Abstract
Objective
In this conceptual review, we propose a novel mechanistic candidate in the etiology of depression with onset in the menopause transition (a.k.a. perimenopausal depression) involving alterations in stress-responsive pathways, induced by ovarian hormone fluctuation.
Methods
The relevant literature in perimenopausal depression was reviewed, including its prevalence, predictors, and treatment with estrogen therapy. Subsequently, the growing evidence from animal models and clinical research in other reproductive mood disorders was synthesized to describe a heuristic model of perimenopausal depression development.
Results
The rate of major depressive disorder and of clinically meaningful elevations in depressive symptoms increases two- to threefold during the menopause transition. While the mechanisms by which ovarian hormone fluctuation might impact mood are poorly understood, growing evidence from basic and clinical research suggests that fluctuations in ovarian hormones and their derived neurosteroids result in altered GABAergic regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Our heuristic model suggests that for some women, failure of the GABAA receptor to regulate overall GABAergic tone in the face of shifting levels of these neurosteroids may induce HPA axis dysfunction, thereby increasing sensitivity to stress, and generating a period of greater vulnerability to depression.
Conclusions
The proposed model provides a basis for understanding the mechanisms by which the changing hormonal environment of the menopause transition may interact with the psychosocial environment of mid-life to contribute to perimenopausal depression risk. Future research investigating this model may inform the development of novel pharmacological treatments for perimenopausal depression and related disorders such as postpartum depression and premenstrual dysphoric disorder.
Keywords: menopause transition, perimenopausal depression, estradiol, progesterone, allopregnanolone, hypothalamic-pituitary-adrenal axis, stress reactivity
Introduction
The rate of major depressive disorder (MDD) in women of reproductive age is double that of men's. Depressive disorders tied to reproductive events may partially account for this increased risk. Premenstrual dysphoric disorder (PMDD) and postpartum depression are two such disorders for which pathophysiological mechanisms include an increased vulnerability to fluctuations in ovarian-derived hormones as well as hypothalamic-pituitary-adrenal (HPA) axis dysregulation. Substantially less research has been conducted on depressive disorders tied to the menopause transition.
The neuroendocrine mechanisms by which the complex hormonal milieu of the menopause transition might trigger depressive symptomology also remain poorly understood, though multiple candidate mechanisms are under investigation. These include, but are not limited to, withdrawal from the anti-inflammatory (1) and neuroprotective (2) effects of estradiol (estradiol) as well as its modulation of the limbic processing (3) and memory (4, 5) of emotionally-relevant information. The primary goal of the current review is to set forth an additional mechanistic hypothesis involving interactions among reproductive steroids, GABAergic neurosteroids, and HPA axis function, which can serve as the basis for further investigation. We will discuss the literature implicating ovarian hormone variability in the development of depression with onset in the menopause transition (a.k.a. perimenopausal depression) and describe a paradigm in which changes in progesterone (progesterone)-derived GABAergic neurosteroids may induce dysfunction of the GABAergic system and, in turn, the HPA axis. To first provide a context in which to discuss this potential mechanism, we briefly describe the following: 1) the endocrine environment characterizing the menopause transition; 2) the prevalence of perimenopausal depression; 3) risk factors for perimenopausal depression; and 4) the evidence for the use of estrogen therapy as a treatment for perimenopausal depression
The Endocrine Environment of the Menopause Transition
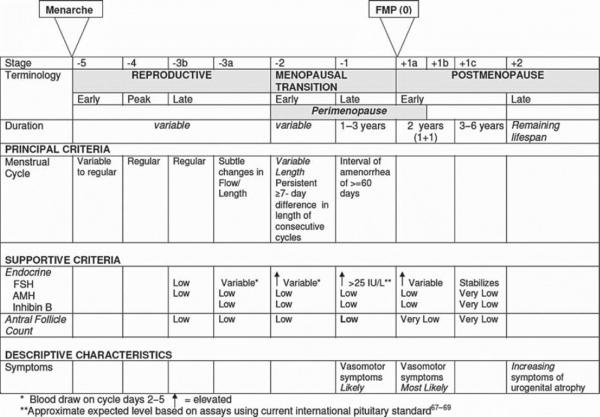
The menopause transition, triggered by a woman's diminishing supply of ovarian follicles, represents the reproductive stage transitioning from reproductively capable ovulatory cycles through the loss of ovulatory function and to the cessation of menses. The latter marks the onset of the menopause. In premenopausal women, antral ovarian follicles produce inhibin B, a protein complex that inhibits follicle stimulating hormone (FSH) release, which stimulates the recruitment and growth of ovarian follicles. As women approach the end of their reproductive years and fewer antral ovarian follicles are available to produce inhibin B, FSH concentrations gradually rise. While FSH levels have historically been used as an endocrine marker of postmenopausal status, FSH is less useful for reproductive staging in the menopause transition due to variability in FSH concentrations at this time. Consequently, standard criteria for reproductive staging are based primarily on menstrual bleeding patterns, which can be corroborated with endocrine data. The Stages of Reproductive Aging Workshop (STRAW), first developed in 2001 and revised in 2011 (6), is among the most commonly used staging systems and divides a woman's reproductive lifespan into stages, using the final menstrual period as the anchor (Figure 1).
Figure 1.
Stages of Reproductive Aging Workshop (STRAW +10). Reprinted with permission from Harlow et al. (2012) (6).
