Abstract
BACKGROUND
— Repolarization abnormality in bundle branch blocks (BBB) is traditionally ignored. This study evaluated the prognostic value of QRS/T angle for mortality in the presence and absence of BBB.
METHODS and RESULTS
— Total 15,408 participants (mean age 54 years, 55.2% women, 26.9% blacks, 2.8% with BBB) were from the Arteriosclerosis Risk in Communities Study. Sex stratified Cox regression models were used to compute hazard ratios (HR) with 95% confidence intervals (CI) for coronary heart disease (CHD) and all-cause mortality for wide spatial QRS/T angle with and without BBB including right BBB (RBBB), left BBB (LBBB) and indetermined-type ventricular conduction defect (IVCD) and RBBB combined with left anterior fascicular block. During a median 22-years follow-up, 4,767 deaths occurred, 728 of them CHD deaths. Using the No-BBB with QRS/T angle below median value as gender-specific reference groups, the mortality risk increase was significant for both women and men with No-BBB and QRS/T angle above the median value. In the pooled ICVD/LBBB group, the risk for CHD death was increased 15.9-fold in women and 6.04 fold in men, and for all-cause deaths 3.01-fold in women and 1.84-fold in men. However, the mortality risk in isolated RBBB group was only significant increase in women but not in men.
CONCLUSION
— A wide spatial QRS/T angle in BBB is associated with increased risk for CHD and all-cause mortality over and above the predictive value for BBB alone. The risk for women is as high as or higher than that in men.
Keywords: Bundle branch block, Electrocardiology, QRS/T angle, Mortality
INTRODUCTION
Wide QRS-T angle has been repeatedly shown to be predictive of adverse cardiovascular disease (CVD) events [1–11] Traditionally, ECG repolarization abnormalities in the setting of bundle branch blocks (BBB) are considered secondary to depolarization changes and of little diagnostic or prognostic utility. Nevertheless, a recent report on predictors of heart failure in the Atherosclerosis Risk In Communities (ARIC) Study showed that concomitant presence of BBB and widened QRS/T angle carries a much higher risk of heart failure than the presence of either predictor alone [11]. These findings suggest that repolarization abnormalities in the setting of BBB may not be merely a benign consequence of BBB. Therefore, we sought to evaluate prognostic significance of the QRS/T angle for coronary heart disease (CHD) and all-cause mortality in persons with and without BBB.
METHODS
Study population and design
The study population consisted of 15,792 men and women aged 45 to 64 years who were participants of the ARIC Study, a prospective epidemiologic study designed to investigate the atherosclerotic disease from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). Eligible participants were interviewed at home and then invited to a baseline clinical examination between 1987 and 1989. Participants attended 3 additional clinical examinations at 3-year intervals and a recent 5th examination completed in 2013 for which data is not included here. Annual telephone contact and surveillance for incident CVD events have been conducted since the baseline visit. The study was approved by each study site’s institutional review board. All participants provided written informed consent. Details of the ARIC Study design, protocol sampling procedures, and selection and exclusion criteria were published previously [12]. For the purpose of this analysis we excluded 384 participants: 201 without ECG, 136 with inadequate quality ECG or ECG diagnosis of external pacemaker or Wolff-Parkinson-White pattern, and 47 who was neither African-American nor white. Therefore 15,408 participants remained and were included in this analysis.
Outcome ascertainment
The outcomes considered in the present investigation were incident fatal CHD event and all-cause mortality that occurred from baseline through December 31, 2010. CHD deaths included fatal myocardial infarction (MI) as well as sudden cardiac death defined as definite or possible CHD death that occurred within one hour after the onset of acute symptoms, or has a history of chest pain within 72 hours before death, or a history of CVD at baseline. All CHD events classification and specific criteria including the adjudication process have been previously described [12–13].
