Abstract
Mild traumatic brain injury (TBI), which is defined as a head trauma resulting in a brief loss of consciousness and/or alteration of mental state, is usually benign, but occasionally causes persistent and sometimes progressive symptoms. Whether a threshold for the amount of brain injury and/or individual vulnerability might contribute to the development of these long-term consequences is unknown. Furthermore, reliable diagnostic methods that can establish whether a blow to the head has affected the brain (and in what way) are lacking. In this Review, we discuss potential biomarkers of injury to different structures and cell types in the CNS that can be detected in body fluids. We present arguments in support of the need for further development and validation of such biomarkers, and for their use in assessing patients with head trauma in whom the brain might have been affected. Specifically, we focus on the need for such biomarkers in the management of sports-related concussion, the most common cause of mild TBI in young individuals, to prevent long-term neurological sequelae due to concussive or subconcussive blows to the head.
Introduction
A blow to the head can result in anything from a superficial skin laceration to severe brain injury. The extremes of this range are easy to recognize by clinical examination and neuroimaging, but whether the brain has been injured by a blow to the head (in the presence of nonspecific symptoms such as dizziness, nausea or headache) is more difficult to assess. The definition of mild traumatic brain injury (TBI) has changed over the past 60 years,1 but the American Congress of Rehabilitation Medicine currently defines mild TBI as head trauma resulting in one of the following: loss of consciousness for less than 30 min, alteration of mental state for up to 24 h (being dazed, confused or disorientated), or loss of memory for events immediately before or after the trauma.2
The terms mild TBI and concussion have historically been used interchangeably to suggest an inconsequential injury; however, mild TBI is far from trivial, since it can induce selective swelling and disconnection of white matter axons.3,4 Furthermore, repeated episodes of mild TBI are associated with chronic and sometimes progressive clinical symptoms and neuropathological changes.5 Although the mechanisms underlying the association between single or repetitive mild TBI and progressive neurodegeneration are not yet understood, we can reasonably assume that accurate biochemical tests of axonal, neuronal and astroglial injury would be helpful to indicate whether a person with head trauma has experienced an injury to the brain, to establish the severity and nature of the injury, and to identify when the injury has resolved.
The detection of brain injury in individuals who have experienced a concussive or subconcussive blow to the head is of particular relevance in sports such as boxing, hockey, rugby and American football. Head injuries are common in players of these sports, and several athletes’ careers have ended because of chronic neurological or psychiatric symptoms.6 An objective test to determine whether an athlete can safely return to their sport would, therefore, be highly desirable, and would reduce the current over-reliance on CT scans (and the associated exposure to ionizing radiation) for this purpose Another group of individuals at risk of brain injury is military personnel, who might be exposed to several types of brain trauma in the battlefield.7 In addition to biomarkers for use in the acute and subacute phases of mild TBI, development of biomarkers that will enable clinical studies of the potential neuropathological cascades in the chronic phase of mild TBI is also important. This statement is valid not only for fluid biomarkers but also for imaging and other markers.
In this Review, we provide an overview of the current research on fluid biomarkers of mild TBI. We describe the biomarkers that are already in clinical use and those that require further development before they can be used in clinical practice.
Pathophysiology of mild TBI
Mild TBI is a complex pathophysiological entity induced by external mechanical forces on the brain. Typically, mild TBI causes no gross pathology, such as haemorrhage or abnormalities that can be seen on a conventional CT scan of the brain,8 but instead causes rapid-onset neurophysiological and neurological dysfunction that, in most patients, resolves in a spontaneous manner over a fairly short period of time. However, approximately 15% of individuals with mild TBI develop persistent cognitive dysfunction.9,10 Mild TBI is usually caused by an impact to the head (contact loading) that induces rotational acceleration of the brain (inertial loading). In some patients, mild TBI occurs without an impact to the head, such as after rapid rotational acceleration of the head in restrained occupants during a motor vehicle crash.11 At a neurophysiological level, these mechanical and inertial forces result in the stretching of white matter axons, leading to diffuse axonal injury.12
Although axonal disconnection rarely occurs at the time of injury, the rapid stretching of axons causes an unregulated flux in ion concentrations, including an efflux of K+ and influx of Na+ from and into the axon that, in turn, causes an increase in intra-axonal Ca2+ concentrations.13,14 As the concentration of Ca2+ increases, the protease calpain becomes activated, triggering calpain-mediated proteolysis of cytoskeletal proteins, which might translate into irreversible axonal pathology. 15 An increase in intra-axonal Ca2+ concentration stimulates glutamate release and glutamate-mediated activation of N-methyl-d-aspartate receptors, resulting in further depolarization of neurons.16,17 Increased activity of various membrane pumps to restore the ionic balance leads to increased glucose consumption, depletion of energy stores, Ca2+ influx into mitochondria, impaired oxidative metabolism, and glycolysis with lactate production, which causes acidosis and oedema.
In addition to these ionic disturbances, ultrastructural studies of axons show mechanical breakage and buckling of microtubules at the time of injury, which can trigger progressive microtubule disassembly.18 These combined pathological processes result in interruption of axonal transport and accumulation of protein products. This accumulation gives rise to the two classic neuropathological phenotypes of axonal swelling: singular axonal bulbs (previously called retraction balls) and axonal varicosities, which occur as a series of protrusions along individual axons.13,19 At a critical threshold of axonal swelling, the axons disconnect at the location of the injury (secondary axotomy).16,17,20,21 Neuronal damage consisting of axonal bulbs and swellings is most commonly located in the deep gyri at the interface between the grey and white matter.13,22 Studies using advanced MRI techniques, such as diffusion tensor imaging, show that the extent of white matter abnormalities after mild TBI correlates with the severity of postconcussion cognitive problems.23–25
Many practicing clinicians have assumed that the axonopathy and metabolic stress in patients with mild TBI is reversed within 1–2 weeks, because this is when clinical symptoms have most often disappeared.26 However, magnetic resonance spectroscopy findings, electro-physiological data and neuropsychological assessments suggest that patients’ physiological parameters return to baseline after 30–45 days.27,28 Moreover, neuropathological analyses indicate that axonopathy might continue for years after TBI.13 Another important consideration affecting the patient’s recovery after mild TBI is their age, since the developing brain seems to be more vulnerable to repeated concussions than is the adult brain,29 owing to differences in the degree of myelination, volume ratio of brain to water, elastic properties, and blood–brain barrier (BBB) integrity.30,31 This knowledge, in conjunction with available biomechanical, radiological and clinical data,32,33 should be communicated to parents with the aim of discouraging the participation of children in contact sports that target the head.
A form of TBI-induced early dementia was first reported in 1928 among professional boxers, years after their careers had ended.34 Initially termed dementia pugilistica or punch-drunk syndrome, the prevalence of this neuropsychiatric manifestation is now estimated at around 20% in former professional boxers.35,36 These observations aroused great interest in the long-term outcomes of patients who developed chronic or progressive symptoms after a single episode or repeated episodes of mild TBI.37 Such symptoms can include changes in cognition (memory and executive functioning), mood (depression, apathy and suicidal thoughts), personality and behaviour (poor impulse control and behavioural disinhibition), and movement (including parkinsonism and symptoms of motor neuron dysfunction), which are similar to those described in ex-boxers.38 Some investigators have started to describe this constellation of symptoms as chronic traumatic encephalopathy (CTE);39–41 however, vigorous debate is ongoing among researchers regarding the definition of CTE from both neuropsychiatric and neuropathological perspectives.