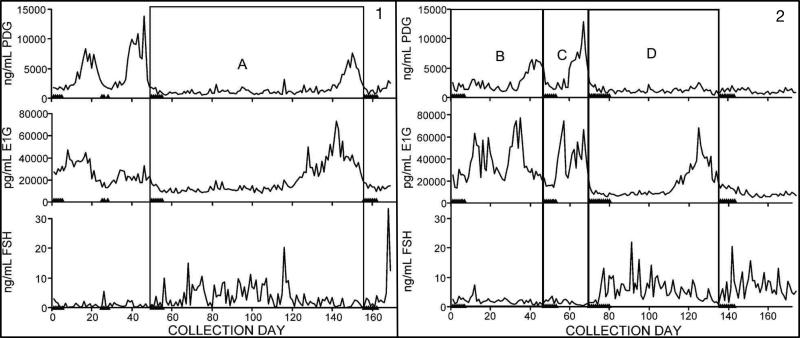
While there are substantial individual differences in the hormonal trajectory through the menopause transition (7, 8), most women experience the following changes (reviewed in (9, 10)), beginning in the early, and progressing into the late menopause transition. First, menstrual cycle length becomes increasingly variable, with long cycles becoming more common as the transition progresses. This is illustrated in Figure 2, showing daily concentrations of urinary FSH and the urinary metabolites of estradiol (estradiol) and progesterone (progesterone) (proxies for estradiol and progesterone concentrations) in two women in the menopause transition. While short cycles are due to early follicular recruitment by intermittently high FSH levels, long cycles can be due to anovulation (Figure 2, Panel 2, Cycle D) or a delayed ovarian response to FSH stimulation, resulting in an extended (low-estradiol) early follicular phase (Figure 2, Panel 1, Cycle A). Second, luteal progesterone also decreases, thought to result from declining dominant follicle quality (Figure 2, Panel 2, Cycle B). Third, appearing in the early and continuing into the late menopause transition are cycles in which concentrations of estradiol are elevated compared to premenopausal concentrations, also thought to result from elevated FSH. In addition to these changes in ovulatory cycles, the late menopause transition is also marked by an increasing frequency of anovulatory cycles (60-70% of cycles), which are characterized by low progesterone and erratic estradiol concentrations. Eventually, estradiol and progesterone production ceases, though recent evidence suggests that the early postmenopausal period is characterized by more ovarian activity than previously believed (8). In summary, most women undergoing a natural transition to menopause are exposed to highly erratic hormonal flux. While an increasing proportion of anovulatory cycles translates to fewer luteal-phase increases in progesterone, variable FSH concentrations cause periods of both hypo- and hyper-estrogenism. This exposure to erratic ovarian hormone concentrations may extend over 5 years (11).
Figure 2.
Daily urinary FSH and urinary metabolites of estradiol (E1G) and progesterone (PDG) in two perimenopausal women illustrating A) a long ovulatory cycle, B) a long ovulatory cycle with low luteal progesterone, C) a normal ovulatory cycle and D) a long anovulatory cycle. Triangles on x-axis represent days of menstrual bleeding. Adapted with permission from O'Connor et al. (2009) (85). PDG, pregnanediol-glucuronide; E1G, estrone-glucuronide; FSH, follicle stimulating hormone.
Prevalence of Perimenopausal Depression
Though the existence of menopause-associated depression has long been debated (12) and several cross-sectional studies find no relationship between the prevalence of MDD and menopausal status (13, 14), longitudinal studies have been more consistent in finding that the menopause transition is associated with a substantial increase in vulnerability to clinically significant depressive symptoms, with odds ratios ranging from 1.33 to 1.79 (15-17); this increased vulnerability is also observed in longitudinal studies in women with no history of MDD (16, 18, 19). Furthermore, studies using the Structured Clinical Interview for DSM Disorders IV to examine the incidence of syndromal MDD in the menopause transition echo the above findings in mixed samples of women with or without a history of MDD (20). For example, in a sub-analysis of 221 initially premenopausal participants from the Study of Women's Health Across the Nation (SWAN), which included women with or without a history of depression who were followed for ten years, the rate of syndromal MDD was doubled during the MT and tripled in the early postmenopausal period (21). In contrast, two longitudinal studies (18, 22) to date suggest that the risk of first-onset syndromal MDD may not be increased in the menopause transition, though additional research to confirm this is warranted.
In all, there is strong evidence that the menopause transition and early postmenopausal period are a time of increased vulnerability to dysphoric mood, though the risk of a major depressive episode may be elevated only in the context of recurrent MDD.
Risk Factors for Perimenopausal Depression
Multiple longitudinal studies have provided insight regarding the factors that are most predictive of the development of depressive symptomology in the menopause transition. These predictors fall into two broad categories, the first relating to traditional psychosocial factors and the second relating to an increased sensitivity to ovarian hormone fluctuations and menopausal symptoms.
A history of MDD is the strongest predictor of both elevated depressive symptoms and syndromal MDD in the menopause transition, with odds ratios of 4-6 (16, 21, 23). Psychosocial stress, including unemployment (16), financial strain (15), lack of social support (15) and stressful life events proximate to the menopause transition (15, 21) also predict increased risk for both depressive symptoms and syndromal MDD. Finally, poor sleep during the menopause transition, independent of night sweats, has been associated with an increased risk of perimenopausal depression (16).
In addition to any role for psychosocial factors in perimenopausal depression, there is evidence to suggest that women vulnerable to perimenopausal depression exhibit a greater “hormonal sensitivity” to the endocrine profile of the menopause transition (24). First, both a history of PMDD and a history of postpartum depression – two disorders for which reproductive hormonal flux may be pathophysiologically relevant (25, 26) – are each strong predictors of perimenopausal depression (16, 17, 19). Second, vasomotor symptoms in the menopause transition are also associated with an increased risk of elevated depressive symptoms (16-18). While the relationship between vasomotor symptoms and depressive symptoms is multifactorial, some evidence suggests that increasingly erratic ovarian hormone fluctuation may represent a shared mediator of risk for both vasomotor symptoms and perimenopausal depression (27). Third, there are data suggesting that the duration of the menopause transition, and therefore a longer exposure to fluctuating hormones of the menopause transition, is positively associated with perimenopausal depression risk (23). A recent report from the Penn Ovarian Aging Study (POAS) (28) found that a more rapid rise in FSH prior to the final menstrual period predicted a decreased risk of elevated depressive symptoms after the final menstrual period; suggesting that a shorter menopause transition may protect against perimenopausal depression.
