin-1L identified a [L/I]2GxL motif that mediates clathrin binding. Interestingly, this motif is also found in many other clathrin binding proteins, although whether it plays a broad role in clathrin binding is currently unknown.
Cell biological approaches have also been used to characterize the functional role of the clathrin binding motifs in β-arrestin-1L. β-arrestin-1L mutants lacking a single clathrin binding motif showed reduced β2AR endocytosis while β-arrestin-1L lacking both clathrin binding motifs effectively disrupted clathrin binding and β2AR endocytosis (Kang et al. 2009). Taken together, these studies demonstrate that β-arrestin interaction with clathrin plays an essential role in endocytosis of many GPCRs while the two independent interactions between β-arrestin-1L and clathrin likely facilitate the formation of a macromolecular complex that regulates the dynamics of receptor endocytosis.
β-arrestin interaction with AP2
Another essential component of CCPs is the adaptor protein AP2. AP2 is a heterotetrameric protein consisting of α, β2, µ2 and σ2 subunits and it functions as a clathrin adaptor and in cargo recruitment to CCPs (Owen et al. 2004). The α-adaptin and β2-adaptin subunits of AP2 function in cargo and adaptor recruitment and are composed of ear (appendage), hinge and trunk domains. The appendage domain of α-adaptin interacts with DP[F/W], FxDxF and WxxF motifs while the appendage domain of β2-adaptin interacts with [D/E]xxFxx[F/L]xxxR. The µ2 subunit of AP2 also binds cargo proteins and interacts with Yxxϕ and [D/E]xxL[L/I] motifs as well as with phosphatidylinositol.
Initial studies from the Caron laboratory identified a direct interaction between β-arrestin and β2-adaptin (Laporte et al. 1999, 2000). They found that deletion of 25 amino acids from the C-terminus of β-arrestin-1 completely disrupted interaction with β2-adaptin while mutation of Arg394 or Arg396 in β-arrestin-2 (equivalent to Arg393 and Arg395 in β-arrestin-1) disrupted β2-adaptin binding. Moreover, a β-arrestin-2-R396A mutant did not co-localize with AP2 in CCPs upon receptor activation, in contrast to wild type β-arrestin-2 (Laporte et al. 2000). Additional studies revealed an essential role for Phe391 and Arg395 in β-arrestin-1 binding to β2-adaptin and showed that F391A and R395E mutants functioned as effective dominant negative mutants in β2AR internalization assays when clathrin binding was also disrupted (Kim and Benovic 2002). Several studies also identified the residues in β2-adaptin that mediate β-arrestin binding and revealed an important role for Arg834, Trp841, Glu849, Tyr888 and Glu902 (Kim and Benovic 2002; Edeling et al. 2006; Schmid et al. 2006).
Based on extensive mutagenesis and biochemical analysis, a β2-adaptin-binding consensus sequence was defined as [D/E]xxFxx[F/L]xxxR in β-arrestins, epsin and autosomal recessive hyper-cholesterolemia protein (ARH) (Edeling et al. 2006; Schmid et al. 2006). Crystallographic studies demonstrate that the appendage domain of β2-adaptin consists of platform and sandwich subdomains. X-ray structures of β2-adaptin crystallized with synthetic peptides containing the [D/E]xxFxx[F/L]xxxR motif from either ARH (Edeling et al. 2006) or β-arrestin-1 (Schmid et al. 2006) show a molecular interface primarily formed by hydrophobic interactions between the C-terminal domain of β-arrestin-1 and the platform domain of β2-adaptin. The center of this interaction is formed by Phe388 and Phe391 in β-arrestin-1 and Tyr888 in β2-adaptin. Interestingly, the β-arrestin-1 region involved in this interaction forms the last β-strand in holo β-arrestin-1 while this region becomes α-helical when bound to β2-adaptin, at least when bound as a peptide. These results suggest a conformational change occurs upon arrestin/adaptin binding and support previous findings that arrestin activation promotes adaptin binding (Kim and Benovic 2002). While these studies suggest a major conformational change occurs in β-arrestin when it binds to β2-adaptin, it will be important to validate such results in β-arrestin complexes with receptor and β2-adaptin.