The brains of former boxers with CTE also display the hallmark pathologies of Alzheimer disease (AD), including neurofibrillary tangles composed of hyperphosphorylated tau and amyloid-β (Aβ) plaques.42,43 Progressive axonopathy in these patients might underlie the rapid formation of Aβ plaques after TBI.44 The risk factors for CTE in ex-boxers are a long career, many bouts, high sparring exposure, many knockouts, poor performance, and being able to tolerate many blows without being knocked out, all of which are associated with cumulative exposure to repetitive brain trauma.40 According to one study, a positive apolipoprotein E ε4 status, commonly associated with AD, is a risk factor for CTE in these individuals.36 Similarly, tau and Aβ pathology, as well as TAR DNA-binding protein 43 (TDP-43) deposition, have been found in the brains of patients with CTE approximately 10 years after professional participation in contact sports such as American football.45 Notably, neuritic Aβ plaques and neurofibrillary tangles have also been found in patients a few years to four decades after a single episode of moderate or severe TBI.46 However, TDP-43 deposition was not found in these patients, suggesting that this pathological feature might be used to distinguish patients with CTE due to a single episode of TBI from those with CTE due to repetitive TBI.47
Currently available fluid biomarkers
CSF biomarkers of acute brain injury
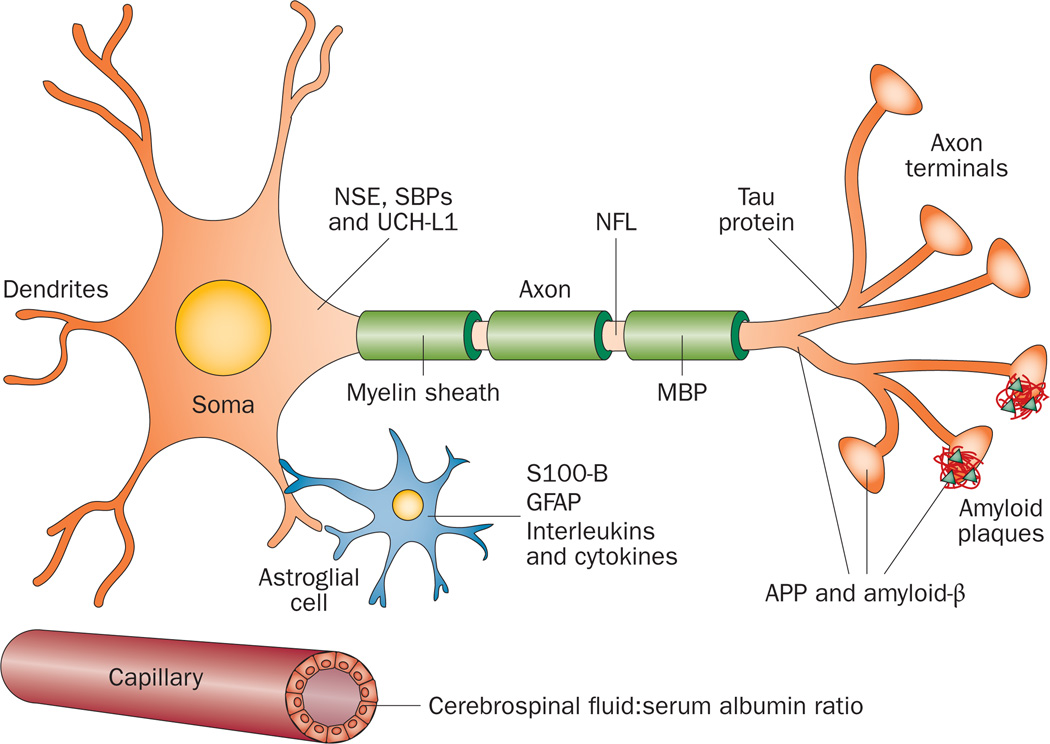
The cerebrospinal fluid (CSF) is in direct contact with the extracellular matrix in the brain, and its composition reflects biochemical changes that occur in this organ.48 For these reasons, the CSF might be considered an optimal source of biomarkers of brain injury. Several CSF biomarkers of brain injury have already been established, including proteins that indicate BBB integrity and neuroinflammation, as well as axonal, neuronal and astroglial damage, as described below (Figure 1, Table 1).
Figure 1.
Possible biomarkers of traumatic brain injury. These molecules include NSE, SBPs and UCH-L1, which are all enriched in the neuronal cytoplasm. NFL is a biomarker of injury to large-calibre myelinated axons. Total tau is a biomarker of injury to thin nonmyelinated axons. APP and amyloid-β are produced in axon terminals and might be involved in synaptic activity and plasticity. Overproduction of amyloid-β in response to trauma could result in formation of diffuse amyloid plaques. Injury to astroglial cells may lead to release of S100-B and GFAP into the extracellular matrix, which might increase S100-B levels in both cerebrospinal fluid and blood. Astrogliosis and post-injury neuroinflammation can result in increased production of interleukins and cytokines. Integrity of the blood–brain barrier is indicated by the cerebrospinal fluid:serum albumin ratio. Abbreviations: APP, amyloid precursor protein; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; NFL, neurofilament light polypeptide; NSE, γ-enolase; SBPs, spectrin breakdown products; UCH-L1, ubiquitin carboxyl-terminal hydrolase isoenzyme L1.
Table 1.
Potential fluid biomarkers of mild TBI
| Marker | Process or structure affected |
Research findings in mild TBI | |
|---|---|---|---|
| Cerebrospinal fluid | Blood | ||
| Cerebrospinal fuid:serum albumin ratio | Blood–brain barrier | Detection method not sensitive enough to changes in concentration53,54 | NA |
| Interleukins and other acute-phase infammatory response proteins | Neuroinfammation | NA | NA |
| Total tau protein | Axon | Levels peak 4–10 days after injury54,71 | Elevated levels in hypoxic brain injury;101,102 studies in mild TBI are lacking |
| Myelin basic protein | Axon | NA | Detection methods not sensitive in mild TBI79 |
| Neuroflament light polypeptide | Axon | Levels peak 4–10 days after injury54,71 | NA |
| Neuroflament heavy polypeptide | Axon | NA | NA |
| γ-Enolase | Neuron | Levels confounded by lysis of red blood cells in blood-contaminated cerebrospinal fuid82 | Serum levels very sensitive to lysis of red blood cells in blood-contaminated cerebrospinal fuid82 |
| S100-B | Astroglial cells | Levels are elevated, but a less sensitive marker than tau protein and neuroflaments71 | Results may be confounded by release from extracerebral tissues77 |
| Glial fbrillary acidic protein | Astroglial cells | Elevated levels, but a less sensitive marker than tau and neuroflament proteins54,71 | Serum levels correlate with changes on brain imaging;118 extracerebral protein has not been detected74 |
| Secreted APP-α and APP-β | Axon | NA | NA |
| Amyloid-β40 and amyloid-β42 | Plaque pathology | No change in levels57,74 | NA |
| Spectrin breakdown products | Neuron | NA | NA |
| Ubiquitin carboxyl-terminal hydrolase isoenzyme L1 | Neuron | NA | One pilot study showed promising results123 |
| Peripheral blood mononuclear small noncoding RNA molecules | Unknown | NA | One pilot study suggests altered expression of three RNA molecules in mild-TBI-exposed military personnel125 |
Abbreviations: APP, amyloid precursor protein; NA, not available; TBI, traumatic brain injury.