Estrogen Therapy in Treating Perimenopausal Depression
Studies demonstrating the efficacy of estradiol to treat perimenopausal depression provide additional support that fluctuating estradiol during the menopause transition may be etiologically relevant to perimenopausal depression. While randomized controlled trials (RCTs) evaluating the efficacy of oral conjugated estrogen for the treatment of perimenopausal depression have been inconsistent in demonstrating a beneficial effect (reviewed in (29)), studies using transdermal estradiol have proven promising. To date, three small RCTs have examined the efficacy of transdermal estradiol as a treatment for perimenopausal depression. In two, remission rates of 68 and 80% were observed compared to rates of approximately 20% and 22%, respectively, in the placebo groups (29, 30). Interestingly, the study obtaining the remission rate of 80% (30) included only patients reporting that the onset of their depressive symptoms coincided with the onset of menstrual irregularity. A third RCT (31) of depressed peri- and postmenopausal women that did not require depression onset to coincide with menstrual irregularity failed to find any differences between transdermal estradiol, the hypnotic zolpidem and placebo on mood. However, this study did find that increases in serum estradiol predicted symptom improvement among depressed peri-, but not postmenopausal, women, suggesting specificity of mood effects of increasing estradiol levels for perimenopausal women. Also of relevance, one open-label study examined the efficacy of transdermal estradiol to treat depression in peri- versus postmenopausal women and reported a greater proportion of perimenopausal women achieved remission compared to postmenopausal women (32). These results are consistent with the study by Morrison et al., which found that transdermal estradiol was an ineffective treatment for depression among postmenopausal women (33).
It is well documented that estradiol “beneficially” modulates pathways implicated in the pathophysiology of depression, including serotonin (34, 35) and norepinephrine pathways, and exerts strong antidepressant effects in animal models (reviewed in (36)). In addition, transdermal estradiol, which can impair ovulation (37) and restore early to mid-follicular phase levels of FSH (38) and estradiol (39), may reduce the degree of ovarian hormone variability to which a perimenopausal woman is exposed. Although the evidence is limited, the efficacy of transdermal estradiol in depression treatment for peri- but not post-menopausal women, therefore suggests that it is stabilization of estradiol during the menopause transition which is effective for mood and, indirectly, that estradiol fluctuation may act as a trigger for perimenopausal depression (31). Clearly there is a need for larger RCTs, including a direct comparison of estradiol therapy and antidepressant medication, as there is an insufficient evidence base with which to inform clinical decisions about treating perimenopausal depression. Treatment studies distinguishing between mid-life women with MDD onset in the menopause transition and those with onset prior to the menopause transition would inform clinical practice and have implications for understanding the pathophysiology of perimenopausal depression.
Mechanisms
While the etiology of perimenopausal depression is not well understood, most studies suggest it is not simply due to low basal hormone concentrations. It has been hypothesized (e.g. (40, 41)) that the ovarian hormone fluctuations that characterize the menopause transition trigger mood disturbances in vulnerable women. To our knowledge, five studies have evaluated the hormone variability hypothesis by examining naturally-occurring fluctuations in ovarian hormones in relation to mood among women in the menopause transition (reviewed in (40)). The first of these, the Massachusetts Women's Health Study (42) measured serum estradiol annually for 3 years in 309 women ranging from premenopausal to postmenopausal (STRAW −3 to +1) and found no association between estradiol variability and CES-D score. In a subset of Seattle Midlife Women's Health Study participants, CES-D score was not associated with a urinary metabolite of estradiol, FSH or testosterone in 131 women in STRAW stages −3 or −2 at baseline (17). In that study, the CES-D was administered annually for 8 years and hormone concentrations were measured monthly for 4 years then quarterly for 4 years. However, only 714 observations were collected in total suggesting that, on average, participants provided 5 samples over the course of the 8-year study. Bromberger et al. (21) also reported that among 3302 SWAN participants, estradiol or FSH variability, calculated across 8 annual measurements, was not associated with depressive symptoms. However, independent of menopausal status, testosterone levels and the change (increase) in testosterone from baseline were positively associated with CES-D score. While, overall, the above studies do not support a relationship between estradiol or FSH variability and mood, the absence of a positive finding may be related to the infrequent hormone sampling intervals. Given the considerable hormonal variation occurring in the menopause transition, such infrequent measurements may be limited in their ability to capture the dynamics of the hormonal environment, characterizing the menopause transition.
In contrast, Freeman et al. (19) found that over 8 years in the Penn Ovarian Aging Study, clinical elevations in depressive symptoms and syndromal MDD were more likely to occur at times when estradiol variability was highest. Ten estradiol and FSH assessment periods occurred over the 8 years; each consisting of two blood draws taken one month apart. estradiol variability at each assessment was calculated as the standard deviation across the two estradiol levels obtained during each assessment period. In cycling women, these measurements were taken in the early follicular phase. Importantly, the relationship between estradiol variability and depressive symptoms continued to be significant after adjustment for increases in poor sleep, which may also accompany periods of increased hormonal flux. This study has several strengths that may explain its ability to detect a relationship between estradiol variability and perimenopausal depression. First, unlike the Massachusetts Women's Health Study and the Seattle Midlife Women's Health Study, the Penn Study included only euthymic participants at baseline, ensuring that they were examining depression with onset in the menopause transition rather than MDD that began prior to, and continued into, the menopause transition. Second, the Penn Study used more frequent hormonal assessments (twice annually, one month apart) than the above-mentioned studies. A study by Daly et al. (43), which also employed more frequent hormone sampling, assessed FSH weekly in 110 women with documented onset of depression in the menopause transition and found that those women who experienced a 50% drop in FSH over 6 weeks, indicating a return to premenopausal ovarian function, experienced a significant decline in depressive symptoms. Future research using more frequent assessments of depressed mood and ovarian hormone concentrations and isolating depression with onset during the menopause transition may therefore more definitively implicate the involvement of hormonal variability in the etiology of perimenopausal depression.