β-arrestin interaction with phosphoinositides
Phosphatidylinositol 4,5-bisphosphate (PIP2) is an important component in clathrin-mediated endocytosis and is mainly enriched at the plasma membrane, although it is also detected in the Golgi, endosomes and endoplasmic reticulum (Watt et al. 2002). Clathrin-mediated endocytosis can roughly be divided into five stages: nucleation, cargo selection, coat assembly, scission, and uncoating (McMahon and Boucrot 2011). PIP2 synthesis is important for the nucleation, cargo selection and coat assembly of CCPs, while scission and uncoating of CCPs is partially dependent on the localized turnover of PIP2 (Antonescu et al. 2011; Zoncu et al. 2007). GPCR trafficking is also dependent on PIP2 since alteration of plasma membrane PIP2 levels significantly affects GPCR endocytosis and recycling (Tóth et al. 2012).
Phosphoinositides also play an important role in β-arrestin-mediated trafficking of GPCRs. β-arrestin-1 and -2 contain a high affinity phosphoinositide-binding site located in the C-domain where three basic residues (Lys-233, Arg-237 and Lys-251 in β-arrestin-2) have been implicated in phosphoinositide binding (Gaidarov et al. 1999). Mutation of these three residues in β-arrestin-2 (β-arrestin-2-KRK/Q) failed to support β2AR recruitment to CCPs and subsequent internalization suggesting that phosphoinositides are important in delivering the receptor-arrestin complex to CCPs. The β-arrestin-2-KRK/Q mutant, however, retains the ability to bind to receptor and clathrin and was recruited to the plasma membrane upon receptor activation (Gaidarov et al. 1999). The binding affinities of various phosphoinositides for β-arrestin-1 and -2 were found to have the following affinities: IP6 (~0.08 µM) > PIP3 (~0.3 µM) > PIP2 (~1.4 µM) > IP4 (~4 µM) > IP3 (~20 µM) (Gaidarov et al. 1999). Interestingly, β-arrestins also appear to regulate the production of PIP2 since β-arrestin-2 binds the enzyme phosphatidylinositol 4-phosphate 5-kinase (PIP5K), which functions in PIP2 production (Nelson et al. 2008). Overexpression of a β-arrestin-2 mutant lacking PIP2 binding abolishes PIP5K interaction and inhibits β2AR internalization. A positive feedback mechanism was proposed where β-arrestin-2 interaction with PIP2 facilitates β2AR internalization by promoting interaction with PIP5K to synergistically produce more PIP2, thereby leading to increased local concentrations of PIP2 (Nelson et al. 2008). Taken together, these results suggest an essential role for phosphoinositides in β-arrestin mediated trafficking of GPCRs.
While PIP2 and PIP3 are the proposed physiological ligands for β-arrestins at the plasma membrane, inositol hexakisphosphate (IP6), a soluble inositol polyphosphate, displays a higher binding affinity for β-arrestins than either PIP2 or PIP3 (Gaidarov et al. 1999). IP6 is abundant in cells with concentrations ranging between 15–100 µM and has been proposed to regulate receptor endocytosis and receptor signaling (Sasakawa et al. 1995). Interestingly, IP6 inhibits both β-arrestin-1 and -2 binding to an activated phosphorylated GPCR (Gaidarov et al. 1999). Moreover, the ability of IP6 to bind to two distinct sites on β-arrestins appears to mediate homo- and hetero-oligomerization of β-arrestin-1 and -2 (Milano et al. 2006). Mutation of either IP6-binding site in β-arrestin-1 disrupted oligomerization while interactions with known binding partners including clathrin, AP2, and ERK2 were maintained. Moreover, subcellular localization studies showed that β-arrestin-1 oligomers and β-arrestin-1/2 hetero-oligomers are primarily cytoplasmic, whereas β-arrestin-1 monomers displayed increased nuclear localization (Milano et al. 2006; Storez et al. 2005). This suggests that IP6 binding to β-arrestins may regulate arrestin localization and function as a negative regulator of arrestin interaction with plasma membrane and nuclear signaling proteins.