ECG processing
Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, Wisconsin) were used at all clinic sites, and resting, 10-second standard simultaneous 12-lead ECGs were recorded in all participants using strictly standardized procedures. All ECGs were processed in a central ECG laboratory (initially at Dalhousie University, Halifax, NS, Canada and later at the EPICARE Center, Wake Forest School of Medicine, Winston-Salem, NC), where all ECGs were visually inspected for technical errors and inadequate quality. Different patterns of BBB were classified according to the Minnesota Code (MC) criteria as follows [14]: complete left BBB (LBBB, MC-7.1), complete right BBB (RBBB, MC-7.2, QRS axis >-45 degree), indetermined-type ventricular conduction defect (IVCD, MC-7.4), and combination of RBBB and left anterior fascicular block (LAFB) (MC-7.8, RBBB and QRS axis between −45 and −120 degree). Global interval measurements were obtained from the spatial magnitude functions of the QRS and T vectors of the quasi-orthogonal XYZ leads. These in turn were computed from the standard 12-lead ECGs using the Kors transform [15]. The algorithm developed for estimation of the onset of the global (from simultaneous quasi-orthogonal leads) T wave was based on combined use of information of the curvature function and ST segment convexity function described previously [8]. Of special concern was securing a reliable estimate of T wave onset time in LBBB because ST segment spatial magnitude starts increasing right after the end of QRS with no distinct “plateau” period. It was observed, however, that the time point of maximum value of the ST segment convexity provided a reasonably consistent estimate for T wave onset. Furthermore, the value of the spatial QRS/T angle between the mean QRS and T vectors is relatively insensitive to variations in the estimates of T wave onset and offset.
Statistical analysis
Frequency distributions of the variables used in analyses were first inspected to rule out anomalies and outliers. Descriptive statistics were used to determine mean values, standard deviations, and percentile distributions for continuous variables, and frequencies and percentages for categorical variables. Cox’s proportional hazards analysis was used to assess the associations of QRS/T angle and BBB (in isolation and combined) with the risk of CHD death and all-cause mortality in incremental models: model-1 unadjusted, and model-2 adjusted for age, sex, race, field center, body mass index, systolic blood pressure, smoking status, hypertension, diabetes mellitus, history of CVD, ratio of total cholesterol/high-density lipoprotein, blood glucose, serum creatinine, and ECG QRS duration.
QRS/T angle was used in the models as a binary variable using gender-specific median as the cut-off point (reference group QRS/T angle <median value) as well as quartiles. In additional analyses, we excluded RBBB from the BBB group given the previous reports RBBB was not prognostically as important as LBBB. Other additional analyses included examining the associations between the spatial QRS/T angle and QRS duration in women and men. All analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Baseline Demographic and ECG Characteristics
The mean age at baseline was 54 years (SD 5.8), 55.2% were women, and 26.9% African American. About one third (35.0%) of the participants had hypertension, 11.9% had diabetes, and 12% had a history of CVD. At baseline, BBBs were present in 429 (2.8%) participants of whom 90 were LBBB, 181 RBBB, 111 IVCD, and 47 bifasicular BBB [RBBB combined with left anterior fascicular block (LAFB, n=28) or RBBB combined with left posterior fascicular block (LPFB, n=19). Study group characteristics by the BBB status (Table 1) show that most of the differences between BBB and No-BBB groups were statistically significant.
Table 1.