To the extent that variability in reproductive steroid hormones may play a role in the etiology of perimenopausal depression, by what mechanism(s) would it do so? Based on the evidence that estradiol modulates serotonergic and noradrenergic function (see (36, 44) for review), combined with the documentation that SSRI and SNRIs are effective in some women with perimenopausal depression, the possibility exists that estradiol fluctuation in the menopause transition may adversely impact the serotonergic and noradrenergic systems (36, 44, 45), though this has never been directly tested.
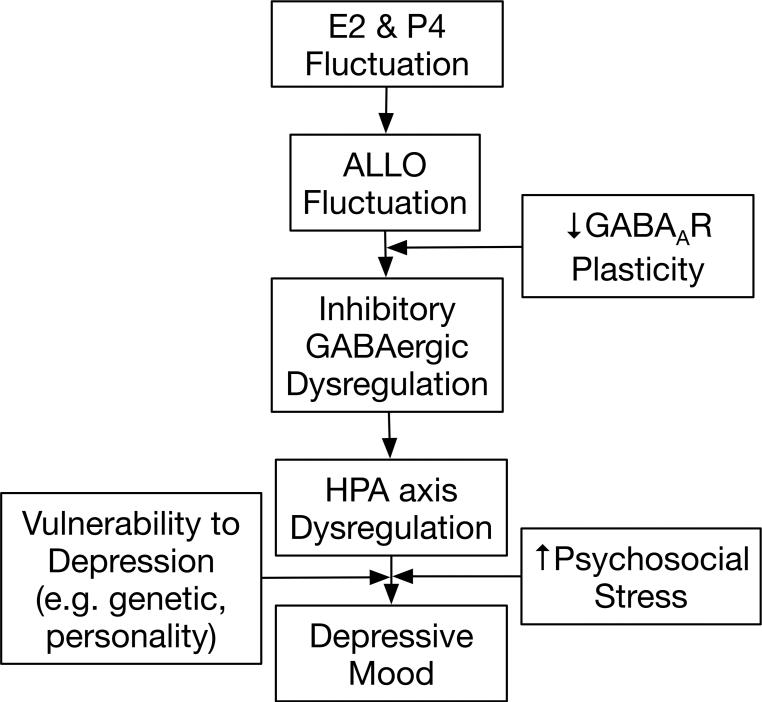
In this review, we discuss the available evidence to support another plausible mechanism – that is, reproductive steroid modulation of GABAergic regulation of the HPA axis. More specifically, we present evidence for the role of progesterone-derived neurosteroids, including allopregnanolone (3α-hydroxy-5α-pregnan-20-one; ALLO), in altering GABAergic modulation of the HPA axis and discuss how these alterations may sensitize perimenopausal women to stress and, consequently, to the development of perimenopausal depression. When this increased stress sensitivity is combined with stressful life events and/or a genetic or dispositional vulnerability to depression, clinical elevations in depressed mood may ensue. This mechanistic candidate is illustrated in Figure 3 and will be expanded upon in the following sections.
Figure 3.
Proposed etiologic model of depression with onset in the menopause transition.
GABA-ergic Neurosteroids in the Menopause Transition
While most studies have focused on the role of estradiol in the risk of perimenopausal depression, evidence from animal models suggests that ovarian hormone fluctuation increases the risk of perimenopausal depression, at least in part, because of the concurrent changes in progesterone-derived neurosteroids. Among the most studied progesterone-derived neurosteroids in humans is ALLO, an A-ring-reduced metabolite of progesterone. ALLO is stress responsive in animals and humans (reviewed in (46)) and serves as a potent, positive allosteric modulator of GABAA receptors via dose-dependent enhancement of GABA-induced Cl-ion channels (47). GABA is the chief inhibitory neurotransmitter in the mammalian central nervous system. The role of GABA in regulating the HPA axis in response to stress by limiting the extent and duration of the HPA axis stress response is well established (48). In part, it is through ALLO's modulation of the GABAA receptor to increase GABAergic transmission that ALLO not only negatively modulates the HPA axis to return it to homeostasis following stress (49), but also exerts profound anxiolytic (50) and antidepressant (51) actions. However, ALLO's anxiolytic properties may also be partially mediated through its effects on the bed nucleus of the stria terminalis, the “relay center” linking stress-responsive pathways such as the HPA axis and limbic structures such as the amygdala (52). Two major sources of ALLO in women of reproductive age are the adrenal glands and the corpus luteum, where ALLO is converted from progesterone (53). Because of ovarian ALLO contributions, ALLO concentrations in premenopausal women are highest in the luteal phase and lowest in the follicular phase (53). However, in postmenopausal women, the adrenal glands become the exclusive source of peripheral ALLO (53). Importantly, peripherally derived ALLO freely crosses the blood-brain barrier (54) and contributes significantly to CNS concentrations (55).
As mentioned earlier, an increasing proportion of anovulatory cycles results in less frequent luteal phases and therefore overall lower levels of progesterone. Although the availability of progesterone is an important determinant of ALLO, estradiol is also likely to positively influence ALLO production through its modulation of the enzymes involved in the conversion of progesterone to ALLO, 5α-reductase and 3α-hydroxysteroid dehydrogenase (56). This is supported by both basic and clinical research. For example, while ovariectomy in animals has been shown to decrease ALLO concentrations in the hippocampus, hypothalamus, pituitary and plasma, transdermal estradiol administration restores pre-ovariectomy brain and plasma ALLO concentrations (see (56) for review). Similarly, transdermal estradiol increases plasma ALLO in postmenopausal women (57, 58). Thus, even in the absence of ovulation and the consequent production of progesterone, intermittent endogenous production of estradiol during the menopause transition may cause fluctuations in the synthesis and release of ALLO.
ALLO fluctuation may have important implications at the GABAA receptor, which is composed of a combination of five (out of 19 existing) subunits. Which subunits a receptor contains will greatly influence its sensitivity to neurosteroids (see (59) for review). For example, the δ subunit has been shown to greatly increase receptor sensitivity to very low concentrations of neurosteroids; mice lacking the δ subunit therefore exhibit greatly reduced neurosteroid sensitivity (60). In contrast, GABAA receptors containing the ε subunit are relatively insensitive to neurosteroids like ALLO. Furthermore, the subunit composition of GABAA receptors is extremely plastic and influenced by neurosteroid levels; for example, during pregnancy when levels of progesterone, and thus progesterone-derived neurosteroids, are extremely elevated, the expression of δ subunits is down-regulated in multiple areas of the brain, thus reducing receptor sensitivity to elevated ALLO levels (61).