Additional interactions involved in β-arrestin-mediated trafficking of GPCRs
While β-arrestin interactions with clathrin, AP2 and phosphoinositides appear critical in arrestin-promoted endocytosis, β-arrestins also bind several additional proteins that regulate GPCR trafficking (Table 1). For example, β-arrestin-2 interacts with endothelial NO synthase which promotes S-nitrosylation of β-arrestin-2 which, in turn, enhances association with CCPs and accelerates GPCR internalization (Ozawa et al. 2008). β-arrestin-1 also interacts with N-ethylmaleimide-sensitive fusion protein (NSF), an ATPase that regulates intracellular transport. Interestingly, β-arrestin-1 interaction with NSF is ATP dependent and overexpression of NSF enhances agonist-promoted internalization of the β2AR (McDonald et al. 1999). β-arrestin-1 interaction with Arf6.GDP and its nucleotide exchange factors, ARNO and EFA6, leads to Arf6 activation and subsequent regulation of GPCR endocytosis and recycling (Mukherjee et al. 2000; Claing et al. 2001; Macia et al. 2012). β-arrestins have also been demonstrated to recruit E3 ubiquitin ligases and de-ubiquitinases to the plasma membrane to regulate GPCR trafficking. For example, β-arrestin-2 is rapidly ubiquitinated by Mdm2 upon β2AR stimulation and depletion of Mdm2 by siRNA or overexpression of dominant-negative Mdm2 attenuates β-arrestin-2 ubiquitination and β2AR internalization (Shenoy et al. 2001, 2009). Moreover, β-arrestin-2 acts as an adaptor between β2AR and the E3 ubiquitin ligase Nedd4 to facilitate β2AR ubiquitination and trafficking (Shenoy et al. 2008; Han et al. 2013). Interestingly, β-arrestin-2 can also be modified by sumoylation at Lys-400 and inhibition of β-arrestin-2 sumoylation attenuates β2AR internalization (Wyatt et al. 2011).
Table 1.
Protein interactions with β-arrestins that function in GPCR trafficking
| Binding Partner |
Region of the binding partner interacting with arrestin |
Region of arrestin interacting with the binding partner |
Functions | References |
|---|---|---|---|---|
| β2-adaptin | The groove between α-helix 1 and the antiparallel β-sheet of the platform sub-domain |
[D/E]xxFxx[F/L]xxxR in the C- terminal tail |
Directly interacts with β-arrestins and facilitates GPCR endocytosis | Laporte et al. 2001 Edeling et al. 2006 Schmid et al. 2006 |
| µ2-adaptin | - | [Y/F]VTL in the N-terminus | Preferentially interacts with β-arrestin2 and facilitates β2AR endocytosis | Marion et al. 2007 |
| Clathrin | Pocket formed by blades 1 and 2 (E89, K96, K98) Hydrophobic pocket formed by blades 4 and 5 |
LØxØ[D/E] in the C-tail [L/I]2GxL (β-arrestin1L) |
Directly interacts with β-arrestins and facilitates GPCR endocytosis |
Goodman et al. 1997 ter Haar et al. 2000 Kang et al. 2009 |
| PIP2 | Phosphate head group | Residues 223-285 (K233, R237, K251 in β-arrestin2) |
Directly interacts with β-arrestins and enhances GPCR endocytosis |
Gaidarov et al. 1999 Nelson et al. 2008 |
| PIP5K-Iα | - | Residues 240-261 | Directly interacts with β-arrestin2 and facilitates β2AR endocytosis | Nelson et al. 2008 |
| IP6 | Phosphate head group | C-domain (K233, R237, K251, K324, K326); N-domain (K157, K160, R161) |
Directly interacts with β-arrestins; inhibits β-arrestin/GPCR interaction, facilitates β-arrestin homo- and hetero-oligomerization and regulates β-arrestin cellular localization |
Gaidarov et al. 1999 Storez et al. 2005 Milano et al. 2006 |
| PI3K | PIK domain | - | Regulates β2AR endocytosis by AP2 recruitment to the β2AR/β-arrestin complex | Naga Prasad et al. 2002 |
| NSF | - | - | Directly interacts with β-arrestin2 and enhances β2AR endocytosis | McDonald et al. 1999 |