Blood–brain barrier integrity
The BBB, which is formed from the endothelial cells that line cerebral capillaries, has an important role in maintaining a regulated microenvironment for reliable neuronal signalling.49 The CSF:serum albumin ratio is a standard biomarker of BBB function.50 Albumin is mainly synthesized in the liver and, consequently, most albumin in CSF is derived from the blood via passage across the BBB. An increase in this ratio indicates BBB damage, which is found in patients with various CNS disorders, such as infections, inflammatory diseases, brain tumours or cerebrovascular diseases.48
Two studies have shown an increase in the CSF:serum albumin ratio in patients with severe TBI associated with a neuroinflammatory response.51,52 By contrast, no such changes have been seen in studies of mild TBI in boxers and military personnel with blast exposure,53,54 suggesting that the BBB remains intact in individuals with mild TBI.
Neuroinflammation
The findings of a large number of studies confirm that an acute inflammatory response occurs within the CNS after severe TBI, which is reflected in the concentrations of various CSF components.51,52,55–64 In general, levels of inflammatory proteins, such as IL-6, IL-8 and IL-10, are increased in CSF in response to severe TBI. The magnitude of the rise correlates with the patient’s outcome, and in some studies also with the extent of BBB dysfunction, as shown by the CSF:serum albumin ratio. This rise is an important confounder, since inflammatory protein levels in plasma are normally much higher than in CSF; passive leakage of inflammatory proteins across an impaired BBB may lead to elevated CSF levels in the absence of neuroinflammation. However, studies on markers of neuroinflammation in patients with mild TBI are lacking. Many of the studies listed above analysed samples of ventricular CSF, which has a different protein composition from lumbar CSF. This approach makes the results of these studies less relevant to patients with mild TBI, in whom CSF samples—if collected at all—tend to be obtained by lumbar puncture.
Acute axonal injury
The two best-established CSF biomarkers of axonal injury are total tau and neurofilament light polypeptide (NFL). These two proteins have distinct regional distributions in the brain, which might be helpful in determining which areas of the brain have been affected by TBI: tau protein is highly expressed in thin, nonmyelinated axons of cortical interneurons,65 whereas NFL is most abundant in the large-calibre myelinated axons that project into deeper brain layers and the spinal cord.66
Initial studies of tau protein as a marker of TBI compared total tau levels in ventricular CSF samples from patients with severe TBI, with the concentration of total tau in samples of CSF obtained by lumbar puncture from various control groups. These studies showed higher levels of total tau in the TBI group than in the control groups,67,68 but did not consider that tau protein levels are normally higher in CSF obtained from brain ventricles than in samples obtained by lumbar puncture.69 Nevertheless, the consensus in the literature is that total tau protein levels in ventricular CSF correlate with lesion size and clinical outcome in patients with TBI, such that high levels are an indication of more-severe injury.67,68,70 Studies in patients with mild TBI, such as amateur boxers, show elevated levels of total tau in CSF obtained by lumbar puncture 4–10 days after a bout, and similar results are found in boxers who have not been knocked out.54,71 Total tau protein levels in these individuals normalize during the 8–12 weeks after a bout, provided that the boxer has not participated in any further bouts.54,71 Total tau protein levels in CSF might, therefore, serve as a marker of axonal damage in grey matter neurons.
Neurofilaments are composed of neuron-specific intermediate filaments.72 Each intermediate filament consists of one light subunit (NFL) plus either a medium subunit (NFM) or a heavy subunit (NFH), arranged head-to-tail.72 High levels of phosphorylated NFH have been demonstrated in the ventricular CSF of patients with severe TBI.73 Similarly, high levels of NFL have been demonstrated in CSF samples obtained by lumbar puncture from amateur boxers with mild TBI after a bout.54,71 The magnitude of the rise in NFL is larger than that for total tau protein, which suggests that mild TBI affects the long myelinated axons in white matter to a greater extent than it affects the short nonmyelinated axons in the cortex.54,71 NFL in CSF seems to be the most sensitive fluid biomarker of axonal injury to date.54,71 Interestingly, the levels of NFL in CSF obtained by lumbar puncture from amateur boxers correlate positively with their exposure to head trauma, such as the number of hits to the head received, and subjective and objective estimates of the intensity of the fight.54,71
Acute neuronal injury
γ-Enolase (also known as NSE, or neuron-specific enolase, despite its presence in erythrocytes, as well as in endocrine cells) is a glycolytic enzyme enriched in neuronal cell bodies.74 The level of NSE was initially analysed in serum and ventricular CSF obtained from patients with severe head trauma and coma, in whom this protein was identified as a promising marker of neuronal damage.75 NSE levels in ventricular CSF correlate with mortality after TBI (levels are higher in non survivors than in survivors) and/or with other TBI severity scores, such as the Glasgow Coma Scale and Glasgow Out come Score, in both adults and children.76–80 Levels of NSE in CSF obtained by lumbar puncture have been suggested as a possible screening tool for inflicted TBI in children,81 but studies on the levels of NSE in CSF samples obtained by lumbar puncture in patients with mild TBI are lacking. The main limitation of using NSE levels in CSF as a biomarker of neuronal injury is its high sensitivity to haemolysis: NSE levels are markedly increased by in vitro lysis of erythrocytes derived from blood contamination of the sample.82
Acute astroglial injury
The S100 proteins are a family of Ca2+-binding proteins that help to regulate intracellular levels of calcium.74 The first S100 protein was identified in 1965,83 and two related homodimeric proteins, S100-A1 (which consists of two α subunits) and S100-B (which consists of two β subunits), were subsequently identified.84 An S100 αβ heterodimer also exists.74 In the biomarker literature, most assays for S100 proteins do not differentiate between the ββ homodimer and the αβ heterodimer; however, in a study in which the different forms could be differentiated, they all showed similar profiles of release into serum after brain trauma.85 S100-B was previously thought to be specific to astrocytes, but has since been detected in oligodendrocytes and various extracerebral cell types; for example, chondrocytes and adipocytes.74 S100-B levels in peripheral blood have been examined extensively in patients with TBI, but studies of the levels of this potential biomarker in CSF are scarce. Amateur boxers have slightly elevated levels of S100-B in CSF samples obtained by lumbar puncture after a bout, but the increase is not as pronounced as that observed in levels of the axonal markers total tau and NFL.71
Similar results (that is, slightly elevated levels in CSF samples obtained by lumbar puncture from amateur boxers after a bout) have been reported for glial fibrillary acidic protein (GFAP),54,71 a CNS-specific intermediate filament protein that is almost exclusively expressed in astroglia.74 The changes in GFAP levels after mild TBI are not as pronounced as the changes in total tau and NFL.54,71 However, when GFAP levels in ventricular CSF were evaluated in patients with severe TBI, the addition of this biomarker to clinical data improved the power of outcome prediction models.86
Amyloid-related processes
Studies in both animals and humans have demonstrated that amyloid precursor protein (APP) accumulates in neurons and axons after brain trauma that causes axonal damage.87–91 In experimental models of TBI, accumulation of APP occurs 2–3 h after the trauma and is also present in patients with mild TBI.3,92 In addition to the accumulation of APP, acute intra-axonal accumulation of Aβ is common in patients with TBI.93–95 Aβ—in particular the Aβ42 isoform, which is prone to aggregation and is associated with the development of AD—is subsequently released into the tissue surrounding damaged axons, where it leads to plaque formation.96–98 Ventricular CSF levels of Aβ40 and Aβ42 increase during the first week after head trauma in patients with severe TBI.99,100 Similar results were found for soluble α and β isoforms of APP.100 However, in samples of CSF obtained by lumbar puncture from patients with mild TBI, no changes in Aβ40 or Aβ42 were seen.54,71 These results suggest that Aβ levels in CSF samples obtained by lumbar puncture are less sensitive to the effects of mild TBI than are NFL and total tau levels.