This ability of the GABAA receptor to change its composition in response to ALLO concentrations is likely to be especially important in times of considerable ALLO flux. Failure of the GABAA receptor to match its composition to an ever-changing hormonal environment could result in either too-high or too-low GABAergic inhibitory tone. In addition, ovarian hormone fluctuation may actually trigger maladaptive changes in GABAA receptor configuration. Evidence supporting this comes from animal models of puberty, in which ovarian hormone fluctuations have been shown to promote the expression of GABAA receptors containing α4, β2 and δ subunits in rodents, the combination of which has been found to transform ALLO's effects from excitatory to inhibitory at the GABAA receptor (62). Thus, rather than positively modulating the GABAA receptor, ALLO inhibits it. In turn, there is an overall decline in GABAergic inhibitory tone. Furthermore, this reduction in GABAergic tone during puberty is also accompanied by an increase in anxiety, as indicated by less time spent in the open arms of the elevated plus maze and more anxiety behavior following a restraint stress (62). Hormone fluctuation across the estrous cycle (63) or progesterone (and therefore ALLO) withdrawal induced in the laboratory (64) have also been shown to result in similar changes in GABAA receptor subunit expression.
Failure of the GABAA receptor to demonstrate adaptive homeostatic plasticity in the context of steroid hormone fluctuations is theorized to be involved in the development of PMDD and postpartum depression (see (65) for review). This may be one mechanism contributing to decreased saccadic eye velocity (SEV), an indirect measure of GABAA receptor sensitivity, in PMDD women during their symptomatic phase (66). In light of this, we propose that insufficient plasticity of the GABAA receptor in the menopause transition, or maladaptive changes to the GABAA receptor, may contribute to mood disturbance during the menopause transition, when concentrations of both estradiol and progesterone become erratic and unpredictable. How GABAA receptors “respond” to fluctuating ALLO concentrations in the menopause transition would be critical in determining overall GABAergic tone, mood, and, theoretically, regulation of the HPA axis. This process, in which hormonal fluctuation can trigger GABAA subunit changes such that ALLO's effects become paradoxical (inhibitory rather than excitatory at the GABAA receptor; anxiogenic rather than anxiolytic), may shed light on the results of a study by Andreen et al. (67). This study of 36 late peri- and early postmenopausal women treated with progesterone supplementation found that women with resultant medium ALLO concentrations reported significantly more negative mood when compared to those women with low ALLO. The possibility exists that the context of the perimenopausal fluctuating hormonal environment to which these women were exposed had contributed to GABAA receptor subunit changes with consequent alterations in ALLO's effects at the receptor, and in turn, its effects on mood (68).
To the extent that GABAergic dysregulation is involved in perimenopausal depression, genes coding for GABAA receptor subunits may be implicated in predisposing some individuals to respond maladaptively to ALLO fluctuations and thus be at increased risk for perimenopausal depression. GABAA receptor subunit gene polymorphisms are differentially associated with risk for other mental disorders such as alcohol dependence (69), MDD, bipolar disorder and schizophrenia (70). In animal models, mice genetically designed to lack the δ subunit do not exhibit ALLO's paradoxical effects at the GABAA receptor during puberty as do wild-type mice (62). An investigation of which GABAA receptor subunit gene polymorphisms are associated with an increased risk for perimenopausal depression may be warranted. Large-scale epidemiologic studies such as SWAN may provide appropriate specimens for such genotyping.
Although ALLO's effects on the GABAergic system are most well-characterized and thus the primary focus of this review, alterations in overall GABAergic tone are likely to impact the release of other neurotransmitters relevant to the development of psychopathology. For example, recent in vitro studies of rodent hippocampal neurons suggest that ALLO, via presynaptic GABAA receptors, modulates glutamate release (71). ALLO's effects on glutamate may be particularly relevant to the study of perimenopausal depression in light of the recent recognition that glutamate transmission is likely a key player in the etiology of MDD and other psychopathologies (72).
HPA Axis Dysregulation in the Menopause Transition
GABA plays a critical role in regulating the HPA axis and limiting HPA activation following exposure to stress (48). As such, any failure of the GABAA receptor to adapt appropriately to the hormonal environment can be expected to have direct consequences for HPA axis activity. For example, if ALLO were to negatively modulate the GABAA receptor in response to hormonal fluctuation (as opposed to serving as a positive allosteric modulator of the GABAA receptor), as is seen in animal models of puberty, this would contribute to an overall increase in HPA axis activity since the GABAergic inhibition of the HPA axis is removed. Under these conditions, because ALLO increases following stress, this could potentiate HPA axis reactivity and prolong recovery in response to stress. In this way, we speculate that menopause transition-related ovarian hormone fluctuation could trigger HPA axis dysregulation.
Dysregulation of the HPA axis in MDD has frequently been described as one of the most consistent findings in psychiatry (73). However, only in the last decade have we begun to view altered HPA axis activity as a risk factor increasing one's susceptibility to depression rather than a consequence or epiphenomenon of depression (74). This view is not only supported by prospective studies identifying elevated cortisol as a precursor to the onset of first-episode MDD (75) as well as relapse (76), but also supported by studies observing increased HPA axis activation among the euthymic relatives of individuals with a history of depression compared to controls with no family history of depression (77). Research on postpartum depression (78, 79) and PMDD (see (80) for review) also suggest that HPA axis dysregulation has pathophysiological relevance to reproductive mood disorders.