Baseline characteristic and outcomes of the study participants (N=15,408)
| Means (SD), or n (%) | No Bundle Branch Block N=14,979 |
Bundle Branch Blocks N=429 |
P*-value |
|---|---|---|---|
| Age (years) | 54 ±5.8 | 57 ±5.4 | <0.001 |
| Body mass index (kg/m2) | 28 ±5.4 | 28 ±5.1 | 0.038 |
| Systolic blood pressure (mmHg) | 121 ±18.8 | 124 ±20.7 | 0.001 |
| Women (%) | 8359 (55.8) | 140 (32.6) | <0.001 |
| African-American (%) | 4026 (26.9) | 114 (26.6) | 0.889 |
| Education ≤ high school (%) | 8401 (56.0) | 266 (62.0) | 0.001 |
| Current smoker (%) | 3906 (26.1) | 122 (28.4) | 0.013 |
| Hypertension (%) | 5181 (34.7) | 181 (42.7) | 0.001 |
| Diabetes (%) | 1754 (11.8) | 67 (15.7) | 0.015 |
| History of CVD† (%) | 1388 (9.3) | 112 (26.1) | <0.001 |
| Antihypertensives (%) | 4529 (30.3) | 190 (44.3) | <0.001 |
| Cholesterol lowering drugs (%) | 428 (2.9) | 15 (3.5) | 0.435 |
| Ratio of total cholesterol/HDL | 4.6 ±1.8 | 5.1 ±1.7 | <0.001 |
| Blood glucose (mg/dL) | 109 ±40.6 | 111 ±36.6 | 0.263 |
| Serum creatinine (mg/dL) | 1.1 ±0.4 | 1.2 ±0.8 | <0.001 |
| Heart rate (/min) | 66 ±10.3 | 64 ±10.7 | <0.001 |
| QRS duration (ms) | 91 ±9.6 | 137 ±15.8 | <0.001 |
| QRS axis-Frontal (°) | 30 ±32.3 | 8.7 ±54.2 | <0.001 |
| T axis-Frontal (°) | 39 ±31.8 | 46 ±58.2 | <0.001 |
| QRS/T angle-Spatial (°) | 69 ±29.2 | 109§ ±38.3 | <0.001 |
| Outcomes (Event rates/1000 person year) | |||
| Coronary heart disease death | 2.4 | 9.4 | <0.001 |
| All-cause mortality | 16.3 | 33.7 | <0.001 |
P values for the mean differences between groups with bundle branch block and no bundle branch block.
History of cardiovascular disease (CVD) = classified by ECG evidence of myocardial infarction according to the Minnesota Code or the NOVACODE criteria, or a self-reported history of a clinical diagnosis of myocardial infarction, angina pectoris, coronary artery bypass surgery, coronary angioplasty, heart failure, or stroke at the time of entry to the study.
Spatial QRS/T angle in the BBB group excluding RBBB 130 ±34° in women and 118 ±35° in men.
Spatial QRS/T angle, bundle branch blocks and mortality risk
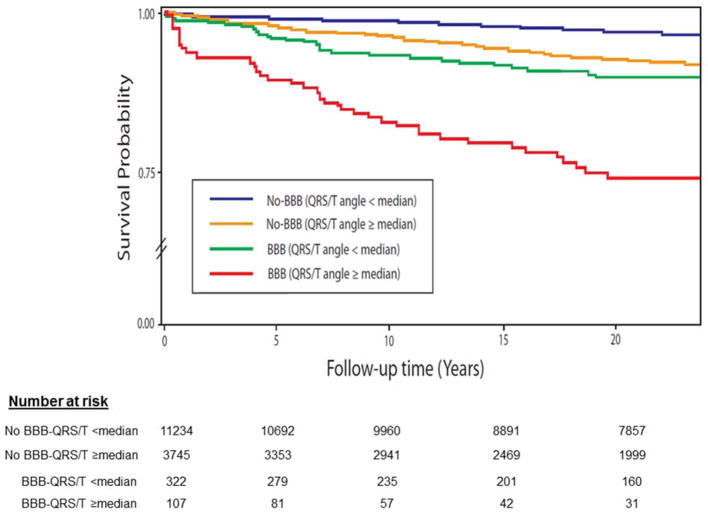
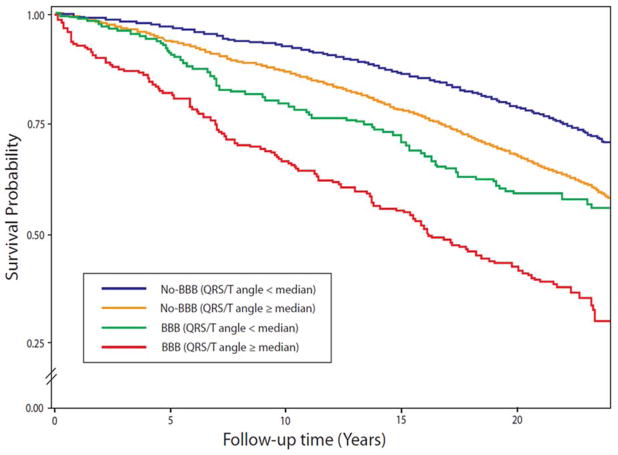