| ARF6 | - | C-tail | GDP-bound form interacts with β-arrestin1; enhances GPCR endocytosis; negatively controls recycling; enhances receptor degradation |
Claing et al. 2001 Houndolo et al. 2005 Macia et al. 2012 |
| ARNO | - | - | Activates ARF6 to facilitate β-arrestin release from LH/CGR | Mukherjee et al. 2000 |
| EFA6 | - | C-tail of β-arrestin1 | β-arrestin1 scaffolds ARF6-GDP and EFA6 to facilitate ARF6 activation leading to β2AR degradation |
Macia et al. 2012 |
| Mdm2 | - | N-domain | Ubiquitinates β-arrestin2 and facilitates β2AR endocytosis | Shenoy et al. 2001 |
| AIP4 | WWI-II domains | N-domain (residues 1-260) | Interacts with β-arrestin2 on early endosomes and facilitates CXCR4 degradation | Bhandari et al. 2007 |
| Nedd4 | - | - | Interacts with β-arrestin to facilitate β2AR ubiquitination and trafficking |
Shenoy et al. 2008 Han et al. 2013 |
| STAM-1 | GAT domain | Residues 25-161 | Interacts with β-arrestin 1 to regulate CXCR4 sorting | Malik and Marchese 2010 |
| USP20 | - | - | Directly deubiquitinates β-arrestin2 and β2AR to prevent receptor degradation | Shenoy et al. 2009 |
| USP33 | - | - | Directly deubiquitinates β-arrestin2 and β2AR to prevent receptor degradation | Berthouze et al. 2009 |
| eNOS | - | N-terminus | Interacts with and s-nitrosylates β-arrestin2 and facilitates β-arrestin2 binding with clathrin and β-adaptin; promotes receptor internalization |
Ozawa et al. 2008 |
Once internalized, GPCRs are either recycled back to the plasma membrane or sorted to the lysosome and degraded (Fig. 1). The dynamic regulation of GPCR ubiquitination by ubiquitin E3 ligases and deubiquitinases plays a crucial role in endocytic sorting (Marchese and Benovic, 2011; Shenoy et al. 2001, 2008, 2009). β-arrestins function to facilitate this process and the stability of β-arrestin ubiquitination and its interaction with a GPCR appear to contribute to whether a receptor is to be recycled or degraded. For example, agonist stimulation of the β2AR induces transient ubiquitination and complex formation with β-arrestin-2 and the receptor is rapidly dephosphorylated and recycled after internalization. In contrast, stimulation of the AT1a receptor promotes sustained binding and ubiquitination of β-arrestin-2 and the receptor is effectively sorted to lysosomes and degraded (Oakley et al. 1999, 2001; Shenoy et al. 2001, 2003). While sustained ubiquitination of GPCRs is important for receptor degradation, deubiquitination of GPCRs regulates receptor recycling back to the plasma membrane. For example, the deubiquitinases USP33 and USP20 have been shown to directly interact with β-arrestin-2 and facilitate both β-arrestin-2 and β2AR deubiquitination. Importantly, a double knockdown of USP20 and USP33 enhances the extent of β-arrestin-2 ubiquitination and increases β2AR degradation (Berthouze et al. 2009; Shenoy et al. 2009).
Once a GPCR is committed to the degradation pathway, it is sorted to the lysosome with the help of the ESCRT complexes (Marchese and Trejo 2013). The presence of a functional β-arrestin has been shown to be essential for effective sorting and degradation of CXCR4. Specifically, β-arrestin-1 colocalizes with atrophin interacting protein 4 (AIP4), an E3 ubiquitin ligase, on early endosomes to facilitate CXCR4 sorting and degradation. Knockdown of β-arrestin-1 inhibits CXCR4 degradation but does not affect CXCR4 ubiquitination or internalization (Bhandari et al. 2007). CXCR4 sorting is also regulated by β-arrestin-1 interaction with signal-transducing adaptor molecule-1 (STAM-1) and disruption of this interaction attenuates agonist-promoted ubiquitination of HRS and enhances sorting to lysosomes (Malik and Marchese 2010).