To date, little is known about HPA axis activity in the menopause transition. Komesaroff et al., 1999 (81), examined cortisol reactivity to the Trier Social Stress Test, a speech and arithmetic stressor battery, following eight weeks of oral estradiol or placebo in women during the menopause transition. It was found that estradiol therapy resulted in an attenuated cortisol response to the stressor. Two additional studies have examined basal cortisol levels in the menopause transition. In the Seattle Midlife Women's Health Study, 91 women provided monthly first-morning urine specimens for cortisol measurement as they transitioned across menopausal stages (early to middle; middle to late and late menopause transition to postmenopause) (82). It was found that 68% of the 22 women transitioning from the middle to late menopause transition exhibited an increase in cortisol, the magnitude of which has previously been associated with a decrement in memory performance in older women (83) and may therefore have clinical significance. While the late menopause transition increase in cortisol was not associated with depressive symptoms in this community sample, this study was limited in that it examined basal cortisol and not cortisol reactivity to stress. A second study by Schmidt et al. (84) found no difference in the basal cortisol levels of 24 women with perimenopausal depression when compared to 26 asymptomatic controls. However, again, this study did not examine cortisol reactivity; it also did not account for STRAW stage. Together, these studies suggest that perimenopausal depression may not be associated with alterations in basal HPA axis hormone concentrations but that ovarian hormones do regulate HPA axis responses to stress in women during the menopause transition. There have been no studies examining HPA axis activation in response to stress in women with perimenopausal depression.
Conclusions and Future Directions
Based on emerging evidence from both animal and clinical research, we propose a heuristic model of perimenopausal depression whereby failure of the GABAA receptor to adapt to fluctuations in ALLO over the course of the menopause transition increases the risk of perimenopausal depression in vulnerable women. Specifically (see Figure 3), in the context of the menopause transition, characterized by fluctuations in ALLO that are consequent to estradiol and progesterone fluctuations, an inability of the GABAA receptor to demonstrate plasticity necessary to maintain GABAergic homeostatic control might exacerbate the response of the HPA axis to stress. Combined with an increased vulnerability to MDD due to personality or genetic factors (e.g. in women with a history of MDD) and/or stressful life events proximate to the menopause transition, the endocrine profile of the menopause transition sets the stage for depressive symptomology. While speculative, this model is consistent with studies linking both HPA axis dysregulation (80) and altered GABAA receptor sensitivity (66) to other reproductive mood disorders. Future research investigating this model has the potential to inform the development of novel pharmacological treatments for perimenopausal depression.
While novel, our model remains speculative as there is virtually no research examining these pathways in the menopause transition. However, the risk factors predictive of perimenopausal depression, including sensitivity to hormonal fluctuations and greater psychosocial stress and/or increased sensitivity to stress, are consistent with this model. Furthermore, evidence from other reproductive mood disorders indirectly suggests that neurosteroid and HPA axis dysregulation may be involved in the etiology of perimenopausal depression. However, we wish to acknowledge that our model is by no means comprehensive. There are likely multiple complex down-stream effects of GABAergic and HPA axis dysregulation as well as entirely separate mechanisms involving serotonin, dopamine and norepinephrine that contribute to the etiology of perimenopausal depression and warrant further investigation.
Our intent is that this model will foster research in perimenopausal depression and its etiological mechanisms. Based on our review of the existing literature, we offer the following recommendations for future research: 1) Studies seeking to clarify the mechanisms involved in perimenopausal depression should confirm that depressive symptomology onset coincides with onset of the menopause transition since a differing etiological mechanism(s) may be involved in perimenopausal depression versus MDD with onset at other life stages; 2) Research aimed at detecting an effect of ovarian hormones on perimenopausal depression and/or its underlying mechanisms should measure hormone concentrations frequently, as once annual assessments of hormones may be insufficient to capture the erratic changes in ovarian hormones occurring in the menopause transition; 3) Examination of ovarian hormone and ALLO variability in relation to mood disturbance before and after the treatment of perimenopausal depression with estradiol therapy will help to advance the proposed etiologic model of perimenopausal depression. To the extent that the etiologic model proposed here is predictive of perimenopausal depression, estradiol and ALLO stabilization with estradiol therapy would be expected to predict clinical outcomes. Couched within a placebo-controlled RCT comparing estradiol therapy and an antidepressant medication, such research would further inform clinical decision-making in the treatment of perimenopausal depression.
Acknowledgements
This work was supported by NIH grant RO1-MH087619. Dr. Gordon is also the recipient of a Postdoctoral Fellowship of the Fonds de la Recherche du Québec – Santé (FRQS). Dr. Clark is supported in part by Grant Number K12 HD055884 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Conflict of Interest Disclosures
Dr. Gordon reports receiving a Pilot Project Grant from the North Carolina Translational and Clinical Sciences Institute; previously she was the recipient of a Canadian Institutes of Health Research (CIHR) Vanier Canada Graduate Scholarship. Dr. Meltzer-Brody has received grant funding from AstraZeneca, the Foundation of Hope, and Sage Pharmaceuticals. Dr. Moses-Kolko has received support from grant HD-067185 from the National Institute of Child Health and Human Development. Dr. Joffe receives research support from Cephalon/Teva, serves as consultant to Noven and Merck, and participates in an advisory board for Merck. Dr. Wisner serves as consultant to Quinn Emanuel Urquhart & Sullivan, L.L.P., which legally represents Pfizer Pharmaceutical Company; the Department of Psychiatry at Northwestern University receives contractual fees for Dr. Wisner's consultation to Quinn Emanuel Urquhart & Sullivan. The other authors report no financial relationships with commercial interests.