Blood biomarkers of acute brain injury
Some proteins that are highly expressed within the CNS are also detectable—albeit at very low concentrations, owing to their dilution in the much larger plasma volume and extracellular matrix of peripheral tissues—in the peripheral blood.101,102 Since collection of peripheral blood samples is considerably easier than collection of CSF in routine clinical practice, many candidate CSF biomarkers of mild TBI have also been assessed in peripheral blood (Table 1). The low concentration of potential biomarkers in peripheral blood is a technical limitation to the use of most standard immunoassays. However, the number of potential biomarkers of brain injury in peripheral blood is steadily increasing as the analytical tools for their detection become ever more sensitive.103
A number of other obstacles to the development of reliable blood biomarkers of mild TBI exist. The BBB, though not as absolute a barrier as the name might suggest,49 hinders the assessment of biochemical changes in the brain by use of biomarkers in the blood (impaired BBB integrity, however, as seen in severe TBI, can increase the levels of brain-derived proteins in the blood). In addition, some potential markers undergo proteolytic degradation in blood, and their levels might be affected by clearance from blood via the liver or kidney. The precision of immunoassays can also be affected by binding of potential biomarkers to carrier proteins, and by extracerebral sources of potential biomarkers. As a consequence, reliable blood biomarkers of neurodegenerative processes (such as those taking place in patients with AD or in the presence of advanced neuro pathology) have been extremely difficult to identify.48 Nevertheless, the literature on potential peripheral blood biomarkers of brain injury in patients with severe TBI is abundant.104
Astroglial injury
S100-B and GFAP have both received considerable attention as peripheral blood markers of astroglial injury. Two reviews of biomarkers for TBI report that the levels of S100-B and GFAP in serum are increased in patients with TBI and correlate with Glasgow Coma Scale scores and neuroradiological findings at hospital admission.104,105 These findings could help to differentiate patients with mild TBI from those with severe TBI, and improve predictions of their outcome.104,105 However, S100-B is also expressed in extracerebral cell types, including adipocytes and chondrocytes.74 For this reason, some researchers have expressed concern that the observed increase in serum levels of S100-B in patients with TBI might be attributable to release of this protein by damaged peripheral tissues, such as fractured bones or injured skeletal muscles. Indeed, elevated serum levels of S100-B have been observed in both patients with multiple trauma and athletes without head injuries.106–110 Moreover, serum S100-B levels may increase in response to BBB dysfunction.111
GFAP might be a better biomarker of mild TBI than is S100-B, because extracerebral expression of GFAP has not been detected. A study of patients with mild TBI and abnormal findings on CT or MRI of the brain showed elevated serum levels of GFAP.112 However, these marker levels did not predict patients’ outcomes at 6 months post-TBI.
Axonal and neuronal injury
Other candidate peripheral blood biomarkers of TBI are NSE, myelin basic protein (MBP) and hyperphosphorylated NFH, although no studies on MBP as a CSF biomarker are currently available. The increase in levels of NSE in CSF observed in response to lysis of erythrocytes is a major limitation of this biomarker in peripheral blood as well as in CSF. MBP levels might be a more specific marker of TBI than are NSE levels (specificity 96% versus 64%, respectively), but its sensitivity is suboptimal (44% for MBP versus 71% for NSE).113 Levels of NFH in serum samples taken from patients with severe TBI increased over 6 consecutive days in patients who eventually died from their injury, but levels of S100-B increased more rapidly than those of NFH.114 However, clinically important differences in biomarker release profiles might also be relevant. For example, NFH levels in serum remained elevated at days 2–4 post-injury in children with TBI who had a poor prognosis, whereas an early drop in serum NFH levels was predictive of improved outcome.115 In a case study, serum concentrations of NFH were initially very high in a patient with fatal TBI induced by severe blast exposure.116 Serum levels of NFH also seem to be fairly independent of BBB integrity.112
Amyloid-related processes
An ultrasensitive digital immunoassay has been developed for quantification of the CNS-specific protein tau in serum.102 Serum levels of tau protein could be measured with a lower limit of detection of 0.02 pg/ml, and ranged from <10 pg/ml to 400 pg/ml in patients who were resuscitated after cardiac arrest; the pattern of changes in serum levels of this protein observed over time correlated with outcome.102 Two peaks in serum concentration were detected: one within the first 24 h after resuscitation, which was seen in almost all patients, and another after 24–48 h. The second peak was highest in patients who eventually died from their injury.114 This is a sensitive detection method and could be useful in the context of mild TBI; further studies are warranted.
Novel fluid biomarkers
A number of potential biomarkers in CSF show increases in levels that correlate with the severity of the brain injury and with predicted outcomes. Longitudinal clinical studies in patients with mild TBI are needed, however, to understand how these biomarkers could be implemented in clinical practice.