References
- Antonescu CN, Aguet F, Danuser G, Schmid SL. Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol Biol Cell. 2011;22:2588–2600. doi: 10.1091/mbc.E11-04-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate β2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitinprotein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- Burtey A, Schmid EM, Ford MGJ, Rapport JZ, Scott MGH, Marullo S, Simon SM, McMahon HT, Benmerah A. The conserved isoleucine-valine-phenylalanine motif couples activation state and endocytic functions of β-arrestins. Traffic. 2007;8:914–931. doi: 10.1111/j.1600-0854.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- Charest PG, Terrillon S, Bouvier M. Monitoring agonist-promoted conformational changes of β-arrestin in living cells by intramolecular BRET. EMBO Reports. 2005;6:334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ. β-arrestinmediated ADP-ribosylation factor 6 activation and β2-adrenergic receptor endocytosis. J Biol Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. Molecular switches involving the AP-2 β2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ferguson SSG, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Gurevich VV, Benovic JL, Keen JH. Arrestin/clathrin interaction: Localization of the arrestin binding locus to the clathrin terminal domain. J Biol Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The molecular acrobatics of arrestin activation. Trends Pharmacol Sci. 2004;25:105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Gurevich VV. The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J Biol Chem. 1998;273:15501–15506. doi: 10.1074/jbc.273.25.15501. [DOI] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of β-arrestin at 1.9 Å: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- Han S-O, Kommaddi RP, Shenoy SK. Distinct roles for β-arrestin2 and arrestin-domain-containing proteins in β2 adrenergic receptor trafficking. EMBO Reports. 2013;14:164–171. doi: 10.1038/embor.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 Å crystal structure of visual arrestin: a model for arrestin's regulation. Cell. 1999;97:257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- Houndolo T, Boulay P, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J Biol Chem. 2005;280:5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Kang DS, Kern RC, Puthenveedu MA, von Zastrow M, Williams JC, Benovic JL. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J Biol Chem. 2009;284:29860–29872. doi: 10.1074/jbc.M109.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Benovic JL. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem. 2002;277:30760–30768. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Goodman OB, Jr, Keen JH, Benovic JL. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The β2-adrenergic receptor/beta-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Partisani M, Paleotti O, Luton F, Franco M. Arf6 negatively controls the rapid recycling of the β2 adrenergic receptor. J Cell Sci. 2012;125:4026–4035. doi: 10.1242/jcs.102343. [DOI] [PubMed] [Google Scholar]
- Malik R, Marchese A. Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell. 2010;21:2529–2541. doi: 10.1091/mbc.E10-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G-protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Marchese A, Trejo J. Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling. Cell Signal. 2013;25:707–716. doi: 10.1016/j.cellsig.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion S, Fralish GB, Laporte S, Caron MG, Barak LS. N-terminal tyrosine modulation of the endocytic adaptor function of the beta-arrestins. J Biol Chem. 2007;282:18937–18944. doi: 10.1074/jbc.M700090200. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Cote NL, Lin F-T, Premont RT, Pitcher JA, Lefkowitz RJ. Identification of NSF as a β-arrestin1-binding protein. J Biol Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Milano SK, Kim Y-M, Stefano FP, Benovic JL, Brenner C. Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J Biol Chem. 2006;281:9812–9823. doi: 10.1074/jbc.M512703200. [DOI] [PubMed] [Google Scholar]
- Milano SK, Pace HC, Kim Y-M, Brenner C, Benovic JL. Scaffolding functions of arrestin-2 revealed by crystal structure and mutagenesis. Biochemistry. 2002;41:3321–3328. doi: 10.1021/bi015905j. [DOI] [PubMed] [Google Scholar]