References
- 1.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Frontiers in Neuroendocrinology. 2008;29(4):507–19. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredemann TM, McMahon LL. 17β Estradiol Increases Resilience and Improves Hippocampal Synaptic Function in Helpless Ovariectomized Rats. Psychoneuroendocrinology. 2014;42(4):77–88. doi: 10.1016/j.psyneuen.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology. 2012;37(3):372–82. doi: 10.1016/j.psyneuen.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barha CK, Dalton GL, Galea LA. Low Doses of 17 alpha-Estradiol and 17 bold italic beta-Estradiol Facilitate, Whereas Higher Doses of Estrone and 17 alpha-and 17 bold italic beta-Estradiol Impair, Contextual Fear Conditioning in Adult Female Rats. Neuropsychopharmacology. 2010;35:547–59. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop+ 10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105–14. doi: 10.3109/13697137.2011.650656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 8.Tepper PG, Randolph JF, Jr, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, Gold EB, Zheng H, Bromberger JT, Sutton-Tyrrell K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women's Health across the Nation (SWAN). The Journal of Clinical Endocrinology & Metabolism. 2012;97(8):2872–80. doi: 10.1210/jc.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hale GE, Robertson DM, Burger HG. The perimenopausal woman: Endocrinology and management. The Journal of Steroid Biochemistry and Molecular Biology. 2014;142(7):121–31. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Randolph JF., Jr Reproductive hormones and the menopause transition. Obstetrics and Gynecology Clinics of North America. 2011;38(3):455. doi: 10.1016/j.ogc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 12.Weissman MM. The myth of involutional melancholia. Jama. 1979;242(8):742–4. [PubMed] [Google Scholar]
- 13.Kaufert PA, Gilbert P, Tate R. The Manitoba Project: a re-examination of the link between menopause and depression. Maturitas. 1992;14(2):143–55. doi: 10.1016/0378-5122(92)90006-p. [DOI] [PubMed] [Google Scholar]
- 14.McKinlay JB, McKinlay SM, Brambilla D. The relative contributions of endocrine changes and social circumstances to depression in mid-aged women. Journal of Health and Social Behavior. 1987;28(4):345–63. [PubMed] [Google Scholar]
- 15.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers M, Randolph JF., Jr Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN). Journal of Affective Disorders. 2007;103(1):267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Archives of General Psychiatry. 2004;61(1):62. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 17.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Menopause. 2008;15(2):223–32. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 18.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Archives of General Psychiatry. 2006;63(4):385. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 19.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006;63(4):375. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. American Journal of Psychiatry. 2004;161(12):2238–44. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 21.Bromberger JT, Kravitz HM, Chang Y-F, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN). Psychological Medicine. 2011;41(9):1879–88. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromberger J, Kravitz H, Matthews K, Youk A, Brown C, Feng W. Predictors of first lifetime episodes of major depression in midlife women. Psychological Medicine. 2009;39(1):55. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression Results from the Massachusetts women's health study. Annals of Epidemiology. 1994;4(3):214–20. doi: 10.1016/1047-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 24.Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harvard review of psychiatry. 2009;17(2):72–86. doi: 10.1080/10673220902899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry. 2000;157(6):924–30. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New England Journal of Medicine. 1998;338(4):209–16. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 27.Freeman EW, Sammel MD, Lin H, Gracia CR, Pien GW, Nelson DB, Sheng L. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstetrics & Gynecology. 2007;110(2, Part 1):230–40. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 28.Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71(1):36–43. doi: 10.1001/jamapsychiatry.2013.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Novaes Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry. 2001;58(6):529. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. American Journal of Obstetrics and Gynecology. 2000;183(2):414–20. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 31.Joffe H, Petrillo LF, Koukopoulos A, Viguera AC, Hirschberg A, Nonacs R, Somley B, Pasciullo E, White DP, Hall JE. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):E1044–E54. doi: 10.1210/jc.2010-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen LS, Soares CN, Poitras JR, Prouty J, Alexander AB, Shifren JL. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. American Journal of Psychiatry. 2003;160(8):1519–22. doi: 10.1176/appi.ajp.160.8.1519. [DOI] [PubMed] [Google Scholar]
- 33.Morrison MF, Kallan MJ, Ten Have T, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biological Psychiatry. 2004;55(4):406–12. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Biegon A, Fischette CT, Rainbow T, McEwen B. Serotonin receptor modulation by estrogen in discrete brain nuclei. Neuroendocrinology. 1982;35(4):287–91. doi: 10.1159/000123396. [DOI] [PubMed] [Google Scholar]
- 35.Biegon A, Reches A, Snyder L, McEwen BS. Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones. Life sciences. 1983;32(17):2015–21. doi: 10.1016/0024-3205(83)90053-x. [DOI] [PubMed] [Google Scholar]
- 36.Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33(1):3–17. doi: 10.1016/j.psyneuen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 37.De Leo V, Lanzetta D, Morgante G, De Palma P, D'Antona D. Inhibition of ovulation with transdermal estradiol and oral progestogens in perimenopausal women. Contraception. 1997;55(4):239–43. doi: 10.1016/s0010-7824(97)00006-1. [DOI] [PubMed] [Google Scholar]
- 38.De Leo V, Lanzetta D, Antona D, De Palma P. Transdermal estrogen replacement therapy in normal perimenopausal women: effects on pituitary-ovarian function. Gynecological Endocrinology. 1996;10(1):49–53. doi: 10.3109/09513599609041270. [DOI] [PubMed] [Google Scholar]
- 39.Girdler SS, Hinderliter AL, Wells EC, Sherwood A, Grewen KM, Light KC. Transdermal versus oral estrogen therapy in postmenopausal smokers: hemodynamic and endothelial effects. Obstetrics & Gynecology. 2004;103(1):169–80. doi: 10.1097/01.AOG.0000103998.48122.0b. [DOI] [PubMed] [Google Scholar]
- 40.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17(4):823–7. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Annals of the New York Academy of Sciences. 2009;1179(1):70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avis NE, Crawford S, Stellato R, Longcope C. Longitudinal study of hormone levels and depression among women transitioning through menopause. Climacteric. 2001;4(3):243–9. [PubMed] [Google Scholar]
- 43.Daly RC, Danaceau MA, Rubinow DR, Schmidt PJ. Concordant restoration of ovarian function and mood in perimenopausal depression. American Journal of Psychiatry. 2003;160(10):1842–6. doi: 10.1176/appi.ajp.160.10.1842. [DOI] [PubMed] [Google Scholar]