Spectrin α chain, non-erythrocytic 1 (also known as αII spectrin) is primarily found in neurons, and is abundantly expressed in axons and presynaptic terminals.117 αII Spectrin is broken down by calpain and caspase-3, which are upregulated in TBI during neuronal necrosis and apoptosis, respectively. Spectrin breakdown products have, therefore, been investigated as potential biomarkers of brain injury in rats and humans.118,119 The levels of spectrin breakdown products in ventricular CSF samples from patients with severe TBI are associated with clinical correlates of the severity of the brain injury, such as Glasgow Coma Scale scores, and could be used to improve the prediction of patient outcomes.86,120,121 For example, in one of these studies, levels of spectrin breakdown products in CSF were measured together with those of another potential marker, UCH-L1 (ubiquitin carboxyl-terminal hydrolase isoenzyme L1),86 a deubiquitinase that is highly expressed in neurons.122 Levels of these two markers contributed clinically relevant prognostic information, in addition to that obtained by routine clinical assessments (such as the International Mission for Progress and Analysis of Clinical Trials in TBI prognostic calculator and the Glasgow Coma Scale).120,121
UCH-L1 and spectrin breakdown product levels increase in a manner similar to S100-B and GFAP in terms of correlation with other measures of the severity of TBI, as well as clinical outcome.104 These breakdown products have also been studied in peripheral blood.86 UCH-L1 levels in blood are, however, increased by compromised BBB integrity.112 As yet, there are no conclusive studies on the potential diagnostic performance of UCH-L1 and spectrin breakdown products as peripheral blood biomarkers in patients with mild TBI, but one prospective cohort study of 96 patients with mild to moderate TBI showed that UCH-L1 is detectable in serum within 1 h of injury and that its level is associated with measures of injury severity, including the Glasgow Coma Scale score, lesions seen on brain imaging, and the need for neurosurgical intervention.123
Proteomic analysis of potential new CSF biomarkers for TBI has not yet identified any such markers that can be used in clinically useful tests.124 A number of proteomics studies on potential biomarkers of TBI in peripheral blood have been published. These studies have replicated the findings from targeted analyses of specific candidate biomarkers, but as yet none of the novel biomarker profiles identified in these studies as being associated with TBI has been validated in independent studies using unrelated, non-proteomic or genomic techniques.104 Exciting preliminary data on the expression profiles of small noncoding RNAs in peripheral blood mononuclear cells from military personnel exposed to mild TBI have been reported; three small RNAs seem to be primarily associated with mild TBI, but the results require replication.125
Fluid biomarkers of chronic injury
Phosphorylated tau
The most prominent neuropathological characteristic of CTE, as reported in studies of former boxers in the 1970s, is neurofibrillary tangles in cortical areas.126 The best-established CSF biomarker to date for tangle pathology, at least in patients with AD, is phosphorylated tau.48 However, some patients with chronic neurodegenerative diseases characterized by abundant tangle pathology—for example, Pick disease and progressive supranuclear palsy127—often have normal CSF levels of phosphorylated tau.128,129 The reason for this discrepancy is at present unknown, but one possible explanation is that the various tauopathies might involve different isoforms of aggregated tau130 that are not detected in equal measure by all assays. The presence and levels of hyperphosphorylated tau isoforms in CSF have not yet been examined in patients with CTE.
TDP-43
Another pathological feature of CTE, namely, inclusions of TDP-43 in neurons and glial cells,47,131,132 is also typical of frontotemporal dementia and amyotrophic lateral sclerosis.133 TDP-43 accumulation in patients with CTE can be widespread and is found in several grey matter structures, such as the brainstem, basal ganglia and cortical areas, as well as in subcortical white matter.47,132 Although assays for measuring TDP-43 levels in CSF have been developed,134 no studies have yet been published on their use in patients with CTE.
Pituitary hormones
The prevalence of chronic pituitary dysfunction, caused by tearing of axons in the pituitary stalk, may be as high as 30–80% in patients 24–36 months after TBI.135 Abnormalities in pituitary hormone levels correlate with the presence of long-term cognitive symptoms after TBI.135 As these hormone disturbances are treatable by hormone replacement therapy, it is important to identify them and monitor the treatment through the measureme nt of pituitary hormone levels.
Interestingly, the occurrence of pituitary dysfunction bears no clear relationship with the severity of TBI.135 Mild-TBI-related chronic pituitary dysfunction has been reported in boxers and kickboxers subjected to repetitive head injury. In a preliminary study, 45% of professional boxers had growth hormone deficiency, although no other pituitary hormone deficiencies were observed.136 In a large study of active and retired boxers, 18% had pituitary hormone deficiencies in one or more of the hypothalamic–pituitary–adrenal and growth hormone–insulin-like growth factor-1 axes.137 An investigation of pituitary dysfunction in amateur kickboxers revealed deficiencies in growth hormone and/or adrenocorticotropin in 27% of the athletes.138 In another study, the concentrations of 12 pituitary and target-organ hormones were measured in two groups of male US combat veterans. Abnormal levels of at least one pituitary hormone were detected in 11 of the 26 participants who had a history of blast-induced concussion.139
Conclusions
A large number of biomarkers of injury to different cell types and structures within the CNS can be detected in CSF and peripheral blood. This Review identifies a number of areas in which further research is needed to establish biomarkers of mild TBI. Several of the biomarker candidates were initially investigated in relation to severe TBI, in which setting they provide clinically relevant information and help to predict patient outcomes. However, longitudinal studies of biomarker levels in patients with clinically relevant mild TBI are scarce. In the absence of such studies, it is difficult to suggest detailed diagnostic algorithms that incorporate fluid biomarkers for use in this setting. The lack of standardized methods to quantify the available biomarkers also precludes the use of validated biomarker cut-off levels to guide clinical decision-making. Achieving validation of such cut-off points is an important goal of current research into biomarkers of mild TBI.
Axons seem to be the structures most vulnerable to damage from mild TBI, and reliable identification of peripheral blood biomarkers for axonal damage is needed. Tau protein and neurofilament proteins are promising biomarkers, but analytical techniques for measurement of this class of biomarkers in blood need to improve in sensitivity, beyond that usually achieved using conventional immunoassays. Apart from assays for S100-B and NSE, clinical assays for quantification of most bio markers do not yet exist, and no certified reference materials or methods for assay calibration are available. Improved animal models that reflect the relevant processes in mild TBI in humans, and in which new novel bio markers might be identified and evaluated, are also needed. Important advances in this regard have been made in the development of a mouse model of blast-induced neurotrauma, which seems to recapitulate key features of the neuro pathology seen in humans with mild TBI.7
A critical challenge in research on the relationship between mild TBI and CTE is the ability to diagnose CTE in living patients. Identification of biomarkers of the neuropathological characteristics of CTE would make it possible to address whether or not individuals exposed to repetitive head trauma are at increased risk of CTE, and whether the extent of this risk can be determined via biomarker levels. The possible existence of a threshold for exposure to repetitive head injury resulting in CTE needs to be resolved, and the contribution of individual vulnerability to the risk of developing CTE also needs to be clarified. Identification of suitable biomarkers could potentially also help to identify preclinical CTE and shed light on whether the pathological process can be halted by appropriate treatment.
Key points.
Biomarkers of neuronal, axonal and astroglial damage could be used to diagnose mild traumatic brain injury (TBI) and predict clinical outcomes of patients with head trauma
Such biomarkers could provide important information for medical counselling of at-risk individuals, such as military personnel and concussed athletes
Cerebrospinal fluid markers are preferred over peripheral blood markers, owing to their increased proximity to the brain and decreased susceptibility to the confounding effects of various extracerebral factors
Ultrasensitive assays are needed for reliable quantification of CNS-specific biomarkers in blood, as their concentrations are below the lower limit of detection by most standard immunoassays
Clinical studies of serial biomarker measurements in conjunction with advanced brain imaging during the acute and subacute phases of mild TBI are warranted
Longitudinal studies of biomarkers in patients with chronic or progressive symptoms after TBI might help to clarify the pathogenesis and clinical course of chronic traumatic encephalopathy
Review criteria.
We searched PubMed for articles in the English language on traumatic brain injury using the keywords “TBI” or “traumatic brain injury”, in combination with “biomarkers”, “CSF”, “plasma”, “serum”, “blood”, “mild traumatic brain injury”, and “chronic traumatic encephalopathy”.
Acknowledgements
The authors’ research work is supported by the Swedish Research Council and Swedish State Support for Clinical Research (H. Zetterberg, K. Blennow), the Wolfson Foundation (H. Zetterberg) and NIH grants R01 NS038104, P01 NS056202 and R03 AG038911 (D. H. Smith).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
H. Zetterberg wrote the article. H. Zetterberg, D. H. Smith and K. contributed equally to researching data for the article, discussion of the content, and review and editing of the manuscript before submission.