Of the 4,767 deaths during a median 22-years follow up, 728 were CHD deaths. Table 2 lists hazard ratios for CHD and all-cause deaths for spatial QRS/T angle dichotomized at median value in each subgroup by gender for the fully-adjusted risk model (Model 2). Using No-BBB with QRS/T angle below median as gender-specific reference groups, the mortality risk increase was significant for both women and men with No-BBB and QRS/T angle above the median value (2-fold in women and 1.72-fold in men). In the pooled ICVD/LBBB group including bifascicular blocks, the risk for CHD death was increased 15.9-fold in women and 6.04 fold in men, and for all-cause deaths 3.01-fold in women and 1.84-fold in men. The CHD death risk in isolated RBBB group with wide QRS/T angle was significant increase in women (hazard ratio (HR) 3.82, 95% confidence interval (CI) 1.18–12.4) but not in men (HR 1.96, 95% CI 0.96–4.48). HRs for No-BBB or BBB with wide QRS/T angle for all-cause deaths were similar to those for CHD deaths although the HR were lower. Overall, the risk levels for wide spatial QRS/T angle in women with BBB were as high as those for men, and for RBBB even higher in women than in men. Figure 1 and Figure 2 show survival probability curves for CHD and all-cause mortality, respectively, in various subgroups of QRS/T angle and BBB. The Figures show progressively increasing risk with the highest risk in the group with combined wide spatial QRS/T angle and BBB.
Table 2.
Hazard ratios with 95% confidence intervals for Coronary heart disease and all-cause mortality for spatial QRS/T angle dichotomized at median values by gender
| Events/1000 person years | Hazard Ratio† (95% CI‡) | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Coronary heart disease death | ||||
| No-BBB* | ||||
| QRS/T <median§ | 0.8 | 2.2 | 1 (reference) | 1 (reference) |
| QRS/T ≥median | 2.1 | 5.3 | 2.00 (1.47–2.72) | 1.72 (1.39–2.13) |
| RBBB | ||||
| QRS/T <median§ | 1.7 | 2.9 | 1.93 (0.26–14.1) | 0.24 (0.05–1.08) |
| QRS/T ≥median | 6.5 | 8.1 | 3.82 (1.18–12.4) | 1.96 (0.96–4.48) |
| LBBB/IVCD# | ||||
| QRS/T <median§ | 1.3 | 9.9 | 1.70 (0.23–12.4) | 2.25 (1.20–4.20) |
| QRS/T ≥median | 13.1 | 26.6 | 15.9 (7.10–35.4) | 6.04 (3.92–9.31) |
| All-cause mortality | ||||
| No-BBB* | ||||
| QRS/T <median§ | 10.6 | 17.5 | 1 (reference) | 1 (reference) |
| QRS/T ≥median | 15.4 | 24.7 | 1.31 (1.20–1.44) | 1.14 (1.05–1.24) |
| RBBB | ||||
| QRS/T <median§ | 17.3 | 25.7 | 1.17 (0.63–2.19) | 0.87 (0.58–1.32) |
| QRS/T ≥median | 34.7 | 39.5 | 1.84 (1.10–3.08) | 1.26 (0.88–1.79) |
| LBBB/IVCD# | ||||
| QRS/T <median§ | 22.6 | 28.9 | 1.65 (1.00–2.71) | 1.10 (0.79–1.54) |
| QRS/T ≥median | 39.2 | 57.1 | 3.01 (1.94–4.65) | 1.84 (1.41–2.41) |
BBB=bundle branch block; RBBB=right bundle branch block; LBBB=left bundle branch block; IVCD=indetermined type ventricular conduction defect.