- 44.Rubinow DR, Schmidt PJ, Roca CA. Estrogen–serotonin interactions: implications for affective regulation. Biological Psychiatry. 1998;44(9):839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 45.Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biological Psychiatry. 1998;44(9):798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 46.Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology. 2014:1–16. doi: 10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. European Journal of Pharmacology. 1987;142(3):483–5. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- 48.Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Structure and Function. 2008;213(1-2):63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- 49.Guo A, Petraglia F, Criscuolo M, Ficarra G, Nappi R, Palumbo M, Trentini G, Purdy R, Genazzani A. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecological Endocrinology. 1995;9(1):1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- 50.Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. Journal of Neuroendocrinology. 1995;7(3):171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 51.Rodrìguez-Landa JF, Contreras CM, Bernal-Morales B, Gutièrrez-Garcìa AG, Saavedra M. Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. Journal of Psychopharmacology. 2007;21(1):76–84. doi: 10.1177/0269881106064203. [DOI] [PubMed] [Google Scholar]
- 52.Toufexis DJ, Davis C, Hammond A, Davis M. Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. The Journal of neuroscience. 2004;24(45):10280–7. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating Levels of Allopregnanolone in Humans: Gender, Age, and Endocrine Influences. The Journal of Clinical Endocrinology & Metabolism. 1998;83(6):2099–103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- 54.Paul SM, Purdy R. Neuroactive steroids. The FASEB Journal. 1992;6(6):2311–22. [PubMed] [Google Scholar]
- 55.Purdy RH, Morrow AL, Moore PH, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of the National Academy of Sciences. 1991;88(10):4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pluchino N, Cubeddu A, Giannini A, Merlini S, Cela V, Angioni S, Genazzani A. Progestogens and brain: an update. Maturitas. 2009;62(4):349–55. doi: 10.1016/j.maturitas.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 57.Pluchino N, Genazzani A, Bernardi F, Casarosa E, Pieri M, Palumbo M, Picciarelli G, Gabbanini M, Luisi M, Genazzani A. Tibolone, transdermal estradiol or oral estrogen-progestin therapies: effects on circulating allopregnanolone, cortisol and dehydroepiandrosterone levels. Gynecological Endocrinology. 2005;20(3):144–9. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- 58.Bernardi F, Pieri M, Stomati M, Luisi S, Palumbo M, Pluchino N, Ceccarelli C, Genazzani A. Effect of different hormonal replacement therapies on circulating allopregnanolone and dehydroepiandrosterone levels in postmenopausal women. Gynecological Endocrinology. 2003;17(1):65–77. [PubMed] [Google Scholar]
- 59.MacKenzie G, Maguire J. The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology. 2014:1–10. doi: 10.1007/s00213-013-3423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proceedings of the National Academy of Sciences. 1999;96(22):12905–10. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maguire J, Mody I. GABA <sub> A</sub> R Plasticity during Pregnancy: Relevance to Postpartum Depression. Neuron. 2008;59(2):207–13. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nature Neuroscience. 2007;10(4):469–77. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lovick T, Griffiths J, Dunn S, Martin I. Changes in GABA< sub> A</sub> receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131(2):397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Griffiths J, Lovick T. Withdrawal from progesterone increases expression of α4, β1, and δ GABAA receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. Journal of Comparative Neurology. 2005;486(1):89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- 65.Maguire J, Mody I. Steroid hormone fluctuations and GABA <sub> A</sub> R plasticity. Psychoneuroendocrinology. 2009;34:S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sundström I, Bäckström T. Patients with premenstrual syndrome have decreased saccadic eye velocity compared to control subjects. Biological Psychiatry. 1998;44(8):755–64. doi: 10.1016/s0006-3223(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 67.Andréen L, Sundström-Poromaa I, Bixo M, Andersson A, Nyberg S, Bäckström T. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology. 2005;30(2):212–24. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABA< sub> A</sub> modulators. Psychoneuroendocrinology. 2009;34(8):1121–32. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Soyka M, Preuss U, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. Journal of psychiatric research. 2008;42(3):184–91. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Fatemi S, Folsom T, Rooney R, Thuras P. Expression of GABAA α2-, β1-and ϵ-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Translational psychiatry. 2013;3(9):e303. doi: 10.1038/tp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwata S, Wakita M, Shin M-C, Fukuda A, Akaike N. Modulation of allopregnanolone on excitatory transmitters release from single glutamatergic terminal. Brain research bulletin. 2013;93:39–46. doi: 10.1016/j.brainresbull.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62(1):63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior. 2003;43(1):60–6. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 74.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Goodyer IM, Tamplin A, Herbert J, Altham P. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. The British Journal of Psychiatry. 2000;177(6):499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- 76.Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJ, Tijssen JG, Wiersinga WM, Schene AH. Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression). Biological Psychiatry. 2006;59(8):696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Mannie Z, Harmer C, Cowen P. Increased waking salivary cortisol levels in young people at familial risk of depression. American Journal of Psychiatry. 2007;164(4):617–21. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- 78.Magiakou M, Mastorakos G, Rabin D, Dubbert B, Gold P, Chrousos G. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. Journal of Clinical Endocrinology & Metabolism. 1996;81(5):1912–7. doi: 10.1210/jcem.81.5.8626857. [DOI] [PubMed] [Google Scholar]
- 79.Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. Journal of Clinical Endocrinology & Metabolism. 2005;90(2):695–9. doi: 10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- 80.Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacology & Therapeutics. 2007;116(1):125–39. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. Journal of Clinical Endocrinology & Metabolism. 1999;84(2):606–10. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- 82.Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopause transition. Menopause. 2006;13(2):212–21. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- 83.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in Urinary Cortisol Excretion and Memory Declines: MacArthur Studies of Successful Aging 1. Journal of Clinical Endocrinology & Metabolism. 1997;82(8):2458–65. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt P, Murphy J, Haq N, Danaceau M, St Clair LS. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. 2002;27(8):907–20. doi: 10.1016/s0306-4530(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 85.O'Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, Weinstein M. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16(6):1178. doi: 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]