References
- 1.Roozenbeek B, Maas AI, Menon DK. Opinion: Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. doi: 10.1038/nrneurol.2013.22. http://dx.doi/org/nrneurol.2013.22. [DOI] [PubMed]
- 2.American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993;8:86–87. [Google Scholar]
- 3.Blumbergs PC, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 4.Browne KD, Chen XH, Meaney DF, Smith DH. Mild traumatic brain injury and diffuse axonal injury in swine. J.Neurotrauma. 2011;28:1747–1755. doi: 10.1089/neu.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baugh CM, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 6.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 2011;30:179–188. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein LE, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003716. 134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCrory P, et al. Consensus statement on concussion in sport—the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Phys. Sportsmed. 2009;37:141–159. doi: 10.3810/psm.2009.06.1721. [DOI] [PubMed] [Google Scholar]
- 9.Røe C, Sveen U, Alvsåker K, Bautz-Holter E. Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study. Disabil. Rehabil. 2009;31:1235–1243. doi: 10.1080/09638280802532720. [DOI] [PubMed] [Google Scholar]
- 10.Williams WH, Potter S, Ryland H. Mild traumatic brain injury and postconcussion syndrome: a neuropsychological perspective. J. Neurol. Neurosurg. Psychiatry. 2010;81:1116–1122. doi: 10.1136/jnnp.2008.171298. [DOI] [PubMed] [Google Scholar]
- 11.Meaney DF, Smith DH. Biomechanics of concussion. Clin. Sports Med. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DH, Meaney DF. Axonal damage in traumatic brain injury. The Neuroscientist. 2000;6:483–495. [Google Scholar]
- 13.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp.Neurol. 2012 doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- 17.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Tang-Schomer MD, Patel AR, Baas PW, Smith DH. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB J. 2010;24:1401–1410. doi: 10.1096/fj.09-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp. Neurol. 2012;233:364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Povlishock JT, Becker DP. Fate of reactive axonal swellings induced by head injury. Lab. Invest. 1985;52:540–552. [PubMed] [Google Scholar]
- 22.Chen XH, et al. Long-term accumulation of amyloid-β, β-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am. J. Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipton ML, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 24.Niogi SN, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilde EA, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 26.New Zealand Guidelines Group Staff, Accident Compensation Corporation (N. Z.) Staff. Traumatic Brain Injury: Diagnosis, Acute Management and Rehabilitation. New Zealand: Accident Compensation Corporation; 2006. [Google Scholar]
- 27.Iverson GL, Gaetz M, Lovell MR, Collins MW. Cumulative effects of concussion in amateur athletes. Brain Inj. 2004;18:433–443. doi: 10.1080/02699050310001617352. [DOI] [PubMed] [Google Scholar]
- 28.Brooks WM, et al. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J. Neurotrauma. 2000;17:629–640. doi: 10.1089/089771500415382. [DOI] [PubMed] [Google Scholar]
- 29.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Recovery of intellectual ability following traumatic brain injury in childhood: impact of injury severity and age at injury. Pediatr. Neurosurg. 2000;32:282–290. doi: 10.1159/000028956. [DOI] [PubMed] [Google Scholar]
- 30.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J. Neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 31.Duhaime AC. Large animal models of traumatic injury to the immature brain. Dev. Neurosci. 2006;28:380–387. doi: 10.1159/000094164. [DOI] [PubMed] [Google Scholar]
- 32.Pinto PS, Meoded A, Poretti A, Tekes A, Huisman TA. The unique features of traumatic brain injury in children Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications, and their imaging findings —part 2. J. Neuroimaging. 2012;22:e18–e41. doi: 10.1111/j.1552-6569.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 33.Pinto PS, Poretti A, Meoded A, Tekes A, Huisman TA. The unique features of traumatic brain injury in children Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings —part 1. J. Neuroimaging. 2012;22:e1–e17. doi: 10.1111/j.1552-6569.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- 34.Martland H. Punch drunk. JAMA. 1928;91:1103–1107. [Google Scholar]
- 35.Roberts AH. Brain Damage in Boxers: a Study of the Prevalence of Traumatic Encephalopathy Among Ex-Professional Boxers. London: Pitman Medical Scientific Publications; 1969. [Google Scholar]
- 36.Jordan BD, et al. Apolipoprotein E ε4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- 37.Stern RA, et al. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Smith DH, Johnson VE, Stewart W. The chronic neuropathologies of single and repetitive traumatic brain injury: potential substrates of dementia? Nat. Rev. Neurol. doi: 10.1038/nrneurol.2013.29. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavett BE, et al. Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Curr. Opin. Neurol. 2011;24:525–531. doi: 10.1097/WCO.0b013e32834cd477. [DOI] [PubMed] [Google Scholar]
- 40.Jordan BD. Chronic traumatic brain injury associated with boxing. Semin. Neurol. 2000;20:179–185. doi: 10.1055/s-2000-9826. [DOI] [PubMed] [Google Scholar]
- 41.Mendez MF. The neuropsychiatric aspects of boxing. Int. J. Psychiatry Med. 1995;25:249–262. doi: 10.2190/CUMK-THT1-X98M-WB4C. [DOI] [PubMed] [Google Scholar]
- 42.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J. Neurol. Neurosurg. Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers’ brains using immunohistochemistry with antibodies to amyloid β-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- 44.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nat. Rev. Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson VE, Stewart W, Trojanowski JQ, Smith DH. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol. 2011;122:715–726. doi: 10.1007/s00401-011-0909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jordan BD. Clinical spectrum of sports-related traumatic brain injury. Nat.Rev.Neurol. doi: 10.1038/nrneurol.2013.33. in press. [DOI] [PubMed] [Google Scholar]
- 48.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 49.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 50.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values Scand. J. Clin. Lab. Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 51.Csuka E, et al. IL-10 levels in cerebrospinal fluid serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-α, TGF-β1 blood-brain barrier function. J. Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 52.Kossmann T, et al. Intrathecal and serum interleukin-6 and the acute-phase response in patients with severe traumatic brain injuries. Shock. 1995;4:311–317. doi: 10.1097/00024382-199511000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Blennow K, et al. No neurochemical evidence of brain injury after blast overpressure by repeated explosions or firing heavy weapons. Acta Neurol. Scand. 2011;123:245–251. doi: 10.1111/j.1600-0404.2010.01408.x. [DOI] [PubMed] [Google Scholar]
- 54.Zetterberg H, et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 2006;63:1277–1280. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 55.Semple BD, Bye N, Rancan M, Ziebell JM, &Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J. Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirchhoff C, et al. Cerebrospinal IL-10 concentration is elevated in non-survivors as compared to survivors after severe traumatic brain injury. Eur. J. Med. Res. 2008;13:464–468. [PubMed] [Google Scholar]