- Moore CAC, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Gurevich VV, Jones JCR, Casanova JE, Frank SR, Maizels ET, Bader M-F, Kahn RA, Palczewski K, Aktories K, Hunzicker-Dunn M. The ADP ribosylation factor nucleotide exchange factor ARNO promotes β-arrestin release necessary for luteinizing hormone/choriogonadotropin receptor desensitization. Proc Natl Acad Sci USA. 2000;97:5901–5906. doi: 10.1073/pnas.100127097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Phosphoinositide 3-kinase regulates β2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J Cell Biol. 2002;158:563–575. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Kovacs JJ, Nobles KN, Whalen EJ, Lefkowitz RJ. β-arrestin scaffolding of phosphatidylinositol 4-phosphate 5-kinase Iα promotes agonist-stimulated sequestration of the β2-adrenergic receptor. J Biol Chem. 2008;283:21093–21101. doi: 10.1074/jbc.M800431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles KN, Guan Z, Xiao K, Oas TG, Lefkowitz RJ. The active conformation of β-arrestin1: direct evidence for the phosphate sensor in the N-domain and conformational differences in the active states of beta-arrestins1 and-2. J Biol Chem. 2007;282:21370–21381. doi: 10.1074/jbc.M611483200. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J Biol Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS. S-nitrosylation of β-arrestin regulates β-adrenergic receptor trafficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Buczyłko J, Imami NR, McDowell JH, Hargrave PA. Role of the carboxyl-terminal region of arrestin in binding to phosphorylated rhodopsin. J Biol Chem. 1991;266:15334–15339. [PubMed] [Google Scholar]
- Sasakawa N, Sharif M, Hanley MR. Metabolism and biological activities of inositol pentakisphosphate and inositol hexakisphosphate. Biochem Pharmacol. 1995;50:137–146. doi: 10.1016/0006-2952(95)00059-9. [DOI] [PubMed] [Google Scholar]
- Schmid EM, Ford MG, Burtey A, Praefcke GJ, Peak-Chew SY, Mills IG, Benmerah A, McMahon HT. Role of the AP2 β-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 2006;4:1532–1548. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of β-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. β-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the β2-adrenergic receptor. J Biol Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. β-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne-Marr R, Gurevich VV, Goldsmith P, Bodine RC, Sanders C, Donoso LA, Benovic JL. Polypeptide variants of β-arrestin and arrestin3. J Biol Chem. 1993;268:15640–15648. [PubMed] [Google Scholar]
- Storez H, Scott MG, Issafras H, Burtey A, Benmerah A, Muntaner O, Piolot T, Tramier M, Coppey-Moisan M, Bouvier M, Labbe-Jullie C, Marullo S. Homo- and heterooligomerization of β-arrestins in living cells. J Biol Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- ter Haar E, Harrison SC, Kirchhausen T. Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc Natl Acad Sci USA. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth DJ, Tóth JT, Gulyás G, Balla A, Balla T, Hunyady L, Varnai P. Acute depletion of plasma membrane phosphatidylinositol 4,5-bisphosphate impairs specific steps in endocytosis of the G-protein-coupled receptor. J Cell Sci. 2012;125:2185–2197. doi: 10.1242/jcs.097279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Gimenez LE, Francis DJ, Hanson SM, Hubbell WL, Klug CS, Gurevich VV. Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biol Chem. 2011;286:24288–24299. doi: 10.1074/jbc.M110.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Paz CL, Schubert C, Hirsch JA, Sigler PB, Gurevich VV. How does arrestin respond to the phosphorylated state of rhodopsin? J Biol Chem. 1999;274:11451–11454. doi: 10.1074/jbc.274.17.11451. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Schubert C, Climaco GC, Gurevich YV, Velez M-G, Gurevich VV. An additional phosphate-binding element in arrestin. J Biol Chem. 2000;275:41049–41057. doi: 10.1074/jbc.M007159200. [DOI] [PubMed] [Google Scholar]
- Watt S, Kular G, Fleming I, Downes C, Lucocq J. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem J. 2002;363:657–666. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt D, Malik R, Vesecky AC, Marchese A. Small ubiquitin-like modifier modification of arrestin-3 regulates receptor trafficking. J Biol Chem. 2011;286:3884–3893. doi: 10.1074/jbc.M110.152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. Activation-dependent conformational changes in β-arrestin 2. J Biol Chem. 2004;279:55744–55753. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
- Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual subtypes. J Mol Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, Camilli PVD. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]