Includes 47 bifascicular blocks [RBBB with left anterior fascicular block (LAFB, n=28) and RBBB with left posterior fascicular block (LPFB, n=19)].
Hazard ratio from risk model adjusted for age, race, field of residence, body mass index, systolic blood pressure, smoking status, education, hypertension, diabetes mellitus, status of cardiovascular disease, ratio of total cholesterol/high density lipoprotein, blood glucose, serum creatinine, and QRS duration at baseline.
CI=confidence interval
Median spatial QRS/T angle cut-off point in No-BBB group 59° for women and 75° for men, in RBBB group 95° for women and 91° for men, and in LBBB/IVCD group 142° for women and 125° for men.
Figure 1.
Survival probability curves for coronary heart disease death across different combinations of bundle branch blocks (BBB) and spatial QRS/T angle using median value as a cut-off point.
Figure 2.
Survival probability curves for all-cause mortality across different combinations of bundle branch blocks (BBB) and spatial QRS/T angle using median value as a cut-off point.
When the spatial QRS/T angle was used in the models as quartiles, the risk of CHD death increased progressively from quartile 1 to quartile 4 in each subgroup, and the combination of BBB and wide QRS/T angle was associated with higher risk than that for each one alone (not shown).
DISCUSSION
The present study evaluated the prognostic value of wide QRS/T angle for CHD and all-cause mortality in men and women in the presence and absence of BBB. Using the No-BBB group with QRS/T angle below median value as the reference group, the mortality risk was increased 2-fold for isolated RBBB in women but the increase was not significant for RBBB in men. The mortality risk increase was significant for both women and men with No-BBB and QRS/T angle above the median value, with a 2-fold in women and 1.72-fold increase in men for CHD death. And in the pooled ICVD/LBBB group including bifascicular blocks there was a profound increase both for CHD and all-cause mortality risk.
The question that often arises is why the normal repolarization in the left lateral wall is crossmural, diagonal from epicardium towards endocardium predominantly in superior-right- posterior direction, in general direction opposite to the spatial direction of depolarization? A plausible explanation is that depolarization currents shorten action potential durations (APD) but the shortening decays with a relatively short time constant. In normal depolarization some LV free wall epicardial region is depolarized last, epicardial APD and repolarization time RT (APDepi and RTepi) is shortest in that region. A teleological notion why reversed repolarization and a narrower QRS/T angle is beneficial is that in the course of evolution repolarization has been optimized so that RTendo is longer than RTepi, and thus papillary muscles and AV valves are protected from acute distension at the onset of rapid ventricular filling.
Clinical utility of prediction the risk of adverse outcomes over a long follow-up period is often questioned because of changing environmental factors and therapeutic interventions such as can be expected in patients with BBB. The counterargument is that the predicted excess risk would likely be even higher without interventions. Preventive actions may take a long period to reduce the risk, and therefore it is desirable to initiate preventive actions as early as possible.
Possible mechanisms for increased mortality risk for wide QRS/T angle in bundle branch blocks
The spatial direction of repolarization sequence in left ventricular lateral wall is predominantly reversed with respect to the direction of depolarization and the spatial QRS/T angle is relatively narrow in normal ventricular depolarization and repolarization. Any deviations in the spatial direction of either depolarization or repolarization or both will increase the QRS/T angle. A wide QRS/T angle represents a larger discordance between depolarization and repolarization [16–17]. With more marked altered depolarization or repolarization direction, repolarization changes gradually from predominantly reverse to predominantly concordant with respect to depolarization. Particularly profound changes are observed in LBBB and in left ventricular hypertrophy with the so called ECG strain pattern. QRS/T angle in these conditions may occasionally approach 180° indicating nearly concordant repolarization sequence [18]. Delayed left ventricular excitation leads into dyssynchrony of ventricular contraction which has become an important consideration in resynchronization therapy [19].