- 57.Goodman JC, Van M, Gopinath SP, Robertson CS. Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir. Suppl. 2008;102:437–439. doi: 10.1007/978-3-211-85578-2_85. [DOI] [PubMed] [Google Scholar]
- 58.Buttram SD, et al. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J. Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- 59.Phillips DJ, et al. Activin A release into cerebrospinal fluid in a subset of patients with severe traumatic brain injury. J. Neurotrauma. 2006;23:1283–1294. doi: 10.1089/neu.2006.23.1283. [DOI] [PubMed] [Google Scholar]
- 60.Maier B, et al. Delayed elevation of soluble tumor necrosis factor receptors p75 and p55 in cerebrospinal fluid and plasma after traumatic brain injury. Shock. 2006;26:122–127. doi: 10.1097/01.shk.0000223127.41641.f4. [DOI] [PubMed] [Google Scholar]
- 61.Shiozaki T, et al. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–410. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- 62.Singhal A, et al. Association between cerebrospinal fluid interleukin-6 concentrations and outcome after severe human traumatic brain injury. J. Neurotrauma. 2002;19:929–937. doi: 10.1089/089771502320317087. [DOI] [PubMed] [Google Scholar]
- 63.Stahel PF, et al. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J. Neurotrauma. 2001;18:773–781. doi: 10.1089/089771501316919139. [DOI] [PubMed] [Google Scholar]
- 64.Bell MJ, et al. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J. Neurotrauma. 1997;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- 65.Trojanowski JQ, Schuck T, Schmidt ML, Lee VM. Distribution of tau proteins in the normal human central and peripheral nervous system. J. Histochem. Cytochem. 1989;37:209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- 66.Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat. Rec. 1970;167:379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- 67.Franz G, et al. Amyloid β1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60:1457–1461. doi: 10.1212/01.wnl.0000063313.57292.00. [DOI] [PubMed] [Google Scholar]
- 68.Zemlan FP, et al. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. doi: 10.1016/s0006-8993(02)02920-7. [DOI] [PubMed] [Google Scholar]
- 69.Blennow K, Nellgård B. Amyloid β1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2004;62:159–160. doi: 10.1212/wnl.62.1.159. [DOI] [PubMed] [Google Scholar]
- 70.Ost M, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 71.Neselius S, et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS ONE. 2012;7:e33606. doi: 10.1371/journal.pone.0033606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Q, et al. Neurofilament proteins in neurodegenerative diseases. Cell. Mol. Life Sci. 2004;61:3057–3075. doi: 10.1007/s00018-004-4268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siman R, et al. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma. 2009;26:1867–1877. doi: 10.1089/neu.2009.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olsson B, Zetterberg H, Hampel H, Blennow K. Biomarker-based dissection of neurodegenerative diseases. Prog. Neurobiol. 2011;4:520–534. doi: 10.1016/j.pneurobio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Scarna H, et al. Neuron-specific enolase as a marker of neuronal lesions during various comas in man. Neurochem. Int. 1982;4:405–411. doi: 10.1016/0197-0186(82)90083-3. [DOI] [PubMed] [Google Scholar]
- 76.Bohmer AE, et al. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery. 2011;68:1624–1631. doi: 10.1227/NEU.0b013e318214a81f. [DOI] [PubMed] [Google Scholar]
- 77.Chiaretti A, et al. NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology. 2009;72:609–616. doi: 10.1212/01.wnl.0000342462.51073.06. [DOI] [PubMed] [Google Scholar]
- 78.Varma S, et al. F2-isoprostane and neuron-specific enolase in cerebrospinal fluid after severe traumatic brain injury in infants and children. J. Neurotrauma. 2003;20:781–786. doi: 10.1089/089771503767870005. [DOI] [PubMed] [Google Scholar]
- 79.Berger RP, et al. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109:E31. doi: 10.1542/peds.109.2.e31. [DOI] [PubMed] [Google Scholar]
- 80.Ross SA, Cunningham RT, Johnston CF, Rowlands BJ. Neuron-specific enolase as an aid to outcome prediction in head injury. Br. J. Neurosurg. 1996;10:471–476. doi: 10.1080/02688699647104. [DOI] [PubMed] [Google Scholar]
- 81.Berger RP, et al. Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: a possible screening tool. Pediatrics. 2006;117:325–332. doi: 10.1542/peds.2005-0711. [DOI] [PubMed] [Google Scholar]
- 82.Ramont L. Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clin. Chem. Lab. Med. 2005;43:1215–1217. doi: 10.1515/CCLM.2005.210. [DOI] [PubMed] [Google Scholar]
- 83.Moore BW, McGregor D. Chromatographic and electrophoretic fractionation of soluble proteins of brain and liver. J. Biol. Chem. 1965;240:1647–1653. [PubMed] [Google Scholar]
- 84.Isobe T, Ishioka N, Okuyama T. Structural relation of two S-100 proteins in bovine brain; subunit composition of S.−100A protein. Eur. J. Biochem. 1981;115:469–474. doi: 10.1111/j.1432-1033.1981.tb06225.x. [DOI] [PubMed] [Google Scholar]
- 85.Nylen K, et al. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir. (Wien) 2008;150:221–227. doi: 10.1007/s00701-007-1489-2. [DOI] [PubMed] [Google Scholar]
- 86.Czeiter E, et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J. Neurotrauma. 2012;29:1770–1778. doi: 10.1089/neu.2011.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McKenzie JE, Gentleman SM, Roberts GW, Graham DI, Royston MC. Increased numbers of β APP-immunoreactive neurones in the entorhinal cortex after head injury. Neuroreport. 1994;6:161–164. doi: 10.1097/00001756-199412300-00041. [DOI] [PubMed] [Google Scholar]
- 88.Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for β-amyloid precursor protein. Acta Neuropathol. 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- 89.Gentleman SM, et al. Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol. 1995;89:537–543. doi: 10.1007/BF00571509. [DOI] [PubMed] [Google Scholar]
- 90.Ahlgren S, Li GL, Olsson Y. Accumulation of β-amyloid precursor protein and ubiquitin in axons after spinal cord trauma in humans: immunohistochemical observations on autopsy material. Acta Neuropathol. 1996;92:49–55. doi: 10.1007/s004010050488. [DOI] [PubMed] [Google Scholar]
- 91.Gleckman AM, Bell MD, Evans RJ, Smith TW. Diffuse axonal injury in infants with nonaccidental craniocerebral trauma: enhanced detection by β-amyloid precursor protein immunohistochemical staining. Arch. Pathol. Lab. Med. 1999;123:146–151. doi: 10.5858/1999-123-0146-DAIIIW. [DOI] [PubMed] [Google Scholar]
- 92.McKenzie KJ, et al. Is β-APP a marker of axonal damage in short-surviving head injury? Acta Neuropathol. 1996;92:608–613. doi: 10.1007/s004010050568. [DOI] [PubMed] [Google Scholar]
- 93.Smith DH, Chen XH, Iwata A, Graham DI. Amyloid β accumulation in axons after traumatic brain injury in humans. J. Neurosurg. 2003;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 94.Uryu K, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp. Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid β plaques despite persistent accumulation of amyloid β in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts GW, Gentleman SM, Lynch A, Graham DI. β A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 97.Graham DI, Gentleman SM, Lynch A, Roberts GW. Distribution of β-amyloid protein in the brain following severe head injury. Neuropathol. Appl. Neurobiol. 1995;21:27–34. doi: 10.1111/j.1365-2990.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 98.Horsburgh K, et al. β-amyloid (Aβ)42(43), Aβ42, Aβ40 and apoE immunostaining of plaques in fatal head injury. Neuropathol. Appl. Neurobiol. 2000;26:124–132. doi: 10.1046/j.1365-2990.2000.026002124.x. [DOI] [PubMed] [Google Scholar]
- 99.Raby CA, et al. Traumatic brain injury increases β-amyloid peptide1–42 in cerebrospinal fluid. J. Neurochem. 1998;71:2505–2509. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- 100.Olsson A, et al. Marked increase of β-amyloid1–42 and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J. Neurol. 2004;251:870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 101.Mortberg E, et al. Plasma tau protein in comatose patients after cardiac arrest treated with therapeutic hypothermia. Acta Anaesthesiol. Scand. 2011;55:1132–1138. doi: 10.1111/j.1399-6576.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 102.Randall J, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. doi: 10.1016/j.resuscitation.2012.07.027. Resuscitation http://dx.doi.org/10.1016/j.resuscitation.2012.07.027. [DOI] [PubMed]
- 103.Rissin DM, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mondello S, et al. Blood-based diagnostics of traumatic brain injuries. Expert Rev. Mol. Diagn. 2011;11:65–78. doi: 10.1586/erm.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kovesdi E, et al. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (Wien) 2010;152:1–17. doi: 10.1007/s00701-009-0463-6. [DOI] [PubMed] [Google Scholar]
- 106.Mussack T, et al. Significance of Elecsys S100 immunoassay for real-time assessment of traumatic brain damage in multiple trauma patients. Clin. Chem. Lab. Med. 2006;44:1140–1145. doi: 10.1515/CCLM.2006.190. [DOI] [PubMed] [Google Scholar]
- 107.Rothoerl RD, Woertgen C. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;49:1490–1493. doi: 10.1097/00006123-200112000-00054. [DOI] [PubMed] [Google Scholar]
- 108.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–1260. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 109.Romner B, Ingebrigtsen T. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 2001;49:1490–1493. doi: 10.1097/00006123-200112000-00053. [DOI] [PubMed] [Google Scholar]
- 110.Stalnacke BM, Ohlsson A, Tegner Y, Sojka P. Serum concentrations of two biochemical markers of brain tissue damage S-100B and neurone specific enolase are increased in elite female soccer players after a competitive game. Br. J. Sports Med. 2006;40:313–316. doi: 10.1136/bjsm.2005.021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blyth BJ, et al. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood–brain barrier function after traumatic brain injury. J. Neurotrauma. 2011;28:2453–2462. doi: 10.1089/neu.2010.1653. [DOI] [PubMed] [Google Scholar]
- 112.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78:1428–1433. doi: 10.1212/WNL.0b013e318253d5c7. [DOI] [PubMed] [Google Scholar]
- 113.Berger RP, et al. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. 2005;103:161–168. doi: 10.3171/ped.2005.103.1.0061. [DOI] [PubMed] [Google Scholar]
- 114.Zurek J, Fedora M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir. (Wien) 2012;154:193–103. doi: 10.1007/s00701-011-1175-2. [DOI] [PubMed] [Google Scholar]
- 115.Zurek J, Bartlova L, Fedora M. Hyperphosphorylated neurofilament NF-H as a predictor of mortality after brain injury in children. Brain Inj. 2011;25:221–226. doi: 10.3109/02699052.2010.541895. [DOI] [PubMed] [Google Scholar]
- 116.Tisdall M, Petzold A. Commenton “chronic traumatic encephalopathy in blast-exposed military veterans a blast neurotrauma mouse model”. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004403. 157le158. [DOI] [PubMed] [Google Scholar]
- 117.Riederer BM, Zagon IS, Goodman SR. Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J. Cell Biol. 1986;102:2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pike BR, et al. Accumulation of non-erythroid α II-spectrin and calpain-cleaved α II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 2001;78:1297–1306. doi: 10.1046/j.1471-4159.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 119.Pineda JA, et al. Clinical significance of α II-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma. 2007;24:354–366. doi: 10.1089/neu.2006.003789. [DOI] [PubMed] [Google Scholar]
- 120.Farkas O, et al. Spectrin breakdown products in the cerebrospinal fluid in severe head injury—preliminary observations. Acta Neurochir. (Wien) 2005;147:855–861. doi: 10.1007/s00701-005-0559-6. [DOI] [PubMed] [Google Scholar]
- 121.Mondello S, et al. α II-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma. 2010;27:1203–1213. doi: 10.1089/neu.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilkinson KD, et al. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 123.Papa L, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 2012;72:1335–1344. doi: 10.1097/TA.0b013e3182491e3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ottens AK, et al. Neuroproteomics in neurotrauma. Mass Spectrom. Rev. 2006;25:380–408. doi: 10.1002/mas.20073. [DOI] [PubMed] [Google Scholar]
- 125.Pasinetti GM, Ho L, Dooley C, Abbi B, Lange G. Select non-coding RNA in blood components provide novel clinically accessible biological surrogates for improved identification of traumatic brain injury in OEF/OIF veterans. Am. J. Neurodegener. Dis. 2012;1:88–98. [PMC free article] [PubMed] [Google Scholar]
- 126.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol. Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 127.Pollock NJ, Mirra SS, Binder LI, Hansen LA, Wood JG. Filamentous aggregates in Pick’s disease, progressive supranuclear palsy, and Alzheimer’s disease share antigenic determinants with microtubule-associated protein tau. Lancet. 1986;2:1211. doi: 10.1016/s0140-6736(86)92212-9. [DOI] [PubMed] [Google Scholar]
- 128.Hampel H, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch. Gen. Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 129.Hall S, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 2012;27:1–8. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 130.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J. Mol. Neurosci. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.King A, et al. Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer’s disease. Neuropathology. 2010;30:408–419. doi: 10.1111/j.1440-1789.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 132.McKee AC, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 134.Geser F, et al. On the development of markers for pathological TDP-43 in amyotrophic lateral sclerosis with and without dementia. Prog. Neurobiol. 2011;95:649–662. doi: 10.1016/j.pneurobio.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guerrero AF, Alfonso A. Traumatic brain injury-related hypopituitarism: a review and recommendations for screening combat veterans. Mil. Med. 2010;175:574–580. doi: 10.7205/milmed-d-09-00189. [DOI] [PubMed] [Google Scholar]
- 136.Kelestimur F, et al. Boxing as a sport activity associated with isolated GH deficiency. J. Endocrinol. Invest. 2004;27:RC28–RC32. doi: 10.1007/BF03345299. [DOI] [PubMed] [Google Scholar]
- 137.Tanriverdi F, et al. Brief communication: pituitary volume and function in competing and retired male boxers. Ann. Intern. Med. 2008;148:827–831. doi: 10.7326/0003-4819-148-11-200806030-00005. [DOI] [PubMed] [Google Scholar]
- 138.Tanriverdi F, et al. Kickboxing sport as a new cause of traumatic brain injury-mediated hypopituitarism. Clin. Endocrinol. Oxf. 2007;66:360–366. doi: 10.1111/j.1365-2265.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 139.Wilkinson CW, et al. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury., Front. Neurol. 2012;3:11. doi: 10.3389/fneur.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]