Altered ventricular depolarization sequence will always induce secondary repolarization abnormalities. Delayed left ventricular repolarization and relaxation and both primary and secondary repolarization abnormalities in BBB may well be independent additional contributing factors to excess mortality risk. It is recognized, however, that the validity of these postulated mechanisms for increased mortality risk remains an open question.
Our results in relation to those by other investigators
In most previous studies the mortality risk for isolated RBBB has not been significant. However, in the extensive Copenhagen Heart Study [20] there was an overall 1.87-fold increase (HR 1.87, 95% CI 1.48–2.36) in cardiovascular mortality, with hazard ratios comparable in men and women with the exception that significant association between RBBB and MI was observed in women only (HR 2.79, 95% CI 1.50–5.22).
A number of studies in men and women with normal ventricular conduction have documented that wide QRS duration is a significant predictor of CVD [7, 10–11, 21–24]. Risk models for evaluating the risk for wide QRS/T angle in our present study were adjusted for QRS duration, and this study extended and demonstrated that a wide spatial QRS/T angle in BBB is associated with increased risk for CHD and all-cause mortality in women and in men.
Clinical implications
BBBs are considered as manifestations of a gradually evolving generalized degenerative process involving not only bundle branches but also structural changes in working myocardium [25]. This suggests that CHD prevention efforts started as early as possible can be expected to improve the overall beneficial impact of intervention. Presentation of a wide QRS/T angle in BBB and already before BBB develops may indicate a more pronounced risk of CHD signaling the need for preventive actions.
Strengths and limitations
The ARIC study is well-designed cohort study with long term follow-up, all data used in the analysis including ECG data were carefully ascertained and/or read at central core labs. However, as in other observational studies, residual confounding remains a possibility despite adjusting for several potential confounders. The population studied was restricted to those with white and African-American ethnicity.
Conclusions
A wide spatial QRS/T angle in BBB is associated with increased risk for CHD and all-cause mortality, and the risk is over and above the predictive value for BBB alone.
Highlights.
Repolarization abnormalities in bundle branch block (BBB) are traditionally ignored.
We examine the prognostic value of QRS/T angle in the presence and absence of BBB.
A wide QRS/T angle in BBB is associated with increased risk for mortality.
Acknowledgments
Funding Sources The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Disclosures The authors have no conflicts of interests to disclose.
References
- 1.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women’s Health Initiative. Circulation. 2006;113(4):473–480. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM for the ARIC Research Group. Comparison of the prognostic significance of the electrocardiographic QRS/Tangles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100:844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L, et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol. 2009;2:548–554. doi: 10.1161/CIRCEP.109.859108. [DOI] [PubMed] [Google Scholar]
- 5.Whang W, Shimbo D, Levitan EB, Newman JD, Rautaharju PM, Davidson KW, et al. Relations between QRS|T angle, cardiac risk factors, and mortality in the third National Health and Nutrition Examination Survey (NHANES III) Am J Cardiol. 2012;109:981–987. doi: 10.1016/j.amjcard.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lown MT, Munyombwe T, Harrison W, West RM, Hall CA, Morrell C, et al. Evaluation of Methods and Management of Acute Coronary Events (EMMACE) Investigators. Association of frontal QRS-T angle--age risk score on admission electrocardiogram with mortality in patients admitted with an acute coronary syndrome. Am J Cardiol. 2012;109:307–313. doi: 10.1016/j.amjcard.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZM, Rautaharju PM, Soliman EZ, Manson JE, Cain ME, Martin LW, et al. Mortality risk associated with bundle branch blocks and related repolarization abnormalities (from the Women’s Health Initiative [WHI]) Am J Cardiol. 2012;110:1489–1495. doi: 10.1016/j.amjcard.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 8.Rautaharju PM, Zhang ZM, Haisty WK, Prineas RJ, Kucharska-Newton AM, Rosamond W, et al. Electrocardiographic predictors of incident heart failure in men and women free from manifest cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Cardiol. 2013;112:843–849. doi: 10.1016/j.amjcard.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautaharju PM, Zhang ZM, Warren J, Gregg RE, Haisty WK, Jr, Kucharska-Newton AM, et al. Electrocardiographic Predictors of coronary heart disease and sudden cardiac deaths in men and women free from cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) Study. J Am Heart Assoc. 2013;2:e000061. doi: 10.1161/JAHA.113.000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang ZM, Rautaharju PM, Soliman ES, Manson JE, Martin LW, Perez M, et al. Different patterns of bundle branch blocks and the risk of incident heart failure in the Women’s Health Initiative (WHI) Study. Circ Heart Fail. 2013;6:655–661. doi: 10.1161/CIRCHEARTFAILURE.113.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZM, Rautaharju PM, Prineas RJ, Loehr L, Rosamond W, Soliman EZ. Usefulness of electrocardiographic QRS/T angles with versus without bundle branch blocks to predict heart failure (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2014;114:412–418. doi: 10.1016/j.amjcard.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 14.Prineas RJ, Crow RS, Zhang ZM. The Minnesota code manual of electrocardiographic findings. 2. Springer; London: 2010. pp. 16–166. [Google Scholar]
- 15.Kors JA, van Herpen G, Sittig AC, van Bemmel JH. Reconstruction of the frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J. 1990;11:1083–1092. doi: 10.1093/oxfordjournals.eurheartj.a059647. [DOI] [PubMed] [Google Scholar]
- 16.Zhu TG, Patel C, Martin S, Quan X, Wu Y, Burke JF, et al. Ventricular transmural repolarization sequence: its relationship with ventricular relaxation and role in ventricular diastolic function. Eur Heart J. 2009;30:372–380. doi: 10.1093/eurheartj/ehn585. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, et al. for MADIT-CRT Executive Committee. Predictors of response to cardiac resynchronization therapy in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;124:1527–1536. doi: 10.1161/CIRCULATIONAHA.110.014324. [DOI] [PubMed] [Google Scholar]
- 18.Rautaharju PM, Soliman EZ. Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: A critical appraisal. J Electrocardiol. 2014;90:1786–1793. doi: 10.1016/j.jelectrocard.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt, Brown M, et al. for MADIT-CRT Investigators. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchorinazitation Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 20.Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J. 2013;34:138–146. doi: 10.1093/eurheartj/ehs291. [DOI] [PubMed] [Google Scholar]
- 21.Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, et al. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circulation Arrhythm Electrophysiol. 2011;4:704–710. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Göteborg, Sweden. Eur Heart J. 2005;26:2300–2306. doi: 10.1093/eurheartj/ehi580. [DOI] [PubMed] [Google Scholar]
- 23.Lewinter C, Torp-Pedersen C, Cleland JG, Køber L. Right and left bundle branch block as predictors of long-term mortality following myocardial infarction. Eur J Heart Fail. 2011;13:1349–1354. doi: 10.1093/eurjhf/hfr130. [DOI] [PubMed] [Google Scholar]
- 24.Haataja P, Nikus K, Kähönen M, Huhtala H, Nieminen T, Jula A, et al. Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population: The Health 2000 Survey. Int J Cardiol. 2013;167:1953–1960. doi: 10.1016/j.ijcard.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Bacharova L, Zhathmary V, Mateasik A. Electrocardiographic patterns of left bundle-branch block caused by inraventricular conduction impairment in working myocardium: a model study. J Electrocardiol. 2011;44:568–70. doi: 10.1016/j.jelectrocard.2011.03.007. [DOI] [PubMed] [Google Scholar]